Abstract
Absent a remission of proteinuria, primary membranous nephropathy (MN) can lead to ESRD over many years. Therefore, use of an earlier end point could facilitate the conduct of clinical trials. This manuscript evaluates complete remission (CR) and partial remission (PR) of proteinuria as surrogate end points for a treatment effect on ESRD in patients with primary MN with heavy proteinuria. CR is associated with a low relapse rate and excellent long–term renal survival, and it plausibly reflects remission of the disease process that leads to ESRD. Patients who achieve PR have better renal outcomes than those who do not but may have elevated relapse rates. How long PR must be maintained to yield a benefit on renal outcomes is also unknown. Hence, available data suggest that CR could be used as a surrogate end point in primary MN, whereas PR seems reasonably likely to predict clinical benefit. In the United States, surrogate end points that are reasonably likely to predict clinical benefit can be used as a basis for accelerated approval; treatments approved under this program must verify the clinical benefit in postmarketing trials. Additional analyses of the relationship between treatment effects on CR and PR and subsequent renal outcomes would inform the design of future clinical trials in primary MN.
Keywords: glomerular disease, membranous nephropathy, nephrotic syndrome, outcomes, surrogate outcomes
Primary membranous nephropathy (MN) is an important cause of nephrotic syndrome in adults, and it is associated with significant morbidity, including the progressive loss of renal function, leading to ESRD.
Primary MN affects patients of all ages and races, but it affects men more commonly than women and adults more commonly than children, with a peak incidence occurring during the fourth and fifth decades of life; 70%–80% of patients with MN present with nephrotic syndrome (proteinuria ≥3.5 g/24 h per 1.73 m2, hypoalbuminemia, hyperlipidemia, and edema).1,2 The remaining 20%–30% of patients present with subnephrotic levels of proteinuria (<3.5 g/24 h) and are usually asymptomatic.3,4
Primary MN has a variable course ranging from spontaneous remission to progressive loss of GFR over several years. Studies of the natural course (without immunotherapy) of idiopathic MN reveal that spontaneous remissions occur in approximately one third of patients, most commonly within the first 2 years of diagnosis.5–9 Although patients who attain a spontaneous remission typically have a good long–term prognosis, patients with heavy and persistent proteinuria are at high risk of progressive loss of renal function, leading to ESRD. In the absence of remission of proteinuria to at least subnephrotic levels, ESRD occurs in about 25% of patients by 8 years and 50% of patients by 10–15 years.9
Given the extended time frame for progression to ESRD, there has been great interest in identifying an earlier end point that could be used in clinical trials to establish the efficacy of therapies for patients with primary MN who are at high risk of developing ESRD. In April of 2012, members of the American Society of Nephrology Glomerular Disease Advisory Group met with members of the US Food and Drug Administration (FDA) to discuss challenges associated with establishing proteinuria as a surrogate end point (a biomarker intended to substitute for a clinical efficacy end point) in drug trials for glomerular diseases. There was general agreement that members from both groups should work together to produce white papers focused on the data supporting proteinuria reduction as a surrogate end point within the context of a specific glomerular disease. This manuscript represents the first of these white papers and evaluates complete remission (CR) and partial remission (PR) of proteinuria as surrogate end points for a treatment effect on ESRD prevention in patients with primary MN at high risk of progression.
Establishing the Effectiveness of Therapies
Before approval in the United States, therapies must be shown to be effective for their intended use. Effectiveness can be established by showing an effect on a clinically meaningful end point or surrogate end point. Clinically meaningful end points include clinical outcomes, such as mortality, need for dialysis, or hospitalization, as well as end points that capture effects on the important symptoms or functional impairments associated with a disease. Surrogate end points are not direct measures of clinical benefit, but instead, they are substitutes for these direct measures. A treatment’s effect on a surrogate end point is expected to predict its effect on a clinically meaningful outcome.
In the United States, surrogate end points that are expected to predict a treatment’s effect can be used as a basis for full approval of a new therapy. Examples of such surrogate end points include BP reduction (used as the basis for approving antihypertensive agents) and doubling of serum creatinine (used as the basis for approving therapies for diabetic kidney disease). BP reduction is accepted as a valid surrogate end point on the basis of the results of placebo–controlled cardiovascular outcome trials, which showed that lowering BP with antihypertensive agents from a variety of pharmacologically distinct drug classes reduced the risk of cardiovascular events. A doubling of serum creatinine is accepted as a valid surrogate end point, because GFR decline is an intermediate step on the pathway to ESRD and because creatinine doubling represents a marked loss of kidney function that is highly predictive of the subsequent development of kidney failure.
In addition to surrogate end points that are used as a basis for full approval of a new therapy, surrogate end points that are deemed reasonably likely to predict a clinical benefit can be used as the basis for accelerated approval of therapies developed to treat serious or life-threatening illnesses when they provide a meaningful therapeutic benefit over existing therapies. Because of the uncertainty about the ability of the surrogate to predict the treatment’s effect on the clinical outcome, postmarketing confirmatory trials are required to verify and describe the clinical benefit of drugs approved under this program.
Considerations Related to the Evaluation of Surrogate End Points
There is no single agreed–on approach for evaluating candidate surrogates. Three factors are generally considered.
(1) Biologic plausibility that what is measured by the candidate surrogate is on the causal pathway to the hard outcome.
(2) Epidemiologic or other data showing a strong and consistent association between the candidate surrogate and the clinical outcome of interest.
(3) Data from interventional trials showing that the effects of various treatments on the candidate surrogate largely account for their effects on the clinical outcome.
Although data that support biologic plausibility and show that an association between the candidate surrogate and outcome is important, such data are generally insufficient to define a surrogate end point; some candidate surrogates that have performed well as prognostic indicators have not performed well in predicting the effect of a treatment on clinical outcomes. For example, lower hemoglobin levels are associated with an increased risk of cardiac disease and mortality in patients with CKD. However, when erythropoiesis-stimulating agents were used to achieve higher versus lower hemoglobin levels in clinical trials, more deaths, strokes, and serious adverse cardiovascular events were seen in patients randomized to higher versus lower hemoglobin targets.10–13 Hence, there is the greatest confidence in a candidate surrogate when there are data showing that drugs (or other interventions) that affect the surrogate also affect the clinical outcome of interest.
Evaluation of Proteinuria Reduction as a Surrogate End Point in Primary MN
Does Proteinuria Alone Contribute to Renal Damage and Decline in GFR?
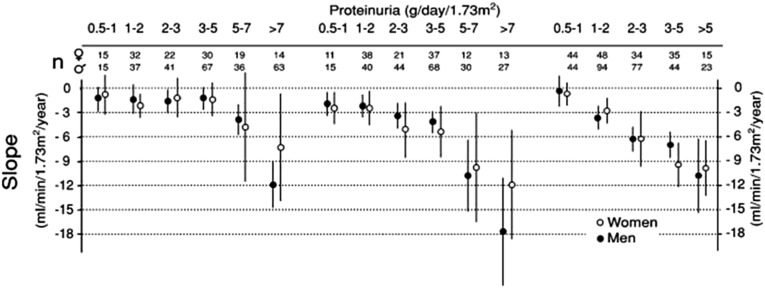
The data supporting a causal role of proteinuria in the progressive loss of renal function are not specific for primary MN. All glomerular diseases, including primary MN, show progressive degrees of interstitial fibrosis and tubular atrophy that parallel glomerular obsolescence and decline in renal function. In vitro and animal in vivo studies suggest that proteinuria contributes to tubulointerstitial injury through various possible mechanisms, including the expression of vasoconstrictive, proinflammatory, or profibrotic molecules14–18 and tubular activation of the complement cascade through the alternative pathway19,20 (reviewed in ref. 21). However, as shown in Figure 1,22 differences among diseases in the level of sustained proteinuria associated with a decline in GFR have been noted, suggesting that factors other than quantity alone must play a role in the relationship between proteinuria and progressive loss of kidney function. Overall, available data suggest that proteinuria contributes to tubulointerstitial injury. Whether proteinuria itself, its molecular composition, severity, and duration directly cause loss of renal function specifically in humans with primary MN remains unknown. Given the limitations of the data supporting the causal role of proteinuria in the progression of kidney disease in primary MN, it is particularly important to look to other sources of data, such as observational studies and interventional trials, when evaluating proteinuria reduction as a surrogate end point in this disease.
Figure 1.
Relation between time-average proteinuria and the rate of renal function decline in MN, FSGS, and IgA nephropathy. Declines in renal function are associated with higher levels of sustained proteinuria in patients with MN (where a decline is observed at levels of sustained proteinuria >5 g/d) than in patients with FSGS (where a decline in renal function is observed with sustained proteinuria around 2–3 g/d) or patients with IgA nephropathy (where a decline in renal function is observed with sustained proteinuria >1 g/d). Thus, the severity of proteinuria alone does not fully explain the rate of loss of renal function, and other disease–specific factors likely play a role. Black circles, men; white circles, women. Reprinted from ref. 22, with permission.
Severity of Proteinuria at Baseline and Its Duration Are Associated with Future Decline in GFR
Observational cohort analyses have consistently shown an association between both the level (severity) and the duration of proteinuria and long-term prognosis. Overall, patients presenting with subnephrotic proteinuria (<3.5 g/d), especially those whose proteinuria never exceeds this subnephrotic range, have a significantly lower risk of progression as defined by a 50% reduction in renal function over 10 years compared with patients presenting with greater degrees of proteinuria.3 In a cohort of 395 patients with primary MN from the Toronto Glomerulonephritis Registry, 287 patients presented with nephrotic-range proteinuria (median=7.4 g/d; range=3.6–31.3) and experienced a mean rate of decline in creatinine clearance of −4.8±9.9 ml/min per year.3 In contrast, 42 patients who presented and remained with subnephrotic proteinuria throughout the duration of follow-up experienced a significantly lower rate of decline in creatinine clearance of −0.9±4.3 ml/min per year, and only 2 patients (5%) suffered a 50% reduction in renal function over 10 years of follow-up.3 The 66 patients who presented with subnephrotic proteinuria but whose proteinuria later increased above 3.5 g/d had a rate of decline in creatinine clearance of −3.5±7.5 ml/min per year—a significantly higher rate of decline than the never nephrotic group but similar to that of patients with nephrotic-range proteinuria at presentation. Approximately 12% experienced a 50% decline in renal function over 10 years compared with approximately 35% of those who presented with nephrotic-range proteinuria at baseline.
Another analysis of the Toronto Glomerulonephritis Registry data performed on 395 patients with MN followed for a minimum of 12 months (mean of 60 months) examined the association between time-averaged proteinuria and the subsequent loss of renal function. For each patient, the average proteinuria level was determined for each 6-month period of follow-up; the time–averaged proteinuria level for a given patient represented the average of every period’s mean.22 In this study, levels of time-averaged proteinuria >4–5 g/d were associated with a more rapid rate of decline in renal function as measured by the slope of creatinine clearance (Figure 1). In contrast, with lower levels of time-averaged proteinuria (averaging from 0.5 to 3–5 g/d), no such relationship was detected (slope of creatinine clearance averaging −1.5 ml/min per year), suggesting a good renal prognosis. These results were independent of sex, BP, and age. Hence, the findings from this analysis emphasize the importance of duration of proteinuria as well as its severity on the risk and rate of decline of renal function. They also suggest that a threshold may exist for the relationship between the level of sustained proteinuria over time and subsequent renal outcomes as judged by the rate of decline in renal function in primary MN.
The interaction between the severity and the duration of proteinuria and progression of renal disease in patients with primary MN is further illustrated by the findings from the Toronto Glomerulonephritis Registry that persistent proteinuria ≥8 g/d for ≥6 months, ≥6 g/d for ≥9 months, or ≥4 g/d for ≥18 months were associated with an increased risk of chronic renal insufficiency defined as a creatinine clearance <60 ml/min per 1.73 m2.23
Finally, the effect of duration of proteinuria on progression of kidney disease is also described in a long–term follow-up analysis of a controlled trial of chlorambucil plus glucocorticoids versus supportive care.24 In this study, treated patients were free from nephrotic syndrome for a significantly greater proportion of the follow-up time than patients in the supportive care group (the ratio of months in remission to total months of follow-up: 0.58 and 0.22, respectively; P<0.001) and had a significantly greater probability of dialysis-free survival at 10 years (92% versus 60%; P=0.004) and a slower rate of decline in GFR.
CR and PR as End Points in Primary MN
In clinical trials to date, treatment success in primary MN has often been defined by a reduction in proteinuria and specifically, the achievement of a CR or PR of proteinuria. A CR in primary MN has been defined as achieving a (essentially) normal level of urinary protein excretion of ≤0.3 g/d or ≤0.2 g/d with a stable GFR.25 The definition of a PR in primary MN has varied over time and across studies. In the past, a PR had been defined as a reduction in proteinuria to below a certain threshold (e.g., <2 but >0.2 g/d).25–28 More recently however, characterizing both the percentage of change and absolute level of proteinuria was found to be a better predictor of long–term renal outcome than either component alone. Thus, in recent studies, a PR has been defined as a 50% reduction in baseline proteinuria and a level <3.5 g/d with a stable GFR.29–31 A stable GFR is usually defined as a GFR that remains unchanged or declines by <15%.32 Except where noted, these definitions are used in the following discussion, which focuses on data from large observational cohort studies and clinical trials linking CR and PR with long–term renal survival and preservation of renal function.
CR and Progression to ESRD
There is strong evidence that patients with nephrotic primary MN who experience a CR have a favorable long–term prognosis.33,34 In the retrospective analysis of 348 patients who were nephrotic with primary MN from the Toronto Glomerulonephritis Registry,30 102 patients attained a CR either spontaneously or with treatment in a mean of 30 months (range=2–195 months). Of these, none reached ESRD over a median follow-up of 87 months (range=14–400 months), and the average slope of decline in creatinine clearance was −0.12±0.4 ml/min per month. These long–term outcome results are better than for the group of patients who attained a PR. In the PR group (n=136), 9% developed ESRD over a median follow-up of 67 months (range=12–376), and the average slope of decline in creatinine clearance was −0.17±0.5 ml/min per month. In comparison, 29% of patients who had no remission (n=110) developed ESRD over a median follow-up of 34 months (range=12–327), and the average slope of decline in creatinine clearance in this group was −0.86±1.1 (P<0.001 for the comparison of slope of decline in creatinine clearance between the PR and no response groups).
In a retrospective multicenter Spanish cohort study of 328 patients with primary MN and nephrotic syndrome (defined as proteinuria >3.5 g/d and hypoalbuminemia <3 g/dl), a spontaneous CR (defined as proteinuria <0.3 g/d in the absence of immunosuppressive therapy or decline in renal function) occurred in 52 (16%) patients over a mean of 39 months (range=4–120 months).9 Of these, none reached ESRD over a mean follow-up of 104 months.
CR and Relapse
Patients in CR may have a recurrence of proteinuria to subnephrotic levels (<3.5 g/d) or experience a recurrence of nephrotic-range proteinuria (>3.5 g/d) with or without full nephrotic syndrome. Analyses of observational studies and clinical trials suggest a low rate of relapse to nephrotic levels of proteinuria in patients who achieve a CR. In the Toronto Glomerulonephritis Registry, relapse to nephrotic-range proteinuria occurred in 23% of 102 patients in CR, with a median time to relapse of 25 (2–164) months.30 In clinical trials, although relapses from CR to subnephrotic-range proteinuria may be common, full relapses from CR to nephrotic-range proteinuria seem to be infrequent. For example, in the study comparing treatment with cyclophosphamide and corticosteroids with adrenocorticotrophic hormone (ACTH),29 20% of patients treated with cyclophosphamide and 30% of patients treated with ACTH in CR had a subnephrotic relapse, but no patient had a relapse to nephrotic-range proteinuria. For patients in CR, the long-term implications of a small increase in proteinuria to subnephrotic levels have not been studied. Whether such relapses from CR to a state of PR significantly affect the likelihood of progression to ESRD is, therefore, unknown.
PR and Progression to ESRD
A PR also represents a marked change in proteinuria, and available data suggest that patients who achieve a PR have improved renal outcomes compared with those who do not achieve a PR. Using the definition of PR that includes a 50% reduction in baseline proteinuria and a level of <3.5 g/d, patients who achieve PR seem to have better kidney survival and an improved slope of creatinine clearance reduction compared with patients who had no remission.30 In the retrospective analysis of 348 patients with MN from the Toronto Glomerulonephritis Registry, the slope of change in creatinine clearance was −0.17±0.50 ml/min per month in the PR group and −0.86±1.1 ml/min per month in the no remission group (P<0.001).30 In addition, the rate of decline in renal function was slower after attaining a PR compared with the period before the PR (slope of decline creatinine clearance of −0.44±0.8 ml/min per month before PR and −0.16±0.7 ml/min per month after attaining PR; n=79; P=0.03).
In a complementary analysis of the same cohort,30 the patient factors associated with improved long–term outcomes were evaluated by univariate and multivariate analyses. Cox proportional hazard regression identified creatinine clearance and proteinuria at baseline, PR, and CR as the only independent predictors of dialysis-free survival. In renal survival analysis, the adjusted hazard ratio (HR) for PR expressed as a time-dependent variable was 0.17 (95% confidence interval [95% CI], 0.09 to 0.33; P<0.001) by univariate analysis and 0.08 (95% CI, 0.03 to 0.19; P<0.001) by multivariate analysis in reference to no remission. In this study, the HR for CR could not be calculated, because no patient in CR reached ESRD.
In the retrospective multicenter Spanish cohort study of 328 patients with primary MN and nephrotic syndrome,9 the long-term outcomes of patients who attained only a PR were not significantly different than those who reached a CR. In that study, PR was defined as proteinuria <3.5 g/d with normal serum albumin in the absence of immunosuppressive therapy or decline in renal function. Compared with patients who did not achieve a spontaneous remission, both groups (CR and PR) had significantly lower rates of ESRD (0% versus 18.7%; P<0.001) and death (1.9% versus 10.7%; P<0.01) over mean follow-ups of 91 and 69 months, respectively.
Additional supportive data are provided by a recent review of 100 patients with primary MN treated with rituximab.35 In this study, eGFR remained stable among patients who achieved a PR or CR (slopes of change in eGFR of 0.07 and 0.08 ml/min per 1.73 m2 per month, respectively), whereas it declined in those without remission with a slope of −0.61 ml/min per 1.73 m2 per month (P=0.001 versus PR).
PR and Relapse
For patients in PR, a relapse is often defined by an increase in proteinuria to ≥3.5 g/d. Rates of relapse after achievement of PR are variable and have been shown to be quite high with some therapies.
In the Toronto Glomerulonephritis Registry, relapses occurred in 46% of 136 patients in PR, with a median time to relapse in the PR group of 8 months (1–147 months).30 Of 62 patients in PR who relapsed, 29 patients had a second remission, and 17 of these (17 of 29) had multiple remissions and relapses. In contrast, in the Spanish cohort study, 6% of patients with a spontaneous PR had a relapse to nephrotic-range proteinuria during follow-up. The mean duration of follow-up for these 52 patients was not reported; however, mean follow-up for patients in CR and PR was 91 months.9 In a recent retrospective analysis of 122 patients with primary MN treated with tacrolimus, patients in PR at the time of the start of the taper had a higher frequency of relapse and a significantly shorter time to relapse than patients in CR (8.5 [3–26] versus 20 months [6–62]; P=0.02). By multivariate analysis, a PR at the onset of tacrolimus taper was independently associated with an increased risk of relapse (HR, 16.58; 95% CI, 1.1 to 249.1; P=0.04).36
Because a longer duration of proteinuria is associated with a poorer renal outcome, both the frequency of relapse and the time in nephrotic proteinuria (time in nonremission) are expected to be correlated with the likelihood of ESRD. For this reason, an analysis of the frequency of relapse and duration in nonremission is likely to provide valuable information on the relationship between the duration of proteinuria and long–term renal survival. In another analysis from the Toronto Glomerulonephritis Registry, patients in PR who never relapsed had a significant improvement in their rate of change of creatinine clearance after remission, which is in contrast to those who relapsed (slope before versus after: −0.40±0.7 and 0.02±0.4 ml/min per month [n=39; P<0.01; paired t test for patients who did not relapse] compared with −0.47±0.9 and −0.34±0.8 ml/min per month, respectively [n=40; NS; paired t test for patients who relapsed]).30
Association of CR and PR with Renal Survival in Interventional Clinical Trials
Numerous interventional clinical studies have been conducted in primary MN (recent review by Chen et al.37). Although most studies reported on the frequency of CR, PR, or CR and PR in response to therapy, many enrolled a very small number of patients (≤20) and/or were of short duration (≤2 years), making them unsuitable to assess the effect of attaining these end points on the occurrence of death, ESRD, or a marked reduction in GFR. For the purpose of this manuscript, 13 clinical studies (11 randomized controlled trials and 2 uncontrolled studies) were reviewed, representing the major proposed therapies for primary MN (corticosteroids, cyclophosphamide, calcineurin inhibitors, ACTH, mycophenolate mofetil, and rituximab). There was marked variability among these studies with respect to the duration of follow-up, with only 11 studies reporting on outcomes beyond 2 years and 7 studies reporting on outcomes ≥3 years.26,27,35,38–41 When possible, these trials compared the treatment arms with respect to the frequency of a 25% or 50% decline in eGFR, ESRD, or death; however, none of the trial publications reported the frequency of these long-term outcomes in relationship to the occurrence of CR, PR, or CR and PR.
Effect of Therapy on CR, PR, or CR and PR and Association with Long-Term Outcome
To date, two major randomized, controlled trials have reported on the outcomes of patients 10 years after treatment.24,26,40 Both studies compared treatment with an alkylating agent (chlorambucil22 or cyclophosphamide35) and corticosteroids with supportive therapy. The trial publications do not discuss the relationship between early treatment effects on CR and/or PR and late treatment effects on long–term renal outcomes; however, data contained within these publications suggest that a relationship exists.
In the trial by Ponticelli et al.,24,26 treatment was associated with CR rates of 29% and 43% at 1 and 2 years, respectively, compared with 0% in the supportive therapy group. For PR, the corresponding rates were 26% and 31% at 1 and 2 years, respectively, in the treatment group compared with 8% and 18% at 1 and 2 years, respectively, in the supportive therapy group.26 At the last follow-up visit, 62% (95% CI, 46% to 76%) of patients in the treatment group were in CR or PR compared with only 33% in the supportive therapy group (95% CI, 19% to 50%).24 These results were associated with a 10-year probability of dialysis-free survival of 92% (95% CI, 83% to 100%) in the treatment group versus 60% (95% CI, 42% to 0.78%) in the supportive care group (P=0.004).24 The study by Jha et al.40 reported CR rates in the treatment group of approximately 11% and 28% at 1 and 2 years, respectively, and CR and PR rates of approximately 34% and 53% at 1 and 2 years, respectively. In the supportive care group, CR rates were approximately 5% at both 1 and 2 years, and CR and PR rates were approximately 8% and 10% at 1 and 2 years, respectively. In this study as well, treatment was associated with an improved 10-year probability of dialysis-free survival in the treatment group (89%) compared with the supportive therapy group (65%; P=0.02).
These data suggest that treatment effects on CR and/or PR seen at 1 and 2 years are associated with and may predict a treatment’s effect on long–term renal survival. Additional analyses of the data from these trials and data from trials of agents with other mechanisms of action would greatly enhance our understanding of CR and PR as surrogate end points.
Summary of Findings
(1) Low-level proteinuria in primary MN is associated with a reduced likelihood of renal failure and a slower rate of decline in GFR. There seems to be a threshold of sustained proteinuria of 4–5 g/d, above which the rate of decline in GFR significantly increases.
(2) Achieving a CR, defined as proteinuria ≤0.3 g/d with a stable GFR, is associated with a favorable long–term renal prognosis and a low risk of relapse.
(3) PR, defined as >50% reduction in proteinuria and proteinuria <3.5 g/d with a stable GFR, is associated with a slower rate of decline in GFR compared with the rate of decline without remission. The rate of relapse after PR is higher than after CR.
(4) A longer duration of PR (as measured by the percentage of follow-up time in PR) is associated with a higher likelihood of renal survival compared with patients without a remission.
(5) Available analyses of clinical trial data suggest that treatment effects on CR and/or PR at 1 and 2 years may predict a treatment’s effect on long–term renal survival; however, additional analyses of these data are needed.
Conclusions
Primary MN has a variable course, but data show that subjects with heavy and persistent proteinuria are at high risk of progressive loss of renal function, leading to ESRD.
CR as an End Point in Primary MN
In patients with primary MN who have heavy and persistent proteinuria, there is strong biologic plausibility that a CR signals remission of the active disease process that leads to the development of ESRD. Analyses to date also suggest that CR is associated with a low relapse rate and excellent long–term renal and patient survival. These data support the use of CR as a surrogate end point for long–term renal outcomes in patients with primary MN with heavy and persistent proteinuria.
PR as an End Point in Primary MN
A PR also represents a marked change in proteinuria, and available data suggest that patients who achieve a PR have better renal outcomes than those who do not. However, the persistence of proteinuria suggests continued disease activity, and rates of relapse after achievement of treatment-induced PR are variable and have been shown to be quite high with some therapies. When taken as a whole, available data suggest that a treatment’s effect on PR may be a suitable basis for accelerated approval. As previously noted, the FDA’s accelerated approval program enables expedited approval of therapies for serious illnesses that provide a meaningful therapeutic benefit over existing therapies on the basis of a treatment effect on a surrogate end point that is deemed reasonably likely to predict clinical benefit. Because of the uncertainty about the ability of the surrogate end point to predict the treatment’s effect on the clinical outcome, however, postmarketing confirmatory trials are required to verify and describe the clinical benefit of drugs approved under this program.
A critical issue related to the use of treatment effects on PR as a basis for accelerated approval is the duration over which a PR must be maintained to provide confidence that the treatment effect on PR is reasonably likely to translate into a treatment effect on renal outcomes. The duration of PR necessary to provide such confidence or whether a threshold of PR duration exists (beyond which the likelihood of relapse diminishes substantially) has not been established. Another critical issue related to the use of PR as a basis for accelerated approval is how to design postmarketing trials to verify a treatment benefit. Potential challenges associated with completing an interpretable trial in the postmarketing setting are shown in Table 1.
Table 1.
Challenges associated with conducting postmarketing confirmatory trials to verify and describe the clinical benefit if PR is used as a basis for accelerated approval
| Even if a trial is enrolled at the time of accelerated approval, drug availability after accelerated approval may interfere with the ability to keep patients assigned to placebo from crossing over to active treatment |
| Rates of relapse after achievement of treatment-induced PR are variable and have been shown to be quite high with some therapies; the use of other therapies and also, the open-label use of the new product in both treatment arms after relapse may complicate interpretation of the data and limit the ability to detect a treatment effect on renal outcomes during long-term follow-up |
| Missing data in subjects who are lost to follow-up or withdraw consent may also make it difficult to interpret a trial’s findings and are a particular concern in trials of very long duration |
Gaps in the Data and Future Research Needs
There are a number of gaps in the data supporting the use of proteinuria as an end point in primary MN. We believe that the following analyses would greatly advance our understanding of proteinuria as an end point in primary MN.
Analyses of existing data from interventional trials exploring the relationship between treatment effects on CR and PR and subsequent renal outcomes.
Analyses of alternative definitions of proteinuria response and their associations with renal outcomes. This is particularly important, because in some patients, the persistence of subnephrotic proteinuria (PR) may reflect glomerular and/or tubulointerstitial scarring rather than the persistence of active MN. Future studies should be considered to assess whether other biomarkers, such as antibodies to phospholipase A2 receptor, could be used to help distinguish between persistent disease activity and chronic scarring in patients with PR.
Analyses that provide a more precise understanding of the quantitative relationship between the duration of PR and subsequent renal outcomes and risk of relapse.
Analyses that address the relationship between relapses from CR to subnephrotic levels of proteinuria and long–term renal survival.
There are other analyses that could help inform the design of clinical trials.
Analyses that speak to the likely size of trials and the likely duration of follow-up needed to evaluate a therapy’s effect on rates of CR in patients with primary MN who are at high risk of progression.
Analyses that speak to the likely size of trials and the likely duration of follow-up needed to observe treatment effects on outcomes of interest in the postmarketing setting should PR be used as a basis for accelerated approval.
The use of proteinuria as an end point in primary MN will likely evolve and be refined over time as a result of additional analyses of existing data, as our understanding of the pathogenesis of this disease improves, and as we gain more experience with this end point in clinical trials of new therapies. We hope that this paper will generate additional discussion and research among members of the nephrology community about the use of proteinuria reduction as a surrogate end point in primary MN and spur additional analyses of existing data.
Disclosures
None.
Acknowledgments
The authors acknowledge the support of the American Society of Nephrology Glomerular Disease Advisory Group.
This article reflects the views of the authors and should not be construed to represent the Food and Drug Administration’s views or policies or those of the American Society of Nephrology.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Erwin DT, Donadio JV, Jr., Holley KE: The clinical course of idiopathic membranous nephropathy. Mayo Clin Proc 48: 697–712, 1973 [PubMed] [Google Scholar]
- 2.Gluck MC, Gallo G, Lowenstein J, Baldwin DS: Membranous glomerulonephritis. Evolution of clinical and pathologic features. Ann Intern Med 78: 1–12, 1973 [DOI] [PubMed] [Google Scholar]
- 3.Hladunewich MA, Troyanov S, Calafati J, Cattran DC, Metropolitan Toronto Glomerulonephritis Registry : The natural history of the non-nephrotic membranous nephropathy patient. Clin J Am Soc Nephrol 4: 1417–1422, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cattran DC: Management of membranous nephropathy. Minerva Urol Nefrol 54: 19–27, 2002 [PubMed] [Google Scholar]
- 5.Noel LH, Zanetti M, Droz D, Barbanel C: Long-term prognosis of idiopathic membranous glomerulonephritis. Study of 116 untreated patients. Am J Med 66: 82–90, 1979 [DOI] [PubMed] [Google Scholar]
- 6.Davison AM, Cameron JS, Kerr DN, Ogg CS, Wilkinson RW: The natural history of renal function in untreated idiopathic membranous glomerulonephritis in adults. Clin Nephrol 22: 61–67, 1984 [PubMed] [Google Scholar]
- 7.MacTier R, Boulton Jones JM, Payton CD, McLay A: The natural history of membranous nephropathy in the West of Scotland. Q J Med 60: 793–802, 1986 [PubMed] [Google Scholar]
- 8.Donadio JV, Jr., Torres VE, Velosa JA, Wagoner RD, Holley KE, Okamura M, Ilstrup DM, Chu CP: Idiopathic membranous nephropathy: The natural history of untreated patients. Kidney Int 33: 708–715, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Polanco N, Gutiérrez E, Covarsí A, Ariza F, Carreño A, Vigil A, Baltar J, Fernández-Fresnedo G, Martín C, Pons S, Lorenzo D, Bernis C, Arrizabalaga P, Fernández-Juárez G, Barrio V, Sierra M, Castellanos I, Espinosa M, Rivera F, Oliet A, Fernández-Vega F, Praga M, Grupo de Estudio de las Enfermedades Glomerulares de la Sociedad Española de Nefrología : Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol 21: 697–704, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besarab A, Goodkin DA, Nissenson AR, Normal Hematocrit Cardiac Trial Authors : The normal hematocrit study—follow-up. N Engl J Med 358: 433–434, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D, CHOIR Investigators : Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R, TREAT Investigators : A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Zoja C, Morigi M, Figliuzzi M, Bruzzi I, Oldroyd S, Benigni A, Ronco P, Remuzzi G: Proximal tubular cell synthesis and secretion of endothelin-1 on challenge with albumin and other proteins. Am J Kidney Dis 26: 934–941, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Zoja C, Benigni A, Remuzzi G: Protein overload activates proximal tubular cells to release vasoactive and inflammatory mediators. Exp Nephrol 7: 420–428, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Yard BA, Chorianopoulos E, Herr D, van der Woude FJ: Regulation of endothelin-1 and transforming growth factor-beta1 production in cultured proximal tubular cells by albumin and heparan sulphate glycosaminoglycans. Nephrol Dial Transplant 16: 1769–1775, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Rangan GK, Tay YC, Wang Y, Harris DC: Induction of monocyte chemoattractant protein-1 by albumin is mediated by nuclear factor kappaB in proximal tubule cells. J Am Soc Nephrol 10: 1204–1213, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Tang S, Leung JC, Abe K, Chan KW, Chan LY, Chan TM, Lai KN: Albumin stimulates interleukin-8 expression in proximal tubular epithelial cells in vitro and in vivo. J Clin Invest 111: 515–527, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaarkeuken H, Siezenga MA, Zuidwijk K, van Kooten C, Rabelink TJ, Daha MR, Berger SP: Complement activation by tubular cells is mediated by properdin binding. Am J Physiol Renal Physiol 295: F1397–F1403, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Biancone L, David S, Della Pietra V, Montrucchio G, Cambi V, Camussi G: Alternative pathway activation of complement by cultured human proximal tubular epithelial cells. Kidney Int 45: 451–460, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Abbate M, Zoja C, Remuzzi G: How does proteinuria cause progressive renal damage? J Am Soc Nephrol 17: 2974–2984, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Cattran DC, Reich HN, Beanlands HJ, Miller JA, Scholey JW, Troyanov S, Genes, Gender and Glomerulonephritis Group : The impact of sex in primary glomerulonephritis. Nephrol Dial Transplant 23: 2247–2253, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Pei Y, Cattran D, Greenwood C: Predicting chronic renal insufficiency in idiopathic membranous glomerulonephritis. Kidney Int 42: 960–966, 1992 [DOI] [PubMed] [Google Scholar]
- 24.Ponticelli C, Zucchelli P, Passerini P, Cesana B, Locatelli F, Pasquali S, Sasdelli M, Redaelli B, Grassi C, Pozzi C, Bizzari D, Banfi G: A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int 48: 1600–1604, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Hogan SL, Muller KE, Jennette JC, Falk RJ: A review of therapeutic studies of idiopathic membranous glomerulopathy. Am J Kidney Dis 25: 862–875, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Ponticelli C, Zucchelli P, Passerini P, Cagnoli L, Cesana B, Pozzi C, Pasquali S, Imbasciati E, Grassi C, Redaelli B, Sasdelli M, Locatelli F: A randomized trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med 320: 8–13, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Ponticelli C, Altieri P, Scolari F, Passerini P, Roccatello D, Cesana B, Melis P, Valzorio B, Sasdelli M, Pasquali S, Pozzi C, Piccoli G, Lupo A, Segagni S, Antonucci F, Dugo M, Minari M, Scalia A, Pedrini L, Pisano G, Grassi C, Farina M, Bellazzi R: A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol 9: 444–450, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Schieppati A, Mosconi L, Perna A, Mecca G, Bertani T, Garattini S, Remuzzi G: Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med 329: 85–89, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Ponticelli C, Passerini P, Salvadori M, Manno C, Viola BF, Pasquali S, Mandolfo S, Messa P: A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis 47: 233–240, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC, Toronto Glomerulonephritis Registry Group : Idiopathic membranous nephropathy: Definition and relevance of a partial remission. Kidney Int 66: 1199–1205, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Praga M, Barrio V, Juárez GF, Luño J, Grupo Español de Estudio de la Nefropatía Membranosa : Tacrolimus monotherapy in membranous nephropathy: A randomized controlled trial. Kidney Int 71: 924–930, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, Maxwell DR, Kunis CL, North America Nephrotic Syndrome Study Group : Cyclosporine in patients with steroid-resistant membranous nephropathy: A randomized trial. Kidney Int 59: 1484–1490, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Ponticelli C, Passerini P, Altieri P, Locatelli F, Pappalettera M: Remissions and relapses in idiopathic membranous nephropathy. Nephrol Dial Transplant 7[Suppl 1]: 85–90, 1992 [PubMed] [Google Scholar]
- 34.Laluck BJ, Jr., Cattran DC: Prognosis after a complete remission in adult patients with idiopathic membranous nephropathy. Am J Kidney Dis 33: 1026–1032, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Ruggenenti P, Cravedi P, Chianca A, Perna A, Ruggiero B, Gaspari F, Rambaldi A, Marasà M, Remuzzi G: Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol 23: 1416–1425, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caro J, Gutiérrez-Solís E, Rojas-Rivera J, Agraz I, Ramos N, Rabasco C, Espinosa M, Valera A, Martín M, Frutos MA, Perea L, Juárez GF, Ocaña J, Arroyo D, Goicoechea M, Fernández L, Oliet A, Hernández Y, Romera A, Segarra A, Praga M, Grupo de Estudio de las Enfermedades Glomerulares de la Sociedad Española de Nefrología (GLOSEN) : Predictors of response and relapse in patients with idiopathic membranous nephropathy treated with tacrolimus. Nephrol Dial Transplant 30: 467–474, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Schieppati A, Cai G, Chen X, Zamora J, Giuliano GA, Braun N, Perna A: Immunosuppression for membranous nephropathy: A systematic review and meta-analysis of 36 clinical trials. Clin J Am Soc Nephrol 8: 787–796, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ponticelli C, Zucchelli P, Passerini P, Cesana B, The Italian Idiopathic Membranous Nephropathy Treatment Study Group : Methylprednisolone plus chlorambucil as compared with methylprednisolone alone for the treatment of idiopathic membranous nephropathy. N Engl J Med 327: 599–603, 1992 [DOI] [PubMed] [Google Scholar]
- 39.Ponticelli C, Zucchelli P, Imbasciati E, Cagnoli L, Pozzi C, Passerini P, Grassi C, Limido D, Pasquali S, Volpini T, Sasdelli M, Locatelli F: Controlled trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med 310: 946–950, 1984 [DOI] [PubMed] [Google Scholar]
- 40.Jha V, Ganguli A, Saha TK, Kohli HS, Sud K, Gupta KL, Joshi K, Sakhuja V: A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol 18: 1899–1904, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Howman A, Chapman TL, Langdon MM, Ferguson C, Adu D, Feehally J, Gaskin GJ, Jayne DR, O’Donoghue D, Boulton-Jones M, Mathieson PW: Immunosuppression for progressive membranous nephropathy: A UK randomised controlled trial. Lancet 381: 744–751, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]