Abstract
Purpose
To report the intraoperative optical coherence tomography findings in idiopathic epiretinal membrane (ERM) with connecting strands and to describe the postoperative outcomes.
Methods
A retrospective, case series study within a prospective observational intraoperative optical coherence tomography imaging study was performed. Epiretinal membranes with connecting strands were characterized on preoperative spectral domain optical coherence tomography images and assessed against corresponding intraoperative (after internal limiting membrane [ILM] peeling) and postoperative spectral domain optical coherence tomography images.
Results
Eleven locations of the connecting strands in 7 eyes were studied. The connecting strands had visible connections from the inner retinal surface to the ERM in all locations, and the reflectivity was moderate in 8 locations and high in 3 locations. After ERM and ILM peeling, disconnected strands were identified in all of the intraoperative optical coherence tomography images. The reflectivity of the remaining intraoperative strands was higher than that of the preoperative lesions and appeared as “finger-like” and branching projections. The remaining disconnected lesions were contiguous with the inner retinal layers. Postoperatively, the intraoperative lesions disappeared completely in all locations, and recurrent formation of ERM was not identified in any eyes.
Conclusion
In ERM eyes with connecting strands, intraoperative spectral domain optical coherence tomography imaging showed moderately to highly reflective sub-ILM finger-like lesions that persist immediately after membrane and ILM peeling. Postoperatively, the hyperreflective lesions disappeared spontaneously without localized nerve fiber layer loss. The sub-ILM connecting strands may represent glial retinal attachments.
Keywords: connecting strands, idiopathic epiretinal membrane, ILM peeling, intraoperative optical coherence tomography, sub-ILM glial proliferation
Idiopathic epiretinal membranes (ERM) commonly develop in elderly patients and can impair visual acuity. Idiopathic epiretinal membranes are composed of nonvascularized tissue that grows along the inner retinal surface in the macular area.1,2
It is believed that migration of retinal glial cells through defects of internal limiting membrane (ILM) into the vitreous cavity causes ERM development on the surface of ILM. Histologic studies of ERM specimens have described fibrocellular proliferations including retinal glial cells, hyalocytes, ganglion cell neurites, and retinal pigment epithelial cells.3,4 However, the exact pathogenesis remains unknown.
Since Puliafito et al5 first introduced optical coherence tomography (OCT) imaging of the macula, it has become a well-established diagnostic and monitoring tool for diseases of the vitreomacular interface, including macular hole, ERM, and vitreomacular traction. As the technology continues to evolve, OCT will become increasingly critical for all aspects of care for patients with macular diseases.6
Preoperative and postoperative OCT imaging plays an essential role in the diagnosis and treatment of ERM. Further research is still required for greater understanding of the pathophysiology underlying the development of ERM.7 Histologic studies using surgically removed specimens show only the epiretinal pathology, not that of the underlying retina.8 However, intraoperative spectral domain OCT (SD-OCT) imaging after membrane peeling in macular surgery can provide additional information on the microscopic morphology of the underlying retina and thus may provide new insight into the sub-ERM morphology and the impact of surgical intervention on anatomical configurations.9–13
Recently, a distinct SD-OCT feature of ERM has been introduced termed nerve fiber layer (NFL) fibrillation or ERM “tethering.” This feature is characterized by “finger-like” projections between the ERM and retinal surface and can be idiopathic or secondary to a previous retinal break or surgery.
Previous studies investigated this characteristic in preoperative SD-OCT images.14–16 However, until this study, a longitudinal investigation with intraoperative and postoperative SD-OCT images has not been performed.
The purpose of this study was to evaluate the intraoperative findings and postoperative morphologic changes at sites of the “connecting strands” after ILM peeling in idiopathic ERM surgery using both intraoperative and postoperative SD-OCT.
Materials and Methods
All subjects included in this study were enrolled in a ClinicalTrials.gov Optical Coherence Imaging study (Intraoperative OCT Guidance of Intraocular Surgery) at Duke University that was approved by the University Institutional Review Board. This is a retrospective case series study using data from the prospective intraoperative OCT (iOCT) imaging study. Subjects participated with full informed consent, and the study adhered to the tenets of the Declaration of Helsinki. For this analysis, only idiopathic ERM patients with connecting strands were included, and eyes with diabetes, retinal pathology (e.g., macular degeneration, retinal vein occlusion), or previous retinal surgery were excluded.
Subjects with ERM surgery were assessed for connecting strands on preoperative SD-OCT imaging. Connecting strands were defined on SD-OCT imaging as the presence of homogeneous material of moderate to high reflectivity interposed between the retinal surface and the ERM. The reflectivity of the homogeneous material was similar to the hyperreflectivity of the ERM and NFL. Idiopathic ERM patients with the distinct connecting strands extending from the retinal surface to the ERM were included in this study. Twenty-five–gauge pars plana vitrectomy surgery was performed in all patients by a single experienced retinal surgeon (C.A.T.) and fellow. Preservative-free triamcinolone was used to visualize the hyaloid in some patients. To stain the ILM, a few drops of indocyanine green solution (0.05%) were added over the posterior pole in the fluid-filled eye. After washing out the dye, the diamond-dusted membrane scraper and/or forceps were used to peel the ILM. Then, at a surgical pause, intraoperative SD-OCT images were obtained.
Optical Coherence Tomography Imaging
Preoperative and postoperative retinal images were obtained using an eye tracking SD-OCT with 61-line horizontal scans (Spectralis; Heidelberg Engineering, Heidelberg, Germany). The postoperative images were captured at an average of 3 months (range, 1–8 months) postoperatively.
Intraoperative SD-OCT images were obtained both using a handheld SD-OCT system (Bioptigen Inc, Research Triangle Park, NC) and a Duke prototype microscope-integrated OCT system with 100-line horizontal scans. The techniques described in our previous studies were used to acquire reliable intraoperative SD-OCT images.9,12 The images were post-processed with the sparsity based simultaneous denoising and interpolation method trained specifically for these imaging sytems.17 Sparsity based simultaneous denoising and interpolation has previously been used for reliable denoising of OCT images in human and murine eyes without distorting image features.17,18 The location of the scans was confirmed with the scanning laser ophthalmoscope infrared images from the Spectralis or the summed voxel projection from the intraoperative images. The locations of preoperative connecting strands for each subject were matched and compared with corresponding locations in intraoperative and postoperative images.
Two independent observers (D.H.N. and P.J.D.) evaluated the presence, reflectivity, and shape of the preoperative connecting strands in the intraoperative SD-OCT images, and the changes of the intraoperative findings in the postoperative SD-OCT images. If there was disagreement among the two observers, another observer (C.A.T.) arbitrated the evaluation.
Results
Of 22 eyes that met the eligibility criteria and for whom we had intraoperative and postoperative OCT imaging, we identified seven eyes of seven patients with connecting strands. All seven patients were white and included five men and two women, and the median age was 65 years (range, 55–82 years). According to the preoperative biomicroscopic findings and SD-OCT images, posterior vitreous detachment at the macula appeared to be present in all patients with connecting strands. In surgery, preservative-free triamcinolone was used to highlight the posterior hyaloid in two patients, and we found vitreous attachment to the optic nerve in only one eye.
Preoperatively, we identified 11 locations of connecting strands in 7 eyes. Examples of preoperative ERMs with connecting strands are shown in Figures 1–3 (first row). Sites of the connecting strands were characterized by ERM separation from the NFL and by connecting strands extending from the inner retinal surface toward the ERM. The connecting strands visibly bridged from the inner retinal surface to the ERM in 10 locations; in one location, the strands extended upward toward the ERM but did not connect to the ERM. All the strands were found 1-disk diameter or more away from the fovea; eight in the superior macula and three in the inferior macula with no distinct nasal or temporal preference. Seven of the 11 sites were at the margin of the ERM. On fundus infrared scanning laser ophthalmoscope imaging, there were striae visible in 7 of 11 locations. The contour of the ERM was smooth (Figures 2 and 3) in 7 sites and convoluted (Figure 4 and in part Figure 1) in 4 locations. The reflectivity of the connecting strands was moderate in eight locations and high in three locations. Intraoperatively, after ERM and ILM peeling, the disconnected strands remained visible and seemed to originate from the inner retinal layers in areas where the ILM had been peeled (Figures 1 and 2 [second row] and Figure 3 [third row]). At the margin of the ILM peel, residual ILM, which was elevated from the retinal surface and sometimes slightly scrolled, was visible on iOCT (Figure 5). On the underside of this partially elevated ILM at the margins of the site of peel, persisting strands often extended from retina to the underside of the ILM (Figure 5). Residual strands at the site of residual ILM or across the areas of ILM removal were always connected to the inner retinal surface in intraoperative SD-OCT images and were never attached to the underside of ILM without a retinal attachment. The reflectivity of all of the residual strands (high in five locations and moderate in six) was equal to or higher than that of the preoperative lesions, which was usually moderate. The shape of the remaining intraoperative lesions appeared as long “finger-like” and branching projections (Figures 1 and 2) which were shorter (Figure 3) in 3 locations.
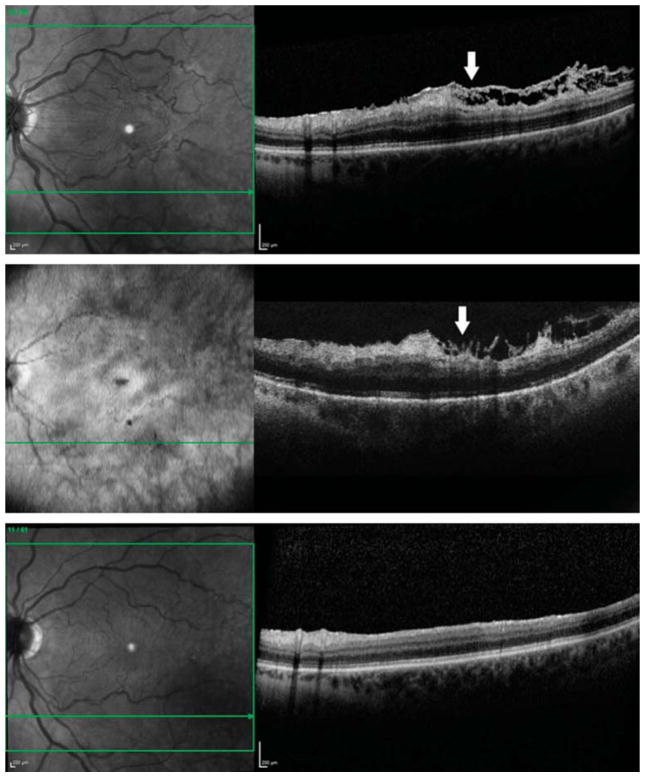
Fig. 1.
Optical coherence tomography images from a 55-year-old white woman with an ERM accompanied by connecting strands. Preoperatively, a horizontal sectional image showed a moderately to highly reflective, connecting lesion under a highly reflective, convolute contour ERM (arrow). The connecting strands had visible but interrupted connections from the inner retinal surface to the ERM and seemed to distort both of them (first row). Although the ERM and the ILM have been removed, an intraoperative horizontal sectional image showed a highly reflective lesion with shadowing of the retina below (arrow). The shape of this lesion appeared as long “finger-like” and branching projections. The remained disconnected lesions were contiguous with the inner retinal surface and seemed to originate from the inner retinal layers (second row). Three months after surgery, the intraoperative remained lesion disappeared completely, and there was no recurrent formation of ERM (third row).
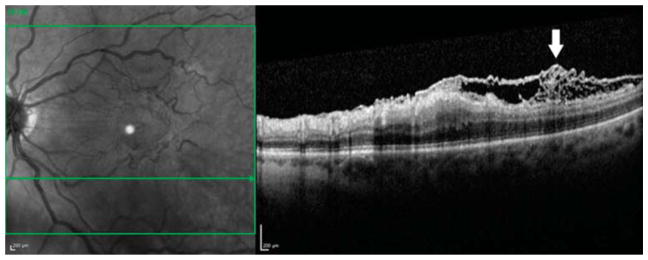
Fig. 3.
Optical coherence tomography images from a 61-year-old white man with an ERM accompanied by connecting strands. Preoperatively, a horizontal sectional image showed a moderately reflective, connecting lesion under a highly reflective, smooth ERM (arrow). The connecting strands had visible connections from the inner retinal surface to the ERM (first row). Before ILM peeling but after ERM removal, the ILM was already absent (it appeared to be removed along with the ERM removal in this area) as demonstrated by the lack of indocyanine green staining (circle) in the region of the preoperative connecting strands (second row). Although the ERM and the ILM have been removed, an intraoperative horizontal sectional image showed a highly reflective lesion (arrow). The shape of this lesion appeared as tufts. The remained disconnected lesions were contiguous with the inner retinal surface and seemed to originate from the inner retinal layers (third row). Three months after surgery, the intraoperative remaining lesion disappeared completely, and there was no recurrent formation of ERM (fourth row).
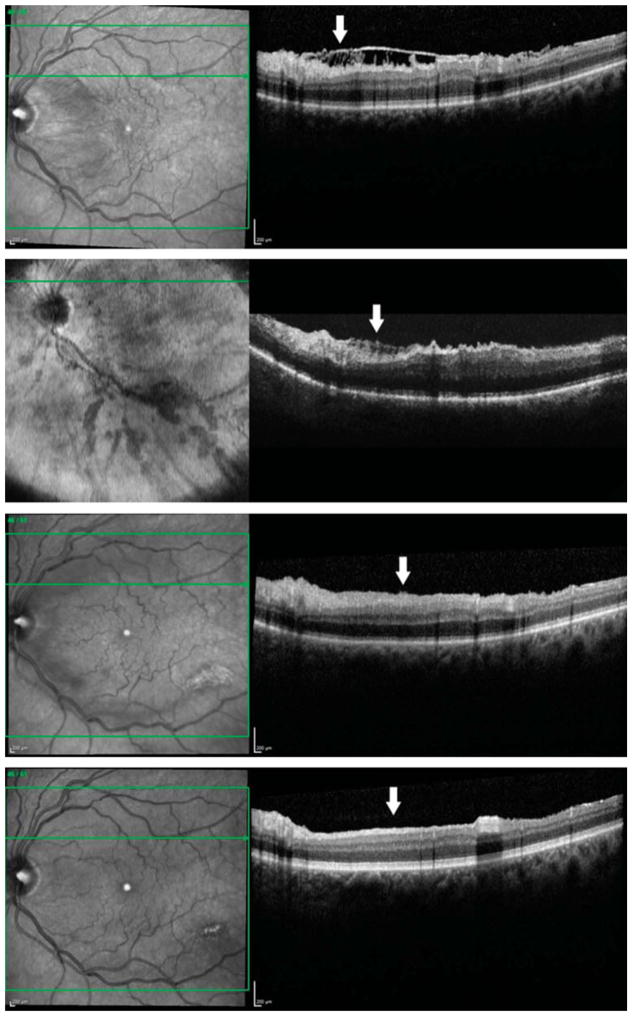
Fig. 2.
Optical coherence tomography images from a 65-year-old white man with an ERM accompanied by connecting strands. Preoperatively, a horizontal sectional image showed a moderately reflective, connecting lesion under a highly reflective, smooth ERM (arrow). The connecting strands had both continuous and discontinuous connections from the inner retinal surface to the ERM (first row). Although the ERM and the ILM have been removed, an intraoperative horizontal sectional image showed a moderately reflective lesion (arrow). The shape of this lesion appeared as “finger-like” and branching projections. The remaining disconnected lesions were contiguous with the inner retinal surface and seemed to originate from the inner retinal layers (second row). Fifteen days after surgery, the remaining strands regressed, with minimal remaining strands and minimal irregularity of the inner retinal surface (arrow) (third row). Then, 3 months after surgery, the intraoperative remained lesion disappeared completely and the inner surface became completely smooth, with no evidence of the previous strands (arrow) (fourth row).
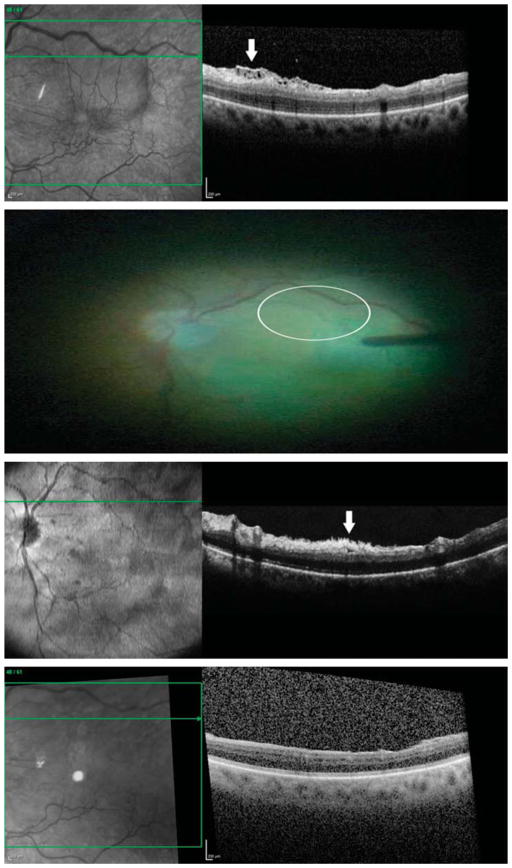
Fig. 4.
The folded contour optical coherence tomography image (arrow) of the ERM– ILM complex in the 55-year-old white woman shown in Figure 1 was consistent with previous histologic studies that showed hyperconvolution of the ILM in ERM eyes.26
Fig. 5.
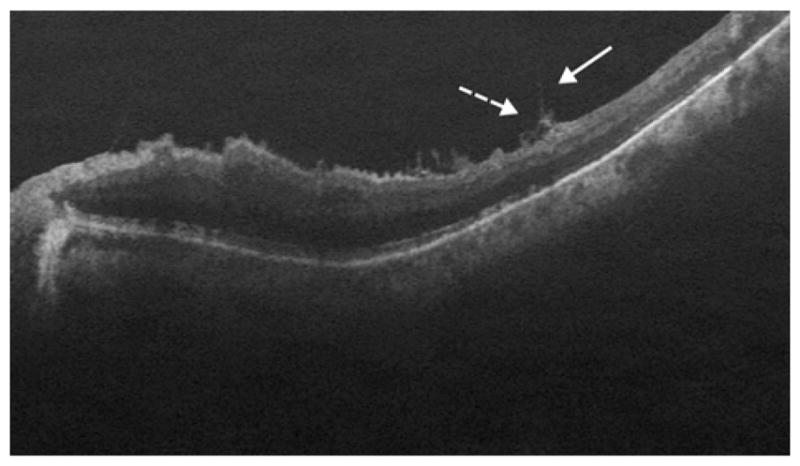
In an 82-year-old white man with visible disconnected strands in areas where the ILM had been peeled, the remaining strands (dotted arrow) also appeared to be located on the underside of the partially elevated ILM (arrow) at the margins of the site of peel.
In all cases, the observed intraoperative prominent “finger-like” and shorter lesions had completely disappeared in all locations, typically at rv3 months (range, 2–8 months). At our earliest followup OCT obtained in 1 patient 15 days after surgery, the remaining strands regressed with minimal irregularity of the inner retinal surface. The inner surface became completely smooth at 3 months after surgery, with no evidence of the previous strands [Figure 2 (third and fourth rows)]. Recurrent formation of ERM was not identified in any eyes [Figures 1–3 (bottom)]. Dissociated optic nerve fiber layer was not present at this location in any eyes.
Discussion
Theories on the pathogenesis of ERM formation suggest that reserve glial cells of the retinal NFL migrate through breaks in the ILM and spread out into a monolayer on the vitreal surface of the ILM.19 Epiretinal membrane is usually discovered in the context of posterior vitreous detachment, but in some cases, the vitreous cortex splits into lamellae and the vitreous is still adherent to the macula. In the majority of cases, separation occurs between the ILM and the posterior hyaloid.20–22 Sigler and colleagues, based on preoperative OCT imaging of ERMs, identified connecting strands and considered these to correspond to glial or inflammatory tissue within a potential space between the ILM and the posterior vitreous cortex, which may be then removed through ILM peeling.16 Because we could view the retina in cross section with SD-OCT immediately after ERM and ILM removal, we were able to identify that the strands had disconnected from the overlying ERM and almost completely remained protruding from the inner retinal surface. Preoperatively, because the connecting strands were located beneath the ERM, a band of highly reflective tissue, the reflectivity of the lesions was usually lower than that of the adjacent ERM and inner retina. After ERM and ILM removal, the connecting strands showed equal or higher reflective properties relative to adjacent tissue on SD-OCT imaging when compared with preoperative images. In postoperative imaging 1 month to 3 months later, the strands had disappeared completely. No recurrence of ERM was observed.
These results suggest several informative findings. First, because the connecting strands remained on the inner retinal surface after ILM peeling in all 11 locations in these 7 eyes, these are strands are likely sub-ILM rather than pre-ILM material. Numerous potential materials could compose these connecting strands. Fibrin, laminin, or other extracellular matrix materials are associated with ERM formation and could be deposited beneath the ILM in strands. Glial cell proliferation could persist and extend between the retina and ILM. Although axons of the NFL could be disrupted with axonal fibers drawn upward, this is less likely because the reflectivity of the strands was different from that of the NFL, and dissociated optic nerve fiber layer was not present in these areas in any eyes.
Formation of the ERM involves glial proliferation, which was considered predominantly epiretinal rather than intraretinal. More recent evidence suggests that there may be a significant intraretinal component.8 Glial cell proliferation has been described under the ILM, and this is a common histological finding in ILM specimens removed from ILM caused by the fibrocellular tissue on the vitreal side of the membrane.4,23,24
Second, the ERM or proliferating glial cells are supposed to be directly, firmly adherent to the ILM, without intermingled vitreous collagen layer. Combined ERM–ILM specimens are reportedly more common than previously recognized, implying that the two membranes are not always distinct and surgically separable.25 Concomitant ILM peeling may contribute to the absence of recurrence and favorable visual acuity outcomes both in our series and previous series. In our series with the connecting strands, in which ILM was peeled in all cases, cleavage occurred between the ILM and the NFL, not between the ILM and the ERM or posterior hyaloid. We inferred preoperative partial detachment of the ILM in all cases of connecting strands. Our findings of hyperconvolution or folding of the ERM–ILM complex was based on both preoperative and iOCT imaging and was consistent with previous histopathology studies that showed folding or hyperconvolution of the ILM in ERM (Figure 4).26,27 With both preoperative and intraoperative SD-OCT, we could identify these structures not previously realized on timedomain OCT. Third, we observed moderately to highly reflective disconnected lesions in iOCT images. The disconnected lesions were contiguous with the inner retinal layers. Both the hyperreflectivity and the contiguity with the inner retinal surface of the sub-ILM lesion most likely represent a glial cell proliferative process originating from the inner retinal layers, which has been reported as a “hyperreflective foveal lesion” in cases of epiretinal membranes with a firm foveal attachment.28 In addition, the reflectivity of the sub-ILM lesions in our study varies from low-medium to moderate reflectivity, matched in part the “lamellar hole-associated epiretinal proliferation” observed at inner retinal defects of lamellar macular hole and other more intense foci were similar to “moderately reflective foveal lesion” observed to fill the foveal defect after macular hole closure.29–31 We recognize that other events such as NFL schisis may also be plausible theories as to the source of this OCT morphology.
Fourth, the shapes of the remaining intraoperative lesions appear as “finger-like” and branching projections. The distinct features of the sub-ILM lesion are compatible with the glial tufts or blooms that were observed in a wholemount immunohistochemical study of human retinas from aged donors. The glial tufts and blooms are reportedly a common subclinical occurrence in aged human retinas.32
Fifth, postoperatively, the intraoperative lesions completely disappeared in all locations. Postoperative disappearance of the intraoperative, highly reflective lesion is consistent with a study that showed postoperative resolution of preoperative fibrillation of the NFL.15 There are 2 possible reasons for why the intraoperative glial lesion disappeared postoperatively. First, Snead et al26 proposed that the primary cause for the ERM (cellophane maculopathy and macular pucker) may originate within the ILM. Therefore, the ILM may be a scaffold for epi-ILM and sub-ILM glial cell proliferation in our series. The disappearance could be the result of surgical removal of the scaffold. Second, the sub-ILM glial cell proliferation may be a consequence of folding and contraction of the ILM caused by the epi-ILM glial cell proliferation. Therefore, the tractional force was released after the removal of the ILM. However, we should not dismiss the hypothesis that this is traction on the NFL from partial ILM separation and the resolution resulted simply from tractional release and normalization of the architecture.
The limitations of this study include the absence of histologic specimens and early postoperative followup OCT imaging evaluations, lack of standardized OCT systems, lack of quantitative analysis, lack of prospective assessment, the use of indocyanine green in every case, and small study size. Practically, however, localizing the part of the connecting strands in surgically removed specimens would be challenging. It would be interesting to see whether iOCT shows a different response immediately after the use of “heavy” (dual) dyes33 or no dyes at all. The strength of this study is that intraoperative SD-OCT imaging provides new insight into the sub-ERM or sub-ILM pathologies after membrane peeling. Therefore, it allows analysis of retinal pathology in situ before postoperative change or remodeling can occur. Additionally, the longitudinal investigation with preoperative, intraoperative and postoperative SD-OCT images demonstrates a dynamic process that involves migration, proliferation, and regression or normalization of anatomy.
In conclusion, in ERM eyes with connecting strands, intraoperative SD-OCT imaging showed moderately to highly reflective sub-ILM lesions after membrane peeling. Postoperatively, the hyperreflective lesions disappeared spontaneously. These findings support our hypothesis that the connecting strands shown in idiopathic ERM eyes are a sub-ILM glial proliferation that is regressed after ILM peeling. However, the NFL traction/schisis is still a possible hypothesis. Although intraoperative SD-OCT imaging study is important in improving our understanding of the pathophysiology of the connecting strands in ERM, future studies should be undertaken to correlate histopathologic analysis of ERM/ILM specimen removed at surgery with the novel intraoperative SD-OCT imaging.
Acknowledgments
Supported in part by the Hartwell Foundation, Andrew Family Foundation, Research to Prevent Blindness, the National Center for Research Resources Grant 1UL1RR024128-01, and the National, Eye Institute Grant 1R01EY023039-01.
C. A. Toth receives research support from Genentech and through an equipment loan from Bioptigen, Inc. She also receives royalties for surgical technology licensed by Duke to Alcon Laboratories. Duke University has an equity interest in Bioptigen, Inc. J.
Footnotes
A. Izatt is a cofounder of Bioptigen, Inc and has corporate, intellectual property, and equity interests in this company. S. Farsiu has a related pending patent corresponding to this technology. None of the other authors have any conflicting interests to disclose.
References
- 1.Aung KZ, Makeyeva G, Adams MK, et al. The prevalence and risk factors of epiretinal membranes: the Melbourne Collaborative Cohort Study. Retina. 2013;33:1026–1034. doi: 10.1097/IAE.0b013e3182733f25. [DOI] [PubMed] [Google Scholar]
- 2.Ng CH, Cheung N, Wang JJ, et al. Prevalence and risk factors for epiretinal membranes in a multi-ethnic United States population. Ophthalmology. 2011;118:694–699. doi: 10.1016/j.ophtha.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao F, Gandorfer A, Haritoglou C, et al. Epiretinal cell proliferation in macular pucker and vitreomacular traction syndrome: analysis of flat-mounted internal limiting membrane specimens. Retina. 2013;33:77–88. doi: 10.1097/IAE.0b013e3182602087. [DOI] [PubMed] [Google Scholar]
- 4.Gandorfer A, Haritoglou C, Scheler R, et al. Residual cellular proliferation on the internal limiting membrane in macular pucker surgery. Retina. 2012;32:477–485. doi: 10.1097/IAE.0b013e3182246e2a. [DOI] [PubMed] [Google Scholar]
- 5.Puliafito CA, Hee MR, Lin CP, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995;102:217–229. doi: 10.1016/s0161-6420(95)31032-9. [DOI] [PubMed] [Google Scholar]
- 6.Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120:2611–2619. doi: 10.1016/j.ophtha.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 7.Steel DH, Lotery AJ. Idiopathic vitreomacular traction and macular hole: a comprehensive review of pathophysiology, diagnosis, and treatment. Eye. 2013;27:S1–S21. doi: 10.1038/eye.2013.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charteris DG, Downie J, Aylward GW, et al. Intraretinal and periretinal pathology in anterior proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol. 2007;245:93–100. doi: 10.1007/s00417-006-0323-5. [DOI] [PubMed] [Google Scholar]
- 9.Dayani PN, Maldonado R, Farsiu S, Toth CA. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina. 2009;29:1457–1468. doi: 10.1097/IAE.0b013e3181b266bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray R, Barañano DE, Fortun JA, et al. Intraoperative microscope-mounted spectral domain optical coherence tomography for evaluation of retinal anatomy during macular surgery. Ophthalmology. 2011;118:2212–2217. doi: 10.1016/j.ophtha.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Pichi F, Alkabes M, Nucci P, Ciardella AP. Intraoperative SD-OCT in macular surgery. Ophthalmic Surg Lasers Imaging. 2012;43:S54–S60. doi: 10.3928/15428877-20121001-08. [DOI] [PubMed] [Google Scholar]
- 12.Hahn P, Migacz J, O’Donnell R, et al. Preclinical evaluation and intraoperative human retinal imaging with a high-resolution microscope-integrated spectral domain optical coherence tomography device. Retina. 2013;33:1328–1337. doi: 10.1097/IAE.0b013e3182831293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehlers JP, Xu D, Kaiser PK, et al. Intrasurgical dynamics of macular hole surgery: an assessment of surgery-induced ultra-structural alterations with intraoperative optical coherence tomography. Retina. 2014;34:213–221. doi: 10.1097/IAE.0b013e318297daf3. [DOI] [PubMed] [Google Scholar]
- 14.Pilli S, Lim P, Zawadzki RJ, et al. Fourier-domain optical coherence tomography of eyes with idiopathic epiretinal membrane: correlation between macular morphology and visual function. Eye. 2011;25:775–783. doi: 10.1038/eye.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartmann KI, Schuster AK, Bartsch DU, et al. Restoration of retinal layers after epiretinal membrane peeling. Retina. 2014;34:647–654. doi: 10.1097/IAE.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sigler EJ, Randolph JC, Calzada JI. Comparison of morphologic features of macular proliferative vitreoretinopathy and idiopathic epimacular membrane. Retina. 2014;34:1651–1657. doi: 10.1097/IAE.0000000000000138. [DOI] [PubMed] [Google Scholar]
- 17.Fang L, Li S, McNabb R, et al. Fast acquisition and reconstruction of optical coherence tomography images via sparse representation. IEEE Trans Med Imaging. 2013;32:2034–2049. doi: 10.1109/TMI.2013.2271904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinivasan PP, Heflin SJ, Izatt JA, et al. Automatic segmentation of up to ten layer boundaries in SD-OCT images of the mouse retina with and without missing layers due to pathology. Biomed Opt Express. 2014;5:348–365. doi: 10.1364/BOE.5.000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foos RY. Vitreoretinal juncture; epiretinal membranes and vitreous. Invest Ophthalmol Vis Sci. 1977;16:416–422. [PubMed] [Google Scholar]
- 20.Gupta P, Yee KM, Garcia P, et al. Vitreoschisis in macular diseases. Br J Ophthalmol. 2011;95:376–380. doi: 10.1136/bjo.2009.175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itakura H, Kishi S. Evolution of vitreomacular detachment in healthy subjects. JAMA Ophthalmol. 2013;131:1348–1352. doi: 10.1001/jamaophthalmol.2013.4578. [DOI] [PubMed] [Google Scholar]
- 22.Rothman AL, Folgar FA, Tong AY, Toth CA. Spectral domain optical coherence tomography characterization of pediatric epiretinal membranes. Retina. 2014;34:1323–1334. doi: 10.1097/IAE.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenawy N, Wong D, Stappler T, et al. Does the presence of an epiretinal membrane alter the cleavage plane during internal limiting membrane peeling? Ophthalmology. 2010;117:320–323. doi: 10.1016/j.ophtha.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Haritoglou C, Schumann RG, Kampik A, Gandorfer A. Glial cell proliferation under the internal limiting membrane in a patient with cellophane maculopathy. Arch Ophthalmol. 2007;125:1301–1302. doi: 10.1001/archopht.125.9.1301-b. [DOI] [PubMed] [Google Scholar]
- 25.DeMarchis EH, Pershing S, Moshfeghi DM. Clinical-pathologic correlation: vitrectomy with epiretinal and internal limiting membrane peel. Ophthalmic Surg Lasers Imaging Retina. 2014;45:218–221. doi: 10.3928/23258160-20140411-01. [DOI] [PubMed] [Google Scholar]
- 26.Snead DR, Cullen N, James S, et al. Hyperconvolution of the inner limiting membrane in vitreomaculopathies. Graefes Arch Clin Exp Ophthalmol. 2004;242:853–862. doi: 10.1007/s00417-004-1019-3. [DOI] [PubMed] [Google Scholar]
- 27.Bovey EH, Uffer S. Tearing and folding of the retinal internal limiting membrane associated with macular epiretinal membrane. Retina. 2008;28:433–440. doi: 10.1097/IAE.0b013e318150d6cf. [DOI] [PubMed] [Google Scholar]
- 28.Koo HC, Shin JA, Lim WI, et al. Hyperreflective foveal lesion observed with optical coherence tomography in cases of epiretinal membranes with a firm foveal attachment. Retina. 2014;34:1824–1832. doi: 10.1097/IAE.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 29.Pang CE, Spaide RF, Freund KB. Epiretinal proliferation seen in association with lamellar macular holes: a distinct clinical entity. Retina. 2014;34:1513–1523. doi: 10.1097/IAE.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 30.Ko TH, Witkin AJ, Fujimoto JG, et al. Ultrahigh-resolution optical coherence tomography of surgically closed macular holes. Arch Ophthalmol. 2006;124:827–836. doi: 10.1001/archopht.124.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakabayashi T, Fujiwara M, Sakaguchi H, et al. Foveal micro-structure and visual acuity in surgically closed macular holes: spectral-domain optical coherence tomographic analysis. Ophthalmology. 2010;117:1815–1824. doi: 10.1016/j.ophtha.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 32.Edwards MM, McLeod SD, Bhutto IA, et al. ARVO. 2014. Idiopathic glial blooms in aged human retinas. paper. [Google Scholar]
- 33.Veckeneer M, Mohr A, Alharthi E, et al. Novel “heavy” dyes for retinal membrane staining during macular surgery: multicenter clinical assessment. Acta Ophthalmol. 2014;92:339–344. doi: 10.1111/aos.12208. [DOI] [PubMed] [Google Scholar]