Abstract
The preventive effects of the American cranberry (Vaccinium macrocarpon) against urinary tract infections are supported by extensive studies which have primarily focused on its phenolic constituents. Herein, a phenolic-free carbohydrate fraction (designated cranf1b-F2) was purified from cranberry fruit using ion exchange and size exclusion chromatography. MALDI-TOF-MS analysis revealed that the cranf1b-F2 constituents are predominantly oligosaccharides possessing various degrees of polymerisation and further structural analysis (by GC-MS and NMR) revealed mainly xyloglucan and arabinan residues. In antimicrobial assays, cranf1b-F2 (at 1.25 mg/mL concentration) reduced biofilm production by the uropathogenic Escherichia coli CFT073 strain by over 50% but did not inhibit bacterial growth. Cranf1b-F2 (ranging from 0.625 - 10 mg/mL) also inhibited biofilm formation of the non-pathogenic E. coli MG1655 strain up to 60% in a concentration-dependent manner. These results suggest that cranberry oligosaccharides, in addition to its phenolic constituents, may play a role in its preventive effects against urinary tract infections.
Keywords: American cranberry, Vaccinium macrocarpon, phenolic, oligosaccharide, biofilm, Escherichia coli
1. Introduction
Urinary tract infections (UTI) commonly occur anywhere from the kidney in the upper urinary tract to the bladder in the lower urinary tract. Although UTIs are generally easy to treat with antibiotics, acute infections can be dangerous for elderly, infant and immunocompromised patients (Jepson, Williams, & Craig, 2012). Some UTI patients can experience frequent recurrent infections and increased susceptibility to drug resistant uropathogens (Jepson et al., 2012; Reid et al., 2001). Over 80% of UTIs are associated with Escherichia coli, which may be transmitted from the bowel to urethra. Biofilms that form on the bladder wall help prevent the bacteria from being eradicated by the immune system and antibiotics (Anderson et al., 2003; Moreno et al., 2008). Evidence suggests that consumption of the American cranberry (Vaccinium macrocarpon Aiton) juice can inhibit the presence of bacteria in urine and reduce UTI symptoms associated with bacteriuria and pyuria (Avorn et al., 1994; Reid et al., 2001). Our group (LaPlante, Sarkisian, Woodmansee, Rowley, & Seeram, 2012), and others (Côté et al., 2011; Iswaldi et al., 2012; Lian, Maseko, Rhee, & Ng, 2012) have studied the antimicrobial effects of the phenolic constituents of cranberries. Some studies (Foo, Lu, Howell, & Vorsa, 2000a, 2000b; Gupta et al., 2012; Howell et al., 2005) have shown that cranberry proanthocyanidins (commonly known as PACs), with at least one A-type linkage, inhibit the adherence of type p-fimbriated E. coli to uroepithelial cells and human red blood cells. The chemistry of cranberry PACs (Lee, 2013) and their absorption and metabolism have been studied (Ou & Gu, 2014). However, the non-phenolic constituents in cranberry have been less investigated (Hotchkiss, Nunez, Khoo, & Strahan, 2013). Herein, we provide the first report describing the structural characterization of a phenolic-free carbohydrate fraction purified from cranberry and its evaluation for inhibition of biofilm formation by both uropathogenic (E. coli CFT073) and non-pathogenic (E. coli MG1655) strains of E. coli.
2. Materials and methods
2.1. Bacterial strains and media
E. coli strains CFT073 and MG1655 were gifts from Dr. Paul Cohen (University of Rhode Island). Luria Bertani (LB) medium (BD, NJ, USA) was supplemented with 5 g/L dextrose. M63 medium (Bioworld, OH, USA) was supplemented with 1 mM MgSO4, 2 g/L dextrose and 5 g/L casamino acid.
2.2. Fractionation of cranberry materials
2.2.1. Purification of crude cranberry hull extract (Cranf1)
Scheme S1 (see Supplementary data) shows the fractionation flow chart of cranberry materials with yields and their total phenolic contents. Briefly, a pectinase (Klerzyme 150, DSM Food Specialties, South Bend, IN, USA) degraded cranberry hull extract (Cranf1) was fractionated using an Agilent 971-FP flash purification system (Agilent Technologies, Santa Clara, CA, USA) with Biotage SNAP KP-C18-HS 120g cartridges (Biotage, Charlotte, NC, USA). 50 mL of Cranf1 aqueous solution (100 mg/mL) was loaded onto the pre-conditioned C18 column cartridge and eluted sequentially with 500 mL of de-ionised H2O, 500 mL of 15% methanol/water, and finally 500 mL of MeOH at 35 mL/min. Fractions eluted with 100% water were pooled as Cranf1W with a yield of 38.1% (w/w), fractions eluted with 15% methanol were pooled as Cranf1b with a yield of 23.8%, and fractions eluted with 100% methanol were pooled as Cranf1M with a yield of 28.1% (see Scheme S1, Supplementary data).
2.2.2. Purification of oligosaccharide enriched fraction Cranf1b
Cranf1b was introduced onto an anion exchange column (Sepharose Q XL 16/10, GE Healthcare Life Sciences, Pittsburgh, PA, USA) and eluted with step-wise gradient of NaCl aqueous solution (0-1 M) at 5 mL/min on a ÄKTA fast protein liquid chromatography (FPLC) system (GE Healthcare Life Sciences). Ten mL fractions were collected and assayed for total carbohydrate content assay.(Masuko et al., 2005) The pooled carbohydrate-containing fractions were freeze-dried and desalted (10×300 mm Bio-gel P2 column; BIO-RAD, Hercules, CA, USA). The constituents that eluted with 100% de-ionised H2O and 0.1 M NaCl were combined and further purified by gel filtration (Sephacryl S-100 HR 16/60, GE Healthcare Life Sciences; elution with de-ionised H2O at 0.25 mL/min), yielding two fractions designated as cranf1b-F1 and cranf1b-F2.
2.3. Biofilm assay
The antibiofilm property of the cranberry materials was measured against E. coli CFT073 and MG1655 using a modified crystal violet staining method in round bottom 96-well microtiter plates (George, 2011; Naves et al., 2008; Niu & Gilbert, 2004). Bacteria colonies from TSA plates were inoculated into LB broth and incubated at 37 °C with 175 rpm shaking for 24 h. The cultures were then diluted 100-fold in M63 medium, distributed in microtiter wells, and treated with a series of two-fold dilutions of test samples (10 - 0.019 mg/mL). The plates were incubated at 37 °C for 6 h or 48 h, gently washed with de-ionised water, and stained with 125 μL of 0.1% crystal violet solution for 15 min. The solution was removed and the wells were again gently washed with de-ionised water and dried for 1 h. 125 μL of 30% acetic acid solution was added to each well and incubated for 15 min. 100 μL from each well was transferred to a flat bottom microtiter plate and the OD550 was measured (Spectramax M2, Molecular devices, Sunnyvale, CA, USA). Percent biofilm formation was calculated as the average OD550 of three replicate treatment wells divided by average OD550 of replicate control wells (30 wells/plate). Each experiment was conducted in duplicate.
2.4. High Performance Size Exclusion Chromatography (HPSEC)
HPSEC was carried out at 40 °C on a TSKgel G3000PW column [7.5 × 300 mm column, Tosoh Bioscience LLC, King of Prussia, PA, USA; Hitachi LaChrom Elite HPLC, Tokyo, Japan; 0.6 mL/min de-ionised water, refractive index (RI) detection]. The molecular weights of compounds were determined by comparison of retention times to a standard curve (Supplementary Fig. S1) generated with standard dextrans of molecular weights ranging from 1000 to 50000 Daltons.
2.5. Glycosyl composition analysis
Sugar composition was determined by GC-MS analysis of monosaccharides (York, Darvill, McNeil, Stevenson, & Albersheim, 1986). Briefly, 100 μg of sample was hydrolysed with 2M TFA for 2 h at 121 °C. The hydrolyte was reduced with sodium borodeuteride (NaBD4) at room temperature for 1.5 h. The reduced monosaccharides were O-acetylated with acetic anhydride at 50 °C for 20 min. The resulting product was extracted with dichloromethane and analysed by GC-MS (DB-1 column, GC Model 6890/MS Model 5973, Agilent Technologies, Santa Clara, CA, USA). The monosaccharide composition was determined by comparison with a GC-MS profile of monosaccharide standards.
2.6. Glycosyl linkage analysis
Partially methylated acetate alditols (PMAAs) of cranf1b-F2 were analysed by GC-MS (Ciucanu & Kerek, 1984; York et al., 1986). Briefly, 600 μg of sample was permethylated with iodomethane and concentrated sodium hydroxide in DMSO. The permethylated oligosaccharide was hydrolysed with 2M TFA and reduced with NaBD4. The sample was then acetylated with acetic anhydride and extracted with dichloromethane. GC-MS analysis was conducted using a Supelco SP2331 column (Sigma-Aldrich, St. Louis, MO, USA). The GC-MS profile was analysed by comparison of retention time and electron-impact fragmentation spectra with PMAA standards.
2.7. NMR analysis
The cranf1b-F2 was deuterium exchanged twice by D2O shake and dissolved in D2O with addition of 1 μL of DMSO as internal reference. 1H, 13C, 2D COSY, TOCSY, NOESY, HSQC and HMBC spectra were obtained on a 500 MHz NMR spectrometer (Varian VNMRS 500MHz, Agilent Technologies) at 25 °C.
2.8. MALDI mass spectrometry
Cranf1b-F2 (1 mg/mL in H2O) was mixed with 2,3-dihydrobenzoic acid (DHB) matrix solution (v/v=1:1). Two μL of the mixture was analysed by MALDI-TOF-MS (Axima Performance, Shimadzu, Kyoto, Japan) in positive reflectron mode with power set at 80kV. 500 profiles were collected for each experiment.
3. Results and discussion
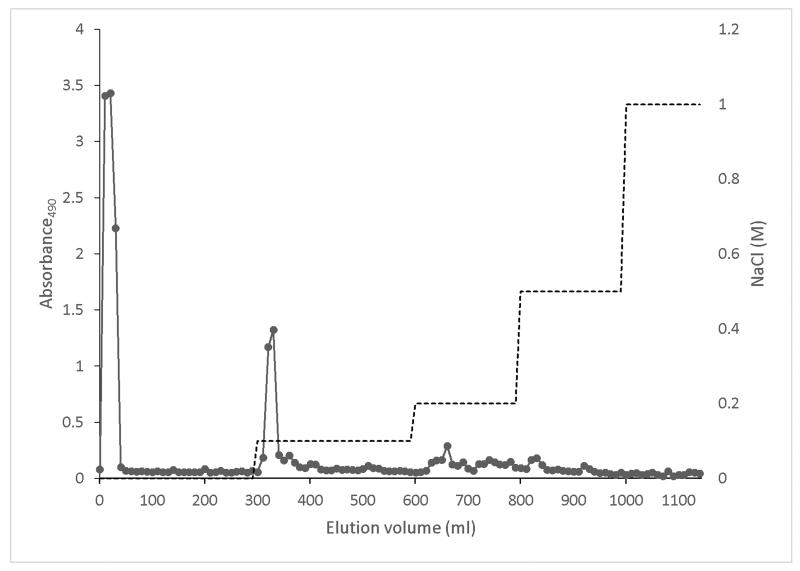
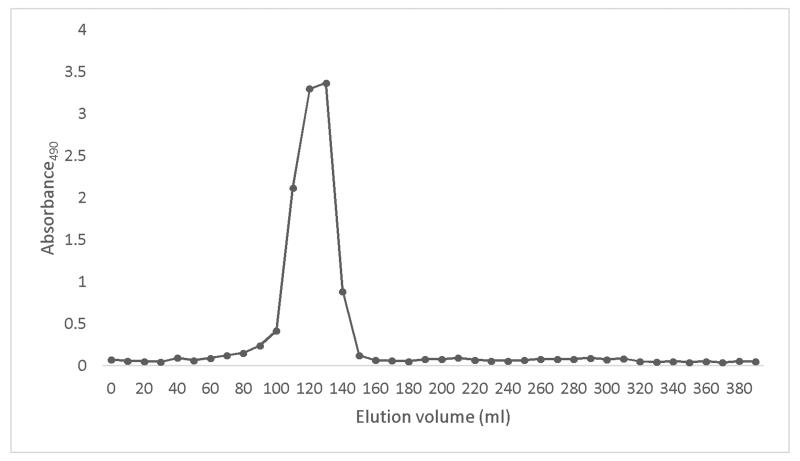
In this study, we investigated a carbohydrate fraction extracted from cranberry and evaluated its inhibitory effect on biofilm formation of two strains of E. coli. The 1H NMR spectra of the original cranberry starting material (Cranf1) and its three major purified fractions namely, Cranf1W, Cranf1b and Cranf1M were obtained (see Supplementary data). The 1H NMR spectrum of Cranf1b showed only trace resonances above 7.0 ppm, indicating that phenolics were mostly removed by C18 column chromatography. The crude cranberry extract cranf1b was purified by anion exchange chromatography and four fractions, cranf1b-F1 (64.0%), cranf1b-F2 (17.5%), cranf1b-F3 (2.5%) and cranf1b-F4 (<1%), were collected (Figure 1a). Due to the limited quantities of the latter fractions, only cranf1b-F1 and cranf1b-F2 were further studied. Cranf1b-F1 and cranf1b-F2 were next purified by gel filtration, resulting in only one peak for each sample (Figure 1b). The homogeneity of cranf1b-F2 was further confirmed by HPSEC profile (Supplementary Fig. S6) and the average molecular size was predicted to be 1370 Da. However, MALDI-TOF MS spectrometry of cranf1b-F2 produced a series of oligosaccharide sodium adduct ions (Supplementary Fig. S7), revealing it to be a mixture of oligomers within a close molecular weight range. The ions at approximately 1055, 1085, 1217, 1247, 1349, 1379, 1511, 1541 can be attributed to Hex3Pen4 (5 hexoses and 4 pentoses), Hex4Pen3, Hex4Pen4, Hex5Pen3, Hex4Pen5, Hex5Pen4, Hex5Pen5 and Hex6Pen4, respectively. Clusters of less abundant ions were observed above 1700 representing oligosaccharides with degrees of polymerisation (DP) larger than 11.
Figure 1a. Elution profile of Cranf1b on Sepharose Q XL 16/10 column, eluted by stepwise gradient of NaCl (0-1 M) (total sugars, -■-).
Figure 1b. Elution profile of Cranf1b-F2 on Sephacryl S-100 HR 16/60 column, eluted by de-ionised water (total sugars, -■-).
The GC-MS profile (Supplementary Fig. S8a) of the monosaccharide acetate alditols (Table 1) indicated that the cranf1b-F2 was primarily composed of arabinose (46%), glucose (40%), xylose (12%) and trace quantities of galactose (2%). The predominance of glucose, xylose and arabinose suggests that cranf1b-F2 is likely a xyloglucan (FRY, 1989; McNeil, Darvill, Fry, & Albersheim, 1984).
Table 1. 13C NMR and 1H NMR chemical shifts (δ in ppm) for cranf1b-F2.
| Residue (Mol %) |
Subunits | Linkages | C1/H1 | C2/H2 | C3/H3 | C4/H4 | C5/H5 | C6/H6 |
|---|---|---|---|---|---|---|---|---|
| Araf (56%) | Arabinan | t-α-Araf | 107.69 | 81.56 | 77.17 | 84.58 | 61.75 | - |
| 5.14 | 4.13 | 3.95 | 4.03 | 3.84 | - | |||
| Arabinan | 3,5-α-Araf | 108.11 | 79.83 | 82.90 | 82.11 | 67.14 | - | |
| 5.11 | 4.28 | 4.09 | 4.30 | 3.83/3.93 | - | |||
| Arabinan | 5-α-Araf | 108.15 | 81.53 | 77.25 | 83.02 | 66.89 | - | |
| 5.08 | 4.12 | 4.02 | 4.21 | 3.88/3.79 | - | |||
| Arabinan | 3-α-Araf | 107.72 | 80.26 | 84.33 | 83.09 | - | - | |
| 5.18 | 4.36 | 3.95 | 4.14 | - | - | |||
| S | t-α-Araf | 109.87 | 81.66 | 77.05 | 84.44 | 61.81 | - | |
| 5.15 | 4.19 | 3.93 | 4.06 | 3.71 | - | |||
|
| ||||||||
| Xylp (14%) | S | 2-α-Xylp | 99.20 | 79.47 | 72.47 | 70.03 | 61.87 | - |
| 5.08 | 3.56 | 3.85 | 3.65 | 3.55 | - | |||
| L | 2-α-Xylp | 98.98 | 81.14 | - | - | - | - | |
| 5.14 | 3.6 | - | - | - | - | |||
| X | t-α-Xylp | 99.48 | 72.06 | 73.67 | 70.14 | - | - | |
| 4.94 | 3.54 | 3.71 | 3.61 | - | - | |||
|
| ||||||||
| Galp (2%) | L | t-β-Galp | 105.10 | - | - | - | - | - |
| 4.60-3.73 | - | - | - | - | - | |||
|
| ||||||||
| Glcp (27%) | _G | t-β-Glclp | 105.24 | - | - | - | - | - |
| 4.53 | 3.62 | - | - | - | - | |||
| G | 4,6-β-Glcp | 103.35 | 73.5 | 74.77 | 79.74 | 74.32 | 67.47 | |
| 4.53 | 3.38 | 3.66 | 3.67 | 3.82 | 3.87/3.80 | |||
| G | 4,6-β-Glcp | 103.20 | 73.5 | 74.77 | 79.64 | 74.32 | 67.04 | |
| 4.52 | 3.37 | 3.66 | 3.69 | 3.82 | 3.93/3.82 | |||
| G | 4-β-Glcp | 103.10 | 73.79 | 76.11 | 79.55 | - | - | |
| 4.51 | 3.3 | 3.49 | 3.54 | - | - | |||
| G_ | α-Glcp | 92.40 | 71.85 | - | - | - | - | |
| 5.21 | 3.57 | 3.82 | 3.64 | 3.94 | 3.86 | |||
| G_ | β-Glcp | 96.34 | 74.44 | 75.32 | 81.15 | 75.41 | 60.52 | |
| 4.65 | 3.28 | 3.63 | 3.62 | 3.59 | 3.80/3.94 | |||
G = -4)-β-D-Glcp-(1-
S = α-L-Araf-(1-2)-a-D-Xylp-(1-6)-β-D-Glcp-(1-
L = β-D-Galp-(1-2)-a-D-Xylp-(1-6)-β-D-Glcp-(1-
X = a-D-Xylp-(1-6)-β-D-Glcp-(1-
G: -4)-β-D-Glcp-(1-
G_: Reducing end glucose
_G: Non-reducing end glucose
Glycosyl linkages of each monosaccharide are listed in Table 1 (GC-MS profile see Supplementary Fig. S8b). In addition to the common glycosyl linkages known for xyloglucan (Fry et al., 1993) 5-α-Arab, 3-α-Arab and 3,5-α-Arab were also found in cranf1b-F2. These additional linkages are consistent with arabinan side chains that are commonly present in cell-wall pectic substances (Caffall & Mohnen, 2009). In xyloglucan nomenclature for side chain subunits (Fry et al., 1993) cranf1b-F2 glycosyl linkages belong to side chain subunits S, L, X and G. 1H and 13C NMR chemical shifts were assigned for the identified cranf1b-F2 subunits (Table 1) based on the recorded 1D NMR and 2D NMR spectra (see Supplementary data) and in consideration of previous reports (Busato et al., 2005; Hoffman et al., 2005; Jia, Cash, Darvill, & York, 2005; Shakhmatov, Toukach, Michailowa, & Makarova, 2014; Watt, Brasch, Larsen, & Melton, 1999).
Although commonly found as separate polymer components of plant cell walls, a portion of xyloglucan and pectic polysaccharides are proposed to be covalently bound (Femenia, Rigby, Selvendran, & Waldron, 1999; Popper & Fry, 2005, 2008; Thompson & Fry, 2000; Vidal, Williams, Doco, Moutounet, & Pellerin, 2003). The putative xyloglucan-pectin complex model was first introduced by Albersheim and coworkers in 1973 (Keegstra, Talmadge, Bauer, & Albersheim, 1973). Thompson and Fry (Thompson & Fry, 2000) observed xyloglucan that co-eluted with anionic pectin during anion exchange chromatography and remained part of the complex after treatment with 8M urea, 6M NaOH and proteinase. Treatment with arabinanase and/or galactanase converted a great portion of the complex into neutral compounds, suggesting that covalent bonding occurs between xyloglucan and the Ara/Gal-rich pectic domain, likely on the arabinan and/or arabinogalactan side chains of a Rhamnogalacturan I region (Abdel-Massih, Baydoun, & Brett, 2003; Popper & Fry, 2005; Thompson & Fry, 2000). However, no NMR spectroscopic evidence for a covalent linkage has yet been reported. In our study, co-elution of the xyloglucan and arabinan components of cranf1b-F2 in every chromatography step, coupled with its slight acidity, (Thompson & Fry, 2000) suggests the existence of a covalent linkage.
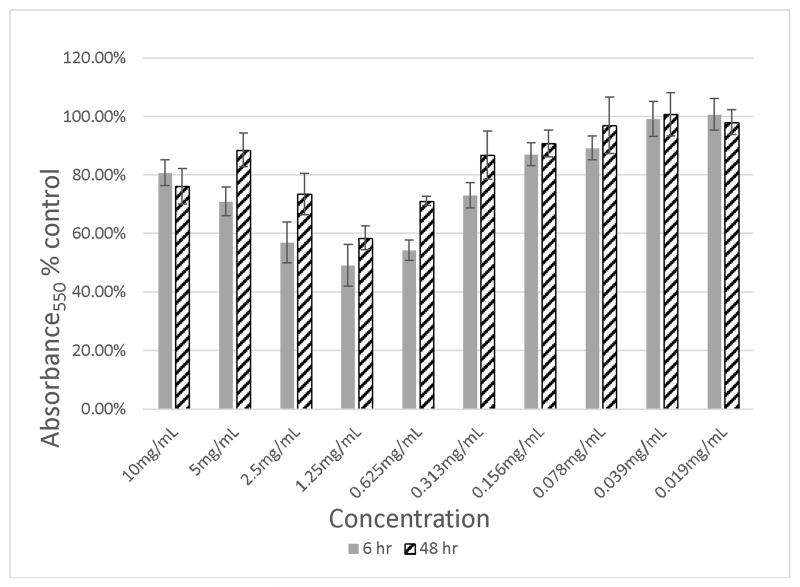
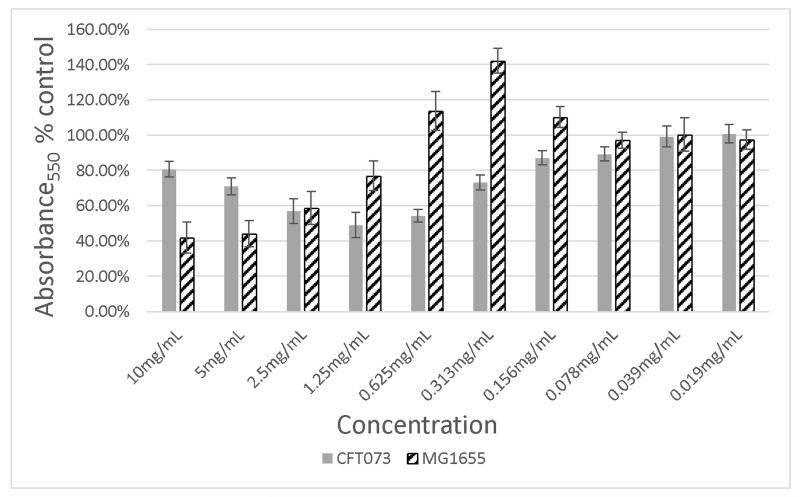
The original cranberry material (Cranf1) and its three major purified fractions, namely, Cranf1W, Cranf1b and Cranf1M were tested for the prevention of biofilm formation against E. coli MG1655, a non-uropathogenic strain, and E. coli CFT073, a well-studied uropathogenic strain (Welch et al., 2002) (see Table S1, Supplementary data). At equivalent concentrations (1.25 mg/mL), Cranf1b showed the most reduction in biofilm formation against the uropathogenic E. coli CFT073 strain, therefore its sub-fractions, Cranf1b-F1 and cranf1b-F2 were further tested against this strain. Although no activity was observed for cranf1b-F1, cranf1b-F2 reduced biofilm formation of E. coli CFT-073 by as much as 50 % at 1.25 mg/mL after 6 h of incubation (Figure 2a). The reductive effect on biofilm formation was maintained for at least 48 h (Figure 2a) with no growth inhibition, demonstrating that the reduced biofilm after 6 h is not merely due to a delay in the initiation of biofilm production. Interestingly, the highest inhibitory effect was not achieved at the highest concentration tested. While the reason for the declining prevention at higher concentration is not yet known, we hypothesise that aggregation of the cranf1b-F2 sample may be partially responsible. HPSEC analysis showed that large particles (>100,000 Da) formed at the higher concentration (Supplementary Fig. S6). Aggregation of oligosaccharides would lead to less concentration of active molecules in solution, hence having a potential impact on the overall activity. Biofilm formation by E. coli MG1655 was also sensitive to the effects of cranf1b-F2 (Figure 2b), but not to cranf1b-F1. A concentration-dependent reduction in biofilm formation was observed between 10 and 0.625 mg/mL; however, an increase in biofilm formation was consistently observed between 0.625 and 0.156 mg/mL of cranf1b-F2. The distinct dose-response patterns between CFT073 and MG1655 may derive from their different abilities to form and sustain biofilms. MG1655 naturally produces much lighter biofilm than CFT073, which likely makes it more vulnerable to biofilm modifying agents.
Figure 2a. Inhibition of E. coli CFT073 biofilm formation by Cranf1b-F2 at concentration from 0.019 mg/mL to 10 mg/mL.
Figure 2b. Inhibition of E. coli MG1655 biofilm formation by Cranf1b-F2 at concentration from 0.019 mg/mL to 10 mg/mL.
As previously discussed, the role of the polyphenols (including PACs) present in cranberries in its preventive effects against urinary tract infections has been extensively studied by several groups (LaPlante, Sarkisian, Woodmansee, Rowley, & Seeram, 2012; Gupta et al., 2012; Howell et al., 2005). Thus, it is possible that the multiple constituents, including polyphenols and oligosaccharides, present in the cranberry whole fruit act additively, complementarily, and/or synergistically in its overall biological effects. Interestingly, in the current study, we did not observe any growth inhibitory and anti-biofilm effects of the Cranf1M fraction (which was enriched in polyphenol constituents) on both of the E. coli strains which was in agreement with our previous report (LaPlante, Sarkisian, Woodmansee, Rowley, & Seeram, 2012). Therefore, while it appears that the phenolic constituents did not contribute to the inhibition of biofilm formation by the uropathogenic E. coli CFT073 strain (based on our bioassays), their overall contribution to the prevention of urinary tract infections by the whole cranberry fruit should not be discounted.
4. Conclusion
In conclusion, our study demonstrates that a phenolic-free, oligosaccharide component of cranberry modifies the biofilm formation of E. coli strains CFT073 and MG1655. Thus, in addition to PACs and other polyphenols, certain carbohydrate components in cranberry may also contribute to its overall anti-infective properties. Further investigation to clarify the structure-activity relationships of these oligosaccharides is currently being pursued by our group.
Supplementary Material
Acknowledgements
This work was supported, in part, by Ocean Spray Cranberries, Inc. (Lakeville-Middleboro, MA, USA). Bacterial strain E. coli CFT073 was a gift from Dr. Paul Cohen (University of Rhode Island). Instruments used for the various chemical analyses were supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (grant number 2 P20 GM103430). NMR and GC-MS data were acquired at a research facility supported in part by the National Science Foundation EPSCoR Cooperative Agreement #EPS-1004057.
Footnotes
Appendix A. Supplementary data
Scheme of fractionation of cranberry materials. Detailed structural analysis data of cranberry materials including Cranf1, Cranf1W, Cranf1b, Cranf1M and cranf1b-F2. Complementary biofilm assay results of Cranf1, Cranf1W, Cranf1b and Cranf1M.
References
- Abdel-Massih RM, Baydoun EA, Brett CT. In vitro biosynthesis of 1,4-beta-galactan attached to a pectin-xyloglucan complex in pea. Planta. 2003;216(3):502–511. doi: 10.1007/s00425-002-0861-y. [DOI] [PubMed] [Google Scholar]
- Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301(5629):105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- Avorn J, Monane M, Gurwitz JH, Glynn RJ, Choodnovskiy I, Lipsitz LA. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. Journal of the American Medical Association. 1994;271(10):751–754. doi: 10.1001/jama.1994.03510340041031. [DOI] [PubMed] [Google Scholar]
- Busato AP, Vargas-Rechia CG, Gorin PA, Petkowicz CL, Tischer CA, Bochicchio R, Reicher F. New 4-O-Substituted Xylosyl Units In The Xyloglucan From Leaves Of Hymenaea Courbaril. International Journal of Biological Macromolecules. 2005;35(5):277–282. doi: 10.1016/j.ijbiomac.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Caffall KH, Mohnen D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydrate Research. 2009;344(14):1879–1900. doi: 10.1016/j.carres.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Ciucanu I, Kerek F. A simple and rapid method for the permethylation of carbohydrates. Carbohydrate Research. 1984;131(2):209–217. [Google Scholar]
- Côté J, Caillet S, Doyon G, Dussault D, Sylvain JF, Lacroix M. Antimicrobial effect of cranberry juice and extracts. Food Control. 2011;22(8):1413–1418. [Google Scholar]
- Femenia A, Rigby N, Selvendran R, Waldron K. Investigation of the occurrence of pectic-xylan–xyloglucan complexes in the cell walls of cauliflower stem tissues. Carbohydrate Polymers. 1999;39(2):151–164. [Google Scholar]
- Foo L, Lu Y, Howell AB, Vorsa N. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry. 2000a;54(2):173–181. doi: 10.1016/s0031-9422(99)00573-7. [DOI] [PubMed] [Google Scholar]
- Foo L, Lu Y, Howell AB, Vorsa N. A-Type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. Journal of Natural Products. 2000b;63(9):1225–1228. doi: 10.1021/np000128u. [DOI] [PubMed] [Google Scholar]
- FRY SC. The structure and functions of xyloglucan. Journal of Experimental Botany. 1989;40(1):1–11. [Google Scholar]
- Fry SC, York WS, Albersheim P, Darvill A, Hayashi T, Joseleau JP, Kato Y, Lorences EP, Maclachlan GA, McNeil M. An unambiguous nomenclature for xyloglucan-derived oligosaccharides. Physiologia Plantarum. 1993;89(1):1–3. [Google Scholar]
- George A. Microtiter dish biofilm formation assay. Journal of Visualized Experiments. 2011;(47) doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Dwivedi M, Mahdi AA, Nagana Gowda GA, Khetrapal CL, Bhandari M. Inhibition of adherence of multi-drug resistant E. coli by proanthocyanidin. Urological Research. 2012;40(2):143–150. doi: 10.1007/s00240-011-0398-2. [DOI] [PubMed] [Google Scholar]
- Hoffman M, Jia Z, Pena MJ, Cash M, Harper A, Blackburn AR, 2nd, Darvill A, York WS. Structural analysis of xyloglucans in the primary cell walls of plants in the subclass Asteridae. Carbohydrate Research. 2005;340(11):1826–1840. doi: 10.1016/j.carres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AT, Nunez A, Khoo C, Strahan GD. Cranberry xyloglucan oligosacharide composition. Patent Application US20130316025 A1. 2013
- Howell AB, Reed JD, Krueger CG, Winterbottom R, Cunningham DG, Leahy M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry. 2005;66(18):2281–2291. doi: 10.1016/j.phytochem.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Iswaldi I, Gomez-Caravaca AM, Arraez-Roman D, Uberos J, Lardon M, Segura-Carretero A, Fernandez-Gutierrez A. Characterization by high-performance liquid chromatography with diode-array detection coupled to time-of-flight mass spectrometry of the phenolic fraction in a cranberry syrup used to prevent urinary tract diseases, together with a study of its antibacterial activity. Journal of Pharmaceutical and Biomedical Analysis. 2012;58:34–41. doi: 10.1016/j.jpba.2011.09.027. [DOI] [PubMed] [Google Scholar]
- Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database of Systematic Reviews. 2012;10:CD001321. doi: 10.1002/14651858.CD001321.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Cash M, Darvill AG, York WS. NMR characterization of endogenously O-acetylated oligosaccharides isolated from tomato (Lycopersicon esculentum) xyloglucan. Carbohydrate Research. 2005;340(11):1818–1825. doi: 10.1016/j.carres.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Keegstra K, Talmadge KW, Bauer W, Albersheim P. The structure of plant cell walls III. A model of the walls of suspension-cultured sycamore cells based on the interconnections of the macromolecular components. Plant Physiology. 1973;51(1):188–197. doi: 10.1104/pp.51.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlante KL, Sarkisian SA, Woodmansee S, Rowley DC, Seeram NP. Effects of cranberry extracts on growth and biofilm production of Escherichia coli and Staphylococcus species. Phytotherapy Research. 2012;26(9):1371–1374. doi: 10.1002/ptr.4592. [DOI] [PubMed] [Google Scholar]
- Lee J. Proanthocyanidin A2 purification and quantification of American cranberry (Vaccinium macrocarpon Ait.) products. Journal of Functional Foods. 2013;5(1):144–153. [Google Scholar]
- Lian PY, Maseko T, Rhee M, Ng K. The antimicrobial effects of cranberry against Staphylococcus aureus. Food Science and Technology International. 2012;18(2):179–186. doi: 10.1177/1082013211415159. [DOI] [PubMed] [Google Scholar]
- Masuko T, Minami A, Iwasaki N, Majima T, Nishimura S-I, Lee YC. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Analytical Biochemistry. 2005;339(1):69–72. doi: 10.1016/j.ab.2004.12.001. [DOI] [PubMed] [Google Scholar]
- McNeil M, Darvill AG, Fry SC, Albersheim P. Structure and function of the primary cell walls of plants. Annual Review of Biochemistry. 1984;53(1):625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- Moreno E, Andreu A, Pigrau C, Kuskowski MA, Johnson JR, Prats G. Relationship between Escherichia coli strains causing acute cystitis in women and the fecal E. coli population of the host. Journal of Clinical Microbiology. 2008;46(8):2529–2534. doi: 10.1128/JCM.00813-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naves P, Del Prado G, Huelves L, Gracia M, Ruiz V, Blanco J, Rodríguez-Cerrato V, Ponte M, Soriano F. Measurement of biofilm formation by clinical isolates of Escherichia coli is method-dependent. Journal of Applied Microbiology. 2008;105(2):585–590. doi: 10.1111/j.1365-2672.2008.03791.x. [DOI] [PubMed] [Google Scholar]
- Niu C, Gilbert E. Colorimetric method for identifying plant essential oil components that affect biofilm formation and structure. Applied and Environmental Microbiology. 2004;70(12):6951–6956. doi: 10.1128/AEM.70.12.6951-6956.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou K, Gu L. Absorption and metabolism of proanthocyanidins. Journal of Functional Foods. 2014;7:43–53. [Google Scholar]
- Popper ZA, Fry SC. Widespread occurrence of a covalent linkage between xyloglucan and acidic polysaccharides in suspension-cultured angiosperm cells. Annals Botany. 2005;96(1):91–99. doi: 10.1093/aob/mci153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper ZA, Fry SC. Xyloglucan-pectin linkages are formed intra-protoplasmically, contribute to wall-assembly, and remain stable in the cell wall. Planta. 2008;227(4):781–794. doi: 10.1007/s00425-007-0656-2. [DOI] [PubMed] [Google Scholar]
- Reid G, Hsiehl J, Potter P, Mighton J, Lam D, Warren D, Stephenson J. Cranberry juice consumption may reduce biofilms on uroepithelial cells: pilot study in spinal cord injured patients. Spinal Cord. 2001;39(1):26–30. doi: 10.1038/sj.sc.3101099. [DOI] [PubMed] [Google Scholar]
- Shakhmatov EG, Toukach PV, Michailowa EA, Makarova EN. Structural studies of arabinan-rich pectic polysaccharides from Abies sibirica L. Biological activity of pectins of A. sibirica. Carbohydrate Polymers. 2014;113:515–524. doi: 10.1016/j.carbpol.2014.07.037. [DOI] [PubMed] [Google Scholar]
- Thompson JE, Fry SC. Evidence for covalent linkage between xyloglucan and acidic pectins in suspension-cultured rose cells. Planta. 2000;211(2):275–286. doi: 10.1007/s004250000287. [DOI] [PubMed] [Google Scholar]
- Vidal S, Williams P, Doco T, Moutounet M, Pellerin P. The polysaccharides of red wine: total fractionation and characterization. Carbohydrate Polymers. 2003;54(4):439–447. [Google Scholar]
- Watt D, Brasch D, Larsen D, Melton L. Isolation, characterisation, and NMR study of xyloglucan from enzymatically depectinised and non-depectinised apple pomace. Carbohydrate Polymers. 1999;39(2):165–180. [Google Scholar]
- Welch R, Burland V, Plunkett G, Redford P, Roesch P, Rasko D, Buckles E, Liou S-R, Boutin A, Hackett J. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proceedings of the National Academy of Sciences. 2002;99(26):17020–17024. doi: 10.1073/pnas.252529799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York WS, Darvill AG, McNeil M, Stevenson TT, Albersheim P. Isolation and characterization of plant cell walls and cell wall components. Methods in Enzymology. 1986;118:3–40. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.