Abstract
Background
Worldwide, there is a high co-endemicity of HIV and H. pylori infection and there is growing evidence that H. pylori co-infection is associated with parameters of HIV disease progression. The objective of this study was to investigate the prevalence of H. pylori infection, and the association with clinical, immunological and virological parameters in a large cohort of HIV-infected individuals and uninfected controls in a West African country.
Methods
HIV-patients (n = 1,095) and HIV-negative individuals (n = 107) were recruited at a university hospital in Ghana. H. pylori status was determined using stool antigen testing. HIV-related, clinical and socio-demographic parameters were recorded and analyzed according to H. pylori status.
Results
The prevalence of H. pylori infection was significantly lower in HIV-positive compared to HIV-negative individuals (51.5 vs. 88%, p<0.0001). In HIV patients, H. pylori prevalence decreased in parallel with CD4+ T cell counts. In ART-naïve HIV-infected individuals, but not in those taking ART, H. pylori infection was associated with higher CD4 cell counts (312 vs. 189 cells/μL, p<0.0001) and lower HIV-1 viral loads (4.92 vs. 5.21 log10 copies/mL, p = 0.006). The findings could not be explained by socio-demographic confounders or reported use of antibiotics. Having no access to tap water and higher CD4+ T cell counts were identified as risk factors for H. pylori infection.
Conclusions
H. pylori prevalence was inversely correlated with the degree of immunosuppression. In ART-naïve individuals, H. pylori infection is associated with favorable immunological and virological parameters. The underlying mechanisms for this association are unclear and warrant investigation.
Introduction
Recently, the interplay between the Human immunodeficiency virus (HIV) and Helicobacter pylori (H. pylori) infection has attracted attention. A number of studies have reported lower H. pylori prevalence rates in HIV-infected compared to HIV-negative individuals [1,2]. This association is unexpected, since usually chronic infections are more commonly found in patients with advanced HIV disease. Furthermore, H. pylori infection is considered a disease of poverty, and poor socioeconomic status has been associated with rather disadvantageous outcomes of HIV infection [3]. The underlying mechanisms for this observed association are unclear. Most existing studies have important limitations such as small sample sizes thus preventing subgroup analyses and robust adjustment for confounders. In particular, information on socio-economic variables, as putative confounders for H.pylori status is sorely lacking. As a consequence, interpretation and comparison of results are difficult and data published to date is partly inconsistent [2].
Considering the significant epidemiological and pathophysiological overlap of HIV and H. pylori infection, the investigation of possible interplay is of interest. Over the past few years it has become clear, that the gastrointestinal tract (GIT) plays an important role in the pathophysiology of HIV/AIDS. Chronic immune activation, associated with intestinal barrier dysfunction, has been identified as central pathomechanism in HIV disease [4]. H. pylori colonize the gastric and duodenal mucosae and induce a specific local and also systemic immune response, involving, among others, CD4+ T cells, dendritic cells, regulatory T cells (Treg) and Th17 cells, with all of these also playing a role in HIV pathogenesis [5–8].
The association of HIV and H. pylori co-infection has not been systematically studied in sub-Saharan Africa, where more than two thirds of HIV-infected individuals live, and where, at the same time, the vast majority of the population gets infected with H. pylori during childhood [9–11]. The objective of this study was to investigate the prevalence of H. pylori infection, and its association with clinical, immunological and virological parameters in a large cohort of HIV-infected individuals and uninfected controls in a West African country.
Materials & Methods
Study setting and recruitment
This cross-sectional study was conducted at the Komfo Anokye Teaching Hospital, a tertiary referral hospital in the Ashanti Region of Ghana. Between November 2011 and November 2012, consecutive adult HIV-infected patients presenting to the HIV outpatient clinic, and HIV-negative blood donors presenting to the blood bank of the hospital, were offered participation in the study. All participants gave a written informed consent prior to enrolment. The study was conducted in conformity with the Helsinki declaration, and was approved by the appropriate ethics committees of the Kwame Nkrumah University of Science and Technology (Ghana) and of the medical association in Hamburg (Germany).
Data collection and measures
Demographic, socioeconomic, and clinical data, as well as a detailed medical history were recorded using standardized questionnaires, which were completed by trained study personnel. In particular, time since diagnosis of HIV infection, duration and kind of antiretroviral therapy (ART), co-medications, and clinical parameters were documented. Routine laboratory parameters were extracted from patient’s folders. EDTA blood samples were obtained for the analysis of CD4/CD8 T cell counts, using a FACSCalibur® flow cytometer (Becton Dickinson, USA). HIV-1 and 2 antibody testing was done using the First Response® HIV-1/2 test (Premier Medical Corporation Limited, India) and the Genscreen® ULTRA HIV Ag-Ab Assay (Bio-Rad, France). EDTA plasma and native stool samples were freshly frozen at -80°C and transported to Germany on dry ice. Stool was tested for H. pylori using the RidaScreen® FemtoLab H. pylori stool antigen test (R-Biopharm AG, Germany). The sensitivity and specificity of this test has been described to be 98% and 96.7% in pediatric patients and 93% and 90% in adult patients [12,13]. HIV-1 viral load was measured using the RealTime HIV-1 PCR system (Abbott Diagnostics, Wiesbaden, Germany) according to the manufacturer’s instructions. The same tests, except HIV-1 viral load analysis, were conducted for cases and controls.
Statistical analysis
Parametric variables were compared using the Student’s t-test, non-parametric variables were compared using the Mann-Whitney U-test. Categorical data were analyzed using Chi-squared or Fisher’s exact test. A multivariable logistic regression model was used to analyze the association between H. pylori infection and other demographic, clinical and laboratory parameters, using only parameters with a significance level of ≤0.05 in bivariate analysis and a correlation coefficient of ≤0.10 in the multivariate regression model. Missing data were excluded from analysis. Statistical analyses were conducted with SPSS version 19 software (IBM, Germany).
Results
Cohort characteristics
We recruited 1,095 HIV-positive individuals and 107 HIV-negative blood donors. Stool samples for H. pylori testing were available for 952 HIV-positive (86.9%) and 100 HIV-negative individuals (93.5%). HIV-positive, compared to HIV-negative individuals, were more often female, significantly older, had a lower BMI, lower socioeconomic status, lower CD4 and higher CD8 T cell counts (Table 1). The majority of HIV-infected participants were female (75.6%), and the mean age was 40 years. Approximately half of HIV-positive individuals (n = 500, 52.5%) were ART-naïve at the time of recruitment, 452 (47.5%) patients were receiving ART for a median duration of 45 months (IQR 19–69). Participants receiving ART, compared to ART-naïve participants, were more likely to be female, had a higher BMI, higher total absolute lymphocyte and CD4 T cell counts compared to ART-naïve HIV-positive participants (S1 Table).
Table 1. Comparison of demographic and laboratory characteristics of HIV-positive and HIV-negative participants.
| Variable | HIV-positive | HIV-negative | p-value |
|---|---|---|---|
| N = 952 | N = 100 | ||
| Female gender, n (%) | 720 (75.6) | 66 (66.0) | 0.04 |
| Age (years), mean ± SD | 40 ± 9.5 | 33 ± 12.3 | <0.0001 |
| Religion, n (%) # | 0.12 | ||
| Christian | 814 (85.5) | 86 (92.5) | |
| Moslem | 120 (12.6) | 6 (6.5) | |
| Traditional African religion | 2 (0.2) | 0 (0.0) | |
| Other | 16 (1.7) | 1(1.0) | |
| Educational level, n (%) # | <0.0001 | ||
| Primary education | 156 (16.4) | 9 (9.7) | |
| Junior Secondary School | 426 (44.7) | 7 (7.5) | |
| Senior Secondary School | 133 (14.0) | 56 (60.2) | |
| Tertiary education | 51 (5.4) | 14 (15.1) | |
| No formal education | 186 (19.5) | 7 (7.5) | |
| Occupation, n (%) # | <0.0001 | ||
| House wife | 13 (1.4) | 1(1.1) | |
| Farmer | 78 (8.2) | 2 (2.2) | |
| Trader | 505 (53.0) | 33 (35.5) | |
| Salary worker | 60 (6.3) | 27 (29.0) | |
| Others | 114 (12.0) | 4 (4.3) | |
| Currently unemployed | 182 (19.1) | 24 (25.8) | |
| Access to tap water, n (%)* | 501 (52.6) | 61 (63.5) | 0.04 |
| H. pylori test result, n (%) | |||
| Positive | 490 (51.5) | 88 (88.0) | <0.0001 |
| Negative | 452 (47.5) | 12 (12.0) | |
| Indeterminate | 10 (1.0) | 0 (0.0) | |
| BMI (kg/m2), mean ± SD | 23.1 ± 4.6 | 24.7 ± 5.0 | 0.002 |
| T-cell populations, median (IQR) | |||
| Total T-cell count/μL | 1,381 (984–1,968) | 1,460 (1,171–1,895) | 0.13 |
| CD4 T-cell count/μL | 380 (173–596) | 958 (786–1,161) | <0.0001 |
| CD8 T-cell count/μL | 914 (620–1,341) | 439 (312–673) | <0.0001 |
BMI, Body mass index
# missing data for 7 participants of the HIV negative group.
* Missing data for 4 participants of the HIV-negative group.
H. pylori infection
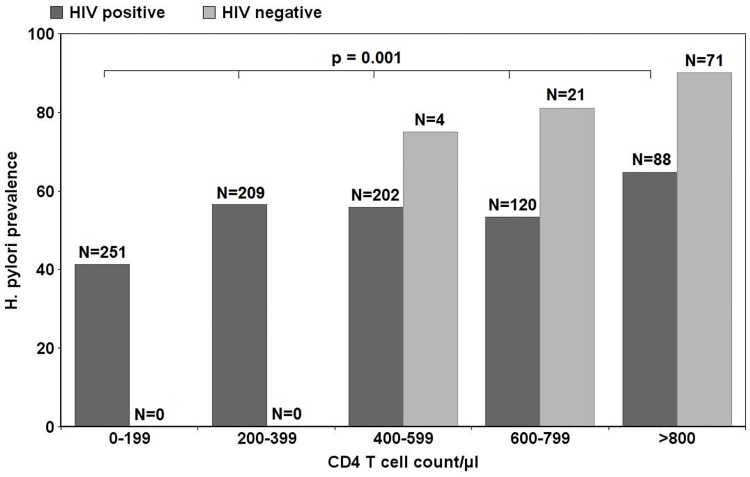
The prevalence of H. pylori infection among HIV-negative individuals was significantly higher compared to HIV-positive individuals (88.0% vs. 51.5%, p<0.0001). In HIV-positive individuals, H. pylori prevalence declined in parallel with CD4+ T cell counts, from 64.8% in patients with more than 800 CD4 T cells/μL, to 41.4% in patients with less than 200 CD4 T cells/μL. The same trend was observed in HIV-negative individuals, without reaching statistical significance (Fig 1).
Fig 1. Comparison of H. pylori prevalence according to CD4 T cell count/μL for HIV-positive participants (p = 0.001, Chi-square test) and for HIV-negative individuals (p = 0.397, Chi-square test); N = Group sizes for CD4 T cell categories including H. pylori positive and negative participants.
The characteristics of HIV-infected individuals according to H. pylori status are shown in Table 2. H. pylori co-infected HIV-positive patients were significantly less likely to have access to tap water (48.8 vs. 58.0%, p = 0.005) and less likely to have attained a tertiary level education (3.7 vs. 7.3%, p = 0.01). There were no significant differences in other demographic variables assessed, or in WHO clinical HIV disease stages (Table 3).
Table 2. Comparison of socio-demographic parameters of HIV-infected participants according to H. pylori status.
| Variable | H. pylori positive | H. pylori negative | p-value |
|---|---|---|---|
| N = 490 | N = 452 | ||
| Female gender, n (%) | 372 (75.9) | 339 (75.0) | 0.74 |
| Age (years), mean ± SD | 40 ± 9.4 | 40 ± 9.6 | 0.97 |
| Religion, n (%)# | |||
| Christian | 410 (83.7) | 396 (87.6) | 0.12 |
| Moslem | 71 (14.5) | 47 (10.4) | |
| Traditional African religion | 2 (0.4) | 0 (0.0) | |
| Other | 7 (1.4) | 9 (2.0) | |
| Educational level, n (%)# | 0.08 | ||
| No formal education | 91 (18.6) | 91 (20.1) | |
| Primary education | 84 (17.1) | 72 (15.9) | |
| Secondary education | 297 (60.6) | 256 (56.6) | |
| Tertiary education | 18 (3.7) | 33 (7.3) | |
| Occupation, n (%)## | 0.75 | ||
| House wife | 6 (1.2) | 7 (1.5) | |
| Farmer | 42 (8.6) | 36 (8.0) | |
| Trader | 257 (52.4) | 242 (53.5) | |
| Salary worker | 27 (5.5) | 33 (7.3) | |
| Others | 64 (13.1) | 48 (10.6) | |
| Currently unemployed | 94 (19.2) | 86 (19.0) | |
| Access to tap water, n (%) | 239 (48.8) | 262 (58.0) | 0.005 |
| Electricity in the household, n (%) | 452 (92.2) | 420 (92.9) | 0.39 |
| Television in household, n (%) | 398 (81.2) | 366 (81.0) | 0.92 |
| Owning a fridge, n (%) | 336 (68.6) | 322 (71.2) | 0.89 |
| Owning a car, n (%) | 35 (7.1) | 51 (11.3) | 0.03 |
Analysis excludes 10 patients with indeterminate H. pylori result.
Table 3. Comparison of clinical and laboratory parameters in HIV-positive and HIV-negative individuals according to H. pylori status.
| Variable | ART-naïve group, n = 494 | ART group, n = 448 | HIV negative group, n = 100 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| H. pylori pos. | H. pylori neg. | p-value | H. pylori pos. | H. pylori neg. | p-value | H. pylori pos. | H. pylori neg. | p-value | |
| N = 239 (48.4%) | N = 255 (51.6%) | N = 251 (56.0%) | N = 197 (44.0%) | N = 88 (88.0%) | N = 12 (12.0%) | ||||
| Time since HIV diagnosis (months), median (IQR) | 0.5 (0.5–3.5) | 0.5 (0.5–1.0) | 0.006 | 53 (24–82) | 53 (25–74) | 0.42 | NA | NA | NA |
| Time on ART (months) median (IQR) | NA | NA | 45 (18–70) | 47 (22–68) | 0.98 | NA | NA | NA | |
| WHO stage # | 0.22 | 0.15 | NA | NA | NA | ||||
| 1 | 109 (45.6) | 118 (46.3) | 139 (55.4) | 117 (59.4) | NA | NA | |||
| 2 | 23 (9.6) | 31 (12.2) | 34 (13.5) | 13 (6.6) | NA | NA | |||
| 3 | 28 (11.7) | 38 (14.9) | 35 (13.9) | 28 (14.2) | NA | NA | |||
| 4 | 0 (0.0) | 2 (0.8) | 2 (0.8) | 4 (2.0) | NA | NA | |||
| No data | 79 (33.1) | 66 (25.9) | 41 (16.3) | 35 (17.8) | NA | NA | |||
| Exposure to TB treatment, n (%) | 21 (8.8) | 41 (16.1) | 0.01 | 24 (9.6) | 17 (8.6) | 0.87 | 0 (0.0) | 0 (0.0) | NA |
| Currently on TB treatment, n (%) | 8 (3.3) | 25 (9.8) | 0.004 | 1 (0.4) | 2 (1.0) | 0.58 | 0 (0.0) | 0 (0.0) | NA |
| Previous TB treatment, n (%) | 13 (5.4) | 16 (6.3) | 0.69 | 23 (9.2) | 15 (7.6) | 0.56 | 0 (0.0) | 0 (0.0) | NA |
| Antibiotic use | NA | ||||||||
| Antibiotic use past six months, n (%) | 3 (1.3) | 2 (0.8) | 0.68 | 0 (0.0) | 1 (O.5) | 0.58 | 0 (0.0) | 0 (0.0) | |
| Current use of co-trimoxazole, n (%) | 62 (25.9) | 80 (31.4) | 0.18 | 49 (19.5) | 48 (24.4) | 0.25 | 0 (0.0) | 0 (0.0) | |
| Self-reported symptoms * | |||||||||
| Epigastric discomfort | 24 (10.0) | 24 (9.4) | 0.81 | 5 (2.0) | 8 (4.1) | 0.20 | 18 (21.4) | 0 (0.0) | 0.08 |
| Anorexia | 6 (2.5) | 12 (4.7) | 0.19 | 2 (0.8) | 5 (2.5) | 0.14 | 0 (0.0) | 0 (0.0) | NA |
| Nausea and vomiting | 9 (3.8) | 16 (6.3) | 0.11 | 2 (0.8) | 6 (3.0) | 0.07 | 2 (2.4) | 0 (0.0) | 0.68 |
| Diarrhea | 23 (9.6) | 23 (9.0) | 0.82 | 5 (2.0) | 1 (0.5) | 0.17 | 12 (14.3) | 2 (16.7) | 0.83 |
| Weight loss | 78 (32.6) | 102 (40.0) | 0.09 | 15 (6.0) | 16 (8.1) | 0.37 | 4 (4.5) | 0 (0.0) | 0.83 |
| Body Mass Index (kg/m2), mean ±SD | 22.4 ± 4.1 | 21.9 ± 4.2 | 0.19 | 24.3 ± 4.5 | 23.8 ± 4.6 | 0.21 | 24.7 ± 5.0 | 24.6 ± 5.6 | 0.96 |
| HIV-1 VL (log 10 c/mL), median (IQR) § | 4.92 (4.09–5.51) | 5.21 (4.59–5.63) | 0.006 | 3.67 (3.10–4.57) | 3.09 (2.31–4.71) | 0.54 | NA | NA | NA |
| T-cell populations, median (IQR) | |||||||||
| Total T-cell count/μL | 1227 (867–1929) | 1253(794–1921) | 0.94 | 1452 (1108–1934) | 1584 (1151–2083) | 0.05 | 1439 (1166–1910) | 1520 (1318–1717) | 0.79 |
| CD4 T-cell count/μL | 312 (128–508) | 189 (75–403) | <0.0001 | 450 (270–643) | 476 (272–654) | 0.78 | 977 (792–1205) | 861 (741–1008) | 0.23 |
| CD8 T-cell count/μL | 832 (564–1336) | 980 (595–1569) | 0.29 | 858 (610–1230) | 990 (697–1356) | 0.02 | 436 (309–637) | 585 (402–758) | 0.18 |
| CD4/CD8 ratio | 0.31 (0.17–0.60) | 0.19 (0.09–0.41) | <0.0001 | 0.55 (0.37–0.84) | 0.49 (0.32–0.81) | 0.07 | 2.12 (1.70–2.91) | 1.80 (1.01–2.42) | 0.13 |
| WBC (x1000/μL), mean ± SD | 4.99 ± 1.98 | 5.28 ± 2.09 | 0.35 | 5.0 (4.0–6.0) | 5.0 (4.0–6.0) | 0.90 | NA | NA | NA |
| Hemoglobin (g/dL), mean ± SD | 11.1 ± 1.79 | 10.4 ± 2.02 | 0.01 | 12.0 (11.0–13.0) | 12.0 (11.0–13.0) | 0.83 | NA | NA | NA |
| Platelets (x1000/μL), mean ± SD | 262.4 ± 97.2 | 314.9 ± 124.2 | 0.003 | 283 (224–333) | 283 (228–330) | 0.84 | NA | NA | NA |
Analysis excludes 10 patients with indeterminate H. pylori result. BMI, Body mass index; WBC, White blood cells; Hgb, Hemoglobin
# WHO clinical stage at recruitment, missing data for 147 patients of the ART-naïve group and 79 of the ART group.
§ Missing viral load data for 14 H. pylori positive and 17 H. pylori negative participants
*Self-reported symptoms in the past 4 weeks, weight loss defined as significant for the patient, or loss of >10% of body weight. Diarrhea was defined as the passage of three or more loose or liquid stools per day
Associations between H. pylori infection and HIV clinical, immunological and virological parameters
Among ART-naïve HIV patients, those with H. pylori co-infection had higher CD4 T cell counts (312 vs. 189 cells/μl, p<0.0001), higher CD4/CD8 ratios (0.31 vs. 0.19, p<0.0001) and lower HIV-1 viral loads (4.92 vs. 5.21 log10 copies/ml, p = 0.006) compared to those without H. pylori co-infection. H. pylori positive patients in this group also had higher mean hemoglobin levels (11.1 vs. 10.4 g/dl, p = 0.01), and lower platelet counts (262.4 vs. 314.9 x1000/μl, p = 0.003), as shown in Table 3. There was no significant difference in the reported use of antibiotics in the 6 months before recruitment between H. pylori positive and negative individuals.
H. pylori infection was also not associated to increased frequencies of gastrointestinal symptoms in H. pylori positive, compared to negative patients, with weight loss (32.6% vs. 40%, p = 0.09), epigastric discomfort (10.0% vs. 9.4%, p = 0.81), and diarrhea (9.6% vs. 9.0%, p = 0.82) being the most common symptoms. In the HIV-infected, ART-exposed group, no significant associations between H. pylori status and CD4+ T cell count, HIV-1 viral load, or the proportion of patients with undetectable viral load were observed. However, significantly lower CD8+ T cell counts (858/μL vs. 990/μL, p = 0.02), and a trend towards higher CD4/CD8 ratios (0.55 vs. 0.49, p = 0.07), as possible indicator of decreased immune activation, were noted among those patients with H. pylori co-infection [14–17].
Among HIV-negative controls, no differences in baseline characteristics, symptoms, or socio-demographic parameters were observed between individuals with and without H. pylori infection. A weak trend towards higher CD4/CD8 ratios was also observed in those HIV-negative individuals with H. pylori infection, compared to those without H. pylori infection (2.12 vs. 1.80, p = 0.13).
Logistic regression analysis of risk factors associated with H. pylori infection in HIV-positive individuals
Using a logistic multivariable regression model including parameters with p≤0.05 in the univariate analysis and a correlation coefficient of ≤0.1 in the regression model, only CD4+ T cell count (aOR 1.06, 95% CI 1.01–1.12, p = 0.012 for every 100 cells/μl higher) and having access to tap water (aOR 0.63, 95% CI 0.47–0.84, p = 0.002) were associated with H. pylori infection (Table 4). Significant predictors of H. pylori co-infection noted in univariate but not in multivariate analysis included use of anti-tuberculous therapy, current use of ART and use of co-trimoxazole. The risk ratio (RR) for H. pylori infection was 0.82 for those patients having access to tap water and 1.37 for those with >200 CD4 T cells/μl within the group of HIV-positive patients. No risk factors were identified to be associated with H. pylori infection in the HIV-negative group (data not shown).
Table 4. Univariate and multivariate logistic regression analysis of factors associated with H. pylori co-infection among HIV-infected individuals.
| Predictor | Unadjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| Female gender | 0.95 (0.71–1.28) | 0.743 | ||
| - | - | |||
| Age | 0.10 (0.99–1.01) | 0.968 | - | - |
| Educational level | 0.94 (0.85–1.03) | 0.167 | - | - |
| Access to Tap water | 0.69 (0.53–0.89) | 0.005 | 0.63 (0.47–0.84) | 0.002 |
| Intake of tuberculosis therapy | 0.66 (0.43–0.10) | 0.049 | 0.72 (0.46–1.12) | 0.142 |
| Use of co-trimoxazole | 0.74 (0.55–0.10) | 0.046 | 0.75 (0.53–1.04) | 0.084 |
| Use of ART | 1.36 (1.05–1.76) | 0.019 | 1.17 (0.86–1.59) | 0.331 |
| Duration on ART | 1.00 (1.00–1.01) | 0.535 | - | - |
| Each 12-month increase | ||||
| T-cell CD4 count | 1.07 (1.03–1.11) | 0.001 | 1.06 (1.01–1.12) | 0.012 |
| Each increase of 100 cells/μL | ||||
| Viral load | 0.91 (0.86–0.98) | 0.007 | - | - |
| Each increase of 1 log c/mL |
Parameters with a p-value ≤0.05 and a correlation coefficient of ≤0.1 between the parameters were included into the multivariate regression model.
Discussion
This is the first and largest study to systematically investigate the interplay between H. pylori and HIV infection in sub-Saharan Africa, where both infections are highly co-endemic. We assessed the prevalence of H. pylori co-infection in a large cohort of unselected adult HIV-infected individuals and HIV-negative controls, and its association with clinical, immunological and virological parameters. We found a graded decrease in H. pylori prevalence in relation to the level of immune competence, being 88% in HIV-negative and 51.5% in HIV-positive individuals. Among HIV positive individuals, H. pylori prevalence declined in tandem with CD4+ T cell counts. A similar trend was observed in HIV-negative individuals, although statistical significance was not attained.
Our results are in accordance with previous epidemiologic studies, indicating a lower H. pylori prevalence in HIV-positive compared to HIV-negative individuals, and also among patients with AIDS compared to matched HIV-infected patients without AIDS [1,2]. However, the interpretation of existing studies is hampered by important limitations, such as small sample sizes which precluded subgroup analyses, and heterogeneous study populations, often including only patients with gastrointestinal symptoms [2]. Information on socio-demographic variables, as putative confounders for H. pylori status, often lacking in previous studies have been explored in the present study. Furthermore, studies including asymptomatic patients used serological tests to determine H. pylori status, which have been shown to be problematic especially in HIV-infected individuals [18]. H. pylori stool antigen tests, as employed in the present study, are non-invasive and have a proven high sensitivity and specificity, making them suitable tools for epidemiologic studies including HIV-infected individuals [12,13].
Although H. pylori is generally considered a disease of poverty and known to be associated with poor hygienic conditions, HIV-negative participants in our study, having a clearly higher H. pylori prevalence, ironically had indicators of a higher socioeconomic status. This suggests that the significant differences in H. pylori prevalence observed between the HIV positive and HIV negative participants may not be explained wholly by socioeconomic disparities. Indeed, the HIV negative participants had more frequent access to tap water compared to HIV-positive individuals, and having no access to tap water was independently associated with the H. pylori infection in our study. Besides indicating poor sanitary conditions, the lack of access to tap water might also directly promote H. pylori acquisition by consumption of contaminated drinking water, e.g. from wells. An association between H. pylori and the consumption of water from wells has previously been reported from India, [19] and H. pylori has also been identified in drinking water samples from Pakistan by PCR [20].
We also found a significant graded decrease in H. pylori prevalence with the progression of immunodeficiency in HIV-positive individuals, with the same trend being observed in HIV-negative individuals, but without reaching statistical significance. The underlying mechanisms responsible for this association between immune competence and H. pylori prevalence are still unclear, although several hypotheses have been offered [2]. The most popular is that more frequent bacterial infections in HIV patients, especially those with advanced disease stages, lead to antibiotic treatment courses, probably resulting in unintended H. pylori eradication [2]. We found no association between H. pylori status and reported intake of antibiotics in the past six months before recruitment. Furthermore, only few patients reported taking antibiotics in this period of time, making it unlikely that the observed differences in H. pylori prevalence are explained by unintended eradication in our study population.
Antibiotic monotherapy has been reported to have only minor efficacy in H. pylori eradication [21]. Using a meta-analysis methodology, a pooled H. pylori eradication rate of 19% for monotherapy regimens has been reported [22]. In our study, Co-trimoxazole prophylaxis and tuberculosis therapy were associated with lower risk of H. pylori status in univariate, but not in multivariate logistic regression analysis. Co-trimoxazole has not been reported to have activity against H. pylori, and a culture medium containing trimethoprim and sulfamethoxazole has been developed to selectively isolate H. pylori from animal samples [23]. In contrast, it is known that rifampicin has activity against H. pylori [24]. A temporary suppression of H. pylori replication by concurrent tuberculosis treatment, or even clearance of the infection, is thus conceivable. However, it is to be noted that HIV patients with advanced disease are often prescribed Co-trimoxazole prophylaxis against opportunistic infections and are also more likely to receive anti-tubercular therapy for tuberculosis hence the observed lack of significant association in multivariate analyses between use of these antibiotics and risk of H. pylori co-infection. These findings suggest that progressive HIV disease rather than antibiotic usage may account for the diminution in frequency of H. pylori co-infection.
Another proposed hypothesis is that the maintenance of H. pylori infection requires an intact mucosal cellular immunity, and that the loss of the CD4+ T cell population in the gastric mucosa may prevent H. pylori persistence [2,25,26]. Hence the parallel decline of H. pylori prevalence with CD4+ T cell count would be consistent with this theory, although there is no evidence that impaired T cell immunity itself might cause a loss of H. pylori infection. CD4+ T cells have been shown to be increased in H. pylori gastritis, but gastric inflammation has been shown to correlate with lower H. pylori bacterial load, and pro-inflammatory genetic profiles are associated to lower H. pylori seroprevalence [27–29]. While Th1 and Th17-polarized effector T cell subsets are critical for the control of H. pylori infection, regulatory T cells have the ability to override this T cell driven immunity [30]. Although the alterations of gastric mucosal T cell immunity in the context of HIV infection are incompletely understood, HIV infection apparently rather impairs regulatory T cell suppressive capacity and is thus unlikely to directly promote H. pylori persistence [31]. Further studies are needed to dissect the interplay between systemic and local mucosal T-cell immunity and H. pylori persistence in the context of HIV infection.
H. pylori infection is linked to a number of adverse clinical effects, such as iron deficiency anemia, childhood growth faltering, other gastrointestinal infections and chronic diarrhea[32–35]. In our study however, H. pylori infection was not associated to the presence of diarrhea, anemia, malnutrition or parasitic diseases (data not shown). Indeed a paradoxical protective effect of H. pylori infection against tuberculosis has been reported [36]. Furthermore, H. pylori infection is associated with enhanced Th1-type immune responses to TB antigens [37]. We have recently shown that H. pylori infection is associated with decreased markers of immune activation in ART-naïve HIV infected patients [38]. Considering that immune activation has been demonstrated to be one of the key mechanisms in HIV pathogenesis [14–17], it is tempting to speculate that H. pylori infection may influence susceptibility to HIV infection or the natural course of HIV disease. A large proportion of HIV-infected individuals worldwide are co-infected with H. pylori, hence such interaction could be relevant for the understanding of HIV immunopathology, and could also have public health implications, especially considering the ongoing efforts to develop an H. pylori vaccine [39].
There are some limitations of our study to be mentioned. The sample size of our HIV-negative group was smaller than that of HIV-positive individuals, and differed in terms of age and gender distribution. However, the main focus of this study was to analyze the effect of H. pylori within the group of HIV patients. Since we included unselected HIV patients, the group was heterogenous, among others, in terms of ART status and clinical stage of HIV disease. The group of patients taking ART in particular was heterogeneous, and we did not record details on the history and efficacy of ART in terms of CD4+ T cell recovery and virological suppression, limiting the informative value of the analysis in this subgroup. Importantly, the cross sectional study design did not allow for the investigation of causal relationships concerning the described associations.
In conclusion, we have shown that H. pylori infection is associated with higher CD4+ T cell counts and lower HIV-1 viral loads in ART-naïve patients. Our findings could not be explained by typical confounders as socioeconomic factors, time since diagnosis of HIV infection or unintended H. pylori eradication by antibiotic use for other infectious conditions. Considering the pathophysiological overlap of both chronic infections, the effects of H. pylori infection on the systemic immune response, and subsequently on the natural course of HIV disease, warrants further investigation employing prospective studies.
Supporting Information
(DOCX)
Acknowledgments
We are grateful to the nurses and physicians of the HIV clinic and the blood bank of the Komfo Anokye Teaching Hospital in Kumasi, Ghana for their support and Mr. Shadrack Osei Assibey for data entry. We thank ESTHER Germany for their continuous support of the partnership with the Komfo Anokye Teaching Hospital.
Data Availability
Data contain sensitive participant information and are available upon request from the corresponding author.
Funding Statement
This work was supported by the German Federal Ministry of Education and Research [grant number 01KA1102 ]. The funders had no role in the design of the study and decision to publish these findings.
References
- 1. Romanelli F, Smith KM, Murphy BS. Does HIV infection alter the incidence or pathology of Helicobacter pylori infection? AIDS patient care and STDs. 2007;21(12):908–919. [DOI] [PubMed] [Google Scholar]
- 2. Nevin DT, Morgan CJ, Graham DY, Genta RM. Helicobacter pylori Gastritis in HIV-Infected Patients: A Review. Helicobacter. 2014;19(5):323–329. [DOI] [PubMed] [Google Scholar]
- 3. Burkey MD, Weiser SD, Fehmie D, Alamo-Talisuna S, Sunday P, Nannyunja J, et al. Socioeconomic determinants of mortality in HIV: evidence from a clinical cohort in Uganda. Journal of acquired immune deficiency syndromes. 2014;66(1):41–47. 10.1097/QAI.0000000000000094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Assimakopoulos SF, Dimitropoulou D, Marangos M, Gogos CA. Intestinal barrier dysfunction in HIV infection: pathophysiology, clinical implications and potential therapies. Infection. 2014. [DOI] [PubMed] [Google Scholar]
- 5. Khamri W, Walker MM, Clark P, Atherton JC, Thursz MR, Bamford KB et al. Helicobacter pylori stimulates dendritic cells to induce interleukin-17 expression from CD4+ T lymphocytes. Infection and immunity. 2010;78(2):845–853. 10.1128/IAI.00524-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitchell P, Germain C, Fiori PL, Khamri W, Foster GR, Ghosh S, et al. Chronic exposure to Helicobacter pylori impairs dendritic cell function and inhibits Th1 development. Infection and immunity. 2007;75(2):810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goll R, Gruber F, Olsen T, Cui G, Raschpichler G, Buset M, et al. Helicobacter pylori stimulates a mixed adaptive immune response with a strong T-regulatory component in human gastric mucosa. Helicobacter. 2007;12(3):185–192. [DOI] [PubMed] [Google Scholar]
- 8. Arnold IC, Hitzler I, Muller A. The immunomodulatory properties of Helicobacter pylori confer protection against allergic and chronic inflammatory disorders. Frontiers in cellular and infection microbiology. 2012;2:10 10.3389/fcimb.2012.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rothenbacher D, Inceoglu J, Bode G, Brenner H. Acquisition of Helicobacter pylori infection in a high-risk population occurs within the first 2 years of life. The Journal of pediatrics. 2000;136(6):744–748. [PubMed] [Google Scholar]
- 10. Goodwin CS, Mendall MM, Northfield TC. Helicobacter pylori infection. Lancet. 1997;349(9047):265–269. [DOI] [PubMed] [Google Scholar]
- 11.UNAIDS. The Gap Report. 2014.
- 12. Makristathis A, Barousch W, Pasching E, Binder C, Kuderna C, Apfalter P, et al. Two enzyme immunoassays and PCR for detection of Helicobacter pylori in stool specimens from pediatric patients before and after eradication therapy. Journal of clinical microbiology. 2000;38(10):3710–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erzin Y, Altun S, Dobrucali A, Asian M, Erdamar S, et al. Comparison of two different stool antigen tests for the primary diagnosis of Helicobacter pylori infection in turkish patients with dyspepsia. Helicobacter. 2004;9(6):657–662. [DOI] [PubMed] [Google Scholar]
- 14. Mussini C, Lorenzini P, Cozzi-Lepri A, Lapadula G, Marchetti G, Nicastri E, et al. for the ICONA Foundation Study Group. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV 2015; e98–e106. 10.1016/S2352-3018(15)00006-5 10.1016/S2352-3018(15)00006-5 [DOI] [PubMed] [Google Scholar]
- 15. Serrano-Villar S, Gutierrez C, Vallejo A, Hernandez-Novoa B, Diaz L, et al. The CD4/CD8 ratio in HIV infected subjects is independently associated with T-cell activation despite long-term viral suppression. J Infect 2013; 66:57–66. 10.1016/j.jinf.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 16. Buggert M, Frederiksen J, Noyan K, Svard J, Barqasho B, et al. Multiparametric Bioinformatics distinguish the CD4/CD8 ratio as a suitable predictor of combined T Cell pathogenesis in HIV infection. J Immunol 2014; 192: 2099–108. 10.4049/jimmunol.1302596 [DOI] [PubMed] [Google Scholar]
- 17. Serrano-Villar S, Sainz T, Lee SA, Hunt PW, Sinclair E, Shacklett BL, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. 2014; 10(5):e1004078 10.1371/journal.ppat.1004078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fabris P, Bozzola L, Benedetti P, Scagnelli M, Nicolin R, Manfrin V, et al. H. pylori infection in HIV-positive patients. A serohistological study. Digestive diseases and sciences. 1997;42(2):289–292. [DOI] [PubMed] [Google Scholar]
- 19. Khan A, Farooqui A, Kazmi SU. Presence of Helicobacter pylori in drinking water of Karachi, Pakistan. Journal of infection in developing countries. 2012;6(3):251–255. [DOI] [PubMed] [Google Scholar]
- 20. Ahmed KS, Khan AA, Ahmed I, Tiwari SK, Habeeb A, Ahi JD, et al. Impact of household hygiene and water source on the prevalence and transmission of Helicobacter pylori: a South Indian perspective. Singapore medical journal. 2007;48(6):543–549. [PubMed] [Google Scholar]
- 21. Gisbert JP, Pajares R, Pajares JM. Evolution of Helicobacter pylori therapy from a meta-analytical perspective. Helicobacter. 2007;12 Suppl 2:50–58. [DOI] [PubMed] [Google Scholar]
- 22. Chiba N, Rao BV, Rademaker JW, Hunt RH. Meta-analysis of the efficacy of antibiotic therapy in eradicating Helicobacter pylori. The American journal of gastroenterology. 1992;87(12):1716–1727. [PubMed] [Google Scholar]
- 23. Stevenson TH, Lucia LM, Acuff GR. Development of a selective medium for isolation of Helicobacter pylori from cattle and beef samples. Applied and environmental microbiology. 2000;66(2):723–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boyanova L, Davidkov L, Gergova G, Kandilarov N, Evstatiev I, Panteleeva E, et al. Helicobacter pylori susceptibility to fosfomycin, rifampin, and 5 usual antibiotics for H. pylori eradication. Diagnostic microbiology and infectious disease. 2014;79(3):358–361. 10.1016/j.diagmicrobio.2014.03.028 [DOI] [PubMed] [Google Scholar]
- 25. Edwards PD, Carrick J, Turner J, Lee A, Mitchell H, Cooper DA. Helicobacter pylori-associated gastritis is rare in AIDS: antibiotic effect or a consequence of immunodeficiency? The American journal of gastroenterology. 1991;86(12):1761–1764. [PubMed] [Google Scholar]
- 26. Panos GZ, Xirouchakis E, Tzias V, Charatsis G, Bliziotis IA, Doulgeroglou V, et al. Helicobacter pylori infection in symptomatic HIV-seropositive and -seronegative patients: a case-control study. AIDS research and human retroviruses. 2007;23(5):709–712. [DOI] [PubMed] [Google Scholar]
- 27. Sayi A, Kohler E, Hitzler I, Arnold I, Schwendener R, Rehrauer H, Muller A. The CD4+ T cell-mediated IFN-gamma response to Helicobacter infection is essential for clearance and determines gastric cancer risk. Journal of immunology. 2009;182(11):7085–7101. [DOI] [PubMed] [Google Scholar]
- 28. Gao L, Weck MN, Nieters A, Brenner H. Inverse association between a pro-inflammatory genetic profile and Helicobacter pylori seropositivity among patients with chronic atrophic gastritis: enhanced elimination of the infection during disease progression? European journal of cancer. 2009;45(16):2860–2866. 10.1016/j.ejca.2009.04.015 [DOI] [PubMed] [Google Scholar]
- 29. Aebischer T, Meyer TF, Andersen LP. Inflammation, immunity, and vaccines for Helicobacter. Helicobacter. 2010;15 Suppl 1:21–28. 10.1111/j.1523-5378.2010.00777.x [DOI] [PubMed] [Google Scholar]
- 30. Salama NR, Hartung ML, Muller A. Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nature reviews. Microbiology. 2013;11(6):385–399. 10.1038/nrmicro3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Angin M, Sharma S, King M, Murooka TT, Ghebremichael M, Mempel TR, et al. HIV-1 Infection Impairs Regulatory T-Cell Suppressive Capacity on a Per-Cell Basis. The Journal of infectious diseases. 2014;210(6):899–903. 10.1093/infdis/jiu188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DuBois S, Kearney DJ. Iron-deficiency anemia and Helicobacter pylori infection: a review of the evidence. The American journal of gastroenterology. 2005;100(2):453–459. [DOI] [PubMed] [Google Scholar]
- 33. Thomas JE, Dale A, Bunn JE, Harding M, Coward WA, Cole TJ, et al. Early Helicobacter pylori colonisation: the association with growth faltering in The Gambia. Archives of disease in childhood. 2004;89(12):1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bravo LE, Mera R, Reina JC, Pradilla A, Alzate A, Fontham E, et al. Impact of Helicobacter pylori infection on growth of children: a prospective cohort study. Journal of pediatric gastroenterology and nutrition. 2003;37(5):614–619. [DOI] [PubMed] [Google Scholar]
- 35. Bhan MK, Bahl R, Sazawal S, Sinha A, Kumar R, Mahalanabis D,et al. Association between Helicobacter pylori infection and increased risk of typhoid fever. The Journal of infectious diseases. 2002;186(12):1857–1860. [DOI] [PubMed] [Google Scholar]
- 36. Perry S, de Jong BC, Solnick JV, de la Luz Sanchez M, Yang S, Lin PL, et al. Infection with Helicobacter pylori is associated with protection against tuberculosis. PloS one. 2010;5(1):e8804 10.1371/journal.pone.0008804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perry S, Chang AH, Sanchez L, Yang S, Haggerty TD, Parsonnet J. The immune response to tuberculosis infection in the setting of Helicobacter pylori and helminth infections. Epidemiology and infection. 2013;141(6):1232–1243. 10.1017/S0950268812001823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eberhardt KA, Sarfo FS, Dompreh A, Kuffour EO, Geldmacher C, Soltau M, et al. Helicobacter pylori coinfection is associated with decreased markers of immune activation in ART-Naïve HIV-positive and in HIV-negative individuals in Ghana. Clin Infect Dis. 2015;61(10):1615–23. 10.1093/cid/civ577 [DOI] [PubMed] [Google Scholar]
- 39. Muller A, Solnick JV. Inflammation, immunity, and vaccine development for Helicobacter pylori. Helicobacter. 2011;16 Suppl 1:26–32. 10.1111/j.1523-5378.2011.00877.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Data contain sensitive participant information and are available upon request from the corresponding author.