Abstract
The uterine myometrium (UT-myo) is a therapeutic target for preterm labor, labor induction, and postpartum hemorrhage. Stimulation of intracellular Ca2+-release in UT-myo cells by oxytocin is a final pathway controlling myometrial contractions. The goal of this study was to develop a dual-addition assay for high-throughput screening of small molecular compounds, which could regulate Ca2+-mobilization in UT-myo cells, and hence, myometrial contractions. Primary murine UT-myo cells in 384-well plates were loaded with a Ca2+-sensitive fluorescent probe, and then screened for inducers of Ca2+-mobilization and inhibitors of oxytocin-induced Ca2+-mobilization. The assay exhibited robust screening statistics (Z´ = 0.73), DMSO-tolerance, and was validated for high-throughput screening against 2,727 small molecules from the Spectrum, NIH Clinical I and II collections of well-annotated compounds. The screen revealed a hit-rate of 1.80% for agonist and 1.39% for antagonist compounds. Concentration-dependent responses of hit-compounds demonstrated an EC50 less than 10μM for 21 hit-antagonist compounds, compared to only 7 hit-agonist compounds. Subsequent studies focused on hit-antagonist compounds. Based on the percent inhibition and functional annotation analyses, we selected 4 confirmed hit-antagonist compounds (benzbromarone, dipyridamole, fenoterol hydrobromide and nisoldipine) for further analysis. Using an ex vivo isometric contractility assay, each compound significantly inhibited uterine contractility, at different potencies (IC50). Overall, these results demonstrate for the first time that high-throughput small-molecules screening of myometrial Ca2+-mobilization is an ideal primary approach for discovering modulators of uterine contractility.
Introduction
The uterine myometrium is a therapeutic target for the inhibition of uterine contractility to delay the early onset of labor, or the stimulation of uterine contractility to induce labor or control postpartum hemorrhage. Current therapeutics used to inhibit premature contractions (termed tocolytics) are associated with detrimental off-target side effects for both infant and mother when used to maintain pregnancy beyond 24–72hrs [1–3]. Conversely, women who develop postpartum hemorrhage as a result of uterine atony and unresponsiveness to contractile agonists (termed uterotonics), frequently require emergency surgical intervention (i.e. hysterectomy). These issues have led to the growing recognition that novel tocolytic and uterotonic agents are urgently needed.
An increase in intracellular Ca2+ in uterine myometrial (UT-myo) cells is the final common pathway controlling myometrial contractions [1,4]. Myometrial contractions during labor are dependent on binding of OT and prostaglandins (PGs) to their receptors on UT-myo cells, stimulating the release of Ca2+ from intracellular stores, promoting Ca2+-entry into cells, and triggering coordinated, synchronous myometrial contractility [1,5–10]. Current tocolytic therapeutics used to inhibit preterm labor includes: atosiban (an OT-receptor antagonist), indomethacin (an inhibitor of PG-synthesis), ritrodine (a beta-adrenergic agonist), nifedipine (a Ca2+-channel blocker), and magnesium sulfate (intracellular-Ca2+ effector) [11–14]. Conversely, oxytocin remains the preferred uterotonic agent for labor induction and prevention of postpartum hemorrhage [15].
Current research tools used for small-scale discovery or testing of tocolytics and uterotonics have utilized either: 1) computational modeling [16,17], 2) fluorescence-based Ca2+-assay [18–20], 3) recording Ca2+-channel currents using whole-cell patch clamp technology [19,21], 4) collagen gel contraction assay [22,23], 5) oxytocin receptor (OTR) assays [24], 6) ex vivo measurements of myometrial tension/contractility [25–31] [formerly referred to as oxytocic bioassay [24]], or 7) in vivo measurements of intrauterine pressure [32–34]. However, to our knowledge there are no reports of large-scale screening for the discovery of new tocolytic or uterotonic compounds.
High-throughput screening (HTS) of small-molecule libraries is the standard approach used in the pharmaceutical industry to discover new lead compounds for drug development. Although a majority of drug discovery efforts are centered around HTS for modulators of molecularly defined, single drug targets, these often ignore the complexity of cell signaling pathways that underlie important physiological processes. HTS of calcium mobilization utilizing fluorescent Ca2+-sensitive probes circumvents this limitation and allows testing of large collections of compounds to identify both agonists and antagonists in a single screen [35]. The benefit of using primary cells in HTS lies in their retention of many in vivo functions and endogenous expression of mechanisms/targets of interests [36]. However, primary cells must be proven reproducible for reliable use in HTS. Here we report the development and validation of a fluorescence-based Ca2+-assay using primary mouse UT-myo cells for identification of uterotonics and tocolytics. Functional annotation analysis of identified hit-compounds provided insight into the pharmacological classes and protein targets that affect both native and OT-induced myometrial Ca2+-mobilization. In a secondary screen using an ex vivo isometric contractility assay, we show the ability and potency of four hit-antagonists to dampen uterine myometrial contractions. Overall, these findings demonstrate that a robust OT-induced Ca2+-mobilization assay can be utilized for screening large compound collections to identify modulators of uterine contractility.
Materials and Methods
Isolation of Murine Uterine Myometrial (UT-Myo) Cells
All animal experiments were approved by the Vanderbilt University Institutional Animal Care and Use Committee and conformed to the guidelines established by the National Research Council Guide for the Care and Use of Laboratory Animals. Adult (8–12wk) CD1 wild-type (Charles River Laboratories) mice were housed in 12h light: 12h dark cycle, with free access to food and water. Timed-pregnancies were performed, and the presence of a vaginal plug was considered day 1 of pregnancy, with the time of expected delivery on d19.5. Mice were euthanized by cervical dislocation under a lethal dose of isoflurane. Upon removal from d19 pregnant mice, uteri were placed into ice-cold Hank’s Buffered Saline Solution (1X HBSS, without Ca2+ or Mg2+), and cut longitudinally along the mesometrial border. After removal of fetuses, placentas, amniotic and endometrial membranes, the myometrium was cut into ~1mm3 pieces and digested in 0.2% Type-II Collagenase (Worthington Biomedicals) in HBSS for 45–60 min at 37°C in 5% CO2 atmosphere. Following tissue digestion, cells were suspended in complete media (phenol-free DMEM supplemented with 10% fetal bovine serum (FBS), 25 mM HEPES, 100 U/ml penicillin-streptomycin) then filtered through 100-micron nylon cell strainers. Isolated cells were centrifuged at 1000rpm for 10 min, then resuspended in complete media and subjected to a differential attachment technique [37] to selectively enrich for uterine myocytes. Specifically, UT-myo cells were plated in 150mm cell culture dishes for 2hr at 37°C in 5% CO2 atmosphere, during which non-myocytes (mostly fibroblasts) attached to the bottom of the cell culture dish. The supernatant, containing the slowly adhering uterine myocytes, was collected and transferred to 150mm cell culture dishes. After 24hrs the media was changed. The cells became near-confluent after 48hrs, at which time the cells were dissociated using 0.25% Trypsin-EDTA. Once resuspended in complete media, cells were plated in either 4-well chamber slides (Tissue-tek) for characterization of the cell population or clear-bottom, black-walled and poly-D-lysine-coated 384-well plates (Grenier Bio-One) for high-throughput screening Ca2+-mobilization assays.
Primary mouse UT-Myo cell characterization
Immunofluorescent labeling of cells for smooth muscle α-actin and calponin antibodies was performed to access the purity and homogeneity of our UT-myo cell cultures, similar to Tribe et al. [38]. After 24 hours, UT-myo cells on chamber slides were washed three times using 1X Phosphate Buffered Saline (PBS) prior to fixation using 4% paraformaldehyde (PFA) for 30 minutes. Additionally, frozen sections (8-micron thickness) of whole mount uterus was collected from day 19 of mouse pregnancy to compare to the isolated UT-myo cell population. Whole-mount uterine sections were fixed with 4% PFA for 2 hours at room temperature, followed by sucrose infiltration and embedding. After washing three times with 1XPBS, blocking solution (10% goat serum, 0.25% Triton X-100 in PBS) was applied for 1 hour at room temperature, followed by an overnight incubation at 4°C in the primary antibody, either: smooth muscle actin (1:250 dilution; Sigma, A 2547) and/or calponin. (1:100 dilution; Abcam ab46794). After washing with 1X-PBS, samples were incubated with fluorescent-labeled secondary antibodies (1:2000; Invitrogen A-11004 and/or A-11008) and DAPI for 3 hours at room temperature. Slides were coverslippped using Aqua Polymount (Polysciences, Inc), and visualized under a fluorescent microscope.
Ca2+ Mobilization Assay Development and Pilot HTS
Assays were performed in the Vanderbilt Institute of Chemical Biology High-Throughput Screening Facility. After 24hr attachment, cells were washed twice with wash buffer [1X HBSS Ca2+ and Mg2+ containing 20mM HEPES and 2.5mM probenecid (Sigma-Aldrich)] using an ELx405™ (BioTek) automated microplate washer. A final aspiration step resulted in 20uL residual volume. The cells were loaded with 20uL of 2X loading buffer [wash buffer containing Fluo-4AM (Invitrogen/Molecular Probes) and 0.023% (w/v) pluronic acid (Invitrogen)] per well using a Multidrop™ Combi (Thermo Fisher Scientific) reagent dispenser. Probenecid was used to block the active transport of the Fluo-4AM out of the cells, while pluronic acid was used to stop Fluo-4AM breakdown by external esterases. After 1hr incubation at 37°C (5% CO2 atmosphere), cells were washed twice with wash buffer (ELx405™) again resulting in 20uL residual volume. The cells were transferred to a Functional Drug Screening System (FDSS 6000; Hamamatsu) to measure baseline fluorescence for 20sec (1Hz; Ex480:Em540) followed by the “compound addition” of 20uL of 0.2% DMSO (0.1% final concentration; Sigma-Aldrich) using the FDSS’ integrated pipettor and continuous measurement of fluorescence for an additional 40 seconds. The plate remained at room temperature, protected from light, for 30min to allow de-esterification/hydrolysis of the Fluo-4AM. Afterwards, the fluorescence was measured by the FDSS for 20 sec (1Hz; Ex480:Em540) prior to recording transient intracellular calcium mobilization for 140 sec following exposure to the “OT addition”: OT (O6379; Sigma-Aldrich,) or vehicle in 10μL wash buffer, for a final concentration of 100 pM to 100 μM. Non-linear regression analyses were performed to generate concentration response curves (CRCs) using a four-parameter logistical equation in Prism 6 (GraphPad). Signal (OT) was normalized to background Max-Min relative fluorescent units (RFU) to determine optimal cell plating density and Fluo-4AM concentration. Comparisons of fit were performed to determine if the non-linear fit lines between the cell densities and Fluo-4AM concentrations were significantly different (p<0.05). Two-way analysis of variance was used to determine significant differences between each cell density and Fluo-4 concentration examined.
Oxytocin CRC to determine EC80
After establishing the optimal cell plating density (8,000 cells/well) and Fluo-4AM concentration (4μM final concentration), additional Ca2+-mobilization assays were performed to obtain the EC80 for OT. During the compound addition, rows A, H and P received only DMSO (0.1% final concentration); while 13-point three-fold dilutions of OT (starting at 100uM final concentration) were performed in the remaining rows. For each well, the mean baseline value (MBV) for Ca2+-fluorescence was calculated during the 0–19 sec timeframe. The max RFU was calculated from the 20–140 sec timeframe, and then subtracted from the baseline. An average Max-MBV RFU was calculated for each OT concentration. Data were analyzed using Prism 6. Non-linear regression analyses were performed to generate concentration response curves and to determine the OT EC80 value for each experimental day (range = 0.82μM to 8.68 μM) of: 1) checkerboard assays for Z´ determination; 2) pilot screen; and 3) confirmation of “hit”-compounds.
Checkerboard Assays for Z´-factor determination
‘‘Checkerboard” assays were performed on 3 separate days to determine the well-to-well uniformity within a plate and day-to-day reproducibility. Calcium-mobilization assays were performed as described above. During the compound addition, every alternate well received 20μL of either 0.2% (0.1% final concentration) DMSO or 20uM (10μM final concentration) atosiban (Sigma-Aldrich A3480). During the OT addition, the entire assay plate received the OT EC80 dose determined on that experimental day using a partial plate (at least 4 columns) of cells. A Z´-factor [39] was calculated for each batch of myometrial cells using:
; SDc+ and SDc−represent standard deviation (SD) for the positive control (DMSO + OT) and negative control (atosiban + OT), respectively. Mc+ and Mc−represent the mean for the positive control and negative control, respectively.
Assessment of DMSO Tolerance
Small-molecule library compounds are dissolved in the solvent DMSO. Since DMSO could have direct effects on the Ca2+-mobilization assay and lead to false-positive hit identification, we examined the assay’s tolerance to DMSO by performing a titration of DMSO ranging from 0.0025% to 1% v/v final concentration. A total of 6 columns were used to examine DMSO tolerance. The DMSO was added during the “compound” addition, followed by OT-EC80 addition 30 minutes later. An average Max-MBV RFU was calculated for each DMSO concentration. Data were analyzed using Prism 6. Non-linear regression analyses were performed to generate concentration response curves
Pilot Screen for Assay Validation
Four columns in each plate used for HTS were filled in a checkerboard pattern with either DMSO control or atosiban during the compound addition, followed by OT addition to all wells, as described above. This was performed in order to calculate a Z´-factor for each pilot screen compound plate. During the pilot screen, 320 test compounds filled columns 3–22 of the 384-well plate. Test compounds in 40 μL of wash buffer were prepared by transferring 80 nL into a 384-well polypropylene plate using the ECHO 555 acoustic liquid handler (Labcyte, Sunnyvale, CA) from a 10mM stock. Test compounds (10μM final concentration) were added during the compound addition, with OT (EC80) added during the OT addition. In this manner, compounds with an inhibitory effect on OT-induced Ca2+-mobilization in UT-myo cells might be identified. The MicroSource Discovery Systems Spectrum Collection (2000 compounds), National Institute of Health Clinical Collection I (446) and II (281) compound libraries were screened using a total of 10 microtiter plates.
A “mean high” value was calculated by averaging the Max-MBV RFU from all of the wells that received Vehicle + OT EC80. A “mean low” value was calculated by averaging the Max-MBV RFU from all of the wells that received atosiban + OT EC80. Percent response was calculated from data collected during the compound addition, using: .
Selection and confirmation of “hit” compounds
A robust Z´-score calculation was used to identify hit-compounds based on whether a test compound’s value was greater than 3 times the mean absolute standard deviations (MADs) away from the median of all the vehicle (VEH) wells.
Finally, all compounds identified as hits were cherry-picked from library plates for confirmation testing and concentration-response titrations using the Ca2+-mobilization assay. Confirmation testing was performed in duplicate at 10 μM concentration to confirm activity all hit-compounds.
Concentration-response titrations of hit-compounds
We examined the potency of the confirmed hit-agonist and antagonist compounds by assaying compounds at three-fold 10-point titrations starting at 30μM. Compounds were transferred to daughter plates using an ECHO acoustic plate reformatter, and tested as described above for the primary screen. Two compounds were purchased from commercial vendors to compare compound potency to those cherry-picked from the compound library plates: benzbromarone (Sigma B5774) and spiperone (MP Biomedicals 0215207283).
Functional annotation in PubChem
Each confirmed hit-compound was searched for in the National Institutes of Health (NIH) PubChem database to obtain its compound identification (CID) number and Medical Subject Heading (MeSH) pharmacological classification(s). Each confirmed hit’s CID was uploaded into BioActivity SAR in PubChem. CIDs were clustered by Compound Activity and Active BioAssay Defined Protein Targets.
Functional Ex Vivo Uterine Isometric Contractility Assay
Uterine myometrial samples were obtained from CD1 wild-type mice on day 19 of pregnancy, on at least three different experimental days. Longitudinal strips of uterine myometrium were prepared by cutting the uterus along the mesometrial (vascularized) border, followed by removal of fetal, placental and connective tissue/membranes. Uterine strips of 4mm X 12mm were attached via silk thread to stainless steel hooks connected to a RadnotiLLC force transducer at one end, while the other end of the tissue was anchored to a glass rod at the base of the tissue bath. Preparations were then submerged in a heated and oxygenated (37°C, 95% O2-5% CO2) RadnotiLLC tissue bath containing Kreb’s Bicarbonate Solution (136.7mM NaCl, 4.7mM KCL, 2.5mM CaCl22H2O, 1.5mM MgCl26H2O, 1.8mM NaH2PO4H2O, 15mM C6H12O6 and 2.52mM NaHCO3). Each strip was placed under 1g tension and allowed to equilibrate in the organ chamber for 60min prior to recording baseline spontaneous contractile activity. Tissue strips that failed to establish a regular amplitude and frequency were excluded from further study. Following the establishment of spontaneous contractions, cumulative doses of either atosiban or confirmed hit-compounds were added to individual organ baths every 10 min. The following hit-compounds tested in the organ bath were purchased from Sigma-Aldrich: atosiban (A3480), benzbromarone (B5774), dipyridamole (D9766) and nisoldipine (N0165); while fenoterol hydrobromide (HBr) was purchased from MP Biomedicals (0215803983). Isometric contractions were recorded using PowerLab/8 SP (ADInstruments) hardware and analyzed with LabChart 7 Pro software (ADInstruments). Contractile activity was assessed by amplitude (cyclic height), frequency (number of contractions/10min) or AUC/sec (area under the curve, which is the sum of the integrals for each contraction divided by the duration of the period assessed). All treatment data were then expressed as a percentage of the baseline spontaneous contractile activity. Data were analyzed using Prism software. Data are expressed as mean±SEM. Non-linear regression analyses were performed to generate CRCs for calculation of IC50 and Emax. Comparisons of fit were performed to determine if the non-linear fit lines between the compounds were significantly different (p<0.05) from DMSO. Two-way analysis of variance was used to determine significant differences between the % response for each concentration of a given compound versus DMSO.
Results
Characterization of Primary Mouse Uterine Myometrial Cells
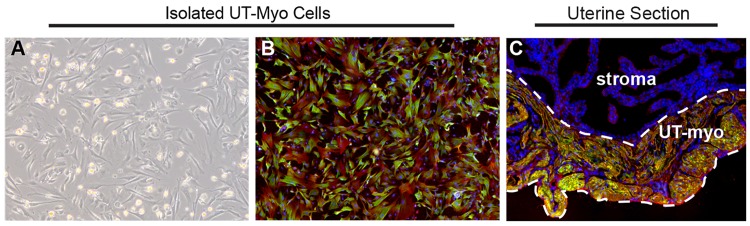
The goal of this investigation was to prepare a UT-myo cell culture that was: 1) homogenous and reproducible for HTS; 2) contained properties comparable to myometrium in vivo. As shown in Fig 1, the majority of isolated UT-myo cells stained positive for two markers of smooth muscle cells, alpha-SMA and calponin (panel B), similar to that of whole uterine tissue collected from day 19 of mouse pregnancy (panel C).
Fig 1. Assessment of primary murine UT-myo cell homogeneity.
A. Representative photomicrograph of UT-myo cells prior to dissociation and use in Ca2+-mobilization or immunofluorescent staining. Representative photomicrograph of UT-myo cells (B) and uterine myometrium (C) stained with smooth muscle cell markers, alpha-SMA (red) and calponin (green), and DAPI (blue). UT-myo cells and whole-mount uterine tissue were collected from day 19 of mouse pregnancy. The placenta and embryo were removed from whole-mount tissue sections.
Development of a Uterine Myometrial Cell Ca2+ Mobilization Assay for HTS
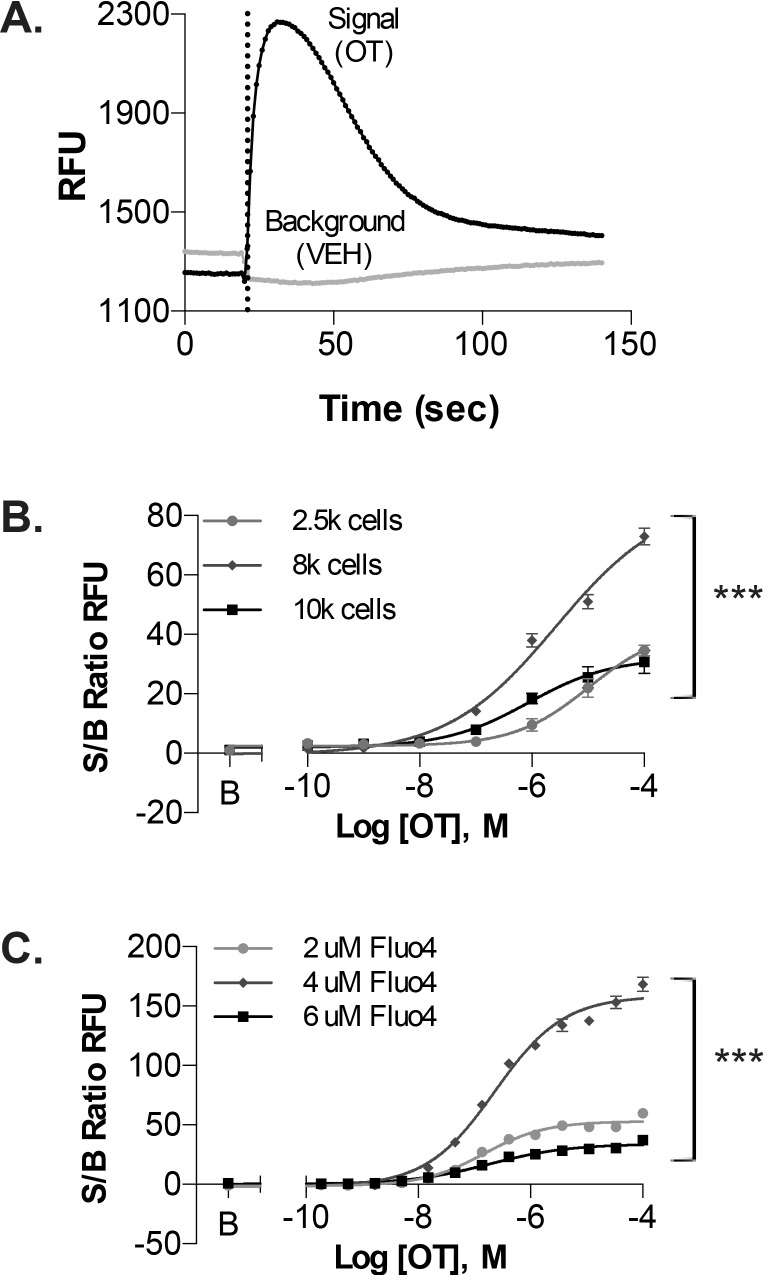
Oxytocin, the most potent endogenous contractile-agonist for induction of labor [40], was selected as the stimulus for intracellular Ca2+-release from UT-myo cells. Treatment of UT-myo cells with OT (100pM to 100μM; indicated by dashed line in Fig 2A) resulted in a robust (~15sec to peak) concentration-dependent increase in intracellular Ca2+-release (indicated by relative fluorescent units; RFUs). By contrast, treatment with vehicle (0.1% DMSO final concentration) had no effect on intracellular Ca2+-release. In order to optimize the Ca2+-mobilization assay for mouse primary UT-myo cells, we performed cell density and Fluo-4AM dye concentration gradients (Fig 2B and 2C, respectively). OT CRCs were used to determine optimal assay conditions based on signal (OT) to background (vehicle) ratios. Based on these results, we concluded that 8,000 cells/well loaded with 4μM Fluo-4AM dye in 384-microtiter plates resulted in optimal assay conditions.
Fig 2. Ca2+-mobilization assay using uterine myometrial cells.
A. Representative recording of OT-induced Ca2+-mobilization from UT-myo cells loaded with Fluo-4AM. Ca2+-mobilization was monitored as an increase in Relative Fluorescent Units (RFUs). Dashed line indicates time of OT or vehicle (VEH) addition. Optimal assay conditions were determined by performing cell density gradient (B) and Fluo-4AM concentration response curves (C), from signal-to-background (S/B) ratios of Max-Min RFU obtained from OT and VEH wells, respectively. Non-linear regression was used to fit the data (Mean±SEM; n = 8 well replicates); significant (***p<0.0001) difference between each fit line.
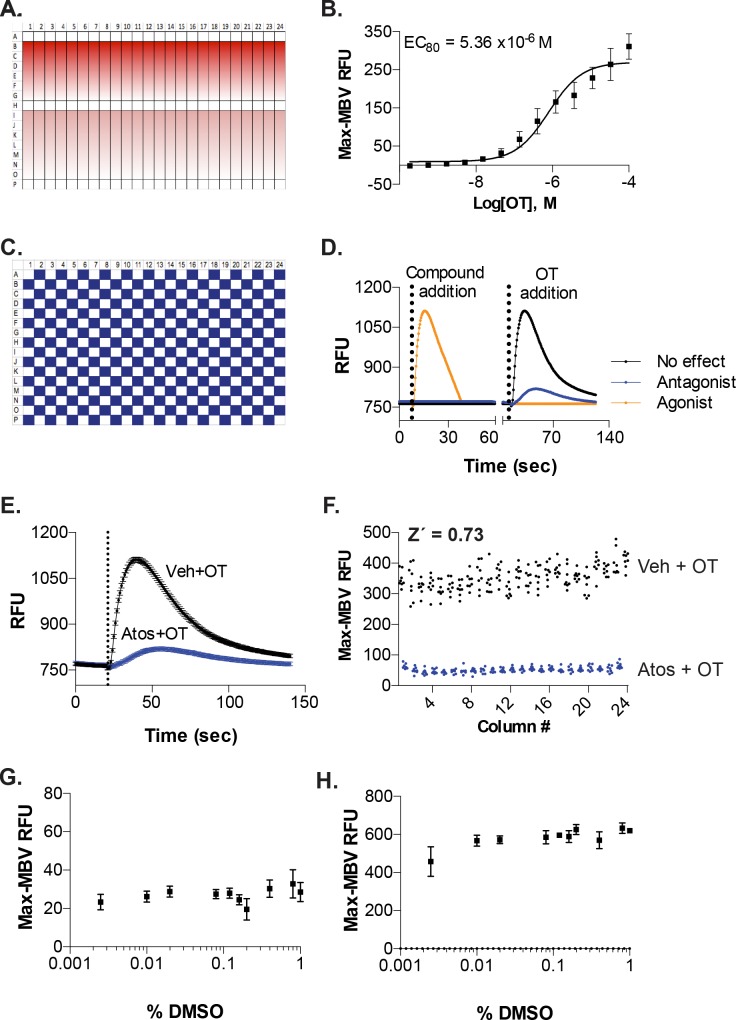
We next performed OT concentration-response experiments to establish the concentration of OT needed to reach 80% of its maximal (EC80) induced-intracellular Ca2+-release from UT-myo cells. A submaximal EC80 dose was chosen for our dual-addition assay format because it provides a robust signal window yet allows ‘‘headroom” for identifying potentiators of OT-induced intracellular-Ca2+ mobilization. We used the platemap shown in Fig 3A, in which three rows of each plate received only 0.1% DMSO, while the remaining rows received 3-fold dilutions of OT (see Methods for details). The concentration response to OT was used to calculate the OT-EC80 for each day of all subsequent assays: 1) checkerboard analyses; 2) pilot screen; and 3) confirmation of hits.
Fig 3. Assay automation and determination of suitability for HTS.
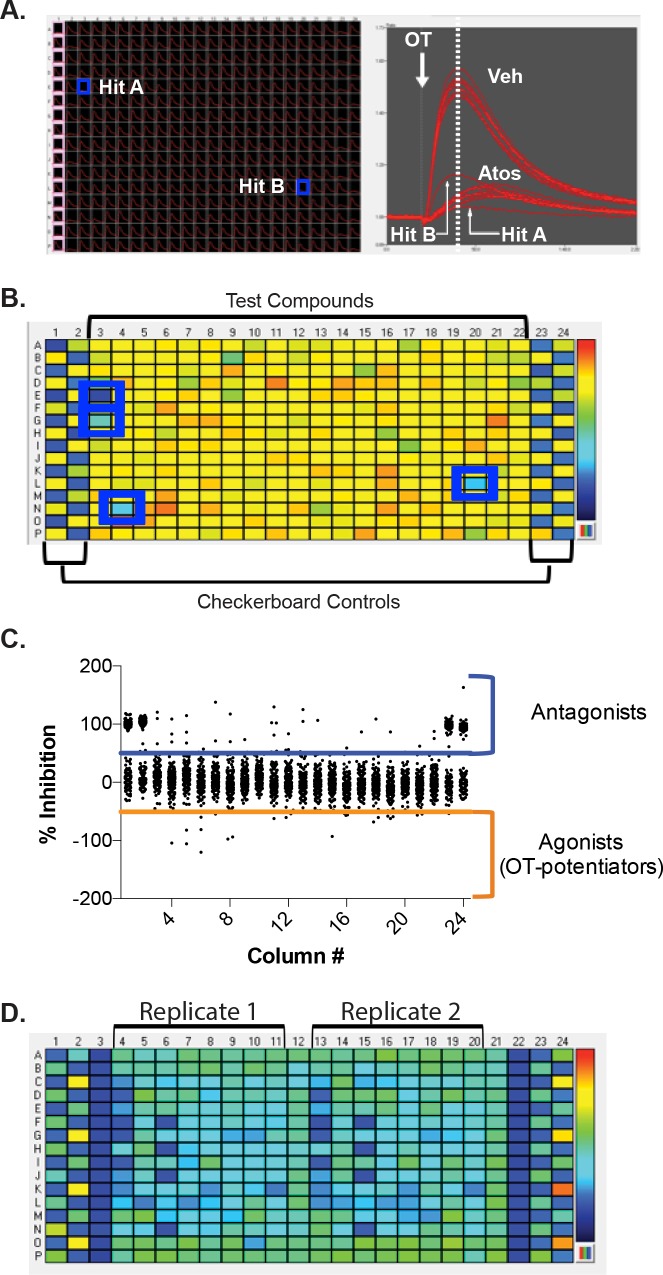
A. Platemap used for CRCs performed for OT-induced Ca2+-mobilization from UT-myo cells. B. Mean OT CRC from all Ca2+-mobilization assays performed for checkerboard analysis and pilot screen (n = 7 independent experiments (batches of primary mouse UT-myo cells) performed in quadruplicate). OT CRCs were used to calculate the OT-EC80 for each day of the checkerboard analyses. Mean ± SEM OT EC80 is shown. C. Platemap used to perform checkerboard analyses. Every other well received either 0.1% DMSO (final concentration vehicle, white boxes) or 10μM atosiban (blue boxes) for 30 min prior to the addition of OT (EC80) to the entire plate. D. Dual-addition assay format for the identification of agonists and antagonists of Ca2+-mobilization from UT-myo cells from a single HTS screen. Compound addition (either atosiban or vehicle) followed by 30min incubation at room temperature, then OT addition. E. Representative graph showing the response of UT-myo cells to either vehicle (Veh) or atosiban (Atos) prior to OT-induced Ca2+-mobilization. F. The Z´-factor was calculated from checkerboard analyses performed on three separate days. Tolerance of UT-myo cells to the compound solvent, DMSO, added during the “compound addition” (G) prior to OT-induced Ca2+-mobilization (H).
Next, we performed a checkerboard analysis to determine whether our assay could identify agonists of Ca2+-mobilization and antagonists of OT-induced Ca2+-mobilization from UT-myo cells. Using the platemap shown in Fig 2C, UT-myo cells received either vehicle (Fig 3C, white boxes) or 10uM atosiban (a known OT-receptor antagonist; Fig 2C blue boxes) during the compound addition (Fig 3D). After a 30-minute incubation of the compound, all cells received the EC80 dose during the OT addition. Inhibition of OT-induced Ca2+-release from UT-myo cells by atosiban was easily distinguished from vehicle (Fig 3E, blue versus black line).
Finally, we calculated the Z´-factor to allow quantification of the suitability of the Ca2+-assay for use in a full-scale HTS. The mean±SEM Z´-factor for the assay plates on three separate days was 0.73±0.04, indicating assay robustness and reproducibility (Fig 3F).
In order for an assay to be useful for high-throughput screening, it is necessary to determine the sensitivity of the assay to DMSO, the solvent used for small-molecule compound libraries. Ca2+-mobilization from UT-myo cells was unaffected at DMSO concentrations up to 0.4% v/v (Fig 3G), and showed no effect on OT-induced Ca2+-mobilization at all concentrations examined (Fig 3H). Thus, DMSO had no effect on Ca2+-mobilization or OT-induced Ca2+-mobilization at the screening concentrations of 0.1% v/v.
Pilot Screening for Assay Validation
A pilot screen using three libraries of compounds was performed to assess the suitability of the screen for HTS and potentially identify lead compounds for immediate development. Specifically, the Spectrum Collection consists of 2,000 compounds with a wide range of biological activity and structural diversity: 50% drug components, 30% natural products, 20% other bioactive components; while the NIH Clinical Collection I and II of contains 730 compounds that have a history of use in human clinical trials.
On each day of screening, the OT EC80 value was calculated to determine the ability of test compounds to inhibit this submaximal concentration of OT. On each screening plate, the outer four columns were used for checkerboard analyses to determine the Z´-factor (Figs 4B and 5B). The test compounds filled the remaining 320 wells, and were screened at a nominal final concentration of 10μM.
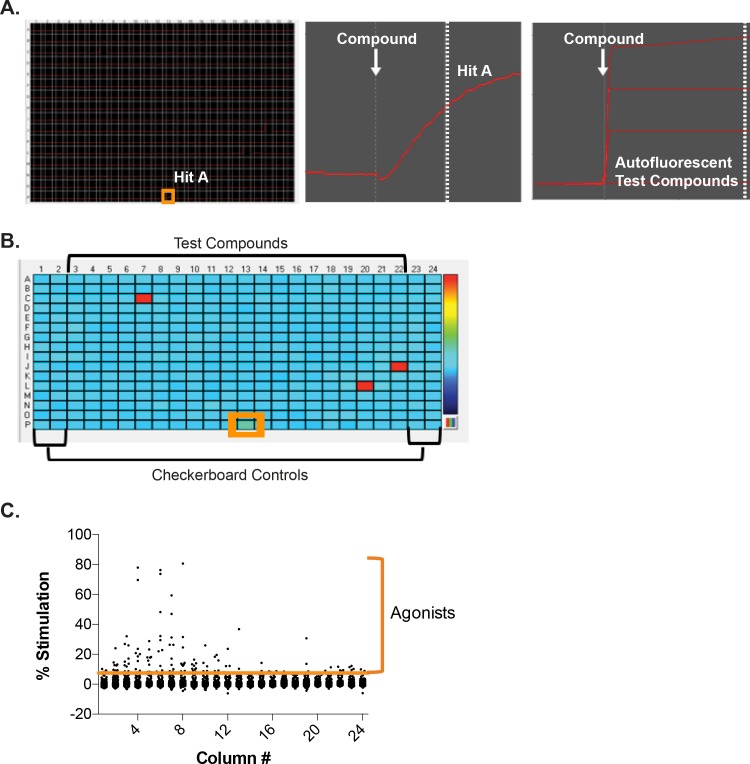
Fig 4. Hit-agonist identification during pilot screen.
A, left panel. Representative image of realtime monitoring of Ca2+-mobilization from UT-myo cells following the compound addition (white arrow, middle panel). The two end columns of each plate were used for checkerboard analyses to determine the Z´-factor for each HTS assay, and received either 0.1% DMSO vehicle or 10uM atosiban during the compound addition. The inner 320 wells received 10μM of test compounds. An example of a “Hit”-agonist is highlighted (orange box). A, right panel. Examples of relative fluorescent recordings from autofluorescent test compounds. B. Plate heatmap of Ca2+-mobilization at the time following compound addition (dashed line, middle panel A). A representative hit-agonist compound (green well) is highlighted by orange box, while autofluorescent compounds are visualized as bright red wells. C. The average cutoff threshold for hit-agonists was 5.85±1.59% stimulation, based on 3*MAD from median (refer to “Materials and Methods” section).
Fig 5. Hit-antagonist identification during pilot screen and confirmation after retesting.
A, left panel. Realtime monitoring of OT-induced Ca2+-mobilization from UT-myo cells. The two end columns (one highlighted in pink) of each plate were used for checkerboard analyses to determine the Z´-factor for each HTS assay. Examples of “Hit”-antagonists are highlighted (blue boxes). A, right panel. OT-induced Ca2+-mobilization of highlighted wells. White arrow indicates the time of OT addition. B. Plate heatmap of Ca2+-mobilization at the time indicated by the dashed line (right panel A). Representative hit-antagonist compounds are highlighted by blue boxes. C. The average cutoff threshold based on 3*MAD from median (refer to “Materials and Methods” section) for “hit”-antagonists was 41.07±3.79% inhibition and “hit”-agonists (OT-potentiators) was 50.02±4.77% activation. D. Representative heatmap of additional Ca2-mobilization assays performed to retest hit-agonists and antagonists in duplicate. Veh = vehicle, Atos = atosiban
During the compound addition, most test compounds had no effect on the stimulation of intracellular Ca2+-release (Fig 4). However, a small number of compounds stimulated Ca2+-mobilization directly (Table 1). Autofluorescent compounds were identifiable by their characteristic immediate (within 1 sec) and sustained effect on fluorescence emission (Fig 4A, right panel compared to middle panel). By highlighting the point at which compounds were added during the Ca2+-assay, we were able to quickly identify potential “hit”-agonist compounds (Fig 4B, highlighted by orange box). The average cutoff for active “hit” agonists was 5.85± 1.59% stimulation, which was based on the mean effect of the minimum control and 3 times the SD (Fig 4C).
Table 1. Calcium-mobilization HTS parameters.
| Agonists | Antagonists | ||||||
|---|---|---|---|---|---|---|---|
| Compound Library | # Compounds | # Hit 1 | Hit-Rate | % Confirmed | # Hit 1 | Hit-Rate | % Confirmed |
| Spectrum | 2000 | 50 | 2.50 | 66.7 | 38 | 1.90 | 86.8 |
| NIH Collection I | 446 | 13 | 2.91 | 75.0 | 5 | 1.12 | 80.0 |
| NIH Collection II | 281 | 11 | 3.91 | 62.5 | 2 | 0.71 | 50.0 |
| Total | 2727 | 74 | 2.71 | 66.7 (n = 49) | 45 | 1.65 | 84.4 (n = 38) |
| 1.80 confirmed hit-rate | 1.39 confirmed hit-rate | ||||||
1 Autofluorescent compounds excluded.
After the OT addition, there was a noticeable inhibition of OT-induced Ca2+-release in UT-myo cells by some of the test compounds examined. A small number of test compounds appeared more effective at inhibiting OT-induced Ca2+-release than atosiban (Fig 5A, Hit A compared to atosiban), while other compounds were less or similarly effective as atosiban (Fig 5A, Hit B compared to atosiban). Similar to the identification of agonists, we were able to quickly identify potential “hit”-antagonist compounds (Fig 5B, highlighted by blue boxes) by highlighting the point at which oxytocin was added during the Ca2+-assay, The % inhibition was calculated in a similar manner as the % stimulation for each compound. The average cut-off for active “hit”-antagonists was 50.02±4.77% inhibition in OT-induced intracellular Ca2+-release at a concentration of 10μM. Table 1 summarizes the number of hits and hit-rates in the pilot screen.
An unexpected observation occurred following the analyses of data collected during the OT addition. As shown in Fig 5C, a second set of test compounds were discovered as potentiators of OT-induced intracellular Ca2+-release. In total, 20 test compounds (0.73% of all compounds screened) were found to potentiate OT-induced calcium mobilization following an average cut-off of -50.83±5.55% inhibition after a median -0.84±0.51% inhibition of control OT wells. None of these “hit”-potentiators of OT-induced calcium mobilization overlapped with the “hit”-agonists discovered during the compound addition. Importantly, one small molecule, N-(3-Trifluoromethylphenyl) Piperazine Hydrochloride (TFMPP) was found as a “hit”-potentiator in two different compound screen plates from two different compound libraries (Spectrum and NIH Clinical I Collection) using two different batches of primary mouse UT-myo cells, with an average 48.84±1.75% activation.
Next, we retested hit-compounds (Fig 5D), and found 67% of compounds were confirmed as hit-agonist compounds capable of inducing calcium mobilization in primary mouse UT-myo cells. Furthermore, we confirmed 84% of compounds as hit-antagonists that were effective for inhibiting OT-induced intracellular Ca2+-release in UT-myo cells (Table 1).
Examining the potency of hit-compounds
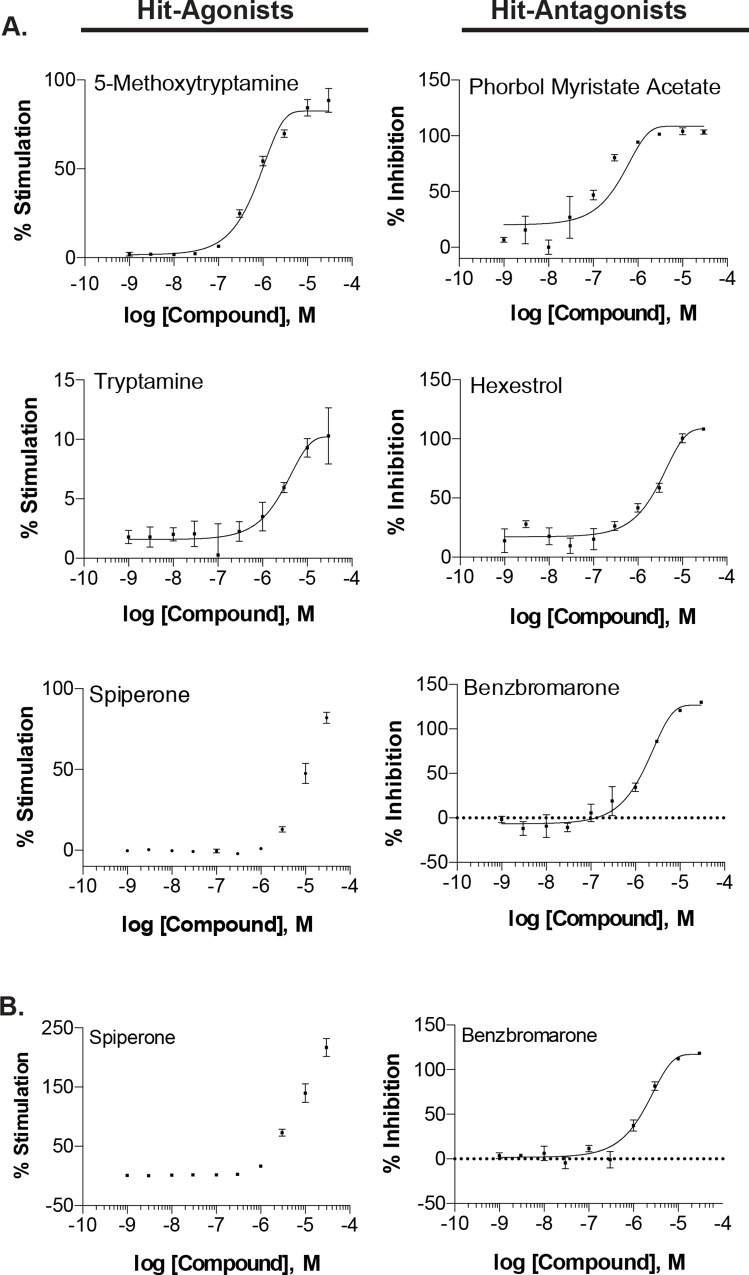
We examined the potency of the confirmed hit-agonist and antagonist compounds by assaying compounds at three-fold 10-point titrations starting at 30μM. The potency of hit-antagonist compounds was generally greater than that of hit-agonist compounds (Table 2). There were 21 hit-antagonist compounds that demonstrated an EC50 less than 10μM, compared to only 7 hit-agonist compounds. Representative concentration-response curves of hit-compounds are shown in Fig 6A. Additionally, we compared the potency of confirmed hit-compounds purchased commercially, to those cherry-picked from the compound library plates. Two representative hit-compounds are shown (Fig 6B vs 6A): spiperone, a hit-agonist and benzbromarone, a hit-antagonist. While the Emax was greater using the spiperone purchased commercially (Emax ± SEM = 217.0 ± 15.24 vs 81.89 ± 3.48) there were no differences in the EC50 value (9.30e-06 vs 1.02e-05) between the two sources of spiperone, respectively. Moreover, no differences were noted in either the Emax (118.4 ± 0.94 vs 130.0 ± 0.19) or EC50 (1.84e-06 vs 1.90e-06) values obtained using the two sources (commercial vs cherry-picked) of benzbromarone, respectively.
Table 2. Concentration-response effect of confirmed hit-compounds.
| Compound ID | Compound Name | EC50 | Emax ± SEM |
|---|---|---|---|
| Agonists | |||
| 1388 | 5-Methoxytryptamine | 7.43e-07 | 90.92 ± 8.1 |
| 3005837 | Pyrithione Zinc | 1.65e-06 | 17.43 ± 0.88 |
| 1150 | Tryptamine | 3.08e-06 | 10.29 ± 2.36 |
| 41684 | Nitazoxanide | 4.20e-06 | 26.9 ± 1.05 |
| 54680693 | Pyrvinium Pamoate | 5.68e-06 | 22.58 ± 0.99 |
| 5649 | Valinomycin | 6.49e-06 | 8.72 ± 1.55 |
| 5265 | Spiperone | 1.02e-05 | 81.89 ± 3.48 |
| 34312 | Oxycarbazepine | 1.10e-05 | 7.44 ± 0.3 |
| 8397 | Xanthopterin | 1.13e-05 | 24.78 ± 3.04 |
| 3598 | Hexachlorophene | 1.39e-05 | 17.79 ± 2.81 |
| 3397 | Flutamide | 2.19e-05 | 15.23 ± 0.74 |
| 5701996 | Isoreserpine | 3.03e-05 | 11.53 ± 1.24 |
| 3386 | Fluoxetine | 4.45e-05 | 21.65 ± 8.32 |
| 4993 | Pyrimethamine | 5.56e-05 | 85.64 ± 2.4 |
| Antagonists | |||
| 3037 | Dichlorophene | 9.44e-07 | 115.28 ± 7.43 |
| 37123 | Diflubenzuron | 9.93e-07 | 35.15 ± 2.99 |
| 52897276 | 7-Desacetoxy-6,7-Dehydrogedunin | 1.07e-06 | 33.83 ± 6.85 |
| 16682730 | Phenylmercuric Acetate | 1.13e-06 | 112.88 ± 3.41 |
| 6083 | Adenosine 5’-monophosphate | 1.16e-06 | 49.74 ± 9.49 |
| 27924 | Phorbol Myristate Acetate | 1.27e-07 | 103.43 ± 1.94 |
| 10205 | Plumbagin | 1.50e-06 | 114.4 ± 3.67 |
| 3352 | Fipronil | 1.70e-06 | 80.65 ± 8.95 |
| 3606 | Hexestrol | 3.36e-06 | 108.18 ± 0.72 |
| 2333 | Benzbromarone | 1.91e-06 | 130.03 ± 0.19 |
| 6377243 | Obtusaquinone | 2.83e-06 | 114.15 ± 8.08 |
| 72385 | Nonoxynol-9 | 4.48e-06 | 123.35 ± 5.55 |
| 3492326 | Dihydromunduletone | 5.03e-06 | 122.17 ± 4.87 |
| 452550 | Tyrothricin | 7.19e-06 | 139.95 ± 13.1 |
| 9556529 | Oxiconazole Nitrate | 7.44e-06 | 80.33 ± 6.91 |
| 3651377 | MK-886 | 7.92e-06 | 152.43 ± 0.93 |
| 2378 | Bifonazole | 8.66e-06 | 61.95 ± 2.77 |
| 4499 | Nisoldipine | 9.43e-06 | 144.42 ± 1.97 |
| 2330 | Benzalkonium Chloride | 9.82e-06 | 115.68 ± 4.9 |
| 3503 | Gossypol | 1.00e-05 | 119.51 ± 0.7 |
| 73357 | Gramicidin | 1.08e-05 | 63.77 ± 4.03 |
Fig 6. Concentration-response effect of confirmed hit-compounds.
A. Representative compound titrations performed to examine the potency of confirmed hit-agonists and hit-antagonists on UT-myo native and OT-induced Ca2+-mobilization. B. Additionally, compound titrations for hit-compounds were performed using commercially purchased compounds. A 10-point three-fold titration of confirmed hit-compounds were added during the compound addition of the Ca2+-mobilization assay. Data is shown as either mean±SEM %stimulation of Ca2+-mobilization or %inhibition of OT-induced Ca2+-mobilization. Non-linear regression was used to fit the data.
Lead hit generation using functional annotation analyses
We performed functional annotation analyses in order to rank confirmed hit-antagonists into leads in order to limit the number of hits examined in our ex vivo isometric contractility assay. The MeSH pharmacological classification of each confirmed hit-compound was obtained from PubChem. As shown in Table 3, the majority of compounds have unknown MeSH pharmacological classification, despite our pilot screen containing well-annotated compound libraries. Surprisingly, a number of our hit-agonist compounds are anti-psychotic agents (3), dopamine antagonists (3), and adrenergic uptake inhibitors (2). As expected, many of our hit-antagonist compounds are known vasodilator agents (3), antihypertensive agents (2), bronchodilator agents (2), Ca2+-channel blockers (2) cardiotonic agents (2), all of which are known to affect intracellular Ca2+-levels in smooth muscle cells. Our screen also identified a known tocolytic agent, fenoterol. Interestingly, several of our confirmed hit-antagonist compounds have anti-inflammatory or immunological pharmacological classifications: anti-Infective agents, local (3), immunologic adjuvants (2), antifungal (2), and anti-bacterial (2) compounds.
Table 3. MeSH pharmacological classifications containing multiple confirmed hit-compounds.
| MeSH Pharmacological Classification | # Compounds |
|---|---|
| Agonists | |
| Unknown | 6 |
| Antineoplastic | 3 |
| Antipsychotic Agents | 3 |
| Dopamine Antagonists | 3 |
| Adrenergic Uptake Inhibitors | 2 |
| Anti-Infective Agents | 2 |
| Antidepressive Agents, Second-Generation | 2 |
| Antineoplastic | 2 |
| Insecticides | 2 |
| Ionophores | 2 |
| Serotonin Uptake Inhibitors | 2 |
| Uncoupling Agents | 2 |
| Antagonists | |
| Anti-Infective Agents, local | 3 |
| Vasodilator | 3 |
| Adjuvants, Immunologic | 2 |
| Antifungal Agents | 2 |
| Antihypertensive Agents | 2 |
| Bronchodilator Agents | 2 |
| Calcium-Channel Blockers | 2 |
| Cardiotonic Agents | 2 |
| Contraceptive Agents | 2 |
The compound identification (CID) number of each confirmed hit compound was uploaded into BioActivity SAR in PubChem, followed by clustering of Compound Activity and Active BioAssay Defined Protein Targets. Shown in Table 4 are the target proteins of prior HTS bioassay studies in which multiple of our confirmed hit compounds were found to be active. Similar to the MeSH pharmacological classifications, a number of hit-agonist compounds have been shown to target the adrenergic, serotonin and dopamine receptors.
Table 4. Target proteins identified by multiple confirmed compounds.
| Target Proteins | # Compounds |
|---|---|
| Agonists | |
| Cytochrome P450, family 1, subfamily A, polypeptide 2 | 8 |
| Alpha-2C adrenergic receptor | 4 |
| 5-hydroxytryptamine receptor 2B | 3 |
| Cytochrome P450 2D6 | 3 |
| E3 ubiquitin-protein ligase Mdm2 isoform MDM2 | 3 |
| Nuclear factor erythroid 2-related factor 2 isoform 1 | 3 |
| 5-hydroxytryptamine receptor 4 | 2 |
| 5-Hydroxytryptamine receptor 5-HT7 | 2 |
| Bromodomain adjacent to zinc finger domain 2B | 2 |
| Chain A, Jmjd2a Tandem Tudor Domains In Complex With A Trimethylated Histone H4-K20 Peptide | 2 |
| Chromobox protein homolog 1 | 2 |
| Histamine H1 receptor | 2 |
| Microtubule-associated protein tau | 2 |
| Nuclear receptor ROR-gamma | 2 |
| Platelet-activating factor acetylhydrolase precursor | 2 |
| Potassium voltage-gated channel subfamily KQT member 2 | 2 |
| Prolyl endopeptidase-like | 2 |
| Putative recombination protein RecB | 2 |
| Serine/threonine-protein kinase mTOR | 2 |
| Antagonists | |
| Vitamin D3 receptor isoform VDRA | 3 |
| Glyceraldehyde-3-phosphate dehydrogenase | 2 |
| Nuclear receptor ROR-gamma | 2 |
| Tumor protein p53 | 2 |
Testing confirmed hit antagonists for tocolytic ability
Based on the percent inhibition and functional annotation analyses, we chose to focus the remainder of the studies on antagonist compounds. We selected 4 confirmed hit-compounds (benzbromarone, dipyridamole, fenoterol HBr and nisoldipine; Table 5) to determine whether any of these inhibitors of UT-myo cell Ca2+-release could prevent uterine myometrial contractions. A well-established ex vivo isometric contractility assay was used as a secondary screen to examine the effect of hit-antagonist compounds on frequency, amplitude and AUC [25–31].
Table 5. Compounds selected for ex vivo uterine myometrial organ bath contractility studies.
| Compound | AVG % Inhibition Ca2+-Assay 1 | MeSH Pharmacological Category | Target protein |
|---|---|---|---|
| Atosiban | 99.67 | Hormone antagonist; Tocolytic agent | OTR, V1aR, V1bR, V2R |
| Benzbromarone | 100.22 | Uricosuric Agent | P450, PPARy, PNR, MCL1, GP120,KCNJ1,SKA, TNF, S1P1 |
| Dipyridamole | 128.11 | Phosphodiesterase Inhibitors; Platelet Aggregation Inhibitor; Vasodilator Agent | PDE3 and 10, P450, ENT1, M1R |
| Fenoterol Hydrobromide | 47.66 | Adrenergic beta-2 receptor agonist; Bronchodilator Agent; Sympathomimetic; Tocolytic Agent | Beta-2AR, DRD1, EP2 |
| Nisoldipine | 63.49 | Antihypertensive Agent; Calcium Channel Blocker; Vasodilator Agent | KCNH2 |
1 Values obtained from primary screen and retesting at 10μM concentration
Spontaneous myometrial contractions were recorded (measured in grams, g, of tension) for 10min, followed by the addition of cumulative doses of vehicle, atosiban, benzobromarone, dipryidamole, fenoterol HBr or nisoldipine (10pM to 1mM; 10min per dose) as shown in representative recordings ( Fig 7A). All compounds examined significantly (p<0.001) inhibited contractile activity (Fig 7B–7D) at different IC50 values (Table 6). Fenoterol and nisoldipine were found to be the most potent (IC50 range = 0.1–0.157 nM and 35.2–87.2nM, respectively) compounds examined based on all parameters: AUC, amplitude and frequency. Fenoterol and nisoldipine also had the greatest efficacy (Emax: -90.47 to -99.12%, and -68.52 to -94.13%, respectively).
Fig 7. Effect of confirmed hit-compounds on ex vivo uterine myometrial contractility.
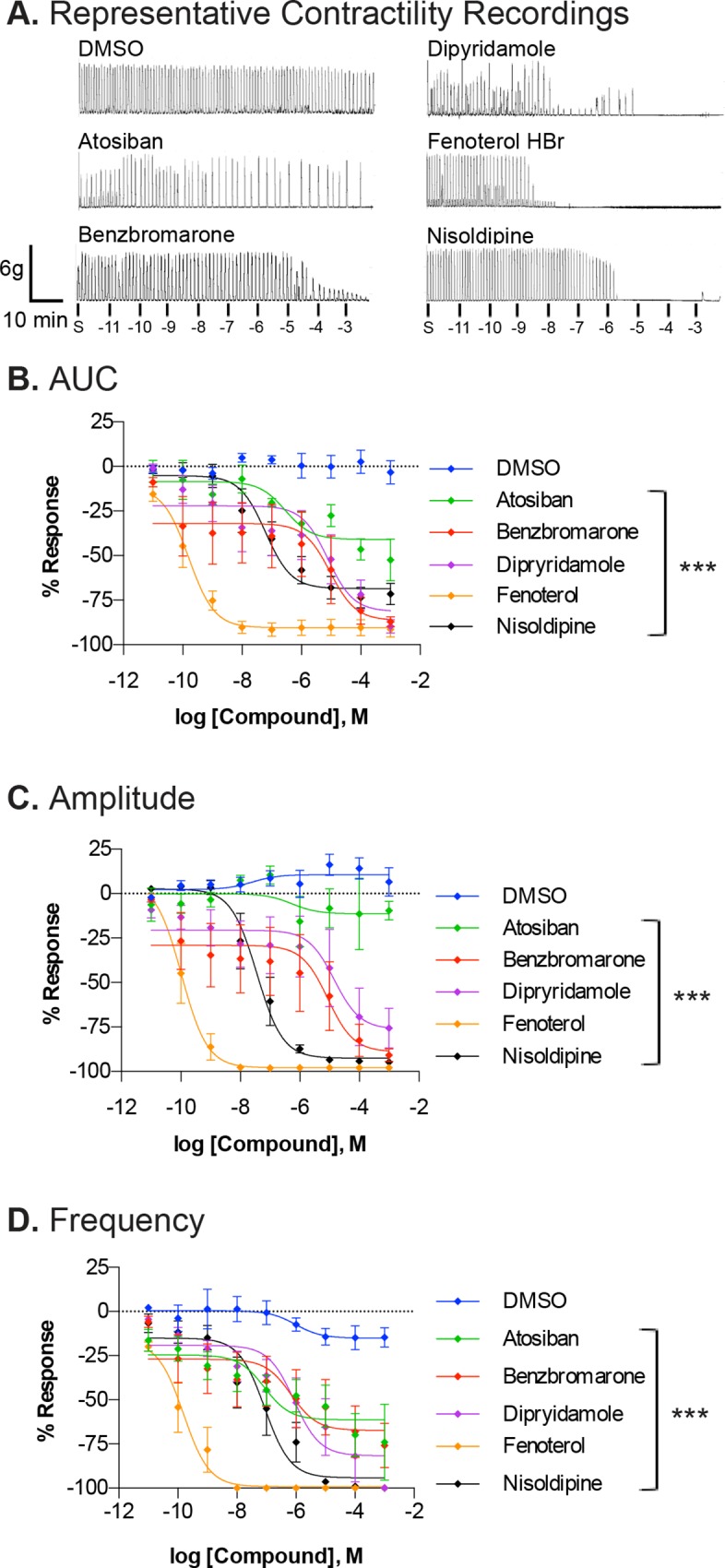
A. Representative recordings of spontaneous contractile activity prior to treatment with increasing concentrations (10pm to 1mM) of either DMSO (vehicle control), atosiban, benzbromarone, dipyridamole, fenoterol HBr or nisoldipine. Isometric tension recordings were analyzed for AUC (B), amplitude (C) and frequency (D) of contractions. Data is shown as mean±SEM % response from baseline (spontaneous contraction) from 5–8 different uterine strips from different mice. Non-linear regression was used to fit the data. Significant (***p<0.0001) difference between each fit line and DMSO is indicated. S = spontaneous contractility
Table 6. Capacity of compounds to inhibit ex vivo uterine myometrial contractility.
| AUC | Amplitude | Frequency | |||||
|---|---|---|---|---|---|---|---|
| Compound | N | IC50 | Emax | IC50 | Emax | IC50 | Emax |
| Atosiban | 5 | 2.875e-07 | -40.93% | 5.340e-07 | -11.36% | 8.499e-08 | -61.31% |
| Benzbromarone | 5 | 9.185e-06 | -86.35% | 8.677e-06 | -88.95% | 6.932e-07 | -67.29% |
| Dipyridamole | 8 | 7.694e-06 | -81.37% | 1.871e-05 | -81.49% | 8.64e-07 | -81.67% |
| Fenoterol HBr | 7 | 1.577e-10 | -90.47% | 1.056e-10 | -97.86% | 1.549e-10 | -99.12% |
| Nisoldipine | 8 | 6.134e-08 | -68.52% | 3.528e-08 | -92.14% | 8.725e-08 | -94.13% |
In order to relate the results of our Ca2+-mobilization assay to results from our secondary ex vivo contractility assay, we compared the inhibitory effects of the compounds between these two assays. We found that the rank order potencies of compounds obtained from the Ca2+-mobilization microtiter plate assay did not necessarily correlate with our secondary contractility assay (Table 7). While fenoterol was the least inhibitory compound in the Ca2+-mobilization assay, it was the most potent compound examined in the isometric contractility assay. Conversely, benzbromarone was one of the least potent compounds to inhibit myometrial contractility, but was selected for examination in this secondary assay based on its high inhibitory capacity in the Ca2+-mobilization assay.
Table 7. Comparison of inhibitory capacity of compounds between calcium-mobilization and ex vivo uterine myometrial contractility assays.
| High-throughput Ca2+ Assay | Ex Vivo Isometric Contractility (AUC) | |||||
|---|---|---|---|---|---|---|
| Compound | N | IC50 | Emax | N | IC50 | Emax |
| Atosiban | 3 | 2.66e-07 | -113.0% | 5 | 2.9e-07 | -40.93% |
| Benzbromarone | 3 | 1.91e-06 | -130.0% | 5 | 9.2e-06 | -86.35% |
| Dipyridamole | 3 | 1.50e-05 | -135.2% | 8 | 7.7e-06 | -81.37% |
| Fenoterol HBr | 3 | 2.25e-05 | -37.76% | 7 | 1.6e-10 | -90.47% |
| Nisoldipine | 3 | 9.43e-06 | -144.4% | 8 | 6.1e-08 | -68.52% |
Discussion
The identification of novel tocolytic or uterotonic compounds remains of paramount importance considering off-target side effects and inefficacy of existing treatments. We report for the first time a robust and reliable uterine myometrial calcium mobilization assay for HTS. The assay was validated for HTS by meeting a series of rigorous performance benchmarks: 1) DMSO-tolerance; 2) assay robustness and reproducibility based on a Z´ = 0.73; 3) a pilot screen that demonstrated the suitability of the assay for HTS, the feasibility of using primary mouse uterine myometrial cells for future large-scale HTS, and identified hit-compounds for immediate testing in a secondary screen; and 4) assay capability of reporting dose-dependent stimulation of calcium-mobilization and inhibition of OT-induced calcium-mobilization. Using a secondary ex vivo assay, we established that four representative hit-antagonists are potent inhibitors of uterine contractility. Collectively, these findings demonstrate the power of the Ca2+-mobilization assay to identify tocolytic compounds.
The assay described here is the first Ca2+-mobilization assay in a 384-well format to screen primary uterine myometrial cells. Our HTS assay identified both agonists of Ca2+-mobilization and antagonists of OT-induced Ca2+-mobilization in uterine myometrial cells in a single-screen. Unexpectedly, it also revealed potentiators of OT, i.e. agonists of OT-induced Ca2+-mobilization. Our assay was found to be robust (Z´-factor = 0.73) and suitable for use in a full-scale HTS campaign to correctly distinguish “hits” from non-hits. Furthermore, our pilot screen using well-annotated compound libraries allowed us to assess assay quality and predict its usefulness in a HTS campaign. Based on the calculated % activation and % inhibition thresholds, the calculated hit-rate prior to confirmation testing was 2.71% for agonists and 1.65% for antagonists, respectively. Large-scale screening for the discovery of new uterotonics or tocolytics is sorely needed. Thus, the robust HTS-ready assay described here may provide a reliable method for identification of novel modulators of uterine Ca2+-mobilization and myometrial contractility.
The cut-off thresholds used to identify hit-compounds during the pilot screen were determined empirically using industry-standard, statistics-based selection criteria. The average hit-cutoff for hit-agonists was 5.85% stimulation. On average, identified hit-agonists were weaker (lower Emax and EC50 values) compared to identified hit-antagonists. Despite this, 49 (or 67%) of the hits were confirmed during retesting, and 7 of those had EC50 values ≤10 uM. In a large-scale HTS, where presumably more potent agonists (and antagonists) will be identified, compounds exhibiting more potent activity can be prioritized over weaker modulators.
False-positives can be a major problem during HTS. Autofluorescence of some test compounds resulted in 22 false positives in the pilot screen. Other false positives were removed during retesting of hit-antagonists as well as titration studies to examine potency. During large-scale HTS campaigns, it is necessary to utilize counterscreen assays in order to remove false-positives and prioritize hit-compounds. Common counterscreen assays include: target or cell selectivity, or compound cytotoxicity. Application of these assays will be important to determine whether any of the hit-compounds identified in this study are: 1) artifacts of detection, 2) specific agonists of uterine Ca2+-mobilization or antagonists of OT-induced uterine Ca2+-mobilization or 3) cytotoxic (relevant to antagonists only). To this end, we have begun developing a comparative screen for UT-myo selectivity using the primary Ca2+-mobilization assay described in this study and primary mouse aorta smooth muscle cells (AOSMCs) [41]. Six hit-agonists were capable of inducing intracellular Ca2+-release from AOSMCs, while 7 hit-agonists have been identified as UT-myo selective and have an EC50≤10μM: 5-methoxytryptamine, tryptamine, nitazoxanide, oxycarbazepine, xanthopterin, fluoxetine and pyrimethamine (from Table 2). In order to test hit-antagonists for uterine-selectivity, we chose to use U46619, instead of OT, to induce intracellular Ca2+-release from AOSMCs since it is established as the most potent agonist of vascular SMCs. Using this strategy, we have identified 6 promiscuous hit-antagonists that are not UT-myo selective: dichlorophene, 7-Desacetoxy-6,7-Dehydrogedunin, plumbagin, tyrothricin (from Table 2). In future studies, we hope to develop comparative counterscreen assays using the primary Ca2+-mobilization assay, described in this study, using mesenteric artery SMCs and fetal ductus arteriosus SMCs, given that these SMC-types (along with AOSMCs) are the most relevant off-targets of current uterotonic and tocolytic therapeutics.
Functional annotation analyses of confirmed agonists and antagonists provided insight into molecular target pathways and pharmacological classes of agents affecting uterine myometrial Ca2+-mobilization. Our pilot screen identified a number of pharmacologic classifications and targets of current uterotonic (alpha-adrenergic receptor agonist) and tocolytic (vasodilators, calcium channel blockers, and beta-2 adrenergic receptor agonists) agents. However, we anticipate that other members of pharmacologic classes of compounds that were uncovered in our analysis could be explored for their potential uterotonic and tocolytic capabilities. To this end, most currently used tocolytics were initially developed for pain or cardiovascular problems, but were later found to be effective tocolytics [13]. Moreover, a recent study identified the inward rectifier potassium channel Kir7.1 as a critical regulator of myometrial cell membrane potential and contractility. It will be important to determine if any of the agonists of calcium signaling identified in the present study act on Kir7.1 to regulate calcium entry [42].
Testing hit-compounds in a tissue assay serves as an excellent transitional screening system between cell-based assays and in vivo models. To this end, ex vivo uterine myometrial contractility assays are a well-established and ideal model for testing the therapeutic capacity to regulate tissue contractility. The results obtained from the uterine myometrial contractility assay showed that all 4 hit-compounds identified from our pilot screen were able to inhibit ex vivo uterine contractions. The IC50 values obtained from the contractility assay were not highly correlated with those obtained from the Ca2+-mobilization assay. We attribute this discrepancy to differences between the tissue strips used in our secondary screen compared to isolated single-cells used in our primary screen. Despite these differences, both screening approaches identified significant inhibitory effects on uterine contractility; their combined use provided additional strength for small molecule screening. Some of these compounds have previously been explored for their inhibitory potential on uterine contractility. Dipyridamole was examined with other phosphodiesterase inhibitors to determine whether they could potentiate 5-hydroxytryptamine inhibition of pig myometrial contractility [43]. Nisoldipine has been previously reported to inhibit spontaneous contractility of non-pregnant human myometrium, as well as OT-induced rat myometrial contractions [44]. The IC50 of nisoldipine reported in that study (6±0.09 μM) was less potent in non-pregnant human myometrial samples compared to what we report (range: 0.04–0.09 μM) for pregnant mouse myometrial samples. Finally, fenoterol has been investigated and shown clinically to inhibit uterine activity, by up to 30%, in women with OT-induced labor [45]. While fenoterol has been shown to be an effective agent for treatment of preterm labor, its use as a tocolytic in women was terminated due to maternal adverse effects [46].
Collectively, this study developed and validated a robust dual-addition assay for HTS to identify agonists and antagonists of Ca2+-mobilization in UT-myo cells. HTS was shown to be an effective tool for discovering small molecular compounds regulating uterine myometrial Ca2+-mobilization. Furthermore, inhibition of myometrial contractions by 4 compounds identified by our HTS suggests that small molecules have the potential to regulate uterine contractility. Together, these data highlight a promising role for large-scale HTS for identifying small molecule molecular modulators and molecular targets of myometrial Ca2+-mobilization, which have high therapeutic potential for women with preterm labor or postpartum hemorrhage/uterine atony.
Acknowledgments
We would like to thank Drs. Dehui Mi and Paige Vinson, and the Vanderbilt Institute of Chemical Biology High Throughput Screening Facility for their technical assistance and expertise used for the HTS assay development. The NIH Clinical Collections of compounds were provided through the National Institutes of Health Molecular Libraries Roadmap Initiative.
Data Availability
All relevant data are within the paper.
Funding Statement
This project was supported by research funds (J. L. H.) from the Vanderbilt Office of Clinical and Translational Scientist Development and CTSA award numbers UL1TR000445 and KL2TR000446 from the National Center for Advancing Translational Sciences. This work was also supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grants HD044741 (B. C. P.), HD081121 to (J. R.)]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Norwitz ER, Robinson JN, Challis JR. The control of labor. N Engl J Med. 1999;341(9):660–6. Epub 1999/08/26. 10.1056/NEJM199908263410906 . [DOI] [PubMed] [Google Scholar]
- 2. Giles W, Bisits A. Preterm labour. The present and future of tocolysis. Best Pract Res Clin Obstet Gynaecol. 2007;21(5):857–68. Epub 2007/04/27. 10.1016/j.bpobgyn.2007.03.011 . [DOI] [PubMed] [Google Scholar]
- 3. de Heus R, Mol BW, Erwich JJ, van Geijn HP, Gyselaers WJ, Hanssens M, et al. Adverse drug reactions to tocolytic treatment for preterm labour: prospective cohort study. BMJ. 2009;338:b744 Epub 2009/03/07. 10.1136/bmj.b744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yasuda K, Nakamoto T, Yasuhara M, Okada H, Nakajima T, Kanzaki H, et al. Role of protein kinase Cbeta in rhythmic contractions of human pregnant myometrium. Reproduction. 2007;133(4):797–806. Epub 2007/05/17. 10.1530/REP-06-0041 . [DOI] [PubMed] [Google Scholar]
- 5. Shynlova O, Tsui P, Jaffer S, Lye SJ. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol. 2009;144 Suppl 1:S2–10. Epub 2009/03/21. 10.1016/j.ejogrb.2009.02.044 . [DOI] [PubMed] [Google Scholar]
- 6. Aguilar HN, Mitchell BF. Physiological pathways and molecular mechanisms regulating uterine contractility. Hum Reprod Update. 2010;16(6):725–44. Epub 2010/06/17. 10.1093/humupd/dmq016 . [DOI] [PubMed] [Google Scholar]
- 7. Kamel RM. The onset of human parturition. Arch Gynecol Obstet. 2010;281(6):975–82. Epub 2010/02/04. 10.1007/s00404-010-1365-9 . [DOI] [PubMed] [Google Scholar]
- 8. Petraglia F, Imperatore A, Challis JR. Neuroendocrine mechanisms in pregnancy and parturition. Endocr Rev. 2010;31(6):783–816. Epub 2010/07/16. 10.1210/er.2009-0019 . [DOI] [PubMed] [Google Scholar]
- 9. Savitsky AG, Savitsky GA, Ivanov DO, Mikhailov AV, Kurganskiy AV, Mill KV. The myogenic mechanism of synchronization and coordination for uterine myocytes contractions during labor. J Matern Fetal Neonatal Med. 2013;26(6):566–70. Epub 2012/11/20. 10.3109/14767058.2012.738261 . [DOI] [PubMed] [Google Scholar]
- 10. Voltolini C, Torricelli M, Conti N, Vellucci FL, Severi FM, Petraglia F. Understanding spontaneous preterm birth: from underlying mechanisms to predictive and preventive interventions. Reproductive sciences. 2013;20(11):1274–92. Epub 2013/03/16. 10.1177/1933719113477496 . [DOI] [PubMed] [Google Scholar]
- 11. Groom KM, Bennett PR, Shennan AH. Randomised, double-blind, placebo controlled pilot study assessing nitroglycerin as a tocolytic. BJOG: an international journal of obstetrics and gynaecology. 2000;107(9):1182–3. Epub 2000/09/26. . [DOI] [PubMed] [Google Scholar]
- 12. Haas DM, Benjamin T, Sawyer R, Quinney SK. Short-term tocolytics for preterm delivery—current perspectives. International journal of women's health. 2014;6:343–9. Epub 2014/04/08. 10.2147/IJWH.S44048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olson DM, Christiaens I, Gracie S, Yamamoto Y, Mitchell BF. Emerging tocolytics: challenges in designing and testing drugs to delay preterm delivery and prolong pregnancy. Expert opinion on emerging drugs. 2008;13(4):695–707. Epub 2008/12/03. 10.1517/14728210802568764 . [DOI] [PubMed] [Google Scholar]
- 14. Vrachnis N, Malamas FM, Sifakis S, Deligeoroglou E, Iliodromiti Z. The oxytocin-oxytocin receptor system and its antagonists as tocolytic agents. Int J Endocrinol. 2011;2011:350546 Epub 2011/12/23. 10.1155/2011/350546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO. World Health Organization (WHO) Guidelines Approved by the Guidelines Review Committee. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. Geneva2012. [PubMed]
- 16. Tong WC, Choi CY, Kharche S, Holden AV, Zhang H, Taggart MJ. A computational model of the ionic currents, Ca2+ dynamics and action potentials underlying contraction of isolated uterine smooth muscle. PloS one. 2011;6(4):e18685 10.1371/journal.pone.0018685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tong WC, Ghouri I, Taggart MJ. Computational modeling of inhibition of voltage-gated Ca channels: identification of different effects on uterine and cardiac action potentials. Frontiers in physiology. 2014;5:399 Epub 2014/11/02. 10.3389/fphys.2014.00399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burghardt RC, Barhoumi R, Sanborn BM, Andersen J. Oxytocin-induced Ca2+ responses in human myometrial cells. Biology of reproduction. 1999;60(4):777–82. . [DOI] [PubMed] [Google Scholar]
- 19. Young RC, Schumann R, Zhang P. Nifedipine block of capacitative calcium entry in cultured human uterine smooth-muscle cells. Journal of the Society for Gynecologic Investigation. 2001;8(4):210–5. Epub 2001/08/30. . [DOI] [PubMed] [Google Scholar]
- 20. Cirillo R, Gillio Tos E, Schwarz MK, Quattropani A, Scheer A, Missotten M, et al. Pharmacology of (2S,4Z)-N-[(2S)-2-hydroxy-2-phenylethyl]-4-(methoxyimino) -1-[(2'-methyl[1,1'-biphenyl]-4-yl)carbonyl]-2-pyrrolidinecarboxamide, a new potent and selective nonpeptide antagonist of the oxytocin receptor. J Pharmacol Exp Ther. 2003;306(1):253–61. 10.1124/jpet.103.049395 . [DOI] [PubMed] [Google Scholar]
- 21. Sawdy R, Knock GA, Bennett PR, Poston L, Aaronson PI. Effect of nimesulide and indomethacin on contractility and the Ca2+ channel current in myometrial smooth muscle from pregnant women. British journal of pharmacology. 1998;125(6):1212–7. Epub 1998/12/24. 10.1038/sj.bjp.0702211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Devost D, Zingg HH. Novel in vitro system for functional assessment of oxytocin action. American journal of physiology Endocrinology and metabolism. 2007;292(1):E1–6. Epub 2006/09/07. 10.1152/ajpendo.00529.2005 . [DOI] [PubMed] [Google Scholar]
- 23. Fitzgibbon J, Morrison JJ, Smith TJ, O'Brien M. Modulation of human uterine smooth muscle cell collagen contractility by thrombin, Y-27632, TNF alpha and indomethacin. Reproductive biology and endocrinology: RB&E. 2009;7:2 Epub 2009/01/10. 10.1186/1477-7827-7-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pak SC, Bertoncini D, Meyer W, Scaunas D, Flouret G, Wilson L Jr. Comparison of binding affinity of oxytocin antagonists to human and rat uterine oxytocin receptors and their correlation to the rat uterine oxytocic bioassay. Biology of reproduction. 1994;51(6):1140–4. Epub 1994/12/01. . [DOI] [PubMed] [Google Scholar]
- 25. Norman JE, Ward LM, Martin W, Cameron AD, McGrath JC, Greer IA, et al. Effects of cGMP and the nitric oxide donors glyceryl trinitrate and sodium nitroprusside on contractions in vitro of isolated myometrial tissue from pregnant women. Journal of reproduction and fertility. 1997;110(2):249–54. Epub 1997/07/01. . [DOI] [PubMed] [Google Scholar]
- 26. Oger S, Mehats C, Barnette MS, Ferre F, Cabrol D, Leroy MJ. Anti-inflammatory and utero-relaxant effects in human myometrium of new generation phosphodiesterase 4 inhibitors. Biology of reproduction. 2004;70(2):458–64. Epub 2003/10/17. 10.1095/biolreprod.103.023051 . [DOI] [PubMed] [Google Scholar]
- 27. Stymiest JL, Mitchell BF, Wong S, Vederas JC. Synthesis of oxytocin analogues with replacement of sulfur by carbon gives potent antagonists with increased stability. The Journal of organic chemistry. 2005;70(20):7799–809. Epub 2005/11/10. 10.1021/jo050539l . [DOI] [PubMed] [Google Scholar]
- 28. Baumbach J, Shi SQ, Shi L, Balducci J, Coonrod DV, Garfield RE. Inhibition of uterine contractility with various tocolytics with and without progesterone: in vitro studies. Am J Obstet Gynecol. 2012;206(3):254 e1-5. 10.1016/j.ajog.2011.12.011 . [DOI] [PubMed] [Google Scholar]
- 29. Robinson H, Wray S. A new slow releasing, H(2)S generating compound, GYY4137 relaxes spontaneous and oxytocin-stimulated contractions of human and rat pregnant myometrium. PloS one. 2012;7(9):e46278 Epub 2012/10/03. 10.1371/journal.pone.0046278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crankshaw DJ, Pistilli MJ, O'Brien YM, Sweeney EM, Dockery P, Holloway AC, et al. The effects of extracellular calcium-sensing receptor ligands on the contractility of pregnant human myometrium in vitro. Reproductive sciences. 2013;20(8):882–90. 10.1177/1933719112468949 . [DOI] [PubMed] [Google Scholar]
- 31. Munglue P, Eumkep G, Wray S, Kupittayanant S. The effects of watermelon (Citrullus lanatus) extracts and L-citrulline on rat uterine contractility. Reproductive sciences. 2013;20(4):437–48. Epub 2012/09/20. 10.1177/1933719112459223 . [DOI] [PubMed] [Google Scholar]
- 32. Fejgin MD, Pak SC, Flouret G, Parsons MT, Wilson L Jr. Comparison of the in vivo activity of different oxytocin antagonists in the pregnant baboon. Journal of the Society for Gynecologic Investigation. 1998;5(5):251–4. Epub 1998/10/17. . [DOI] [PubMed] [Google Scholar]
- 33. Buhimschi CS, Saade GR, Buhimschi IA, Gokdeniz R, Boyle MB, Garfield RE. Effect of stimulatory and inhibitory drugs on uterine electrical activity measured noninvasively from the abdominal surface of pregnant rats. Am J Obstet Gynecol. 2000;183(1):68–75. 10.1067/mob.2000.105348 . [DOI] [PubMed] [Google Scholar]
- 34. Pierce SL, Kutschke W, Cabeza R, England SK. In vivo measurement of intrauterine pressure by telemetry: a new approach for studying parturition in mouse models. Physiological genomics. 2010;42(2):310–6. Epub 2010/05/13. 10.1152/physiolgenomics.00058.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prudencio AB. Cell-Based High-Throughput Screening Assay for Identification of G-Protein-Coupled Receptors Agonists and Antagonists. dianas. 2013;2(1):e20130302. [Google Scholar]
- 36. Eglen R, Reisine T. Primary cells and stem cells in drug discovery: emerging tools for high-throughput screening. Assay Drug Dev Technol. 2011;9(2):108–24. 10.1089/adt.2010.0305 . [DOI] [PubMed] [Google Scholar]
- 37. Kasten FH. Functional capacity of neonatal mammalian myocardial cells during aging in tissue culture. Adv Exp Med Biol. 1975;53:389–420. . [DOI] [PubMed] [Google Scholar]
- 38. Tribe RM, Moriarty P, Poston L. Calcium homeostatic pathways change with gestation in human myometrium. Biology of reproduction. 2000;63(3):748–55. . [DOI] [PubMed] [Google Scholar]
- 39. Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. Journal of biomolecular screening. 1999;4(2):67–73. Epub 2000/06/06. . [DOI] [PubMed] [Google Scholar]
- 40. Mitchell BF, Aguilar HN, Mosher A, Wood S, Slater DM. The uterine myocyte as a target for prevention of preterm labor. FVV in ObGyn. 2013;5(1):72–81. [PMC free article] [PubMed] [Google Scholar]
- 41. Miller FJ Jr., Filali M, Huss GJ, Stanic B, Chamseddine A, Barna TJ, et al. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res. 2007;101(7):663–71. 10.1161/CIRCRESAHA.107.151076 . [DOI] [PubMed] [Google Scholar]
- 42. McCloskey C, Rada C, Bailey E, McCavera S, van den Berg HA, Atia J, et al. The inwardly rectifying K+ channel KIR7.1 controls uterine excitability throughout pregnancy. EMBO Mol Med. 2014;6(9):1161–74. 10.15252/emmm.201403944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kitazawa T, Kubo O, Satoh M, Taneike T. Involvement of 5-hydroxytryptamine7 receptors in inhibition of porcine myometrial contractility by 5-hydroxytryptamine. British journal of pharmacology. 1998;123(2):173–82. Epub 1998/03/07. 10.1038/sj.bjp.0701583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li FF, Fu SX, Li YS. [Effects of m-nisoldipine and nisoldipine on isolated uterine muscle]. Zhongguo yao li xue bao = Acta pharmacologica Sinica. 1992;13(1):59–62. Epub 1992/01/01. . [PubMed] [Google Scholar]
- 45. Lipshitz J. The uterine and cardiovascular effects of oral fenoterol hydrobromide. British journal of obstetrics and gynaecology. 1977;84(10):737–9. Epub 1977/10/01. . [DOI] [PubMed] [Google Scholar]
- 46. Nonnenmacher A, Hopp H, Dudenhausen J. [Effectiveness and safety of atosiban vs. pulsatile administration of fenoterol in the treatment of preterm labour]. Zeitschrift fur Geburtshilfe und Neonatologie. 2009;213(5):201–6. Epub 2009/10/27. 10.1055/s-0029-1225640 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.