Abstract
The prevalence of Helicobacter pylori infection in Indonesia is still controversial and mainly investigated in the largest ethnic group, Javanese. We examined the prevalence of H. pylori infection using four different tests including culture, histology confirmed by immunohistochemistry and rapid urease test. We also analyzed risk factors associated with H. pylori infection in five largest islands in Indonesia. From January 2014–February 2015 we consecutively recruited a total of 267 patients with dyspeptic symptoms in Java, Papua, Sulawesi, Borneo and Sumatera Island. Overall, the prevalence of H. pylori infection was 22.1% (59/267). Papuan, Batak and Buginese ethnics had higher risk for H. pylori infection than Javanese, Dayak and Chinese ethnics (OR = 30.57, 6.31, 4.95; OR = 28.39, 5.81, 4.61 and OR = 23.23, 4.76, 3.77, respectively, P <0.05). The sensitivity and specificity for RUT and culture were 90.2%, 92.9% and 80.5%, 98.2%, respectively. The patients aged 50–59 years group had significantly higher H. pylori infection than 30–39 years group (OR 2.98, P = 0.05). Protestant had significantly higher H. pylori infection rate than that among Catholic (OR 4.42, P = 0.008). It was also significantly lower among peoples who used tap water as source of drinking water than from Wells/river (OR 9.67, P = 0.03). However only ethnics as become independent risk factors for H. pylori infection. Although we confirmed low prevalence of H. pylori in Javanese; predominant ethnic in Indonesia, several ethnic groups had higher risk of H. pylori infection. The age, religion and water source may implicate as a risk factor for H. pylori infection in Indonesia.
Introduction
Helicobacter pylori infection has been recognized as one of the most common chronic bacterial infections in humans and associated with peptic ulcer disease, gastric adenocarcinoma, and primary gastric B-cell lymphoma [1]. The overall prevalence varies globally from one geographical region to another with occurs mainly in developing countries. Indonesia is a developing country located between South China Sea (Pacific Ocean, in North) and the Indian Ocean (in South); it is an archipelago of more than 13,600 islands with Sumatra, Papua, Kalimantan (Borneo), Sulawesi and Java as five main islands. There are around 300 distinct native ethnic groups in Indonesia, and 742 different languages which most of them belonging to the geographically dispersed Austronesian-speaking family [2]. Javanese is the largest ethnic group who comprise 40.2% of the total population, followed by Sundanese, Batak, Madurese and Betawi (Statistics Indonesia, http://www.bps.go.id/).
Table 1 is the summarizes of previous studies that examined the prevalence of H. pylori in Indonesia (Table 1). Although many researchers have investigated the prevalence in Indonesia, the results are controversial and contradictory (0–68%) [3,4] probably due to the different study populations and different tests for H. pylori diagnosis [5]. Moreover these studies mainly investigated only the largest ethnic group, Javanese [3,4,6–10]. In our previous study, we confirmed that the prevalence of H. pylori infection in Surabaya (Java island) was low, only 11.5% using five different methods to diagnose H. pylori infection [5], the data were concordance with the low age-standardized incidence rate of gastric cancer in Indonesia among Asian countries (2.8/100,000; GLOBOCAN2012, http://globocan.iarc.fr/). We also found that the highest prevalence of H. pylori was observed in patients from the Chinese Indonesian population instead of patients from the Javanese population. Another our study also found unexpected result about the prevalence of H. pylori infection in minor group in North Sulawesi. The overall H. pylori prevalence was only 14.3% for adults and 3.8% for children [11]. However our results cannot be generalized across Indonesia due to the difference of host factor and environmental condition. Further investigation from all Indonesia is necessary to elucidate the reasons of low gastric cancer rate in Indonesia.
Table 1. Summary previous Helicobacter pylori prevalence studies in Indonesia.
| Author | Study period | Area | n | Average age (range) | Test | Positive rate |
|---|---|---|---|---|---|---|
| Syam AF [6] | 2001 | Jakarta | 63 | 42.4 (16–73) | Stool antigen | 66.7% (42/63) |
| RUT | 4.8% (3/63) | |||||
| Histology | 11.1% (7/63) | |||||
| Tokudome S [10] | 2003 | Yogyakarta | 91 | 48.0 for men | UBT | 4% in men and 0% in women |
| 46.6 for women | Serum antibody | 5% in men and 4% in women | ||||
| Tokudome S [3] | 2005 | Semarang | 171 | 57.4 for men | UBT | 0% in men and 0% in women |
| 49.2 for women | Serum antibody | 2% in men and 2% in women | ||||
| Syam AF [14] | 2003–2004 | 6 cities | 550 | 44.98 (15–82) | Histology | 10.2% (56/550) |
| Saragih JB [7] | 1998–2005 | Jakarta | 2903 | no information | Histology | 12.8% (52/407) in 1998 |
| 2.9% (50/403) in 2005 | ||||||
| Aulia D [8] | 2007 | Jakarta | 70 | 47.6 (18–79) | Histology | 5.7% (4/70) |
| Abdullah M* [4] | 1998–1999 | Jakarta | 125 | 50.3 (18–82) | RUT | 68% (85/125) in the antrum4% (5/125) in the corpus |
| Culture | ||||||
| Histology | ||||||
| Arinton IG [9] | 2005 | Purwokerto | 81 | 56.8 (45–75) | PCR | 41.9% (34/81) |
| Zhao Y [15] | 2007 | Mataram | 294 | 34.0 (6–74) | UBT | 11.2% (33/294) |
| Miftahussurur M [11] | 2011–2012 | Manado | 251 Adults | 46.2 (14–88) | Urine test | Adults 14.3% (36/251) |
| 131 Children | 8.47 (6–12) | Children 3.8% (5/131) | ||||
| Miftahussurur M [5] | 2012 | Surabaya | 78 | 49.1 (14–77) | Urine test | 5.1% (4/78) |
| RUT | 9.0% (7/78) | |||||
| Culture | 6.4% (5/78) | |||||
| Histology + IHC | 7.7% (6/78) | |||||
| Overall | 11.5% (9/78) |
UBT, urea breath test; PCR, polymerase chain reaction; RUT, rapid urease test
*This study tested for H. pylori by histology, culture, and rapid urease test
The presence of H. pylori in saliva, dental plaque [12], and feces [13] indicated that person-to-person spreading is probably a major transmission mechanism of H. pylori. A number of studies have found poor hygiene standards, crowded households and deficient sanitation are important to both acquisition of infection in childhood and spreading of this disease. Lower social economic status, non-filtered water, and smoking to be a risk factor for H. pylori [16]. On the other hand, the improvement of hygiene conditions has significantly decreased the prevalence of this infection in many parts of North America and Europe [17]. In Japan, the prevalence of H. pylori infection was higher among individuals born before 1950 and lower in those born thereafter; the data indicated a rapid change in the sanitary conditions and standard of living in Japan after the World War II, and clean public water systems were introduced in Japan in the 1950s. Therefore, sanitary conditions, such as a full equipment rate of water and sewage, are considered to be important factors for H. pylori infection [18].
To our knowledge, very few reports had investigated H. pylori in non-Javanese ethnics [11,14,15] and no report had examined the prevalence of H. pylori infection from several islands in Indonesia using same methods. In this study, we examined the prevalence of H. pylori infection in five largest islands using four different tests. We also identified and analyzed environmental factors on different ethnics in Indonesia.
Materials and Methods
Study population
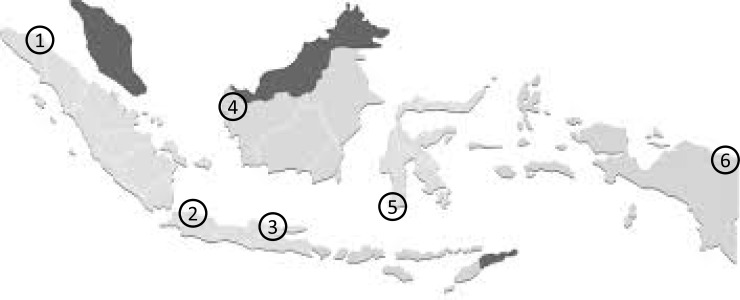
We performed prospective study from January 2014 until February 2015. The survey took place on Jakarta (n = 31) and Surabaya (n = 50) in Java island, Jayapura (n = 21) in Papua island, Makassar (n = 30) in Sulawesi island, Pontianak (n = 64) in Borneo island and Medan (n = 71) in Sumatera island (Fig 1). Experienced endoscopists collected four gastric biopsy specimens during each endoscopy session: three samples from the lesser curvature of the antrum approximately 3 cm from the pyloric ring and one sample from the greater curvature of the corpus. To minimize the potential bias, we used the same experienced pathologist (TU) performed the experiments, who also performed experiments for Myanmar, Vietnam, Bhutan, Dominican Republic and Indonesia [5,19–23]. Biopsy specimens for culture were immediately placed in transport media at -20°C, and stored at -80°C within a day of collection until used for culture testing. Three antral specimens were used for H. pylori culture, rapid urease test (RUT), and histological examination. One corporal specimen was used for histological examination. Peptic ulcers and erosive gastritis were identified by endoscopy. Written informed consent was obtained from all participants, and the study protocol was approved by the Ethics Committee of Dr. Cipto Mangunkusumo Teaching Hospital (Jakarta, Indonesia), Dr. Soetomo Teaching Hospital (Surabaya, Indonesia), Dr. Wahidin Sudirohusodo Teaching Hospital (Makassar, Indonesia) and Oita University Faculty of Medicine (Yufu, Japan).
Fig 1. Map of collecting area in Indonesia.
A total of 267 consecutive patients were obtained biopsy specimen at the five largest islands in Indonesia; (1) Medan (Sumatera island), (2) Jakarta (Java island), (3) Surabaya (Java island), (4) Pontianak (Borneo island), (5) Makassar (Sulawesi island), and Jayapura (Papua island).
H. pylori infection status
To maximize diagnostic accuracy, H. pylori infections were diagnosed based on the combined results of three methods from four different tests; culture, histology confirmed by immunohistochemistry (IHC) and rapid urease test (CLO test, Kimberly-Clark, USA). For H. pylori culture, one antral biopsy specimen was homogenized and directly inoculated onto Mueller Hinton II Agar medium (Becton Dickinson, NJ, USA) supplemented with 7% horse blood without antibiotics. The plates were incubated for up to 10 days at 37°C under microaerophilic conditions (10% O2, 5% CO2, and 85% N2). H. pylori were identified on the basis of colony morphology, Gram staining results, and positive reactions for oxidase, catalase, and urease. Isolated strains were stored at -80°C in Brucella Broth (Difco, NJ, USA) containing 10% dimethylsulfoxide and 10% horse serum.
All biopsy materials for histological testing were fixed in 10% buffered formalin and embedded in paraffin. Serial sections were stained with hematoxylin and eosin as well as May–Giemsa stain. Samples with bacterial loads greater than or equal to grade 1 by updated Sydney system were considered positive for H. pylori. IHC was also performed as previously described [24]. Briefly, after antigen retrieval and inactivation of endogenous peroxidase activity, tissue sections were incubated with α-H. pylori antibody (DAKO, Denmark) overnight at 4°C. After washing, the sections were incubated with biotinylated goat antirabbit IgG (Nichirei Co., Japan), followed by incubation with an avidin-conjugated horseradish peroxidase solution (Vectastain Elite ABC kit; Vector Laboratories Inc., Burlingame, CA, USA). Peroxidase activity was detected using an H2O2/diaminobenzidine substrate solution.
Statistical analysis
Discrete variables were tested using the chi-square test; continuous variables were tested using the Mann-Whitney U and t-tests. A multivariate logistic regression model was used to calculate the odds ratios (OR) of the clinical outcomes that included age, sex, H. pylori infection status, demographic and environment information. All determinants with P values of < 0.10 were entered together into the full logistic regression model, and the model was reduced by excluding variables with P values of > 0.10. The OR and 95% confidence interval (CI) were used to estimate the risk. A P value of < 0.05 was accepted as statistically significant. The SPSS statistical software package version 18.0 (SPSS, Inc., Chicago, IL) was used for all statistical analyses.
Results
Prevalence of H. pylori infection and accuracy several tests
A total of 267 patients with dyspeptic symptoms (143 female and 124 male; mean age of 47.5 ± 14.6 years; range, 17–80 years) were recruited including 39 patients aged ≤29 years, 40 patients aged 30–39 years, 67 patients aged 40–49 years, 57 patients aged 50–59 years, and 64 patients aged ≥60 years. Based on ethnic group they were consisted of 70 Batak subjects, 54 Chinese Indonesian, 42 Javanese, 30 Buginese, 40 Dayak, 21 Papuan, three Madurese, two Acehnese, two Sundanese, one Banjarese, one Balinese, and one Ambonese subject. Among three tests, RUT showed higher positive rate compared with other tests (both P <0.001).
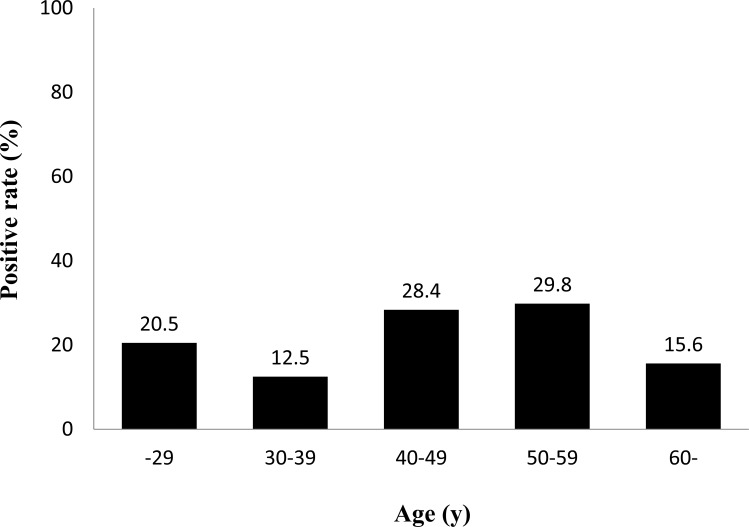
Table 2 shows H. pylori-positive rates for each test. Thirty-two patients were positive by all three tests. Fourteen patients were positive only by the RUT. Three and two patients were positive only by histology and culture, respectively. Using histology confirmed IHC as a gold standard, the sensitivity and specificity of RUT and culture were 90.2%, 92.9% and 80.5%, 98.2%, respectively. Negative predictive value (NPV) and positive predictive value (PPV) were 98.1%, 69.8% and 96.5%, 89.2%, respectively. Overall accuracy rates were 92.5% and, 95.5%, respectively. Using histology confirmed IHC, the prevalence of H. pylori infection was 15.4% (41/267), whereas using culture it was 13.9% (37/267) (S1 Table). However when patients were considered to be H. pylori positive in case at least one test showed positive, the prevalence of H. pylori infection was 22.1% (59/267). In the subsequent analyses, patients were considered to be negative for H. pylori infection when all test results were negative, whereas patients with at least one positive test result were considered positive for H. pylori infection (Fig 2).
Table 2. Prevalence of Helicobacter pylori infection in each diagnostic test n (%).
| -29 | 30–39 | 40–49 | 50–59 | 60- | Total | |
|---|---|---|---|---|---|---|
| n | 39 | 40 | 67 | 57 | 64 | 267 |
| RUT | 7 (17.9) | 5 (12.5) | 18 (26.9) | 13 (22.8) | 10 (15.6) | 53 (19.9) |
| Culture | 7 (17.9) | 4 (10.0) | 10 (14.9) | 8 (14.0) | 8 (12.5) | 37 (13.9) |
| Histology confirmed by IHC | 6 (15.4) | 4 (10.0) | 13 (19.4) | 12 (21.1) | 6 (9.4) | 41 (15.4) |
| Positive if at least one test result positive | 8 (20.5) | 5 (12.5) | 19 (28.4) | 17 (29.8) | 10 (15.6) | 59 (22.1) |
Fig 2. Prevalence of Helicobacter pylori infection in Indonesia by age group.
Three different methods were used to test for H. pylori infection, including culture, histology confirmed by immunohistochemistry and rapid urease test. Patients were considered negative for H. pylori when all test results were negative; H. pylori-positive status required at least one positive test result.
Symptoms, endoscopic findings and H. pylori infection rate
Epigastric pain and bloating were the highest symptoms. There were no significant difference between positivity of H. pylori infection with variables relating to gastrointestinal symptoms and past illness history (P = 0.36 and P = 0.74, respectively) (Table 3). In endoscopic diagnosis, gastric and duodenal ulcer was found among 4 cases (1.5%) and 29 cases (10.9%), respectively. Seven patients have both gastric and duodenal ulcer (2.6%). Gastric cancer was found in 1 case (0.4%); however H. pylori infection was negative. Among 19 subjects with normal endoscopy, 5 (26.3%) were infected with H. pylori. There was no difference the proportion of patients were infected with H. pylori in gastritis (44, 18.2%) and peptic ulcer group (10, 25.0%) (P = 0.66).
Table 3. Gastrointestinal symptoms and past illness history classified by H. pylori results.
| n | H. pylori-negative (n = 208) | H. pylori-positive (n = 59) | |
|---|---|---|---|
| Symptoms | |||
| • Epigastric pain | 156 | 120 (57.7%) | 36 (61.0%) |
| • Heart burn | 10 | 8 (3.8%) | 2 (3.4%) |
| • Abdominal pain | 22 | 21 (10.1%) | 1 (1.7%) |
| • Bloating | 37 | 26 (12.5%) | 11 (18.6%) |
| • History of hematemesis/melena | 16 | 11 (5.3%) | 5 (8.5%) |
| • Nausea/vomiting | 18 | 14 (6.7%) | 4 (6.8%) |
| Illness history | |||
| • Diabetes mellitus | 21 | 17 (8.2%) | 4 (6.8%) |
| • Hypertension | 50 | 39 (18.8%) | 11 (18.6%) |
| • dyslipidemia | 4 | 4 (1.9%) | 0 (0.0%) |
| • Asthma | 2 | 1 (0.5%) | 1 (1.7%) |
| • Hepatitis/Chronic liver disease | 6 | 1 (0.5%) | 5 (8.5%) |
| • Tuberculosis | 2 | 2 (1.0%) | 0 (0.0%) |
Ethnic groups and H. pylori infection rate according to demographic, sanitation and socio-cultural factors
There were significant difference on the prevalence of H. pylori infection related with ethnics group (P <0.001). The highest prevalence of H. pylori infection was Papuan patients; nine of 21 (42.9%) were positive for H. pylori. Among 70 Batak patients, 28 (40.0%) was positive for H. pylori. H. pylori infection was found in 11 of 30 Buginese (36.7%) patients. On the other hand, seven of 54 (13.0%) Chinese were positive for H. pylori infection; Chinese-Surabaya (15.4%, 4/26) was no significance difference of H. pylori infection compared to Chinese Pontianak (12.5% (3/24), P = 0.93). Three Dayak (7.5%) were positive for H. pylori infection. Among 42 Javanese patients, only one (2.4%) was positive for H. pylori infection. Madurese, Acehnese, Sundanese, Banjarese, Balinese and Ambonese patients were negative for H. pylori infection.
Table 4 shows the prevalence of H. pylori infection in the sixth largest ethnics number group according to various range age groups. Interestingly when we just analyzed Papuans, Batak and Buginese, the youngest group aged ≤29 had high prevalence of H. pylori infection contrary with the low H. pylori prevalence group (Javanese, Dayak, and Chinese). The ethnic groups also had significant difference with religion, monthly income, source of water, type of latrine, history of drugs, smoking habit and alcohol consumption (Table 5). Papuan ethnic significantly had high prevalence of Protestant, high subjects with low socio-economic (monthly family income IDR 2.500.000 = 193.31 USD), high smokers and alcohol users, but low mineral water source. On the other hand, Chinese ethnic had high prevalence of Protestant and mineral water source, but low prevalence of low socioeconomic, smokers and alcohol users.
Table 4. Prevalence of Helicobacter pylori infection in the sixth largest ethnics number group (%).
| Papuan | Batak | Buginese | Chinese | Dayak | Javanese | |
|---|---|---|---|---|---|---|
| n | 21 | 70 | 30 | 54 | 40 | 42 |
| -29 | 2 (50.0) | 4 (66.7) | 2 (66.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 30–39 | 2 (33.3) | 1 (12.5) | 1 (16.7) | 1 (14.3) | 0 (0.0) | 0 (0.0) |
| 40–49 | 2 (28.6) | 11 (52.4) | 2 (28.6) | 1 (7.7) | 2 (20.0) | 1 (35.7) |
| 50–59 | 2 (100) | 10 (55.6) | 2 (50.0) | 2 (20.0) | 1 (10.0) | 0 (0.0) |
| 60- | 1 (50) | 2 (11.8) | 4 (40.0) | 3 (18.8) | 0 (0.0) | 0 (0.0) |
| Total | 9 (42.9) | 28 (40.0) | 11 (36.7) | 7 (13.0) | 3 (7.5) | 1 (2.4%) |
Table 5. Details of subjects classified by ethnic group (%).
| Variable | Papuan | Batak | Buginese | Chinese | Dayak | Javanese |
|---|---|---|---|---|---|---|
| Number | 21 | 70 | 30 | 54 | 40 | 42 |
| Age (years)** | 41 (23–63) | 49.5 (24–80) | 48 (22–76) | 49.0 (17–77) | 43.0 (18–77) | 48.5 (18–70) |
| Body Mass Index (kg/m2)*** | 23.9 ± 3.26 | 22.0 ± 2.52 | 23.0 ± 3.90 | 23.0 ± 3.75 | 21.1 ± 3.64 | 23.3 ± 4.11 |
| Sex (% males) | 52.4 | 41.4 | 53.3 | 44.4 | 57.5 | 45.2 |
| Majority religion (%)* | Protestant(100) | Protestant(75.7) | Muslim(86.7) | Protestant(35.2) | Catholic(52.5) | Muslim(90.5) |
| Monthly income <192.31 USD (%)* | 66.6 | 77.1 | 26.7 | 25.9 | 67.5 | 50.0 |
| Mineral water (%)* | 57.1 | 38.6 | 70.0 | 75.9 | 22.5 | 30 |
| Latrine non-toilet (%) | 4.8 | 0.0 | 3.3 | 0.0 | 2.5 | 0.0 |
| Smokers (%)* | 28.6 | 31.4 | 13.3 | 3.7 | 45.0 | 19.1 |
| Alcohol user (%)* | 23.8 | 18.6 | 6.7 | 9.3 | 42.5 | 9.5 |
| H. pylori positive (%) | 42.9 | 40.0 | 36.7 | 13.0 | 7.5 | 2.4 |
*P <0.05
** Median (minimum-maximum)
*** Mean ± standard deviation
Adjusted OR were calculated for H. pylori infection rate with multivariate analysis (Table 6). We entered all determinants with P values of < 0.10 by bivariate analysis (age, sex, religion, ethnics and source of drinking water) into the full logistic regression model. Papuan, Batak and Buginese had higher risk for H. pylori infection than Javanese (P <0.05). Moreover the next analysis on the sixth largest ethnics in this study; Papuan, Batak and Buginese ethnics had higher risk for H. pylori infection than Javanese, Dayak and Chinese ethnics (OR = 30.57, 6.31, 4.95; OR = 28.39, 5.81, 4.61 and OR = 23.23, 4.76, 3.77, respectively, P <0.05) after adjusted for age and sex. The patients aged 50–59 years group had significantly higher H. pylori infection rate than 30–39 years group (Fig 2). The prevalence of H. pylori infection among the Protestant was significantly higher than that among Catholic (OR 4.42, P = 0.008). It was also significantly lower among peoples who used tap water as source of drinking water than from Wells/river although after adjusted age and sex. However final model analysis found only ethnics was significantly as independent risk factor for H. pylori infection (OR = 11.48 [CI 1.12–118.24], OR = 13.32 [CI 1.54–114.96] and OR = 23.47 [2.76–199.51], P <0.05 for Papuan, Batak, Buginese, respectively than Javanese). There were no statistically significant relationship between H. pylori infection rate and gender, social economic status, type of occupation, marital status, body mass index, type of symptoms, type of latrine, history of drugs, smoking habit and alcohol consumption.
Table 6. Association of demographic and sanitation with H. pylori infection status.
| Variable | Total (+H. pylori%) | Crude OR | 95% CI for OR | P |
|---|---|---|---|---|
| Age | ||||
| ≤29 | 8 (20.5%) | 1.81 | 0.54–6.10 | 0.34 |
| 30–39 | 5 (12.5%) | 1.00 | ||
| 40–49 | 19 (28.4%) | 2.77 | 0.94–8.14 | 0.06 |
| 50–59 | 17 (29.8%) | 2.98 | 1.00–8.90 | 0.05 |
| ≥60 | 10 (15.6%) | 1.30 | 0.41–4.11 | 0.66 |
| Gender | ||||
| Males | 32 (25.8%) | 1.49 | 0.84–2.67 | 0.18 |
| Females | 27 (18.9%) | 1.00 | ||
| Religion | ||||
| Muslim | 13 (13.8%) | 1.36 | 0.42–4.49 | 0.61 |
| Catholic | 4 (10.5%) | 1.00 | ||
| Protestant | 40 (34.2%) | 4.42 | 1.46–13.32 | 0.008 |
| Others | 2 (11.1%) | 1.06 | 0.18–6.42 | |
| Ethnic | ||||
| Javanese | 1 (2.4%) | 1.00 | ||
| Papuan | 9 (42.9%) | 30.75 | 3.53–267.68 | 0.002 |
| Batak | 28 (40.0%) | 27.33 | 3.55–210.32 | 0.001 |
| Buginese | 11 (36.7%) | 23.74 | 2.85–197.39 | 0.003 |
| Dayak | 3 (7.5%) | 3.32 | 0.33–33.37 | 0.31 |
| Tionghoa | 7 (13.0%) | 6.11 | 0.72–51.73 | 0.97 |
| Others | 0 (0.0%) | 0.0 | 0.00 | 0.99 |
| Social economic status | ||||
| < Rp.2.500.000 (192.31 USD) | 35 (24.3%) | 1. 59 | 0.83–3.03 | 0.16 |
| Rp.2.500.000–5.000.000 | 17 (16.8%) | 1.00 | ||
| > Rp. 5.000.000 | 7 (31.8%) | 2.31 | 0.82–6.51 | 0.12 |
| Occupation | ||||
| Government job | 12 (31.6%) | 3.23 | 0.36–29.28 | 0.30 |
| Health workers | 1 (12.5%) | 1.00 | ||
| Student | 0 (0.0%) | 0.00 | 0.00 | 0.99 |
| Housewife | 12 (16.2%) | 1.36 | 0.15–12.04 | 0.79 |
| Farmer | 15 (46.9%) | 6.18 | 0.68–56.15 | 0.11 |
| Private job | 17 (19.1%) | 1.65 | 0.19–14.35 | 0.65 |
| Unemployed | 2 (16.7%) | 1.40 | 0.11–18.62 | 0.80 |
| Marital status | ||||
| Unmarried | 6 (16.7%) | 1.00 | ||
| Married | 53 (22.9%) | 1.49 | 0.59–3.77 | 0.40 |
| Body Mass Index | ||||
| <18.5 | 5 (17.9%) | 1.30 | 0.13–13.37 | 0.82 |
| 18.5–24.9 | 44 (25.0%) | 2.00 | 0.23–17.07 | 0.53 |
| 25–29.9 | 8 (14.5%) | 1.02 | 0.11–9.65 | 0.99 |
| >30 | 1 (14.3%) | 1.00 | ||
| Source of drinking water | ||||
| Mineral | 26 (17.6%) | 3.74 | 0.48–29.30 | 0.21 |
| PAM | 3 (21.4%) | 4.91 | 0.45–53.27 | 0.19 |
| Wells/river | 29 (35.4%) | 9.67 | 1.23–76.12 | 0.03 |
| Tap water | 1 (4.3%) | 1.00 | ||
| Latrine | ||||
| Toilet | 58 (22.0%) | 1.00 | ||
| Non toilet | 1 (33.3%) | 1.78 | 0.16–19.93 | 0.64 |
| History of drugs | ||||
| No | 29 (19.0%) | 1.64 | 0.46–5.86 | 0.45 |
| PPI, H2blockers, antibiotics | 27 (30.0%) | 3.00 | 0.83–10.91 | 0.10 |
| Others | 3 (12.5%) | 1.00 | ||
| Smokers | ||||
| Yes | 18 (30.0%) | 1.64 | 0.85–3.16 | 0.14 |
| No | 38 (20.8%) | 1.00 | ||
| Alcohol consumption | ||||
| No | 41 (20.8%) | 1.00 | ||
| Yes | 15 (32.6%) | 1.84 | 0.91–3.73 | 0.09 |
Discussion
Although culture remains a reference method due to ability to directly detect H. pylori organisms, it have limited sensitivity. Moreover guideline mentioned there was no single test can be considered as the gold standard for the diagnosis of H. pylori infection [25]. In the present study, we used four different H. pylori tests to increase diagnostic accuracy as well as to compare results among tests. We confirmed that the H. pylori infection prevalence in five largest islands in Indonesia using combination of the diagnostic tests to be 22.1%, contrast with several Southeast Asian countries with high H. pylori infection prevalence such as Thailand and the Philippines (54.1 to 76.1% and 60%, respectively) [26,27], but almost similar with Malaysia which also low incidence of gastric cancer country. The prevalence of H. pylori infection in Orang Asli, the aboriginal community, residing in the state of Kelantan, Malaysia has been reported to be 19% [28].
However as a wide country with spans almost 2 million square kilometers between Asia and Australia and consist of 300 distinct native ethnic groups, the prevalence of H. pylori in Indonesia should be observed by considering ethno-geographic group. Moreover, several ethnics had higher prevalence of H. pylori infection than Chinese Indonesian, the highest prevalence ethnic was reported in previous study [5]. The highest prevalence of H, pylori ethnic, Papuans, is various indigenous peoples of Papua island and neighboring islands. They are speakers of the Papuan languages and often distinguished ethnically and linguistically from Austronesians. Most of them quite maintain their traditions, especially who living in central mountainous region/highland zones [29]. The high prevalence of H. pylori infection in Papuans concordance with previous study which reported the prevalence was 58% in Papua New Guinea, eastern part of Papua island [30]. It will be interesting to know the genotypes of H. pylori strains of Papuans peoples which may have similarity with New Guinea and Aborigines Australia strains. The eastern sections of Indonesia, especially Papua, were geographically connected to Australia as a single continent (Sahul) about 60,000 years ago.
The lower prevalence of Chinese descent than that of Chinese non-immigrants was reported in previous studies [5,31]. Chow et al. reported that seroprevalence of Chinese which born in Malaysia/Singapore (43.1%) were lower than those born in China/Hong Kong (68.2%) [31]. By multivariate analysis they also found that the higher risk for H. pylori infection in chopsticks users which suggests person-to-person transmission of H. pylori via the oral-oral route with ethno-specific food practices an important risk factor. Environmental factors might contribute to the lower H. pylori infection rate in Chinese Indonesians. Beside use Chinese cuisine legacy, Chinese Indonesian also modified some of the dishes with addition of Indonesian local ingredients [32] which might associated with the low prevalence of H. pylori as same as ‘budu’ or local anchovy sauce, and ‘pegaga’ or centenella asiatica have also been reported to be associated with the low prevalence of H. pylori in Malaysia [33].
Another interesting result we found was that Buginese, a majority numerous ethnic in the southern part of Sulawesi had also high prevalence of H. pylori (36.7%), however still lower than that in Philippines [27]. Sulawesi and the Philippines except for Palawan is assumed to be zoogeographical separated with Sundaland (mainland of Asia) which is supported by distributional patterns [34]. Alfred Russell Wallace designated a faunal boundary organisms demarcating the transition between Asian and Melanesian features (Lombok eastwards, Sulawesi, the Moluccas and Philippines-but not Palawan). Most of the languages of the Wallace region belonging to the extensive Austronesian language family but with more distantly related Papuan languages occurring in the Far Eastern provinces, especially in areas where Melanesian features predominate [35]. Contrary with this study, our previous study found the prevalence of H. pylori infection in North Sulawesi was very low (14.3%) by urine test confirmed with serology [11]. Recently we also confirmed these data with five different diagnostic tests (unpublished data). It is still unclear why there was difference of H. pylori prevalence within Sulawesi island. We should remark that Sulawesi island consist of various indigenous ethnic groups which have different phenotype. Although we observed hspMaori type in North Sulawesi, a subpopulation of East Asian type, often isolated from native Taiwanese and Maori tribe as well as some subjects in Philippines [36].
The extremely low prevalence of H. pylori infection in Javanese group also confirmed our previous study in Surabaya, Java island [5]. Javanese had a low H. pylori prevalence as well as Malay ethnic group in Malaysia which have the similar host genetic factors that reduced susceptibility to H. pylori infection [37,38]. In the last ice age, the central and western sections of the Indonesian archipelago were connected by dry land to the Asian mainland (Sundaland) including Java, Sumatera and Borneo island. Therefore it is not surprising that Dayak ethnic also had low prevalence of H. pylori infection. Dayak is the indigenous peoples of Borneo which was categorized on Malayo-Polynesian linguistic subgroup speakers. Europeans created the term `Dayak`to refer to the non-Malay inhabitants of Borneo [39].
However it is still questionable why Batak ethnic in North Sumatera had high prevalence of H. pylori infection. Genotyping of H. pylori strains and host factors analysis from Batak ethnic may partly explain the reason of these differences. The transmission routes of H. pylori are still not entirely understood, but human-to-human spread through oral-oral or faecal-oral routes are considered the most plausible routes for infection [18]. Therefore intra-racial or intra-community spread such as transmission from mother to child might contribute to these racial differences in H. pylori infection rates.
Our data showed there were difference of several demographic and environmental factors between ethnic groups; age, religion and the source of drinking water were associated with increased risk of infection. Several ethnics showed the age-related prevalence pattern of H. pylori infection in developing countries that H. pylori infections occur earlier in life and with high frequency [18]. We also found the prevalence of H. pylori infection in Protestant was higher than that in Catholic. The religious beliefs and practices might be as important factors for spreading H. pylori in Indonesia. However it may also due to that in this study, the majority of Protestant were Papuan and Batak ethnics which have the highest prevalence of H. pylori. The use of well or river water was associated with increased risk of infection. Therefore, consistent with several previous studies [33,40], H. pylori could survive and contaminate the local water supplies to be the most plausible. However in fact, only ethnics as independent risk factor for H. pylori infection. Further studies in each group is needed to clarify the most influenced variables of demographic and sanitation which related prevalence patterns of H. pylori infection in Indonesia, especially in high prevalence area.
Similar with our previous study [5], rapid urease test showed higher positive rate compared with other tests. Compared with histology and culture, RUT is faster, cheaper, and has comparable sensitivity and specificity even in Indonesia. The number of samples in this study were relatively low, certainly become the limitation in this study. In addition, we included only patients with dyspepsia in our study population. In general, the prevalence of H. pylori infection is higher in dyspeptic patients than in the general population. Currently we are still continuing surveys to add the sample numbers and expand to other islands, including collecting serum. A larger sample size is necessary to elucidate the prevalence of H. pylori in Indonesia. We just performed survey in 1–2 cities every island. Most of the cities is a capitol of province which may have better sanitary and socio-economic condition than the rural part. Therefore, our results cannot be generalized across Indonesia. A study to investigate genotypes of H. pylori strains in Indonesia is now in progress. Genotyping information may partly explain the differences of H. pylori infection among ethnic group in Indonesia.
Conclusion
Several ethnics group have higher risk for H. pylori infection than Javanese group, predominantly ethnic which reported have low prevalence of H. pylori infection in previous study. The age, religion and water source may implicated as a risk factor for H. pylori infection in Indonesia. Improving the sanitary conditions to decrease the prevalence of H. pylori in Indonesia is important.
Supporting Information
When patients were considered to be H. pylori positive in case at least one test showed positive, the prevalence of H. pylori infection was 22.1% (59/267).
(PDF)
Abbreviations
- IHC
immunohistochemistry
- RUT
rapid urease test
Data Availability
The relevant underlying data are presented in the paper and its Supporting Information files. Additional detailed data requests about questionnaires available on request (yyamaoka@oita-u.ac.jp).
Funding Statement
This report is based on work supported in part by grants from the National Institutes of Health (DK62813) (YY), and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (24406015, 24659200, 25293104, 26640114, 15H02657 and 221S0002) (YY), (26440198) (RS). This work was also supported by the Japan Society for the Promotion of Science (JSPS) Institutional Program for Young Researcher Overseas Visits and the Strategic Funds for the Promotion of Science and Technology from Japan Science and Technology Agency (JST).
References
- 1. Peek RM Jr., Blaser MJ (2002) Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2: 28–37. [DOI] [PubMed] [Google Scholar]
- 2. Tumonggor MK, Karafet TM, Hallmark B, Lansing JS, Sudoyo H, et al. (2013) The Indonesian archipelago: an ancient genetic highway linking Asia and the Pacific. J Hum Genet 58: 165–173. 10.1038/jhg.2012.154 [DOI] [PubMed] [Google Scholar]
- 3. Tokudome S, Samsuria Soeripto WD, Triningsih FX, Suzuki S, Hosono A, et al. (2005) Helicobacter pylori infection appears essential for stomach carcinogenesis: observations in Semarang, Indonesia. Cancer Sci 96: 873–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abdullah M, Ohtsuka H, Rani AA, Sato T, Syam AF, et al. (2009) Helicobacter pylori infection and gastropathy: a comparison between Indonesian and Japanese patients. World J Gastroenterol 15: 4928–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miftahussurur M, Shiota S, Suzuki R, Matsuda M, Uchida T, et al. (2015) Identification of Helicobacter pylori infection in symptomatic patients in Surabaya, Indonesia, using five diagnostic tests. Epidemiol Infect 143: 986–996. 10.1017/S095026881400154X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Syam AF, Rani AA, Abdullah M, Manan C, Makmun D, et al. (2005) Accuracy of Helicobacter pylori stool antigen for the detection of Helicobacter pylori infection in dyspeptic patients. World J Gastroenterol 11: 386–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saragih JB, Akbar N, Syam AF, Sirait S, Himawan S, et al. (2007) Incidence of Helicobacter pylori infection and gastric cancer: an 8-year hospital based study. Acta Med Indones 39: 79–81. [PubMed] [Google Scholar]
- 8. Aulia D, Manz GO, Simadibrata M (2009) Pepsinogen I concentration in organic dyspepsia patients at Gastroenterology Division, Department of Internal Medicine, Cipto Mangunkusumo Hospital. Acta Med Indones 41: 107–114. [PubMed] [Google Scholar]
- 9. Arinton IG (2011) Adjustment of cut-off values in ELISA for detection of Helicobacter pylori infection. Acta Med Indones 43: 88–91. [PubMed] [Google Scholar]
- 10. Tokudome S, Soeripto, Triningsih FX, Ananta I, Suzuki S, et al. (2005) Rare Helicobacter pylori infection as a factor for the very low stomach cancer incidence in Yogyakarta, Indonesia. Cancer Lett 219: 57–61. [DOI] [PubMed] [Google Scholar]
- 11. Miftahussurur M, Tuda J, Suzuki R, Kido Y, Kawamoto F, et al. (2014) Extremely low Helicobacter pylori prevalence in North Sulawesi, Indonesia and identification of a Maori-tribe type strain: a cross sectional study. Gut Pathog 6: 42 10.1186/s13099-014-0042-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al-Hawajri AA, Keret D, Simhon A, Zlotkin A, Fishman Y, et al. (2004) Helicobacter pylori DNA in dental plaques, gastroscopy, and dental devices. Dig Dis Sci 49: 1091–1094. [DOI] [PubMed] [Google Scholar]
- 13. Parsonnet J, Shmuely H, Haggerty T (1999) Fecal and oral shedding of Helicobacter pylori from healthy infected adults. JAMA 282: 2240–2245. [DOI] [PubMed] [Google Scholar]
- 14. Syam AF, Abdullah M, Rani AA, Nurdjanah S, Adi P, et al. (2006) Evaluation of the use of rapid urease test: Pronto Dry to detect H pylori in patients with dyspepsia in several cities in Indonesia. World J Gastroenterol 12: 6216–6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao Y, Wang J, Tanaka T, Hosono A, Ando R, et al. (2012) Association between HLA-DQ genotypes and haplotypes vs Helicobacter pylori infection in an Indonesian population. Asian Pac J Cancer Prev 13: 1247–1251. [DOI] [PubMed] [Google Scholar]
- 16. Mhaskar RS, Ricardo I, Azliyati A, Laxminarayan R, Amol B, et al. (2013) Assessment of risk factors of Helicobacter pylori infection and peptic ulcer disease. J Glob Infect Dis 5: 60–67. 10.4103/0974-777X.112288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elitsur Y, Dementieva Y, Rewalt M, Lawrence Z (2009) Helicobacter pylori infection rate decreases in symptomatic children: a retrospective analysis of 13 years (1993–2005) from a gastroenterology clinic in West Virginia. J Clin Gastroenterol 43: 147–151. 10.1097/MCG.0b013e318157e4e7 [DOI] [PubMed] [Google Scholar]
- 18. Goh KL, Chan WK, Shiota S, Yamaoka Y (2011) Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter 16 Suppl 1: 1–9. 10.1111/j.1523-5378.2011.00874.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vilaichone RK, Mahachai V, Shiota S, Uchida T, Ratanachu-ek T, et al. (2013) Extremely high prevalence of Helicobacter pylori infection in Bhutan. World J Gastroenterol 19: 2806–2810. 10.3748/wjg.v19.i18.2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shiota S, Murakami K, Fujioka T, Yamaoka Y (2010) Population-based strategies for Helicobacter pylori-associated disease management: a Japanese perspective. Expert Rev Gastroenterol Hepatol 4: 149–156. 10.1586/egh.10.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shiota S, Cruz M, Abreu JA, Mitsui T, Terao H, et al. (2014) Virulence genes of Helicobacter pylori in the Dominican Republic. J Med Microbiol 63: 1189–1196. 10.1099/jmm.0.075275-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nguyen TL, Uchida T, Tsukamoto Y, Trinh DT, Ta L, et al. (2010) Helicobacter pylori infection and gastroduodenal diseases in Vietnam: a cross-sectional, hospital-based study. BMC Gastroenterol 10: 114 10.1186/1471-230X-10-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nguyen LT, Uchida T, Tsukamoto Y, Trinh TD, Ta L, et al. (2010) Clinical relevance of cagPAI intactness in Helicobacter pylori isolates from Vietnam. Eur J Clin Microbiol Infect Dis 29: 651–660. 10.1007/s10096-010-0909-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uchida T, Kanada R, Tsukamoto Y, Hijiya N, Matsuura K, et al. (2007) Immunohistochemical diagnosis of the cagA-gene genotype of Helicobacter pylori with anti-East Asian CagA-specific antibody. Cancer Sci 98: 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chey WD, Wong BC, Practice Parameters Committee of the American College of G (2007) American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 102: 1808–1825. [DOI] [PubMed] [Google Scholar]
- 26. Sahara S, Sugimoto M, Vilaichone RK, Mahachai V, Miyajima H, et al. (2012) Role of Helicobacter pylori cagA EPIYA motif and vacA genotypes for the development of gastrointestinal diseases in Southeast Asian countries: a meta-analysis. BMC Infect Dis 12: 223 10.1186/1471-2334-12-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Destura RV, Labio ED, Barrett LJ, Alcantara CS, Gloria VI, et al. (2004) Laboratory diagnosis and susceptibility profile of Helicobacter pylori infection in the Philippines. Ann Clin Microbiol Antimicrob 3: 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rahim AA, Lee YY, Majid NA, Choo KE, Raj SM, et al. (2010) Helicobacter pylori infection among Aborigines (the Orang Asli) in the northeastern region of Peninsular Malaysia. Am J Trop Med Hyg 83: 1119–1122. 10.4269/ajtmh.2010.10-0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Utsumi T, Lusida MI, Yano Y, Nugrahaputra VE, Amin M, et al. (2009) Complete genome sequence and phylogenetic relatedness of hepatitis B virus isolates in Papua, Indonesia. J Clin Microbiol 47: 1842–1847. 10.1128/JCM.02328-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Richens J, West B, Turner H (1989) Campylobacter pylori in patients undergoing gastroscopy at Goroka Base Hospital. P N G Med J 32: 23–26. [PubMed] [Google Scholar]
- 31. Chow TK, Lambert JR, Wahlqvist ML, Hsu-Hage BH (1995) Helicobacter pylori in Melbourne Chinese immigrants: evidence for oral-oral transmission via chopsticks. J Gastroenterol Hepatol 10: 562–569. [DOI] [PubMed] [Google Scholar]
- 32. Sugimoto M, Ohno T, Graham DY, Yamaoka Y (2011) Helicobacter pylori outer membrane proteins on gastric mucosal interleukin 6 and 11 expression in Mongolian gerbils. J Gastroenterol Hepatol 26: 1677–1684. 10.1111/j.1440-1746.2011.06817.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee YY, Ismail AW, Mustaffa N, Musa KI, Majid NA, et al. (2012) Sociocultural and dietary practices among Malay subjects in the north-eastern region of Peninsular Malaysia: a region of low prevalence of Helicobacter pylori infection. Helicobacter 17: 54–61. 10.1111/j.1523-5378.2011.00917.x [DOI] [PubMed] [Google Scholar]
- 34. Perger R (2013) Did the genus Parandrocephalus Heller, 1916 (Coleoptera, Cerambycidae, Callichromatini) cross the Wallace line? The taxonomic status of Parandrocephalus blairi Bentanachs & Vives 2009 and a new subgenus of Hexamitodera Heller, 1896, with notes on convergent evolution and secondary sexual characters. Zookeys: 77–89. 10.3897/zookeys.293.5133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karafet TM, Hallmark B, Cox MP, Sudoyo H, Downey S, et al. (2010) Major east-west division underlies Y chromosome stratification across Indonesia. Mol Biol Evol 27: 1833–1844. 10.1093/molbev/msq063 [DOI] [PubMed] [Google Scholar]
- 36. Moodley Y, Linz B, Yamaoka Y, Windsor HM, Breurec S, et al. (2009) The peopling of the Pacific from a bacterial perspective. Science 323: 527–530. 10.1126/science.1166083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maran S, Lee YY, Xu S, Rajab NS, Hasan N, et al. (2013) Gastric precancerous lesions are associated with gene variants in Helicobacter pylori-susceptible ethnic Malays. World J Gastroenterol 19: 3615–3622. 10.3748/wjg.v19.i23.3615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee YY, Mahendra Raj S, Graham DY (2013) Helicobacter pylori infection—a boon or a bane: lessons from studies in a low-prevalence population. Helicobacter 18: 338–346. 10.1111/hel.12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sugimoto M, Furuta T, Yamaoka Y (2009) Influence of inflammatory cytokine polymorphisms on eradication rates of Helicobacter pylori . J Gastroenterol Hepatol 24: 1725–1732. 10.1111/j.1440-1746.2009.06047.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dube C, Tanih NF, Ndip RN (2009) Helicobacter pylori in water sources: a global environmental health concern. Rev Environ Health 24: 1–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
When patients were considered to be H. pylori positive in case at least one test showed positive, the prevalence of H. pylori infection was 22.1% (59/267).
(PDF)
Data Availability Statement
The relevant underlying data are presented in the paper and its Supporting Information files. Additional detailed data requests about questionnaires available on request (yyamaoka@oita-u.ac.jp).