Abstract
Superantigens (SAgs) are important virulence factors in S. aureus. Recent studies identified their presence in animal coagulase-negative staphylococci (CNS). The emergence of human-associated SAg+ CNS would mark a prodigious shift in virulence capabilities. We examined CNS isolates from healthy human nares and diseased individuals, and determined that no known SAgs were present.
Introduction
Staphylococcal superantigens (SAgs) are an important group of exotoxins. The SAg family consists of 23 members which includes toxic shock syndrome toxin-1 (TSST-1), the cause of nearly all cases of menstrual TSS and 50% of non-menstrual cases, staphylococcal enterotoxins (SEs), common causes of food poisoning and non-menstrual TSS, and SE-like (SEl) SAgs which lack or have not been tested for emetic activities, and whose roles in disease are under investigation [1]; SEs include SEA-SEE and SEG, and the SEl SAgs include SEl-H to X.
SAgs derive their name from their capacity to induce T-cell activation [2,3]. Classical antigens stimulate 0.01% of T-cells, while SAgs can stimulate 30% or more of T-cells [1–4]. SAgs recognize a specific subset of Vβ-chains of the T-cell receptor (Vβ-TCR) and crosslink the Vβ-TCRs and α and/or β-chains of major histocompatibility complex (MHC) class II molecules on antigen presenting cells (APCs) [1–3]. The crosslinking activates the T-cells and APCs leading to proliferation and massive release of cytokines [1–4].
Nearly all S. aureus strains produce one or more SAgs [1,5]. However, there are only a few reports that discuss SAg production by coagulase-negative staphylococci (CNS) [6–11]. In the early 1980s, a study concluded that human CNS did not produce the SAg TSST-1 [6]. However, more recent studies indicate that CNS isolated from veterinary sources and food may produce typical S. aureus SAgs [11,12]. These data led us to examine the presence or expression of SAgs in human CNS from multiple sources.
Materials and Methods
Ethics statement
The collection of staphylococcal isolates from 50 healthy human volunteer donors was performed under approved University of Minnesota IRB protocol 1103M97296. All volunteer donors gave written informed consent to participate. All other staphylococcal isolates that were evaluated in this study were submitted by physicians treating toxic shock syndrome patients. IRB approval to receive and test these specimens was not required since these were clinical samples submitted at the request of treating physicians and not part of a human clinical study.
Staphylococcal isolates
Fifty healthy adult volunteers were analyzed for staphylococcal nasal colonization. Sterile cotton swabs were used to obtain individual samples from both nares of the volunteers. Staphylococcal isolates were identified by colony color on mannitol salt agar plates and morphology (gram-positive, cluster-forming cocci) and catalase activity. S. aureus and coagulase negative staphylococci were differentiated based on their coagulase activities (using rabbit plasma and slide coagulase test). Fourteen nasal S. aureus isolates were cultured from these healthy individuals. Additionally, 48 nasal CNS isolates were obtained from these same individuals. CNS typing of the 48 nasal organisms was performed using the bioMérieux api Staph identification system according to manufacturer’s instructions. Briefly, bacterial strains were plated on TSA blood agar plates and grown at 37°C overnight for 20 hrs. A homogenous bacterial suspension was prepared in API Staph medium to a turbidity of 0.5 McFarland and used to inoculate the api strip. Strips were read after incubation 37°C for 24 hrs. All 48 organisms were determined to be S. epidermidis.
Fifty-seven coagulase negative staphylococci from human patients with TSS as defined by the treating physician were submitted for SAg testing to the Schlievert laboratory between 1980 and 2007. We have previously shown that physicians have high accuracy in identifying TSS cases based on patient symptoms and signs of disease [13]. All of these submitted 57 CNS isolates were S. epidermidis as determined by the clinical microbiology laboratories of submitting medical centers. These isolates were tested for TSST-1, SEB, and SEC by antibody-based assays [14]. In multiple studies, we have shown that this antibody-based assay reliably detects production of these three SAgs [14–16]. This latter collection of isolates was not available for SAg gene testing by PCR as they were discarded after antibody testing with negative results for TSST-1, SEB, and SEC. However, 3 more recently submitted S. epidermidis isolates from TSS patients were available for SAg gene testing by PCR.
Collectively, three groups of isolates were compared for the presence of SAgs or their genes. Group 1 included the 14 S. aureus isolates from the nares of healthy individuals; these also served as controls for SAg detection. Group 2 included the 48 nasal S. epidermidis isolates from healthy individuals. Group 3 (clinical isolates) included 60 total S. epidermidis isolates recovered from the bloodstream of patients with TSS, and an additional 10 clinical S. lugdunensis isolates from the University of Iowa hospitals and clinics. Thus, we tested a total of 118 CNS for SAgs by antibody tests or their genes by PCR.
Antibody and PCR assays for SAgs and their genes
CNS isolates from TSS patients (57) were tested by an antibody-based test for the three major SAg causes of TSS, notably TSST-1, SEB, and SEC. Briefly, the isolates were cultured at 37°C with shaking (200 rpm) overnight in 50 ml Todd Hewitt broth and then treated with 4 volumes of absolute ethanol to precipitate SAgs. Subsequently, the precipitates were resuspended in 0.1 ml of distilled water (500 times concentrated) and clarified by centrifugation (10,000 x g, 5 min). The clarified supernates were tested by double immunodiffusion for reaction with hyperimmune rabbit antisera specific for each toxin. The lower limit of detection of SAgs by this method was 0.01 ug/ml original culture fluid. In tests of thousands of S. aureus strains, TSST-1 is typically produced at 3.5 ug/ml, whereas SEB and SEC are produced at 25–100 ug/ml.
For all other isolates, including the three more recent CNS (S. epidermidis) isolates from TSS patients, the organisms were cultured in 10 mL of Todd Hewitt broth and then subjected to DNA extraction for use in PCR [17].The primers used and PCR amplification reactions were done as previously published [17].
Results
Nasal colonization
Of fifty healthy volunteers, 48 (96%) carried staphylococci in their anterior nares. Among the volunteers, 48 of 48 (100%) individuals were positive for CNS, and 14 out of 48 (29%) individuals were positive for both CNS and S. aureus. API strip tests determined that all nasal CNS isolates were S. epidermidis species. There was no individual who was colonized by coagulase-positive S. aureus in the absence of CNS.
SAg gene profile of Staphylococcal isolates
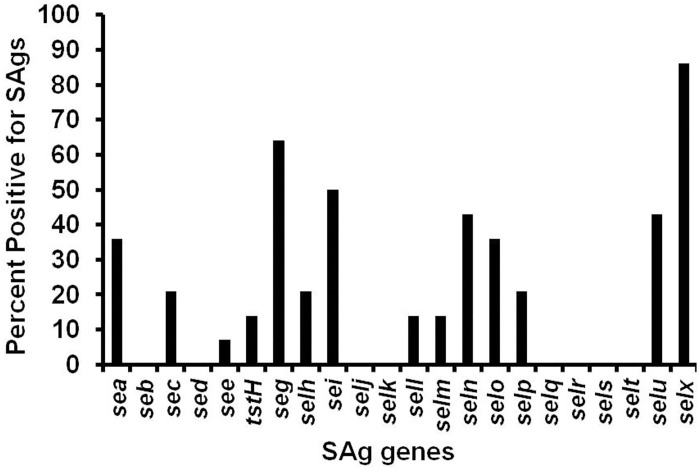
Conventional PCR was used to screen for the presence of canonical SAg genes sea-seg, tstH, and selh-selx in three groups of staphylococcal isolates: (Group 1) nasal S. aureus isolates from 14 healthy individuals, (Group 2) nasal CNS isolates from healthy individuals (48 S. epidermidis isolates), and (Group 3) clinical CNS isolates (60 S. epidermidis and 10 S. lugdunensis) (Table 1). As expected, all S. aureus strains, in Group 1, carried genes encoding for SAgs with an average of 5 SAg genes per isolate (Table 2). Among them, selx was the most prevalent with 86% of distribution. seg, seli, seln, and selu also appeared in high frequency (greater than 40%). There was no detection of SEB, SED, SEl-J, SEl-K, SEl-Q, SEl-R, SEl-S, and SEl-T (Fig 1). Data for individual strain production of SAgs is shown in S1 Dataset. Unlike in S. aureus isolates, there was no known SAg gene detected among the 118 CNS (108 S. epidermidis and 10 S. lugdunensis) isolates in both Groups 2 and 3 regardless of their origin (Table 2). None of the 57 S. epidermidis isolates submitted for TSS-associated SAg testing to the Schlievert laboratory between 1980 and 2007 produced detectable TSST-1, SEB, or SEC, with lower limit of detection being 0.01 ug/ml. These isolates were not available for PCR testing in the current study. Three additional TSS S. epidermidis isolates from TSS patients were available for PCR analysis, and none of the organisms contained any of the known SAg genes; thus a total of 60 S. epidermidis isolates from TSS patients were negative for the three (TSST-1, SEB, SEC) SAgs known to cause most cases of TSS.
Table 1. Species and sources of the staphylococcal isolates.
| Species (no. of isolates) | Sources (no. of isolates) |
|---|---|
| S. aureus (14) | Anterior nares a (14) |
| S. epidermidis (48) | Anterior nares a (48) |
| S. epidermidis (60) | TSS (60) |
| S. lugdunensis (10) | Endocarditis (3), Breast abscess (1), Finger Pulp (2), Others (4) |
a Samples obtained from nasal swabs of healthy volunteers.
Table 2. Prevalence of SAg genes in staphylococcal isolates.
| Group | Total no. of isolates | No. of isolates producing known SAgs or having SAg genes | Average number of known SAgs or SAg genes per isolate |
|---|---|---|---|
| 1 (S. aureus) | 14 | 14 | 5 ± 2 |
| 2 (S. epidermidis) | 48 | 0 | 0 |
| 3 (S. epidermidis and S. lugdunensis) | 60 and 10 | 0 and 0 | 0 and 0 |
Fig 1. SAg gene profile of nasal S. aureus isolates.
Conventional PCR and specific primers for known SAg genes were utilized [17].
Discussion
When TSST-1 was first described in 1981 [16,18], one of the original published manuscripts indicated that TSST-1 could be produced by CNS (S. epidermidis) [18]. A subsequent publication showed that at least some of those CNS that tested positive were in fact S. aureus strains, noting that TSST-1 positive S. aureus may give weak coagulase test reactions and appearing instead to be CNS [6]. However, the remaining CNS in that initial study were shown to be TSST-1 negative, with the original test results being false positives. Subsequent to the initial description of TSST-1, the Centers for Disease Control and Prevention (CDC) hypothesized that the TSST-1 gene originated in CNS and was transferred by bacteriophages to S. aureus. The Schlievert and Bergdoll (now deceased) laboratories both tested a sampling of S. epidermidis strains from unused tampons that had been isolated by the CDC to test their hypothesis. Both the Schlievert and Bergdoll laboratories showed that none of the S. epidermidis strains were positive for TSST-1 [6]. It was thus concluded that CNS strains do not produce TSST-1 and do not cause TSS in humans. It is noteworthy that despite these findings, CNS remain the number one cause of bacteremia in humans, many with symptoms that resemble TSS [19,20].
The original defining immunobiological activities of SAgs (formerly called pyrogenic toxins) are ability to cause fever and enhance host susceptibility to lethal endotoxin shock by up to 106-fold in rabbits [21]. Subsequently, it was shown that these two activities depended on the ability of SAgs to stimulate T cells and antigen-presenting cells to produce massive amounts of cytokines; the origin of the term SAg to describe the toxins [3]. Because of the TSS-like symptoms observed in some patients with CNS infections, the Schlievert laboratory tested 22 S. epidermidis, 10 times concentrated culture fluids, for ability to induce fever and enhance endotoxin shock in rabbits; all 22 strains were negative, suggesting these CNS did not produce known or yet to be described SAgs that caused the TSS symptoms [6].
Recent studies indicate that known SAgs present in nearly all S. aureus can be occasionally present in CNS [7,10]. These results are in opposition with the above previous studies that established that CNS of human origin, primarily S. epidermidis, do not encode S. aureus SAgs [6]. In one study of a single S. epidermidis strain originally provided by the Bergdoll laboratory, the organism has been clearly established as both CNS and verified to produce S. aureus SAgs [7]. There have also been occasional other studies noting SAg production by CNS, particularly of animal or food origin [8,11]. Additionally, one study showed that 6.6% of 136 human CNS strains produced known SAgs (TSST-1 and/or SEA-SED) [10]. Interestingly, none of 35 S. epidermidis and none of 12 S. lugenunensis strains in that study produced known SAgs, in agreement with the findings from the 1980s [6] and our current study, wherein we showed that none of 118 S. epidermidis and S. lugdunensis strains produce any of the known SAgs. Thus, with one exception strain (FRI909) [7], the major CNS (S. epidermidis and S. lugdunensis) that infect humans do not produce known or unknown SAgs, despite their abilities to cause human diseases.
In a prior study, we tested a small number of CNS that only occasionally cause human infections, for example S. hominis and S. capitis, and showed none produced TSST-1, SEB, and/or SEC [6]. In contrast, in a recent study of human CNS, researchers in Japan showed that 10% of S. hominis and capitis strains from humans produce one or more of these same three SAgs [10]. These data differences make three important points: 1) CNS in Japan may be different from those isolated in the U. S., where to date no confirmed TSS cases have been associated with SAgs production by CNS; 2) even if CNS strains in Japan produce known SAgs, the SAgs do not cause TSS (none of the affected patients showed TSS symptoms); and 3) there are no data to suggest that CNS strains from humans in the U.S. should be routinely tested for SAgs as causative factors in TSS.
Our study indicates, as expected, that both S. aureus and CNS can be isolated from anterior nares of humans. While only 24% of tested individuals were positive for S. aureus, 96% were positive for S. epidermidis CNS. Interestingly, individuals colonized with S. aureus were also co-colonized with CNS, but when dually present, S. aureus dominated. This is in agreement with a previous study where we reported that S. aureus and CNS are inversely correlated in colonization of mucosal surfaces [22]. When we examined the CNS strains for the presence of SAgs, none of the isolates encoded for SAg genes, whereas S. aureus isolates encoded an average of 5 SAgs per strain. To date, this is the only study to evaluate a large collection of S. epidermidis strains for the presence of any of the 23 known staphylococcal SAg genes.
Supporting Information
These include the 14 S. aureus strains and 51 S. epidermidis strains tested by PCR, 10 S. lugdunensis strains tested by PCR and 57 S. epidermidis isolates from TSS patients tested by antibody reaction against hyperimmune serum raised specifically against TSST-1, SEB, or SEC.
(XLS)
Acknowledgments
We thank Dr. Alexander R. Horswill for providing the S. lugdunensis strains used in this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the University of Iowa Start-up funds for PMS. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Spaulding AR, Salgado-Pabon W, Kohler PL, Horswill AR, Leung DY, Schlievert PM. (2013) Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev 26: 422–447. 10.1128/CMR.00104-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kotzin BL, Leung DY, Kappler J, Marrack P (1993) Superantigens and their potential role in human disease. Adv Immunol 54: 99–166. [DOI] [PubMed] [Google Scholar]
- 3. Marrack P, Kappler J (1990) The staphylococcal enterotoxins and their relatives. Science 248: 705–711. [DOI] [PubMed] [Google Scholar]
- 4. Brosnahan AJ, Schlievert PM (2011) Gram positive bacterial superantigen outside-in signaling causes toxic shock syndrome. FEBS J 278: 4649–4667. 10.1111/j.1742-4658.2011.08151.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vu BG, Stach CS, Salgado-Pabon W, Diekema DJ, Gardner SE, Schlievert PM. (2014) Superantigens of Staphylococcus aureus from patients with diabetic foot ulcers. J Infect Dis 210:1920–1927. 10.1093/infdis/jiu350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kreiswirth BN, Schlievert PM, Novick RP (1987) Evaluation of coagulase-negative staphylococci for ability to produce toxic shock syndrome toxin 1. J Clin Microbiol 25: 2028–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Madhusoodanan J, Seo KS, Remortel B, Park JY, Hwang SY, Fox LK et al. (2011) An enterotoxin-bearing pathogenicity island in Staphylococcus epidermidis . J Bacteriol 193: 1854–1862. 10.1128/JB.00162-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park JY, Fox LK, Seo KS, McGuire MA, Park YH, Rurangirwa FR et al. (2011) Detection of classical and newly described staphylococcal superantigen genes in coagulase-negative staphylococci isolated from bovine intramammary infections. Vet Microbiol 147: 149–154. 10.1016/j.vetmic.2010.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crass BA, Bergdoll MS (1986) Involvement of coagulase-negative staphylococci in toxic shock syndrome. J Clin Microbiol 23: 43–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Akiyama H, Yamasaki O, Tada J, Arata J (2000) The production of superantigenic exotoxins by coagulase-negative staphylococci isolated from human skin lesions. J Dermatol Sci 24: 142–145. [DOI] [PubMed] [Google Scholar]
- 11. Zell C, Resch M, Rosenstein R, Albrecht T, Hertel C, Gotz F. (2008) Characterization of toxin production of coagulase-negative staphylococci isolated from food and starter cultures. Int J Food Microbiol 127: 246–251. 10.1016/j.ijfoodmicro.2008.07.016 [DOI] [PubMed] [Google Scholar]
- 12. Park JY, Fox LK, Seo KS, McGuire MA, Park YH, Rurangirwa FR et al. (2011) Comparison of phenotypic and genotypic methods for the species identification of coagulase-negative staphylococcal isolates from bovine intramammary infections. Vet Microbiol 147: 142–148. 10.1016/j.vetmic.2010.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schlievert PM, Kim MH (1991) Reporting of toxic shock syndrome Staphylococcus aureus in 1982 to 1990. J Infect Dis 164: 1245–1246. [DOI] [PubMed] [Google Scholar]
- 14. Schlievert PM (1988) Immunochemical assays for toxic shock syndrome toxin-1. Methods Enzymol 165: 339–344. [DOI] [PubMed] [Google Scholar]
- 15. Bonventre PF, Weckbach L, Staneck J, Schlievert PM, Thompson M (1983) Production of staphylococcal enterotoxin F and pyrogenic exotoxin C by Staphylococcus aureus isolates from toxic shock syndrome-associated sources. Infect Immun 40: 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schlievert PM, Shands KN, Dan BB, Schmid GP, Nishimura RD (1981) Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis 143: 509–516. [DOI] [PubMed] [Google Scholar]
- 17. Salgado-Pabon W, Case-Cook LC, Schlievert PM (2014) Molecular analysis of staphylococcal superantigens. Methods Mol Biol 1085: 169–185. 10.1007/978-1-62703-664-1_10 [DOI] [PubMed] [Google Scholar]
- 18. Bergdoll MS, Crass BA, Reiser RF, Robbins RN, Davis JP (1981) A new staphylococcal enterotoxin, enterotoxin F, associated with toxic- shock-syndrome Staphylococcus aureus isolates. Lancet 1: 1017–1021. [DOI] [PubMed] [Google Scholar]
- 19. Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, et al. (1999) Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis 29: 239–244. [DOI] [PubMed] [Google Scholar]
- 20. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, et al. (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39: 309–317. [DOI] [PubMed] [Google Scholar]
- 21. Bohach GA, Fast DJ, Nelson RD, Schlievert PM (1990) Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol 17: 251–272. [DOI] [PubMed] [Google Scholar]
- 22. Schlievert PM, Case LC, Strandberg KL, Tripp TJ, Lin YC, et al. (2007) Vaginal Staphylococcus aureus superantigen profile shift from 1980 and 1981 to 2003, 2004, and 2005. J Clin Microbiol 45: 2704–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
These include the 14 S. aureus strains and 51 S. epidermidis strains tested by PCR, 10 S. lugdunensis strains tested by PCR and 57 S. epidermidis isolates from TSS patients tested by antibody reaction against hyperimmune serum raised specifically against TSST-1, SEB, or SEC.
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.