Abstract
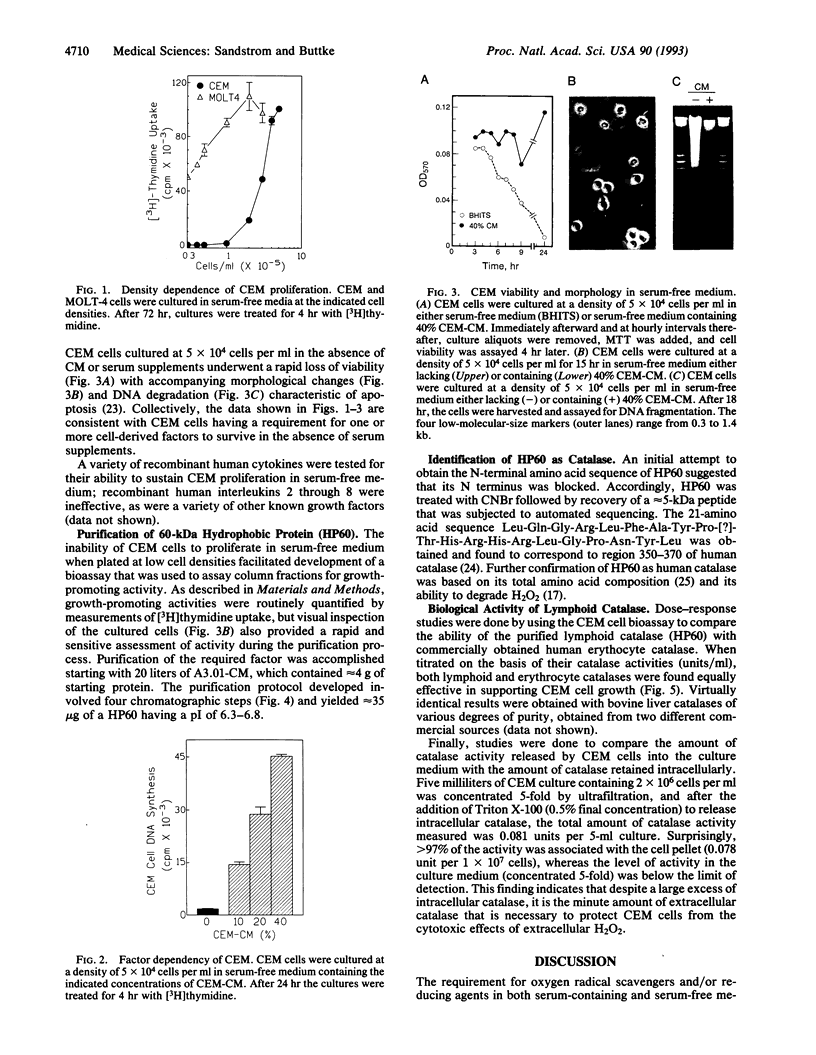
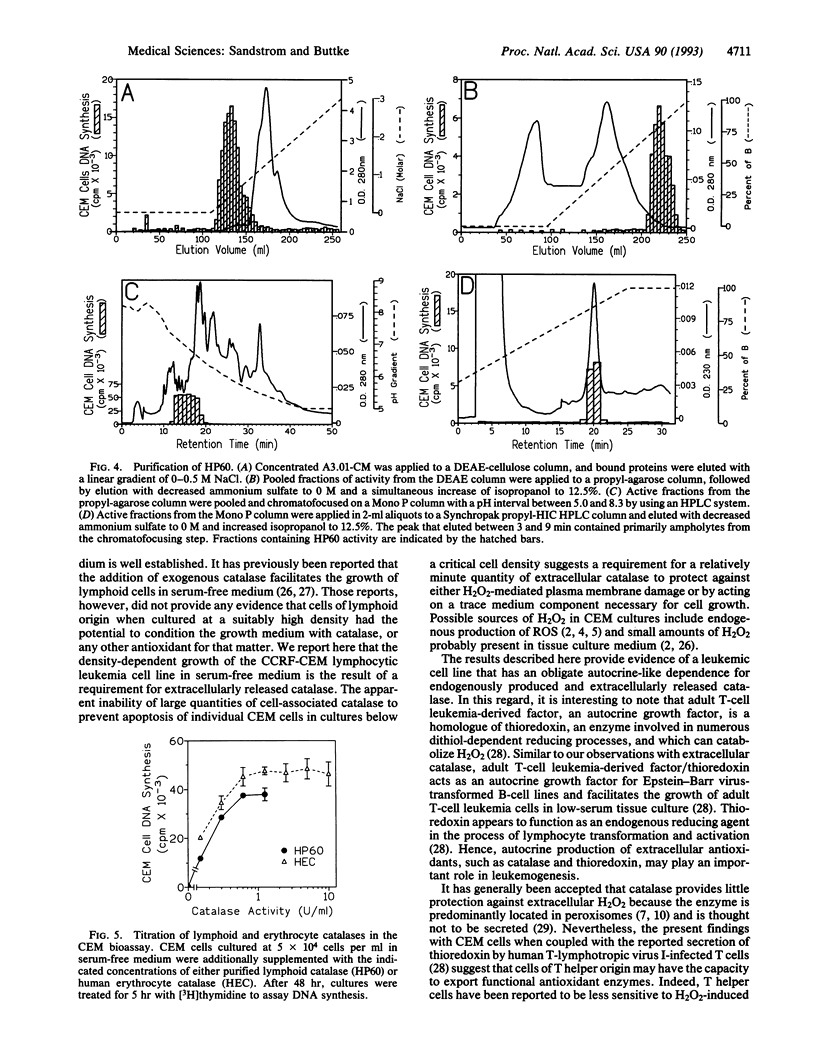
CCRF-CEM is a human T-cell line originally isolated from a child with acute lymphoblastic leukemia. At cell densities > 2 x 10(5) cells per ml, CEM cells grow in serum-free medium, but at lower cell densities the cultures rapidly undergo apoptosis, or programmed cell death. The viability of low-density CEM cells could be preserved by supplementing the serum-free medium with "conditioned" medium from high-density CEM cultures, but a variety of known growth factors and lymphokines were ineffective. Fractionation of conditioned medium by sequential chromatography on DEAE-cellulose, propyl agarose, chromatofocusing, and hydrophobic-interaction HPLC resulted in the isolation of a 60-kDa protein capable of sustaining CEM growth in the absence of serum. The active protein was identified as human catalase based on its amino acid sequence and composition and was subsequently shown to exhibit catalase activity and to be replaceable by human erythrocyte catalase or bovine liver catalase. Comparison of the level of intracellular catalase activity with the amount released into the culture medium demonstrated that the latter accounted for < 3% of the total catalase activity present in the cell culture. These findings show that, despite its low amount, the catalase released by CEM cells, and perhaps by T cells in general, provides a critical first line of defense against hydrogen peroxide (H2O2) present in the extracellular milieu.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amstad P., Peskin A., Shah G., Mirault M. E., Moret R., Zbinden I., Cerutti P. The balance between Cu,Zn-superoxide dismutase and catalase affects the sensitivity of mouse epidermal cells to oxidative stress. Biochemistry. 1991 Sep 24;30(38):9305–9313. doi: 10.1021/bi00102a024. [DOI] [PubMed] [Google Scholar]
- Bonaventura J., Schroeder W. A., Fang S. Human erythrocyte catalase: an improved method of isolation and a reevaluation of reported properties. Arch Biochem Biophys. 1972 Jun;150(2):606–617. doi: 10.1016/0003-9861(72)90080-x. [DOI] [PubMed] [Google Scholar]
- Brown R. L., Griffith R. L., Ruscetti F. W., Rabin H. Modulation of interleukin 2 release from a primate lymphoid cell line in serum-free and serum-containing media. Cell Immunol. 1985 Apr 15;92(1):14–21. doi: 10.1016/0008-8749(85)90060-7. [DOI] [PubMed] [Google Scholar]
- Buttke T. M., Folks T. M. Complete replacement of membrane cholesterol with 4,4',14-trimethyl sterols in a human T cell line defective in lanosterol demethylation. J Biol Chem. 1992 May 5;267(13):8819–8826. [PubMed] [Google Scholar]
- Cantin A. M., Fells G. A., Hubbard R. C., Crystal R. G. Antioxidant macromolecules in the epithelial lining fluid of the normal human lower respiratory tract. J Clin Invest. 1990 Sep;86(3):962–971. doi: 10.1172/JCI114798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri G., Clark I. A., Hunt N. H., Cowden W. B., Ceredig R. Effect of antioxidants on primary alloantigen-induced T cell activation and proliferation. J Immunol. 1986 Oct 15;137(8):2646–2652. [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C., Fadok V. A., Sellins K. S. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- Darfler F. J., Insel P. A. Clonal growth of lymphoid cells in serum-free media requires elimination of H2O2 toxicity. J Cell Physiol. 1983 Apr;115(1):31–36. doi: 10.1002/jcp.1041150106. [DOI] [PubMed] [Google Scholar]
- Duprez V., Lenoir G., Dautry-Varsat A. Autocrine growth stimulation of a human T-cell lymphoma line by interleukin 2. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6932–6936. doi: 10.1073/pnas.82.20.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Folks T., Benn S., Rabson A., Theodore T., Hoggan M. D., Martin M., Lightfoote M., Sell K. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (AIDS)-associated retrovirus. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4539–4543. doi: 10.1073/pnas.82.13.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Hogquist K. A., Nett M. A., Unanue E. R., Chaplin D. D. Interleukin 1 is processed and released during apoptosis. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8485–8489. doi: 10.1073/pnas.88.19.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. DNA damage and oxygen radical toxicity. Science. 1988 Jun 3;240(4857):1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lennon S. V., Martin S. J., Cotter T. G. Dose-dependent induction of apoptosis in human tumour cell lines by widely diverging stimuli. Cell Prolif. 1991 Mar;24(2):203–214. doi: 10.1111/j.1365-2184.1991.tb01150.x. [DOI] [PubMed] [Google Scholar]
- Masutani H., Naito M., Takahashi K., Hattori T., Koito A., Takatsuki K., Go T., Nakamura H., Fujii S., Yoshida Y. Dysregulation of adult T-cell leukemia-derived factor (ADF)/thioredoxin in HIV infection: loss of ADF high-producer cells in lymphoid tissues of AIDS patients. AIDS Res Hum Retroviruses. 1992 Sep;8(9):1707–1715. doi: 10.1089/aid.1992.8.1707. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Limited N-terminal sequence analysis. Methods Enzymol. 1990;182:602–613. doi: 10.1016/0076-6879(90)82047-6. [DOI] [PubMed] [Google Scholar]
- McCubrey J., Holland G., McKearn J., Risser R. Abrogation of factor-dependence in two IL-3-dependent cell lines can occur by two distinct mechanisms. Oncogene Res. 1989;4(2):97–109. [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murrell G. A., Francis M. J., Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J. 1990 Feb 1;265(3):659–665. doi: 10.1042/bj2650659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novogrodsky A., Ravid A., Rubin A. L., Stenzel K. H. Hydroxyl radical scavengers inhibit lymphocyte mitogenesis. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1171–1174. doi: 10.1073/pnas.79.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan F., Korneluk R. G., Tropak M. B., Gravel R. A. Isolation and characterization of the human catalase gene. Nucleic Acids Res. 1986 Jul 11;14(13):5321–5335. doi: 10.1093/nar/14.13.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush D. N., McKenna R. M., Walker S. M., Bakkestad-Legare P., Jeffrey J. R. Catalase increases lymphocyte proliferation in mixed lymphocyte culture. Transplant Proc. 1988 Dec;20(6):1271–1273. [PubMed] [Google Scholar]
- Sagone A. L., Jr, Husney R., Guter H., Clark L. Effect of catalase on the proliferation of human lymphocytes to phorbol myristate acetate. J Immunol. 1984 Sep;133(3):1488–1494. [PubMed] [Google Scholar]
- Schreck R., Baeuerle P. A. A role for oxygen radicals as second messengers. Trends Cell Biol. 1991 Aug;1(2-3):39–42. doi: 10.1016/0962-8924(91)90072-h. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Staite N. D., Messner R. P., Zoschke D. C. Inhibition of human T lymphocyte E rosette formation by neutrophils and hydrogen peroxide. Differential sensitivity between helper and suppressor T lymphocytes. J Immunol. 1987 Oct 1;139(7):2424–2430. [PubMed] [Google Scholar]
- Yodoi J., Uchiyama T. Diseases associated with HTLV-I: virus, IL-2 receptor dysregulation and redox regulation. Immunol Today. 1992 Oct;13(10):405–411. doi: 10.1016/0167-5699(92)90091-K. [DOI] [PubMed] [Google Scholar]
- Zoschke D. C., Staite N. D. Suppression of human lymphocyte proliferation by activated neutrophils or H2O2: surviving cells have an altered T helper/T suppressor ratio and an increased resistance to secondary oxidant exposure. Clin Immunol Immunopathol. 1987 Feb;42(2):160–170. doi: 10.1016/0090-1229(87)90003-1. [DOI] [PubMed] [Google Scholar]




