Abstract
While the steps in the action of botulinum neurotoxin (BoNT) are well known, the factors underlying the timing of these steps are not fully understood. After toxin is injected into a muscle, it resides in the extracellular space and must be taken up into the nerve terminals. More toxin will be taken up if near the endplate. Toxin is distributed mainly by convection and there is likely little diffusion. Toxin that is not taken up will go into the general circulation where it may have a slight systemic effect. The uptake is activity and temperature dependent. Encouraging the unwanted muscle contractions after injection should be helpful. Cooling will decrease the uptake. The times for washout from the extracellular space and uptake of the toxin are not well established, but are likely measured in minutes. Toxin in the general circulation has a long half time. The time from injection to weakness is determined by how long it takes to get sufficient damage of the SNARE proteins to interfere with synaptic release. Toxins are zinc dependent proteases, and supplemental zinc may produce a greater effect. There will be weakness as long as there is residual toxin in the nerve ending.
Keywords: Endplate, Uptake, Duration of action, Cooling, Muscle activity, Zinc
The botulinum neurotoxins (BoNTs) are commonly used for many different indications. The clinical effects are certainly well known, but a detailed explanation of all the phenomena is still lacking. In particular, while many of the steps in the action of the BoNTs are well understood, the factors underlying the timing of these steps are less so. In relation to effects at the neuromuscular junction, initial weakening does not occur for several days and the peak occurs in the order of several weeks. Effects subside at 2 months and strength generally returns to normal by 3 months. Improved knowledge of the physiology should have clinical implications to improve efficacy in the use of toxins. This article will review the sequential steps in toxin action, pointing out what is known and what is not, and will try to indicate clinical implications of these issues.
After toxin is injected into a muscle, it resides in the extracellular space and must be taken up into the nerve terminals. As this process occurs only at the endplate, clearly more toxin will be taken up if the injection is near the endplate. Endplates are generally located in the middle of muscle fibers, but as fibers are organized differently in different muscles, anatomical knowledge must guide the injector. Physiologically, the motor point is the place on the muscle where the stimulation intensity is least to evoke a muscle response. Sometimes this is the endplate zone, but sometimes not, so this is not reliable (Guzman-Venegas et al., 2014). By EMG criteria, endplate spikes certainly are indicative of being in the right place, but these cannot always be found. If the motor unit configurations show initial negative phases, this means that the muscle action potentials are arising near the recording needle, and the endplate must be nearby. High density surface EMG can also define the endplate zone (Lapatki et al., 2011). If attention is paid to injecting at the endplate, the effect will be greater (Delnooz et al., 2014, Gracies et al., 2009).
Toxin will be distributed in the muscle belly by means of convection, that is, the bulk movement of the fluid determined by the fluid volume and the force of the injection. Subsequently there might be diffusion; that is, spread from the initial site by Brownian motion determined by the concentration gradient and molecular size. Fascial boundaries between muscles do not appear to be significant barriers (Shaari et al., 1991), so nearby muscles can be affected depending on the accuracy of needle placement and the volume of the injection. Diffusion takes time, and there may not be much time because there is continuous wash out from the extracellular space.
BoNT in the muscle has been imaged by MRI (Elwischger et al., 2014). Toxin distributes along the long axis of muscle fibers and does not change much in a 10 minute period. The volume of distribution is similar, but might be slightly less, in spastic versus normal muscle. In fact, as modelled in mouse muscle, the volume of distribution becomes rather quickly less (Figure 1) (Tang-Liu et al., 2003). By two hours it is appreciably smaller and by 12 hours, there is hardly any to be seen. The different marketed brands of BoNT exist in different forms and complexed with different amounts of protein, but the region of effect does not seem to differ if similar volumes are used for injection (Carli et al., 2009).
Figure 1.
Autoradiographs of sections through the gastrocnemius muscle injected with 125I-BoNT/A-complex, from four separate rats, (A) immediately after injection, (B) 2 h postinjection, (C) 6 h postinjection, and (D) 12 h postinjection. Sections correspond to the central plane of the injection and range from 5.6 to 8.3 mm below the surface of the preparation. Autoradiographs of sections through the gastrocnemius muscle injected with high-dose free-125I-BoNT/A, from four separate rats, (E) immediately after injection, (F) 2 h postinjection, (G) 6 h postinjection, and (H) 12 h postinjection. Sections correspond to the central plane of the injection and range from 3.6 to 4.9 mm below the surface of the preparation. From (Tang-Liu et al., 2003) with permission.
Taken together, the evidence seems fairly clear that the distribution is determined primarily by convection, which in practical terms is the volume of the injection.
As noted already, there is continuous washout from the extracellular space. The half-life may only be a few minutes, but is at most a few hours as seen the mouse model (Tang-Liu et al., 2003). It is difficult to find any studies about residual drug in the muscle after intramuscular injection as most studies report the opposite – how fast drug gets into the general circulation. One study of digoxin showed a washout halftime of 4 minutes (Hess and Muller, 1982). Hence, it is clear that any toxin taken up will have to be in a very short time. This time differs considerably from the time to effect.
Toxin that is not taken up into the nerve endings will be washed out and will go into the general circulation where it may have a slight systemic effect. There is a long half time in the circulation, perhaps several days (Simpson, 2013). It will have the opportunity to be taken up in other muscles or other presynaptic terminals elsewhere in the body. That there is only slight systemic effect is because there is so little toxin compared with the size of the body. It is not certain what happens to toxin not taken up, but it might be removed by the liver (Simpson, 2013).
Since the toxin is in the muscle for only a short time, it is fortunate that the uptake is rapid. In the rat hemidiaphagm, the halftime for binding is about 12 minutes and for subsequent internalization 5 minutes (Simpson, 1980).
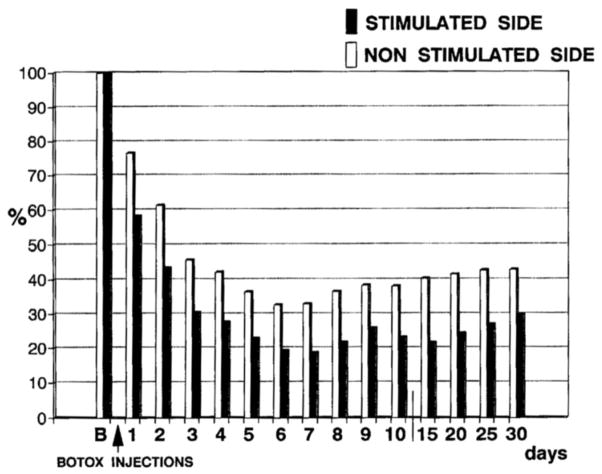
The uptake on BoNT is activity dependent. The more activity, the more uptake. Activity dependence appears to occur because activity will give rise to more opportunities for binding of the toxin to the synaptic membrane. This phenomenon was first demonstrated in the rat diaphragm (Hughes and Whaler, 1962), but then subsequently in human extensor digitorum brevis muscle (Figure 2) (Eleopra et al., 1997). Clinically, it has been demonstrated that activity after injection will increase the amount of weakness produced, but it was not possible to show an increased therapeutic effect (Chen et al., 1999). Activity dependent uptake is likely partly responsible for the often seen clinical situation of a greater clinical effect than weakness effect. Uptake will be greater in the nerve terminals of those muscle fibers involved in the unwanted muscle spasms. Clinically, it makes sense to encourage the involuntary movements in the few minutes following the injection. This should produce more uptake and uptake more selectively in the desired nerve terminals.
Figure 2.
The normalized compound muscle action potential (CMAP) from extensor digitorum brevis muscles on the two sides at various times after onabotulinumtoxinA injection. Injections were similar on the sides, but after injection the peroneal nerve was stimulated only on one side. Beginning at one day and lasting to 30 days, the CMAP amplitude was less on the stimulated side. From (Eleopra et al., 1997) with permission.
Uptake is also temperature dependent (Poulain et al., 1992). Muscle temperature is generally homeostatically controlled. Sometimes skin cooling is used to reduce the superficial pain of the injection (Irkoren et al., 2014). To the extent that this will reduce muscle temperature, it should reduce uptake and reduce the effect of the injection.
In the presynaptic terminal, released light chains of BoNT enzymatically destroy one of the SNARE proteins. The SNARE proteins are being continuously made. Hence the time from injection to weakness must depend on how long it takes to get sufficient damage to interfere with synaptic release. This time must be days.
Toxins are zinc dependent proteases, and the question has arisen as to whether the intracellular zinc concentration might be limiting toxin action (Koshy et al., 2012). It is difficult to find clear information on this point. Somewhat old data suggest that roughly half of persons in North America over 50 years old consume less zinc than federally recommended, and nearly 30% of these individuals may show overt signs of zinc deficiency (Briefel et al., 2000, Prasad et al., 1993). If this is the case, then supplemental zinc may produce a greater effect. There is some evidence that supplemental zinc can produce a greater effect in patients not assessed for zinc deficiency (Koshy et al., 2012). In a double blind, placebo controlled, cross over study of 77 patients with multiple disorders treated with three different toxin preparations, there was a subjective benefit of supplemental zinc given together with phytase. Phytase is given with the zinc to increase its bioavailability and absorption (Rimbach et al., 1998). This study has been called into question, however, because of scientific rigor and the matter remains controversial (Cohen, 2014). The distribution of body zinc is not clear. Logically, supplemental zinc should be of value only if a muscle is zinc deficient, and it might be that muscle zinc is maintained even in the face of slight serum deficiency. Or, of course, it could be the other way around.
There will be weakness as long as there is residual toxin in the nerve ending damaging the SNARE proteins. The SNARE proteins are constantly resynthesized, but this process apparently cannot keep up with the rate of lysis. Toxin appears to remain in the terminal for a matter of months, and this determines its duration of action (Rossetto et al., 2014). The BoNTs are likely metabolized mainly by the proteasome pathway, but information on this is limited (Rossetto et al., 2014).
To summarize the clinical implications:
Toxin should be injected near the motor endplate and there are electrophysiological methods for identifying the endplate
Accurate needle placement is needed to avoid affecting neighboring muscles
Avoid cooling the injection site
Encourage the unwanted muscle spasms in the minutes after the injection; probably after about an hour this is no longer of benefit
Consider zinc supplementation at the time of injection if a patient is zinc deficient
Clearly there is more to learn about the method of action of BoNT, and further insights may well lead to other ways to maximize clinical effects.
Highlights.
After botulinum toxin is injected, it is taken up into nerve terminals, so injection into endplate zones is optimal.
Botulinum toxin uptake is activity dependent.
Injection of botulinum toxin into muscle distributes the toxin mainly by convection.
Toxin not taken up into the nerve terminals is washed out of the muscle into the general circulation.
Toxin is active in the presynaptic terminal until metabolized.
Acknowledgments
Dr. Hallett’s work is supported by the NINDS Intramural Program.
Footnotes
Conflict of interest
Dr. Hallett serves as Chair of the Medical Advisory Board for and receives honoraria and funding for travel from the Neurotoxin Institute. He may accrue revenue on US Patent #6,780,413 B2 (Issued: August 24, 2004): Immunotoxin (MAB-Ricin) for the treatment of focal movement disorders, and US Patent #7,407,478 (Issued: August 5, 2008): Coil for Magnetic Stimulation and methods for using the same (H-coil); in relation to the latter, he has received license fee payments from the NIH (from Brainsway) for licensing of this patent. He is on the Editorial Board of 20 journals, and received royalties and/or honoraria from publishing from Cambridge University Press, Oxford University Press, John Wiley & Sons, Wolters Kluwer, Springer, and Elsevier. He has received honoraria for lecturing from Columbia University. Dr. Hallett’s research at the NIH is largely supported by the NIH Intramural Program. Supplemental research funds have been granted by the Kinetics Foundation for studies of instrumental methods to monitor Parkinson’s disease, BCN Peptides, S.A. for treatment studies of blepharospasm, Medtronics, Inc., for studies of deep brain stimulation, Parkinson Alliance for studies of eye movements in Parkinson’s disease, UniQure for a clinical trial of AAV2-GDNF for Parkinson Disease, Merz for treatment studies of focal hand dystonia, and Allergan for studies of methods to inject botulinum toxins.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Briefel RR, Bialostosky K, Kennedy-Stephenson J, McDowell MA, Ervin RB, Wright JD. Zinc intake of the U.S. population: findings from the third National Health and Nutrition Examination Survey, 1988–1994. The Journal of nutrition. 2000 May;130(5S Suppl):1367S–73S. doi: 10.1093/jn/130.5.1367S. [DOI] [PubMed] [Google Scholar]
- Carli L, Montecucco C, Rossetto O. Assay of diffusion of different botulinum neurotoxin type a formulations injected in the mouse leg. Muscle Nerve. 2009 Sep;40(3):374–80. doi: 10.1002/mus.21343. [DOI] [PubMed] [Google Scholar]
- Chen R, Karp BI, Goldstein SR, Bara-Jimenez W, Yaseen Z, Hallett M. Effect of muscle activity immediately after botulinum toxin injection for writer’s cramp. Mov Disord. 1999;14(2):307–12. doi: 10.1002/1531-8257(199903)14:2<307::aid-mds1016>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Cohen JL. Scientific skepticism and new discoveries: an analysis of a report of zinc/phytase supplementation and the efficacy of botulinum toxins in treating cosmetic facial rhytides, hemifacial spasm and benign essential blepharospasm. Journal of cosmetic and laser therapy : official publication of the European Society for Laser Dermatology. 2014 Oct;16(5):258–62. doi: 10.3109/14764172.2014.948882. [DOI] [PubMed] [Google Scholar]
- Delnooz CC, Veugen LC, Pasman JW, Lapatki BG, van Dijk JP, van de Warrenburg BP. The clinical utility of botulinum toxin injections targeted at the motor endplate zone in cervical dystonia. Eur J Neurol. 2014 Dec;21(12):1486–e98. doi: 10.1111/ene.12517. [DOI] [PubMed] [Google Scholar]
- Eleopra R, Tugnoli V, De Grandis D. The variability in the clinical effect induced by botulinum toxin type A: the role of muscle activity in humans. Mov Disord. 1997;12(1):89–94. doi: 10.1002/mds.870120115. [DOI] [PubMed] [Google Scholar]
- Elwischger K, Kasprian G, Weber M, Meyerspeer M, Linder C, Auff E, et al. Intramuscular distribution of botulinum toxin--visualized by MRI. J Neurol Sci. 2014 Sep 15;344(1–2):76–9. doi: 10.1016/j.jns.2014.06.028. [DOI] [PubMed] [Google Scholar]
- Gracies JM, Lugassy M, Weisz DJ, Vecchio M, Flanagan S, Simpson DM. Botulinum toxin dilution and endplate targeting in spasticity: a double-blind controlled study. Arch Phys Med Rehabil. 2009 Jan;90(1):9–16. e2. doi: 10.1016/j.apmr.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Guzman-Venegas RA, Araneda OF, Silvestre RA. Differences between motor point and innervation zone locations in the biceps brachii. An exploratory consideration for the treatment of spasticity with botulinum toxin. Journal of electromyography and kinesiology : official journal of the International Society of Electrophysiological Kinesiology. 2014 Dec;24(6):923–7. doi: 10.1016/j.jelekin.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Hess P, Muller P. Extracellular versus intracellular digoxin action on bovine myocardium, using a digoxin antibody and intracellular glycoside application. J Physiol. 1982 Jan;322:197–210. doi: 10.1113/jphysiol.1982.sp014032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R, Whaler BC. Influence of nerve-ending activity and of drugs on the rate of paralysis of rat diaphragm preparations by Cl. botulinum type A toxin. J Physiol (Lond) 1962;160:221–33. doi: 10.1113/jphysiol.1962.sp006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irkoren S, Ozkan HS, Karaca H. A Clinical Comparison of EMLA Cream and Ethyl Chloride Spray Application for Pain Relief of Forehead Botulinum Toxin Injection. Annals of plastic surgery. 2014 Dec 19; doi: 10.1097/SAP.0000000000000121. [DOI] [PubMed] [Google Scholar]
- Koshy JC, Sharabi SE, Feldman EM, Hollier LH, Jr, Patrinely JR, Soparkar CN. Effect of dietary zinc and phytase supplementation on botulinum toxin treatments. Journal of drugs in dermatology : JDD. 2012 Apr;11(4):507–12. [PubMed] [Google Scholar]
- Lapatki BG, van Dijk JP, van de Warrenburg BP, Zwarts MJ. Botulinum toxin has an increased effect when targeted toward the muscle’s endplate zone: a high-density surface EMG guided study. Clin Neurophysiol. 2011 Aug;122(8):1611–6. doi: 10.1016/j.clinph.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Poulain B, de Paiva A, Dolly JO, Weller U, Tauc L. Differences in the temperature dependencies of uptake of botulinum and tetanus toxins in Aplysia neurons. Neurosci Lett. 1992 May 25;139(2):289–92. doi: 10.1016/0304-3940(92)90573-p. [DOI] [PubMed] [Google Scholar]
- Prasad AS, Fitzgerald JT, Hess JW, Kaplan J, Pelen F, Dardenne M. Zinc deficiency in elderly patients. Nutrition. 1993 May-Jun;9(3):218–24. [PubMed] [Google Scholar]
- Rimbach G, Walter A, Most E, Pallauf J. Effect of microbial phytase on zinc bioavailability and cadmium and lead accumulation in growing rats. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 1998 Jan;36(1):7–12. doi: 10.1016/s0278-6915(97)00117-8. [DOI] [PubMed] [Google Scholar]
- Rossetto O, Pirazzini M, Montecucco C. Botulinum neurotoxins: genetic, structural and mechanistic insights. Nature reviews Microbiology. 2014 Aug;12(8):535–49. doi: 10.1038/nrmicro3295. [DOI] [PubMed] [Google Scholar]
- Shaari CM, George E, Wu BL, Biller HF, Sanders I. Quantifying the spread of botulinum toxin through muscle fascia. Laryngoscope. 1991 Sep;101(9):960–4. doi: 10.1288/00005537-199109000-00006. [DOI] [PubMed] [Google Scholar]
- Simpson L. The life history of a botulinum toxin molecule. Toxicon. 2013 Jun;68:40–59. doi: 10.1016/j.toxicon.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Simpson LL. Kinetic studies on the interaction between botulinum toxin type A and the cholinergic neuromuscular junction. J Pharmacol Exp Ther. 1980 Jan;212(1):16–21. [PubMed] [Google Scholar]
- Tang-Liu DD, Aoki KR, Dolly JO, de Paiva A, Houchen TL, Chasseaud LF, et al. Intramuscular injection of 125I-botulinum neurotoxin-complex versus 125I-botulinum-free neurotoxin: time course of tissue distribution. Toxicon. 2003 Oct;42(5):461–9. doi: 10.1016/s0041-0101(03)00196-x. [DOI] [PubMed] [Google Scholar]