Abstract
Purpose
To evaluate the feasibility and utility of intraoperative OCT (iOCT) during pars plana vitrectomy surgery (PPV) for dense vitreous hemorrhage (VH).
Methods
The Prospective Assessment of Intraoperative and Perioperative OCT For Ophthalmic Surgery (PIONEER) study examined the utility of iOCT in ophthalmic surgery. Intraoperative scanning was performed with a microscope-mounted spectral domain OCT system. This report is a case series of those eyes undergoing PPV for dense central VH that precluded preoperative OCT assessment. iOCT images were qualitatively evaluated for retinal abnormalities that might impact intraoperative or perioperative management. Clinical variables were collected and assessed. Surgeon assessment of iOCT utility was also evaluated.
Results
Twenty-three eyes were identified and included. The etiology for the VH was proliferative diabetic retinopathy (19 eyes, 82.6%), horseshoe retinal tear (1 eye, 4.3%), retinal vein occlusion with neovascularization (1 eye, 4.3%), presumed polypoidal choroid vasculopathy (1 eye, 4.3%), and presumed retinal arterial macroaneurysm (1 eye, 4.3%). iOCT revealed epiretinal membrane (14 eyes, 60.9%), macular edema (14 eyes, 60.9%), posterior hyaloidal traction (1 eye, 4.3%), and retinal detachment (1 eye, 4.3%). Surgeon feedback suggested that iOCT impacted surgical decision-making in eyes where membrane peeling was performed.
Conclusion
iOCT during PPV for VH may provide physicians with clinically relevant information that may impact surgical management, perioperative management, and patient outcomes.
Keywords: diabetic retinopathy, intraoperative, intraoperative OCT, iOCT, intraoperative optical coherence tomography, non-clearing vitreous hemorrhage, optical coherence tomography, retinal surgery, vitreous hemorrhage
Introduction
Vitreous hemorrhage (VH) is a common cause of reversible vision loss and blindness among patients with vascular diseases such as diabetic retinopathy.1 When a VH persists despite conservative management, pars plana vitrectomy (PPV) is the treatment of choice.2,3 Optical coherence tomography (OCT) is an imaging technology that has revolutionized the diagnosis of retinal pathology and is a critical diagnostic test in the evaluation of eyes with significant retinal vascular disease.4 VH patients present a unique challenge for surgical planning because central hemorrhages overlying the macula limit the utility of clinical examinations and preclude preoperative OCT evaluation.
Utilization of OCT technology in the operating room has the potential to provide surgeons with information on retinal architecture that was unavailable preoperatively due to media opacities. Recent research has documented the utility of intraoperative OCT (iOCT) in the management of numerous macular pathologies including macular hole, vitreomacular traction, retinal detachment, and epiretinal membrane.5-12 The purpose of this study is to evaluate iOCT during PPV for dense VH and to determine the prevalence of various macular pathologies discovered intraoperatively and to assess its potential utility for intraoperative (e.g., membrane peeling) and perioperative management (e.g., early identification of macular edema that may benefit from intravitreal pharmacotherapy).
Methods
The Prospective Assessment of Intraoperative and Perioperative OCT For Ophthalmic Surgery (PIONEER) study is a single center, prospective, multi-surgeon study on the feasibility and utility of iOCT for both anterior and posterior segment ophthalmic surgery.12 This report focuses on those eyes in PIONEER that underwent PPV for dense central VH and the potential feasibility and utility of iOCT in those cases. This study was approved by the Cleveland Clinic Foundation Institutional Review Board and was conducted in accordance with the Declaration of Helsinki. All patients gave written informed consent to participate in the PIONEER study.
For this report, the primary inclusion criterion was that the main indication for enrollment in the PIONEER study was non-clearing VH that prevented visualization of the fovea on OCT during the sixty days prior to surgery. The primary exclusion criterion was a lack of iOCT imaging during surgery following vitrectomy. Assessment of preoperative variables, including fundus photos and fundus drawings, were reviewed to ensure that VH was the primary indication for surgery and that the VH was central and prevented visualization of the posterior pole. Clinical variables, intraoperative procedures, and postoperative management data were collected and evaluated. Twenty-three eyes of 21 patients were identified that met inclusion/exclusion criteria for this study.
Surgeon feedback questionnaires were performed immediately following surgery on all cases of membrane peeling (e.g., epiretinal membrane, fibrovascular proliferations, internal limiting membrane). These targeted surveys from the PIONEER study focused on the role of iOCT in surgical decision-making and the agreement of iOCT with the surgical impression of tissue configurations (e.g., residual membranes). In addition, the total time surgery was halted for iOCT scans to be completed was recorded.
iOCT Scanning System
In the PIONEER study, a standardized imaging protocol was utilized with a microscope-mounted Bioptigen Envisu SDOIS (Bioptigen, Research Triangle Park, NC) portable OCT (Figure 1), as previously reported.5,6,12 iOCT images were acquired after the core vitrectomy and/or after membrane peeling in all eyes. Scan patterns included 10 mm radial scans, 10 mm × 10 mm, and 10 mm × 5 mm oversampled volume scans. The 10 mm × 10 mm and the 10 mm × 5 mm cubic scans were completed at both 0° and 90°.
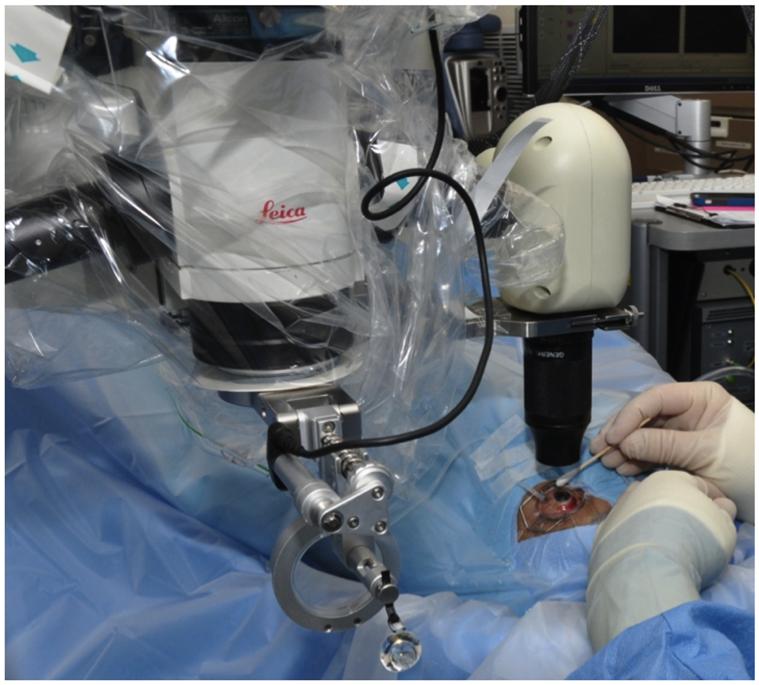
Figure 1.
A customized microscope-mounted system with a portable optical coherence tomography probe enables physicians to use the surgical microscope foot pedal controls to adjust the probe location during image acquisition.
iOCT Image Analysis
Two independent expert reviewers (JFG and JPE) analyzed iOCT images from each case. Images were qualitatively analyzed for the following variables: posterior hyaloidal traction, macular hole, epiretinal membrane (ERM), macular edema, and retinal detachment. Eyes were classified as having an ERM if there was a linear hyperreflective zone on the inner surface of the retina involving the fovea or away from the fovea that resulted in foveal/retinal architectural alterations (e.g., striae). Eyes were not classified as having an ERM if a similar, linear hyperreflective layer overlay the optic disk.
Results
Clinical and Surgical Characteristics
Of the 23 eyes in the study, mean age was 61.5 (range: 42-93 years old). Nineteen of 23 eyes (82.6%) had VH due to proliferative diabetic retinopathy. The etiologies of VH in the other four eyes were retinal tear (n=1, 4.3%), retinal vein occlusion with neovascularization (n=1, 4.3%), presumed polypoidal choroidal vasculopathy (n=1, 4.3%), and presumed retinal arterial macroaneurysm (n=1, 4.3%). All eyes underwent a standard 3-port small-gauge PPV (23- or 25-gauge) and 21 eyes (91.3%) had endolaser, 5 eyes (21.7%) underwent epiretinal membrane peeling. Two eyes received intravitreal anti-vascular endothelial growth factor (VEGF) injections at the conclusion of surgery.
Qualitative iOCT Analysis and Time Impact on Surgery
All 23 eyes (100%) had adequate iOCT images for qualitative analysis. Fourteen eyes (60.9%) had macular edema, 14 eyes (60.9%) had an ERM, one eye (4.3%) had a retinal detachment, and one eye (4.3%) had posterior hyaloidal traction (Figures 2-4). There were no eyes with a macular hole. Three eyes (13%) had no macular abnormalities (Figure 5). The mean time surgery was halted for iOCT image acquisition was 4.4 minutes (range: 2-10.2 minutes, n =21).
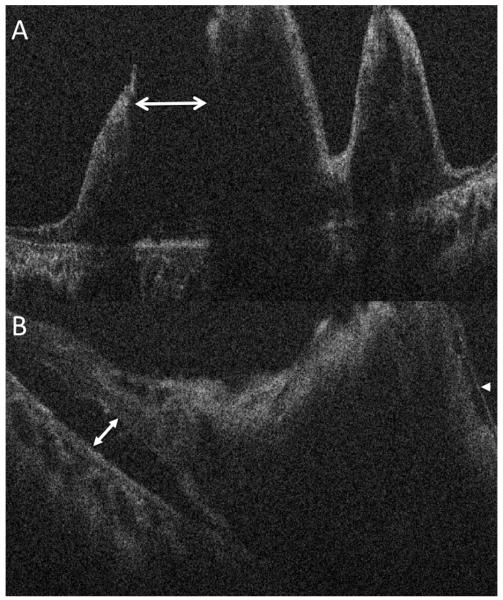
Figure 2.
Intraoperative optical coherence tomography (OCT) reveals an underlying combined rhegmatogenous/tractional retinal detachment. (A) Intraoperative OCT identifies a large full-thickness retinal break (double arrow) between areas of traction. (B) Intraoperative OCT in another area of the macula identifies residual epiretinal membrane (arrowhead) and subtle retinal detachment (double arrow).
Figure 4.
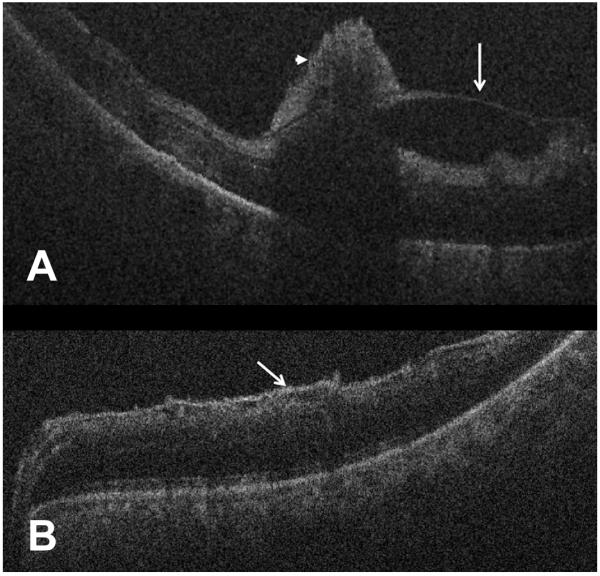
Intraoperative optical coherence tomography demonstrating preretinal membranes following removal of vitreous hemorrhage. (A) B-scan shows macular edema, posterior hyaloidal traction (white arrow), and pre-retinal hemorrhages (arrowhead, Case 2). (B) B-scan reveals epiretinal membrane without significant retinal thickening (white arrow).
Figure 5.
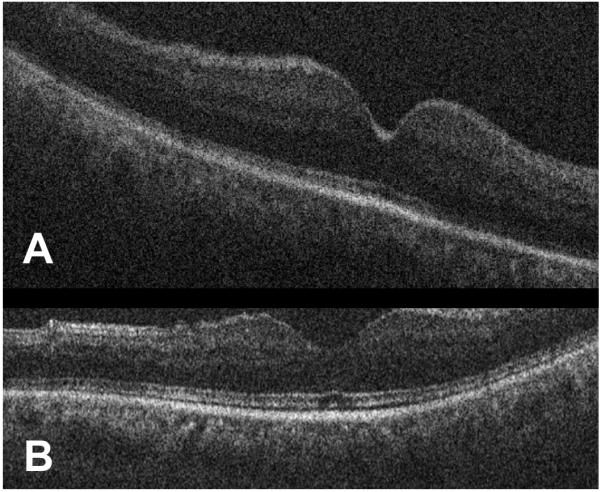
Intraoperative optical coherence tomography demonstrates a normal fovea l architecture. (A) B-scan with normal foveal contour and no macular edema (Case 3). (B) Second example of normal foveal architecture on B-scan with minimal attenuation of the ellipsoid zone.
Impact of iOCT on Surgical Decision-Making and Postoperative Management
Specific surgeon feedback was obtained in those eyes that underwent epiretinal membrane peeling at the time of surgery. Surgeon feedback was completed in 6 of 7 eyes that underwent membrane peeling (e.g., posterior hyaloid peeling, epiretinal membrane peeling). Surgeons reported that iOCT imaging impacted the surgical plan for four of the six cases (66%). In these four cases, the surgeon was unsure of peel completeness in 3 cases. In these cases, iOCT demonstrated completion for two cases (66%) and demonstrated an incomplete peel for one case (33%), helping facilitate surgical completion of the objectives. In one case the surgeon believed the peel to be incomplete, however the iOCT demonstrated the peel was complete preventing unnecessary surgical maneuvers.
Role of iOCT for Postoperative Management of Macular Edema
Overall, six of 23 eyes (26.1%) were treated postoperatively with intravitreal pharmacotherapy for macular edema. Macular edema was noted on iOCT for five of these six patients (83.3%). All six eyes received anti-VEGF therapy. Three of these 6 eyes (50%) received anti-VEGF therapy in the early postoperative period (< 90 days).
Representative Case Examples
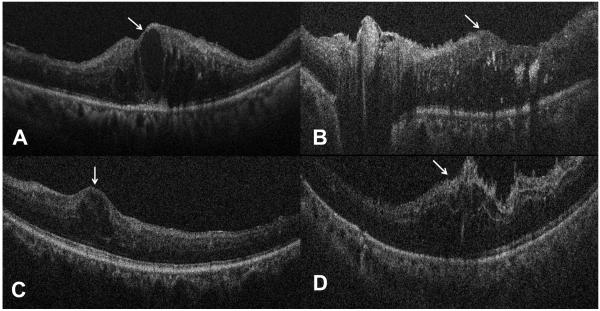
Case 1 is an 80 year-old female with PDR who presented with a non-clearing VH for one year. iOCT results demonstrated that her retina was attached with pre-retinal hemorrhages, exudates, and macular edema (Figure 3). Following surgery, she underwent anti-VEGF therapy.
Figure 3.
Intraoperative optical coherence tomography examples demonstrating macular edema that was not previously diagnosed until obtaining intraoperative imaging. (A) B-scan demonstrates an epiretinal membrane, exudates, and macular edema (white arrow, Case 1). (B) B-scan demonstrates a membrane over the nerve, retinal thickening (white arrow) with associated intraretinal fluid and hyperreflective foci corresponding to hard exudate. (C) B-scan reveals isolated foveal cystic changes (white arrow) with minimal extrafoveal epiretinal membrane. (D) B-scan following vitreous hemorrhage removal identifies extensive intraretinal fluid with associated macular edema (white arrow).
Case 2 is a 58 year-old female with PDR and a non-clearing VH for six weeks. She underwent a PPV, membrane peel, endolaser, and gas tamponade. iOCT results demonstrated that her retina was attached with macular edema, posterior hyaloid traction, and pre-retinal hemorrhages (Figure 4).
Case 3 is a 60 year-old male with a non-clearing VH for four months who had a history of trauma but also presented with proliferative diabetic retinopathy (PDR) in the fellow eye. He underwent a PPV, endolaser, and air-fluid exchange. iOCT results demonstrated that his retina was attached with a normal foveal contour and no macular edema (Figure 5). He did not require any postoperative therapy.
Discussion
Surgical management of VH is unique because media opacities reduce the ability of the surgeon to assess the architecture of the retina preoperatively. To date, there are no published studies examining the role of iOCT during PPV for VH. iOCT for VH patients may provide important information to surgeons to facilitate optimal surgical decision-making and perioperative management. In addition, iOCT results may help surgeons determine visual prognosis and educate patients about their visual potential.
This study demonstrates that eyes undergoing PPV for central VH have significant retinal pathologies. In fact, only 13% of eyes have no evidence of retinal pathology on iOCT. 61% of eyes have macular edema, 61% of eyes have an ERM, and 4.3% of eyes have a retinal detachment.
Recent iOCT studies have documented microarchitectural changes that occur during surgical manipulation for macular hole, ERM, vitreomacular traction syndrome, and retinal detachment repair surgeries.5-8,10,12-14 iOCT before and after peeling the ILM can provide evidence of peel completion as part of macular hole and ERM surgery.8,12-14 A small study of iOCT for rhegmatogenous retinal detachment repair identified microarchitectural changes associated with poor visual outcomes.6 Subclinical changes observed using iOCT for macular hole repair have also been associated with anatomic and visual outcomes.5 Future studies are needed to determine whether intraoperative findings such as macular edema or posterior hyaloidal traction are associated with functional or visual outcomes. In this study, fourteen eyes (60.9%) were noted to have macular edema intraoperatively. This knowledge may impact postoperative decision-making in regards to pharmacologic therapies and OCT surveillance. The use of anti-VEGF therapy to maintain or improve visual acuity in patients with diabetic macular edema is well established.15-18 In fact, 6 eyes were treated postoperatively with anti-VEGF therapy and 83% of these cases were noted to have macular edema intraoperatively. Further research is needed to determine whether macular edema discovered on iOCT is predictive of postoperative macular edema and whether iOCT should inform anti-VEGF treatment decisions.
One advantage of iOCT is the ability to provide surgeons with timely information about retinal architecture during surgery and to give feedback prior to leaving the operating room. The identification and characterization of ERM with iOCT may have important implications for intrasurgical management. Further research is needed to determine whether iOCT can help surgeons identify clinically significant ERMs that would otherwise be unrecognized and whether intraoperative recognition and simultaneous surgical treatment affects patient outcomes.
Recent developments have made iOCT image acquisition more efficient with limited changes to surgical workflow. The average surgical delay for image acquisition in this study was 4.4 minutes. Initial iOCT research utilized hand-held OCT probes.14 The current study utilized a microscope-mounted system that enables the surgeon to control the scan location using the operating microscope pedals.5-8,13 New systems include microscope-integrated OCT systems that can provide real-time surgeon feedback on tissue manipulation and instrument movements without pausing surgery.19-23 Microscope-integrated systems may increase iOCT technology adoption by limiting the extra time required to obtain iOCT scans.
Recognition of certain retinal pathologies intra-operatively may alter surgical decision-making. Surgeon feedback in this study revealed that in select cases iOCT can prevent further peeling when iOCT confirms completion of surgical objectives in discordance with surgeon impressions. Additionally, in many of these PDR cases, the retinal anatomy is often distorted and complex. This study also supports the potential that iOCT may provide clarity for the surgeon regarding the architectural status of the macula when the surgeon is unsure of the presence of membranes that require peeling. This has been reflected in other studies where iOCT appears to definitively alter surgical decision-making in approximately 15% of cases and inform the surgeon regarding the surgical anatomy in a much larger percentage.12
This report has some limitations that should be recognized. The current study sample size is small and limits correlations of iOCT findings and long-term visual outcomes. Additional limitations include a lack of masking, randomization, and a comparison group. In addition, the imaging system utilized in this study is not integrated into the microscope, which may impact overall functionality of iOCT in these cases.
This study demonstrates the feasibility of iOCT during PPV for VH and documents the significant prevalence of underlying macular pathologies in this population. Further research is needed to validate the frequency of macular pathologies seen in this patient population. Additional studies are needed to definitively determine if iOCT results alter surgical management and whether these results ultimately affect visual outcomes.
Summary Statement.
Intraoperative OCT (iOCT) identified a high frequency of retinal pathologies in eyes with dense, central vitreous hemorrhage that precluded preoperative OCT evaluation of the fovea within 60 days of surgery. Findings included epiretinal membranes, macular edema, posterior hyaloidal traction, and retinal detachment. iOCT results may impact surgical decision-making and perioperative care in these cases.
Acknowledgments
The authors would like to acknowledge Jamie Reese, RN at Cole Eye Institute, our lead intraoperative OCT research coordinator, and the entire intraoperative OCT research team for helping to make this study possible.
Financial Support: NIH/NEI K23-EY022947-01A1 (JPE); Ohio Department of Development TECH-13-059 (JPE, SKS). The funding organizations had no role in the design or conduct of this research.
Footnotes
Disclosures:
JE: Bioptigen (C, P), Thrombogenics (C, S, R), Synergetics (P), Genentech (R), Leica (C), Carl Zeiss Meditec (C), Alcon (C)
JG: Pfizer (O)
SS: Bausch and Lomb (C, R); Bioptigen (P); Allergan (R); Synergetics (P); Leica (C), Carl Zeiss Meditec (C)
References
- 1.Spraul CW, Grossniklaus HE. Vitreous Hemorrhage. Surv Ophthalmol. 1997 Jul-Aug;42(1):3–39. doi: 10.1016/s0039-6257(97)84041-6. [DOI] [PubMed] [Google Scholar]
- 2.The Diabetic Retinopathy Vitrectomy Study Research Group Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Two-year results of a randomized trial. Diabetic Retinopathy Vitrectomy Study report 2. JAMA Ophthalmol. 1985 Nov;103(11):1644–1652. [PubMed] [Google Scholar]
- 3.Randomized clinical trial evaluating intravitreal ranibizumab or saline for vitreous hemorrhage from proliferative diabetic retinopathy. JAMA Ophthalmol. 2013 Mar;131(3):283–293. doi: 10.1001/jamaophthalmol.2013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virgili G, Menchini F, Murro V, et al. Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy. Cochrane Database Syst Rev. 2011;7 doi: 10.1002/14651858.CD008081.pub2. CD008081. [DOI] [PubMed] [Google Scholar]
- 5.Ehlers JP, Xu D, Kaiser PK, et al. Intrasurgical dynamics of macular hole surgery: an assessment of surgery-induced ultrastructural alterations with intraoperative optical coherence tomography. Retina. 2014 Feb;34(2):213–221. doi: 10.1097/IAE.0b013e318297daf3. [DOI] [PubMed] [Google Scholar]
- 6.Ehlers JP, Ohr MP, Kaiser PK, Srivastava SK. Novel microarchitectural dynamics in rhegmatogenous retinal detachments identified with intraoperative optical coherence tomography. Retina. 2013 Jul-Aug;33(7):1428–1434. doi: 10.1097/IAE.0b013e31828396b7. [DOI] [PubMed] [Google Scholar]
- 7.Ehlers JP, Tam T, Kaiser PK, et al. Utility of intraoperative optical coherence tomography during vitrectomy surgery for vitreomacular traction syndrome. Retina. 2014 Jul;34(7):1341–1346. doi: 10.1097/IAE.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ray R, Baranano DE, Fortun JA, et al. Intraoperative microscope-mounted spectral domain optical coherence tomography for evaluation of retinal anatomy during macular surgery. Ophthalmology. 2011 Nov;118(11):2212–2217. doi: 10.1016/j.ophtha.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Hahn P, Migacz J, O’Connell R, et al. The use of optical coherence tomography in intraoperative ophthalmic imaging. Ophthalmic Surg Lasers Imaging. 2011 Jul 1;42(4):S85–94. doi: 10.3928/15428877-20110627-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wykoff CC, Berrocal AM, Schefler AC, et al. Intraoperative OCT of a full-thickness macular hole before and after internal limiting membrane peeling. Ophthalmic Surg Lasers Imaging. 2010 Jan-Feb;41(1):7–11. doi: 10.3928/15428877-20091230-01. [DOI] [PubMed] [Google Scholar]
- 11.Pichi F, Alkabes M, Nucci P, Ciardella AP. Intraoperative SD-OCT in macular surgery. Ophthalmic Surg Lasers Imaging. 2012 Nov-Dec;43(6 Suppl):S54–60. doi: 10.3928/15428877-20121001-08. [DOI] [PubMed] [Google Scholar]
- 12.Ehlers JP, Dupps WJ, Kaiser PK, et al. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg With Optical CoherEncE TomogRaphy (PIONEER) Study: 2-Year Results. Am J Ophthalmol. 2014 Jul 29; doi: 10.1016/j.ajo.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Binder S, Falkner-Radler CI, Hauger C, et al. Feasibility of intrasurgical spectral-domain optical coherence tomography. Retina. 2011 Jul-Aug;31(7):1332–1336. doi: 10.1097/IAE.0b013e3182019c18. [DOI] [PubMed] [Google Scholar]
- 14.Dayani PN, Maldonado R, Farsiu S, Toth CA. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina. 2009 Nov-Dec;29(10):1457–1468. doi: 10.1097/IAE.0b013e3181b266bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012 Apr;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 16.Elman MJ, Qin H, Aiello LP, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology. 2012 Nov;119(11):2312–2318. doi: 10.1016/j.ophtha.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt-Erfurth U, Lang GE, Holz FG, et al. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology. 2014 May;121(5):1045–1053. doi: 10.1016/j.ophtha.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 18.Rajendram R, Fraser-Bell S, Kaines A, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. JAMA Ophthalmol. 2012 Aug;130(8):972–979. doi: 10.1001/archophthalmol.2012.393. [DOI] [PubMed] [Google Scholar]
- 19.Ehlers JP, Tao YK, Farsiu S, et al. Integration of a spectral domain optical coherence tomography system into a surgical microscope for intraoperative imaging. Invest Ophthalmol Vis Sci. 2011 May;52(6):3153–3159. doi: 10.1167/iovs.10-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hahn P, Migacz J, O’Donnell R, et al. Preclinical evaluation and intraoperative human retinal imaging with a high-resolution microscope-integrated spectral domain optical coherence tomography device. Retina. 2013 Jul-Aug;33(7):1328–1337. doi: 10.1097/IAE.0b013e3182831293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehlers JP, Kaiser PK, Srivastava SK. Intraoperative optical coherence tomography using the RESCAN 700: preliminary results from the DISCOVER study. Br J Ophthalmol. 2014 Apr 29; doi: 10.1136/bjophthalmol-2014-305294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehlers JP, Tao YK, Farsiu S, et al. Visualization of real-time intraoperative maneuvers with a microscope-mounted spectral domain optical coherence tomography system. Retina. 2013 Jan;33(1):232–236. doi: 10.1097/IAE.0b013e31826e86f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahn P, Migacz J, O’Connell R, et al. Unprocessed real-time imaging of vitreoretinal surgical maneuvers using a microscope-integrated spectral-domain optical coherence tomography system. Graefes Arch Clin Exp Ophthalmol. 2013 Jan;251(1):213–220. doi: 10.1007/s00417-012-2052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehlers JP, Yuan A, Kaiser PK, et al. Trans-tamponade optical coherence tomography: postoperative imaging in gas-filled eyes. Retina. 2013 Jun;33(6):1172–1178. doi: 10.1097/IAE.0b013e31827b6565. [DOI] [PubMed] [Google Scholar]