Abstract
Patients with chronic kidney disease (CKD) have a substantial risk of developing coronary artery disease. Traditional cardiovascular disease (CVD) risk factors such as hypertension and hyperlipidemia do not adequately explain the high prevalence of CVD in CKD. Both CVD and CKD are inflammatory states and inflammation adversely impacts lipid balance. Dyslipidemia in CKD is characterized by elevated triglycerides and high density lipoprotein that is both decreased and dysfunctional. This dysfunctional high density lipoprotein becomes pro-inflammatory and loses its atheroprotective ability to promote cholesterol efflux from cells, including lipid-overloaded macrophages in the arterial wall. Elevated triglycerides result primarily from defective clearance. The weak association between low density lipoprotein cholesterol level and coronary risk in CKD has led to controversy over the usefulness of statin therapy. This review examines disrupted cholesterol transport in CKD, presenting both clinical and pre-clinical evidence of the impact of the uremic environment on vascular lipid accumulation. Preventative and treatment strategies are explored.
Keywords: cholesterol transport, chronic kidney disease (CKD), atherosclerosis, high-density lipoprotein (HDL), low-density lipoprotein (LDL), inflammation, dyslipidemia, cardiovascular disease (CVD), uremic toxins, reactive oxygen species (ROS), lipid-lowering therapy, statin therapy, nontraditional risk factor
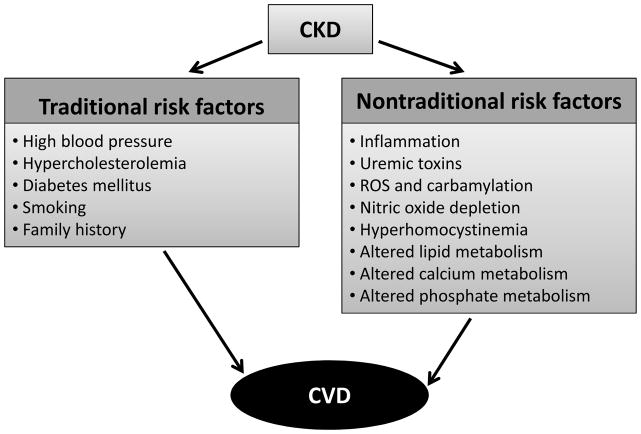
Chronic kidney disease (CKD) impacts approximately 10–13% of the general population. CKD accelerates development of atherosclerosis and increases risk of cardiovascular disease (CVD) (1, 2). CVD is the primary cause of morbidity and mortality in these patients. Most patients with CKD die of cardiovascular events before reaching end stage renal disease (ESRD) (3). Cardiovascular damage starts early in the progression of kidney disease. Even mildly decreased glomerular filtration rate (GFR) or moderately increased albuminuria (microalbuminuria) may be independent predictors for CVD or stroke (4, 5). Incidence and severity of CVD increase as GFR declines (6). While Framingham risk factors including hypertension, hypercholesterolemia, and diabetes mellitus (DM), are common in CKD patients (Fig. 1), they do not fully account for the magnitude of CVD risk in CKD (7–9). Exhaustive interventions to improve risk profile, such as controlling levels of low-density lipoprotein (LDL) -cholesterol or C-reactive protein (CRP), fail to improve cardiovascular outcomes
Figure 1. CKD and CVD risk factors and their interplay.
Traditional risk factors are found in both the CKD and non-CKD population. Nontraditional risk factors may result from or be worsened by CKD and negatively impact the cardiovascular system in the CKD population.
Nontraditional risk factors probably help to promote development of atherosclerosis in the presence of decreased kidney function (10, 11) (Fig. 1). These include inflammation, uremic toxins, oxidative stress, carbamylation, nitric oxide (NO) depletion, hyperhomocystinemia, and altered metabolism of lipids, calcium and phosphate (12, 13). Endothelial dysfunction is a common feature of CKD that occurs early and is a predictor of CVD (14, 15).
Pathology studies have demonstrated that coronary atherosclerosis and calcification are more frequent and advanced in CKD patients compared with non-CKD patients (16, 17). Soft tissue mineralization in the form of arterial calcification is a marker of atherosclerosis and a strong predictor of cardiovascular events (18). A recent imaging study comparing non-culprit coronary atherosclerotic plaques from CKD and non-CKD patients in the Massachusetts General Hospital Optical Coherence Tomography Registry showed more prevalent calcification and cholesterol crystals in plaques of CKD patients (19). Cholesterol crystals trigger inflammation and destabilize plaque. Greater severity of atherosclerosis in CKD patients may be associated with impaired lipid and calcium phosphate metabolism.
Atherosclerosis is thought to be accelerated in CKD due to accumulation of atherogenic oxidation-prone lipoproteins and small dense LDL particles, along with high density lipoprotein (HDL) paucity and qualitative dysfunction, oxidative stress and inflammation. This review will consider the multiple lipid-related mechanisms that contribute to cardiovascular risk in CKD beyond serum cholesterol levels.
The Lipid Profile in CKD and its Relationship with CVD
Dyslipidemia is common in CKD. Epidemiological studies have shown that the incidence of CKD is associated with increased plasma triglycerides and very low density lipoprotein (VLDL) cholesterol as well as decreased HDL cholesterol (20, 21). Intermediate-density lipoproteins and chylomicron remnants may accumulate. Total and LDL cholesterol levels are often within normal limits or somewhat reduced (22). LDL cholesterol levels are less predictive of cardiovascular risk in CKD, especially in those with lower GFR. Despite this, the benefit of lipid lowering in this population has been demonstrated (23, 24). While total cholesterol in the general population is linked to the risk of developing and dying from CVD, data from the CKD population have been less clear. In fact, a ‘reverse epidemiology phenomenon’ has been found in dialysis patients, with lower cholesterol levels associated with higher mortality rates, possibly reflecting the profound malnutrition and inflammatory status present in this population (25). While these findings with advanced kidney disease and ESRD collectively suggest alterations in the atherogenic mechanisms compared with the general population, the discussion here will focus on patients with non-dialysis-dependent CKD, as the underlying processes may not be identical in dialysis patients compared to earlier stages of kidney disease.
Perhaps more important than any quantitative change in cholesterol is the alteration in lipoprotein structure. The lipoproteins found in CKD patients are disproportionately modified, with LDL that is enriched in triglycerides and an increased proportion of small-dense LDL (Table 1). LDL is comprised of a heterogeneous range of particles with variable atherogenic potency. LDL particles vary in size, buoyant density and structure. Small dense LDL is believed to be markedly pro-atherogenic and this is attributed to its ability to infiltrate the vessel wall and its increased susceptibility to oxidative modification (26–30). Small dense LDL is able to breach the endothelial monolayer and bind to proteoglycans of the extracellular matrix in the arterial intima (31). The small dense LDL particles then remain trapped in the intima where they can be oxidized by NADPH (reduced nicotinamide adenine dinucleotide phosphate) oxidases or myeloperoxidase (MPO) or via nonenzymatic oxidation. Studies link small dense LDL to CVD risk even with normal total LDL (32, 33). Shoji et al (34) demonstrated that small dense LDL is a clinically relevant marker of carotid atherosclerosis useful in atherosclerotic risk assessment. Accumulation of small dense LDL particles has been observed in CKD patients (35, 36).
Table 1.
The Lipid Profile in CKD.
| Type of lipid abnormality | Implication in atherosclerotic CVD | Reference |
|---|---|---|
| Presence of small-dense LDL | Small dense LDL are highly susceptible to oxidative modification and serve as a marker of carotid atherosclerosis | [25–36] |
| Decreased levels of endothelium-bound LPL | Decreased clearance of remnant LDL: VLDLs are not converted to IDLs and LDLs and accumulate in the blood stream; this results in increased systemic LDL level and elevated cholesterol and triglyceride levels | [20, 21, 22, 35–40] |
| Elevated level of Lp(a) | Lp(a) accumulates in the vascular wall in atherosclerotic lesions and is resistant to lipid lowering drugs; an elevated level of Lp(a) is an independent risk factor for CVD | [41–44] |
| Upregulated activity of HMG-CoA reductase and ACAT-2 | Promotes accumulation of cholesterol esters in the fat droplets, reduced capacity to absorb cholesterol, and overproduction of Apo-B-containing lipoproteins (LDL, VLDL) | [49–52] |
| Reduction in activity of LCAT and PON1 | Leads to accumulation of nascent preβ-HDL due to impaired maturation of HDL particles, conversion of lipid-rich HDL2 to lipid-poor HDL3, and limited HDL-mediated clearance of cholesterol from extrahepatic tissues | [49–56, 80–82, 85, 86] |
Abbreviations: CKD, chronic kidney disease; CVD, cardiovascular disease; LDL, low-density lipoprotein; HDL, high-density lipoprotein; LPL, lipoprotein lipase; VLDL, very low density lipoprotein; IDL, intermediate density lipoprotein; Lp(a), Lipoprotein(a); HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A; acylcoenzyme A:cholesterol acyltransferase-2; apoB, apolipoprotein B; LCAT, lecithin-cholesterol acyltransferase; PON1, paraoxonase 1.
High triglycerides is one of the most common lipid abnormalities in CKD patients (37). The ratio of triglyceride to cholesterol is also increased in both LDL and HDL particles in these patients. Increased serum triglyceride levels indicate the presence of increased chylomicron remnants, which can penetrate vascular endothelium and lead to atherosclerosis. Normally, triglyceride-rich VLDL released from the liver is converted to intermediate density lipoprotein by lipoprotein lipase (LPL)-catalyzed hydrolysis of the lipids. In CKD, there is an upsurge in triglyceride-rich lipoproteins such as VLDL because of decreased levels of endothelium-bound LPL and mounting levels of apolipoprotein (Apo)-CIII, a competitive inhibitor of the activity of LPL (38). LPL anchors to the capillary endothelial surface and catalyzes the hydrolysis of triglycerides and phospholipids within VLDL and chylomicrons (39). CKD leads to downregulation of LPL and the VLDL receptor in adipose tissue, skeletal muscle and cardiac muscle (22). When the VLDL receptor is reduced in adipocytes and myocytes, they are less able to clear VLDL and its transformation into intermediate-density lipoprotein and LDL is limited. Remnant lipoproteins (intermediate-density lipoprotein and VLDL) are the primary plasma carriers of cholesterol for delivery to all tissues and their accumulation has been shown to promote atherosclerosis progression (40).
Lipoprotein(a) (Lp(a)) is a modified LDL which has a highly glycosylated Apo(a) linked to ApoB100 by a disulfide bridge. Elevated levels of small isoforms of Lp(a) are considered a risk factor for CVD and elevated Lp(a) concentrations are resistant to lipid lowering drugs. Lp(a), which may have atherothrombotic properties (41), accumulates in the vascular wall in human atherosclerotic lesions. Serum Lp(a) is increased in persons with kidney failure, possibly because the kidney degrades Lp(a) (42,43). Upon restoration of kidney function by transplantation, Lp(a) levels decrease (44).
Cholesterol levels amongst patients with CKD may not have the same relationship with CVD as holds in the general population. Applying guideline based LDL cholesterol targets may be less useful in the CKD population. Despite this, convincing cardiovascular event risk reduction benefits of statins have been demonstrated in the non-dialysis-dependent CKD population though the benefits are dampened compared to the general population and appear more uncertain with more advanced stages of CKD (45, 46). In a data analysis of the Modification of Diet in Renal Disease (MDRD) Study, a multicenter randomized clinical trial with mainly stage 3 and 4 CKD patients, hyperlipidemia was found not to be independently associated with all-cause mortality, CVD mortality or CKD progression (47). In this study, 840 adults aged 18–70, mean arterial pressure<125mmHg and elevated creatinine (1.4~7.0mg/dL in men and 1.2~7.0mg/dL in women) were enrolled. Patients with insulin dependent DM, prior kidney transplantation, known renal artery stenosis, Class III or IV New York Heart Assocation congestive heart failure, and those with frequent hospitalizations were excluded. A majority of patients (95%) did not have DM. Mean concentrations of HDL and non-HDL cholesterol were 40±14 and 177±45 mg/dL, respectively, and median triglyceride level was 140 mg/dL, with an interquartile range of 115 mg/dL. Statins were used in 4% of the cohort, and 9% of the total were on a cholesterol-lowering regimen. During the median 10 year follow-up, no association between any of the lipid variables and all-cause mortality was found in unadjusted or adjusted analyses. In an analysis of the data in which HDL cholesterol, non-HDL cholesterol, and log-transformed triglycerides were continuous variables, no association with all-cause mortality or CVD mortality was found. In another study by Shlipak, involving an elderly cohort (mean age 75±6) with mild to moderate CKD (mean 50 ± 10 [SD] mL/min), hyperlipidemia was again found not to be associated with CVD mortality over the average follow-up of 8.6 years (48).
Nephrotic syndrome, characterized by proteinuria of 3.5 g/d, anasarca and hypoalbuminemia, is associated with lipid derangements. A pattern of elevated LDL, Apo-B and triglyceride levels is frequently observed due to a combination of increased hepatic lipoprotein synthesis and reduced catabolism. Reduced clearance as well as overproduction of Apo-B has been demonstrated in nephrotic syndrome. Experiments performed in puromycin-induced nephrotic rats indicate upregulated activity of HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) reductase (Table 1), the rate-limiting enzyme in cholesterol biosynthesis, which leads to increased LDL production. In this animal model, acquired LDL receptor deficiency and lack of LDL clearance by hepatic cholesterol 7α-hydroxylase combined with enhanced LDL production increases systemic LDL level (49, 50). In addition, acyl coenzyme A:cholesterol acyltransferase 2 (ACAT-2), which facilitates the formation of cholesterol esters in hepatic and intestinal cells, is upregulated. Elevated ACAT-2 levels contribute to overproduction of Apo-B-containing lipoproteins, including LDL (51). Thus, nephrotic rats exhibit a 3- to 4-fold increase in liver tissue ACAT-2 mRNA and protein expression and enzymatic activity, corresponding to a significant reduction of free cholesterol in hepatic tissue (52).
CKD is associated with an alteration in the HDL fraction (49). Thus, reduction in the level of cardio-protective HDL2 and an increase in lipid-poor HDL3 is noted in CKD (53–55). Derangements in the ratio of cardio-protective, lipid-rich HDL2 to lipid-poor HDL3 may limit the HDL-mediated clearance of cholesterol from extrahepatic tissues. This unbalanced HDL ratio may be a consequence of severe proteinuria with urinary loss of lecithin-cholesterol acyltransferase (LCAT), a critical enzyme in HDL maturation (53, 56).
It has been postulated by Chawla and others (47) that the atherogenic effects of hyperlipidemia are diluted by the presence of other competing CVD risk factors or that CKD itself may lead to cardiac disease mortality from cardiomyopathy, small vessel coronary disease and arteriosclerosis rather than the typical large vessel coronary artery disease associated with hyperlipidemia. Additional observations of worsened cardiovascular death risk without clear association to cholesterol may be explained by the increased role of inflammation and the altered role of adenosine triphosphate binding cassette (ABC) transporters with advanced CKD (21).
The concurrent presence of the metabolic syndrome amplifies the inflammatory state at various stages of CKD, as extrapolated from NHANES (National Health and Nutrition Examination Survey) III data (57). The accumulation of pro-inflammatory markers in the uremic milieu is further associated with inflammation-malnutrition syndrome characterized by oxidative stress, inflammatory reaction, elevated oxidized LDL and endothelial injury promoting atherosclerosis (58).
Impaired Reverse Cholesterol Transport
Reverse cholesterol transport is a process responsible for clearing excess cholesterol from the arterial wall and is therefore considered a crucial defense mechanism against atherosclerosis. Cellular cholesterol homeostasis is exquisitely maintained so that cholesterol inflow is balanced by cholesterol outflow. Influx of oxidized lipids and lipoproteins occurs mainly though scavenger receptors. Cholesterol efflux occurs through multiple pathways. In reverse cholesterol transport, excess cholesterol is moved from peripheral cells, such as lipid-overloaded macrophages found in atherosclerotic plaque, to the plasma and finally to the liver for excretion into feces either as neutral sterols or after metabolic conversion into bile acids (59).
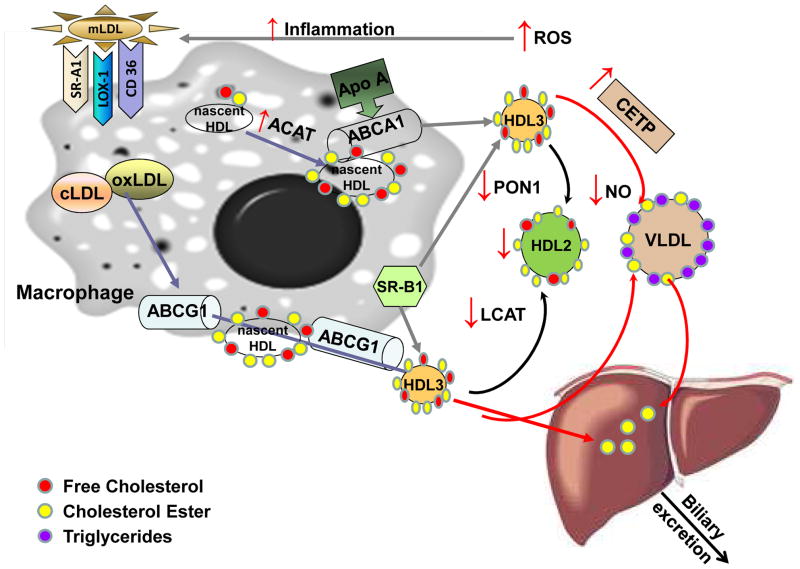
Reverse cholesterol transport begins with cholesterol export from macrophages to serum lipoproteins, which serve as extracellular cholesterol acceptors. Cholesterol removal from macrophages can proceed via the ABC transporters ABCA1 and ABCG1, the scavenger receptor (SR)B1 and through passive aqueous diffusion (60). ABCA1 promotes the efflux of cholesterol and phospholipid from macrophages to lipid-poor Apo-AI, the major protein component of HDL. Apo-AI takes up the transported cholesterol and phospholipids to form nascent HDL (Fig. 2) (61). The critical role of ABCA1 is apparent in Tangier disease, a severe HDL deficiency disorder caused by mutations in ABCA1. Tangier disease is characterized by rapid turnover of plasma Apo-AI, accumulation of cholesterol esters in many tissues, and prevalent atherosclerosis (62). Alternatively, cholesterol can be effluxed by ABCG1 with more mature spherical HDL particles serving as acceptor molecules (Fig. 2). ABCA1 and ABCG1 show additive activity in promoting macrophage reverse cholesterol transport in vivo (63, 64). SRB1 is bidirectional and transfers cholesterol both to and from extracellular HDL/Apo-AI to cells. It may be less critical than ABCA1 in the macrophage (65).
Figure 2. Reverse cholesterol transport in chronic kidney disease.
CKD alters lipoprotein composition through multiple mechanisms, not all of which are understood. Loss of protein is thought to contribute, as is augmented production of ROS. Inflammation and ROS may lead to accumulation of modified LDL (mLDL), such as highly oxidized LDL (oxLDL) or carbamylated LDL (cLDL). Internalization of modified LDL in macrophages occurs via the major scavenger receptors (CD36, SRA-1, LOX1) and contributes to foam cell formation. The presence of mLDL enhances expression of the ABCG1 transporter. In CKD, an elevated level of ACAT-2 facilitates formation and domination of cholesterol esters. Removal of free cholesterol from macrophages proceeds via SR-B1, which contributes to HDL formation through both ABC transporters (ABC) A1 and ABCG1. Cholesterol and phospholipids are eliminated through formation of nascent HDL from circulating Apo-AI. ABCA1 and ABCG1 show additive activity in promoting macrophage reverse cholesterol transport. Nascent HDL is generated when Apo-AI interacts with ABCA1. Uptake of free cholesterol and its conversion to cholesterol ester is mediated by LCAT and results in transformation of HDL3 to HDL2. In kidney disease, conversion of HDL3 to HDL2 is impaired because of LCAT deficiency. CETP mediates transfer of cholesterol ester from HDL to triglyceride rich lipoproteins - VLDL. In CKD, increased activity of CETP is detected, which contributes to low plasma HDL. In uremic patients on maintenance hemodialysis, cholesterol efflux capacity of HDL is markedly reduced when compared to HDL from healthy subjects. Moreover, anti-oxidative and antiinflammatory functions of HDL are impaired due to reduced activity of PON1. HDL from CKD patients loses its vasoprotective properties, inhibiting NO production and increasing vascular cell adhesion Oxidative modifications of HDL limit the ability of HDL to bind to SR-B1 to unload esterified cholesterol to the liver.
Abbreviations: ABCA1 and G1, ATP-binding cassette sub-family A1 and G1 members; ACAT, acyl coenzyme A:cholesterol acyltransferase; apoA-I; apolipoprotein A-I; CETP, cholesteryl ester transfer protein;; HDL, high density lipoprotein; LCAT, lecithin cholesterol acyltransferase; LOX1, lectin-like oxidized LDL receptor 1; NO, nitric oxide; PON1, serum paraoxonase/arylesterase 1; ROS, reactive oxygen species; SRA1/B1, scavenger receptor class A member 1/class B member 1; VLDL, very low density lipoprotein.
The plasma enzyme LCAT is activated by Apo-AI on nascent HDL to form cholesteryl ester from free cholesterol, prompting maturation from discoidal to spherical HDL. Esterification of cholesterol by LCAT is a key step in reverse cholesterol transport (54). Cholesteryl ester transfer protein (CETP) mediates transfer of cholesterol ester from HDL to triglyceride rich lipoproteins (Fig. 2). Low CETP activity leads to higher HDL and may be cardioprotective (66, 67).
CKD adversely impacts reverse cholesterol transport at multiple levels. Cultured human coronary arterial endothelial cells exposed for 48 hours to 20% plasma from CKD patients exhibit decreased ABCA1 and ABCG1 expression versus controls (68). Plasma Apo-AI and HDL cholesterol content are significantly reduced in CKD likely due to impaired synthesis of Apo-AI by the liver and low LCAT activity (69). Patients with decreased kidney function frequently have LCAT deficiency, and the enzyme that is present shows reduced activity (53–55). Further contributing to low HDL in CKD is increased activity of CETP (56). HDL is reduced in CKD independent of confounders such as body mass index and diabetes (70). Low HDL in CKD was found to be associated with an increase in intermediate monocytes, a type of monocyte with poor cholesterol efflux capacity, low ABCA1 and elevated cytokine production. Higher levels of intermediate monocytes predict cardiovascular events in subjects at elevated cardiovascular risk (71).
HDL in CKD: Loss of Cardioprotective Properties
Under normal circumstances, circulating HDL acts to protect against atherosclerosis through reverse cholesterol transport. It also has anti-inflammatory and anti-coagulant properties (72). Further, HDL behaves as an anti-oxidant by removing oxidant molecules from the arterial wall, thus limiting oxidative modification of LDL and reducing exposure of macrophages to oxidized lipids (73). Elevated HDL is generally believed to protect from atherosclerosis (74). However, drugs or genetic polymorphisms that increase HDL fail to decrease cardiovascular events and it is now recognized that the relationship between HDL and atherosclerosis is complex (74, 75). HDL quality may be a more accurate indicator of cardiovascular risk than plasma concentration (76), and improperly functioning HDL may not be atheroprotective (77).
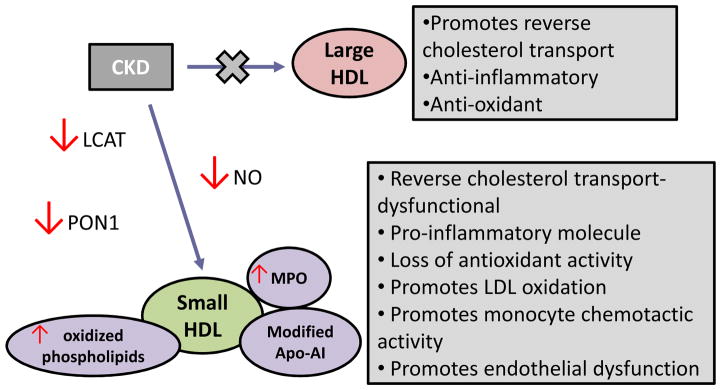
CKD is a pathological state associated with inflammation that alters the biological activity of HDL particles (12) (Fig. 3). HDL particle composition in individuals with CKD is abnormal, with depletion of large buoyant HDL and relative enrichment of small dense HDL (78, 79). In CKD, anti-oxidative and anti-inflammatory functions of HDL are impaired, possibly due to reduced activity of HDL-associated enzymes, such as paraoxonase 1 (PON1), nitric oxide (NO) synthase (NOS) and LCAT (80, 81). These dysfunctional HDL particles promote phospholipid oxidation, accumulation of serum amyloid A and CRP, and induce cytokine production by monocytes and myeloid dendritic cells in patients with CKD (82, 83). Displacement of Apo-AI in HDL by serum amyloid leads to lower circulating Apo-AI levels due to an increase in the fast catabolism of free Apo-AI by the kidney.
Figure 3. Changes in HDL functions mediated by CKD.
Small dense HDL particles dominate in individuals with CKD. Chronic pro-inflammatory conditions activate macrophages, releasing myeloperoxidase (MPO). MPO-derived oxidants modify HDL, which impairs the functioning of HDL-associated enzymes, such as paraoxonase 1 (PON1), nitric oxide (NO) synthase and lecithin cholesterol acyltransferase (LCAT). These enzymes are essential for anti-oxidative, anti-inflammatory and vasoprotective properties of unmodified HDL.
One of the potential pathways of converting an antiatherogenic HDL into its atherogenic forms in CKD and other pro-inflammatory conditions involves myeloperoxidase (MPO), an enzyme expressed by macrophages in the human artery wall (84). Modification of HDL causes specific loss of cholesterol acceptor activity, interferes with endothelial NOS activity, and impairs the ability of HDL to activate LCAT and the major HDL-associated anti-inflammatory enzyme paraoxonase 1 (PON1) (80) (Fig. 2, 3).
PON1 activity is an essential mechanism by which HDL inhibits LDL oxidation. An increasing body of evidence suggests that PON1, an esterase associated with Apo-AI and clusterin in HDL, offers protection against oxidation of LDL (82, 85). PON1 also enhances cholesterol efflux from macrophages by forming lysophosphatidylcholine through lactonase activity on phospholipids which, in turn, promotes HDL binding mediated by ABCA1 (86).
Amongst the variables affecting the cellular handling of cholesterol and its accumulation in cells, HDL plays an important role in cholesterol efflux (87, 88). Given that the accumulation of cholesterol in cells is a factor which promotes atherogenesis, the ability of HDL to facilitate the efflux of cholesterol from cells may be able to modulate the risk of cardiovascular events. Recent work by Rohatgi et al provides strong evidence that this may be the case (88). In a study of patients from the Dallas Heart Study the authors used a method to measure the efflux of cholesterol labeled with the fluorescent marker boron dipyrromethene difluoride and determined hazard ratios of cardiovascular events according to the quartile of cholesterol efflux capacity. This approach correlated moderately with a radiolabeled cholesterol methodology. They found an inverse association between cholesterol efflux capacity and cardiovascular events, with 67% fewer cardiovascular events in the highest versus lowest quartile of cholesterol efflux capacity (88). These findings suggest that measurement of cholesterol efflux might be a useful clinical tool to improve assessment of cardiovascular risk. This may have important applicability to patients with CKD who have dysfunctional HDL. For example, Yamamoto et al have shown that the HDL from patients receiving maintenance hemodialysis has a markedly reduced ability to mediate cholesterol efflux from macrophages compared to HDL from healthy controls with matching clinical and demographic characteristics (89). It should be noted that the assays in these two studies utilized murine and human macrophage cell lines, respectively, and there may be differences between macrophage behavior in these experimental settings and the behavior of macrophages in vivo. Nevertheless, these findings suggest that reduced cholesterol efflux from macrophages mediated by abnormal HDL may, at least in part, be responsible for the increased cardiovascular event rate in patients with kidney disease and that cholesterol efflux assays may be useful as biomarkers for CVD.
HDL normally promotes repair of endothelium and increases bioavailability of NO by activating endothelial NOS (90). However, HDL from CKD patients loses its vasoprotective properties and may have adverse effects on vascular endothelium by inhibiting NO production (Fig. 2, 3), promoting superoxide production, and increasing vascular cell adhesion molecule 1 (VCAM-1) expression (91, 92). Abnormal HDL may contribute to the high levels of VCAM-1 found in patients with CKD and reduced GFR (93, 94).
Uremic Toxins
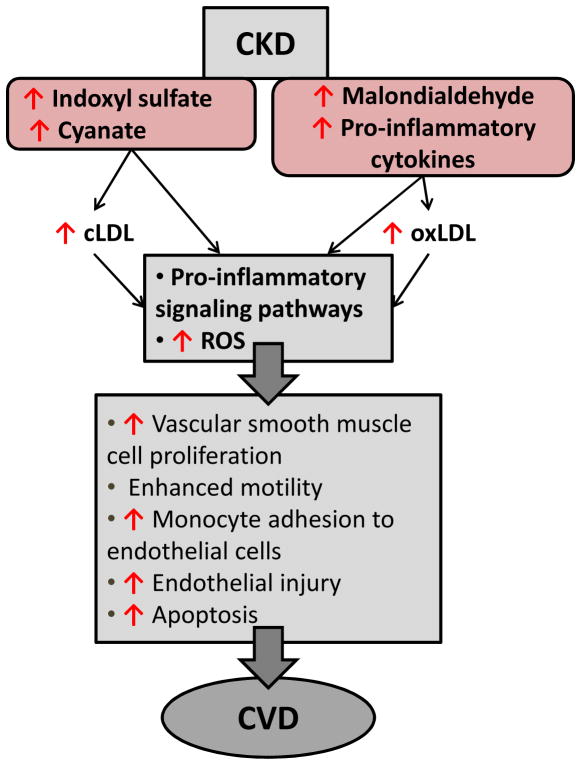
Indoxyl sulfate, a uremic toxin derived from dietary protein via metabolism by intestinal bacteria, accumulates in the serum of CKD patients due to decreased kidney clearance. Indoxyl sulfate is a powerful inducer of free radicals that lead to oxidative stress (95). It is a nephrotoxin involved in the progression of CVD in CKD (96). Indoxyl sulfate affects endothelial function by upregulating adhesion molecules such as VCAM-1 and inhibiting endothelial cell proliferation (97, 98). It also stimulates vascular smooth muscle cell proliferation (99–101) (Fig. 4).
Figure 4. CKD-mediated progression of atherosclerosis.
Pro-inflammatory cytokines and uremic toxins are elevated in the serum of CKD patients. These substances directly modify LDL, and either directly or through modified LDL, activate inflammatory pathways. Enhanced uremic toxins and modified LDL – carbamylated or oxidized (cLDL or oxLDL), mediate the release of adhesion molecules and reactive oxygen species (ROS). Activation of pro-inflammatory signaling pathways leads to endothelial injury and dysfunction, vascular smooth muscle cell proliferation and CVD progression.
Malondialdehyde, a byproduct of peroxidation of polyunsaturated fats, is a widely used measure of reactive oxygen species (ROS) status and its level is raised in CKD (102) (Fig. 2). Malondialdehyde-modified LDL, which is believed to be an indicator of the overall level of oxidized LDL, is a marker of coronary artery disease severity (103).
Uremic conditions lead to an increase in carbamylated LDL, which is produced through post-translational modification of LDL by cyanate derived from urea or thiocyanate. Carbamylated LDL has atherogenic properties (104, 105), triggers ROS production, and causes endothelial dysfunction with impaired vascular relaxation (106, 107). It is cytotoxic to endothelium and induces adhesion molecules that attract monocytes while also promoting smooth muscle proliferation, all critical events in the atherogenic process (108, 109).
Treatment Approaches
Information on treatment and prevention of CVD in CKD is scarce, but studies indicate that CVD treatments that are effective in non-CKD patients may not improve outcome in CKD. Physicians should also be more cautious in using cardiac medications when kidney function is decreased and may need to adjust dosages (110).
Role of Lipid-Lowering Therapy
KDIGO (Kidney Disease: Improving Global Outcomes) strongly recommends statin therapy for all patients older than 50 years with CKD not treated with maintenance dialysis or kidney transplantation, and statin treatment in those younger than 50 years with atherosclerotic CVD risk above 10% over 10 years. Statins should not be initiated in patients on hemodialysis. KDIGO does not recommend using LDL as a determinant of need for treatment or as a treatment target (111, 112). However, benefits of statins in CKD remain a point of controversy. The Study of Heart and Renal Protection (SHARP), a randomized trial of 6247 patients with CKD of various stages and 3023 dialysis patients without a known history of myocardial infarction or coronary revascularization, evaluated therapy with simvastatin 20 mg daily plus ezetimibe 10 mg daily versus placebo. Over the median follow up 4.9 years, the study showed no significant difference between the two groups for coronary deaths, but the treatment group showed lower LDL and a significant 17% reduction in the risk of combined major atherosclerotic events, such as non-fatal myocardial infarction, ischemic stroke or coronary revascularization procedures. Overall mortality was unaffected (23, 24). The study was underpowered to detect the effects separately between dialysis and non-dialysis patients but a trend to reduced benefit of simvastatin/ezetimibe from Stage 3 successively to Stage 5 disease and ESRD was observed.
A meta-analysis of 50 studies compiled by Palmer et al showed that statins are effective as primary prevention in reducing cardiovascular events and death in adults with non-dialysis-dependent CKD (45). Another meta-analysis encompassing 31 clinical trials supported the benefit of statin therapy, demonstrating a 23% reduction in risk of cardiovascular events as well as a reduction in all-cause deaths, but this data also suggested similarly dampened benefit of statins with advancing CKD stages (113). In statin-treated CKD patients, a meta-analysis of 50 trials concluded that statins significantly reduce lipid concentrations and cardiovascular outcomes in patients with non-dialysis-dependent CKD, but failed to improve all-cause mortality (114). Efflux capacity to HDL of ESRD patients, measured in cultured human macrophages, was not improved by statins (89).
Fibric acid derivatives (fibrates, such as fenofibrate and gemfibrozil) are used to reduce triglycerides and increase HDL in CKD patients (115). However, fibrates can elevate creatinine and increase risk for myopathy and rhabdomyolysis (116). KDIGO recommends against use of combination of statin and fibrates in CKD patients due to increased adverse events. Use of niacin to lower triglycerides and raise HDL can also be considered although its effectiveness in improving cardiovascular outcome has not been shown (117, 118).
Non-Lipid Lowering Effects of Statins and Novel Approaches to Reducing Inflammation and Oxidative Stress
As previously noted, the standard cholesterol profile does not correlate with overall mortality and cardiovascular mortality risk in advanced CKD patients, yet the data suggest that statins are associated with improved outcomes if ESRD has not been reached. While much of the statin effect may be related to the LDL reduction in the general population, it is conceivable that the pleiotropic effects of statins may play a greater role in CKD, especially related to their associated reduction in inflammatory markers, interleukin 6 and CRP. The observed trend of diminishing benefits with statin treatment with progressive advancement in CKD suggests the possibilities of modifications to the cholesterol structure and function, alteration of cholesterol transport balance and the predominance of other yet undefined series of competing risk factors with advancing CKD. The changes encountered in CKD may be on the basis of accumulating uremic toxins directly or the increase in systemic inflammation and appear to shift the pivotal point of treatment away from HMG-CoA reductase inhibition. Dietary and lifestyle interventions may represent another approach to reducing adverse outcomes in CKD (119). For example, following a high fiber diet (23 grams per day) for six weeks was found to improve the lipid profile in CKD patients, leading to significantly decreased LDL, total cholesterol, and cholesterol-HDL ratio (120).
Our group has found that the dietary supplement resveratrol, found in some foods and herbal medicines with large consumption worldwide, improves lipid handling and impedes atherosclerotic processes in murine and cell culture models (121). Toklu and colleagues used a hypertensive rat model to show that resveratrol is cardioprotective through improvement of oxidant status and inhibition of lipid peroxidation (122). Resveratrol has a favorable safety record and is well-tolerated; it is also inexpensive and can be combined with other therapies without contraindication (123), and thus may merit evaluation as an adjunct treatment in CVD in the setting of decreased kidney function (124).
Future Directions
The traditional cholesterol profile focuses on the systemic and hepatic metabolism of lipids. This traditional CVD risk profile is useful in the general population but in CKD it is conceivable that the effects of more localized atherosclerotic factors in arterial endothelial cells and intimal macrophages become predominant, negating the systemic circulatory levels. The levels of several genes, referred to here as the “cholesterol metabolic signature,” are pivotal in cholesterol movement and may influence the atherosclerotic burden, especially in CKD patients. Influx of cholesterol is regulated by the LDL receptor, scavenger receptors (CD36, SRA1, and SRB1), and the lectin-like oxidized LDL receptor (LOX-1). Efflux via reverse cholesterol transport is controlled by 27-hydroxylase, ABCA1, ABCG1, liver X receptor (LXR)-α/β. Animal models of CKD show upregulation of LXR mRNA and its gene products, but lipid accumulation is noted, further demonstrating the collective dysregulation. Murine models of decreased kidney function show decreased ABCA1 and impaired macrophage cholesterol efflux (122). Exploring the contribution of these cholesterol movers to abnormal lipid balance in CKD may lead to novel targeted approaches to prevention and treatment in this vulnerable population.
Conclusions
CKD is a worldwide public health issue and has been identified as a major risk factor for atherosclerotic CVD (125, 126). In the early stages of CKD patients are more likely to die of CVD than they are to eventually progress to ESRD (127). Thus, CKD can be defined as a pro-atherogenic state (128). The atheroma–promoting CKD milieu fosters dyslipidemia, impaired HDL function and disrupted reverse cholesterol transport. Factors contributing to this atherogenic environment include the presence of uremic toxins, persistent inflammation and oxidative stress.
In addition to its relationship to overt CVD, CKD may lead to clinically silent myocardial injury (129). Detection of subclinical CVD may lead to successful treatment and better outcome. At present, concerted medical management (including lifestyle modifications) remains the treatment approach of choice in CKD-related CVD. Novel approaches targeted to the CKD population to restore HDL quality, reduce oxidative damage and improve cholesterol handling require new initiatives.
Acknowledgments
Support: This work was supported by R21 AT007032-01A1 from The National Center for Complementary and Integrative Health by the Elizabeth Daniell Research Fund. We thank Janet and Robert Buescher for their generous support through the foundation bearing their name.
Footnotes
Financial Disclosure: The authors declare that they have on relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42(5):1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 2.Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Alberta Kidney Disease Network. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 4.Manjunath G, Tighiouart H, Ibrahim H, MacLeod B, Salem DN, Griffith JL, Coresh J, Levey AS, Sarnak MJ. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41(1):47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Chertow GM, Fan D, McCullock CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 6.Chonchol M, Whittle J, Desbien A, Orner MB, Petersen LA, Kressin NR. Chronic kidney disease is associated with angiographic coronary artery disease. Am J Nephrol. 2008;28(2):354–360. doi: 10.1159/000111829. [DOI] [PubMed] [Google Scholar]
- 7.Sarnak MJ, Coronado BE, Greene T, et al. Cardiovascular disease risk factors in chronic renal insufficiency. Clin Nephrol. 2002;57(5):327–335. doi: 10.5414/cnp57327. [DOI] [PubMed] [Google Scholar]
- 8.Zoccali C. Traditional and emerging cardiovascular and renal risk factors: an epidemiologic perspective. Kidney Int. 2006;70(1):26–33. doi: 10.1038/sj.ki.5000417. [DOI] [PubMed] [Google Scholar]
- 9.Weiner DE, Tabatabai S, Tighiouart H, et al. Cardiovascular outcomes and all-cause mortality: Exploring the interaction between CKD and cardiovascular disease. Am J Kidney Dis. 2006;48(3):392–401. doi: 10.1053/j.ajkd.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Carrero JJ, Stenvinkel P. Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: a hypothesis proposal. Clin J Am Soc Nephrol. 2009;4(1):S49–S55. doi: 10.2215/CJN.02720409. [DOI] [PubMed] [Google Scholar]
- 11.Shik J, Parfrey PS. The clinical epidemiology of cardiac disease in chronic kidney disease. Curr Opin Nephrol Hypertens. 2005;14(6):550–557. doi: 10.1097/01.mnh.0000170752.64150.88. [DOI] [PubMed] [Google Scholar]
- 12.Menon V, Gul A, Sarnak MJ. Cardiovascular risk factors in chronic kidney disease. Kidney Int. 2005;68(4):1413–1418. doi: 10.1111/j.1523-1755.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 13.Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimburger O, Massy Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol. 2008;3(2):505–521. doi: 10.2215/CJN.03670807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malyszko J. Mechanism of endothelial dysfunction in chronic kidney disease. Clinica Chimica Acta. 2010;411(19–20):1412–1420. doi: 10.1016/j.cca.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Stam F, van Guldener C, Becker A, et al. Endothelial dysfunction contributes to renal function-associated cardiovascular mortality in a population with mild renal insufficiency: the Hoorn study. J Am Soc Nephrol. 2006;17(2):537–545. doi: 10.1681/ASN.2005080834. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura S, Ishibashi-Ueda H, Niizuma S, Yoshihara F, Horio T, Kawano Y. Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin J Am Soc Nephrol. 2009;4(12):1892–1900. doi: 10.2215/CJN.04320709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakano T, Ninomiya T, Sumiyoshi S, et al. Association of kidney function with coronary atherosclerosis and calcification in autopsy samples from Japanese elders: the Hisayama study. Am J Kidney Dis. 2010;55(1):21–30. doi: 10.1053/j.ajkd.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 18.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71(5):438–441. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 19.Kato K, Yonetsu T, Jia H, et al. Nonculprit coronary plaque characteristics of chronic kidney disease. Circ Cardiovasc Imaging. 2013;6(3):448–456. doi: 10.1161/CIRCIMAGING.112.000165. [DOI] [PubMed] [Google Scholar]
- 20.Schaeffner ES, Kurth T, Curhan GC, et al. Cholesterol and the risk of renal dysfunction in apparently healthy men. J Am Soc Nephrol. 2003;14(8):2084–2091. doi: 10.1681/ASN.V1482084. [DOI] [PubMed] [Google Scholar]
- 21.Vaziri ND. Lipotoxicity and impaired high density lipoprotein-mediated reverse cholesterol transport in chronic kidney disease. J Ren Nutr. 2010;20(Suppl):S35–S43. doi: 10.1053/j.jrn.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol. 2006;290(2):F262–F272. doi: 10.1152/ajprenal.00099.2005. [DOI] [PubMed] [Google Scholar]
- 23.Baigent C, Landray MJ, Reith C, et al. SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harbin M, Amadio A, Tejani A. Critical appraisal of the SHARP trial: the results may be dull. Clin Ther. 2014;36(12):2112–2117. doi: 10.1016/j.clinthera.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Coresh J, Eustace JA, et al. Association between cholesterol level and mortality in dialysis patients: Role of inflammation and malnutrition. JAMA. 2004;291(4):451–459. doi: 10.1001/jama.291.4.451. [DOI] [PubMed] [Google Scholar]
- 26.Chait A, Brazg RL, Tribble DL, Krauss RM. Susceptibility of small, dense, low-density lipoproteins to oxidative modification in subjects with the atherogenic lipoprotein phenotype, pattern. B Am J Med. 1993;94(4):350–356. doi: 10.1016/0002-9343(93)90144-e. [DOI] [PubMed] [Google Scholar]
- 27.Kotani K, Tsuzaki K, Traniguchi N, Sakane N. LDL particle size and reactive oxygen metabolities in dyslipidemic patients. Int J Prev Med. 2012;3(3):160–166. [PMC free article] [PubMed] [Google Scholar]
- 28.Tribble DL, Rizzo M, Chait A, Lewis DM, Blanche PJ, Krauss RM. Enhanced oxidative susceptibility and reduced antioxidant content of metabolic precursors of small, dense low-density lipoproteins. Am J Med. 2001;110(2):103–110. doi: 10.1016/s0002-9343(00)00700-2. [DOI] [PubMed] [Google Scholar]
- 29.Kwiterovich PO., Jr Clinical relevance of the biochemical, metabolic, and genetic factors that influence low-density lipoprotein heterogeneity. Am J Cardiol. 2002;90(8A):30i–47i. doi: 10.1016/s0002-9149(02)02749-2. [DOI] [PubMed] [Google Scholar]
- 30.Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276(11):875–881. [PubMed] [Google Scholar]
- 31.Galeano NF, Al-Haideri M, Keyserman F, Rumsey SC, Deckelbaum RJ. Small dense low density lipoprotein has increased affinity for LDL receptor-independent cell surface binding sites: a potential mechanism for increased atherogenicity. J Lipid Res. 1998;39(6):1263–1273. [PubMed] [Google Scholar]
- 32.Williams PT, Superko HR, Haskell WL, et al. Smallest LDL particles are most strongly related to coronary disease progression in men. Arterioscler Thromb Vasc Biol. 2003;23(2):314–321. doi: 10.1161/01.atv.0000053385.64132.2d. [DOI] [PubMed] [Google Scholar]
- 33.Williams PT, Zhao XQ, Marcovina SM, Otvos JD, Brown BG, Krauss RM. Comparison of four methods of analysis of lipoprotein particle subfractions for their association with angiographic progression of coronary artery disease. Atherosclerosis. 2014;233(2):713–720. doi: 10.1016/j.atherosclerosis.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shoji T, Hatsuda S, Tsuchikura S, et al. Small dense low-density lipoprotein cholesterol concentration and carotid atherosclerosis. Atherosclerosis. 2009;202(2):582–588. doi: 10.1016/j.atherosclerosis.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 35.Chu M, Wang AY, Chan IH, Chui SH, Lam CW. Serum small-dense LDL abnormalities in chronic renal disease patients. Br J Biomed Sci. 2012;69(3):99–102. [PubMed] [Google Scholar]
- 36.Deighan CJ, Caslake MJ, McConnell M, Boulton-Jones JM, Packard CJ. The atherogenic lipoprotein phenotype: small dense LDL and lipoprotein remnants in nephrotic range proteinuria. Atherosclerosis. 2001;157(1):211–220. doi: 10.1016/s0021-9150(00)00710-3. [DOI] [PubMed] [Google Scholar]
- 37.Ritz E, Wanner C. Lipid changes and statins in chronic renal insufficiency. J Am Soc Nephrol. 2006;17 (12 Suppl 3):S226–S30. doi: 10.1681/ASN.2006080919. [DOI] [PubMed] [Google Scholar]
- 38.Ginsberg HN, Le NA, Goldberg IJ, et al. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. J Clin Invest. 1986;78(5):1287–1295. doi: 10.1172/JCI112713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mead JR, Irvine SA, Ramji DP. Lipoprotein lipase: structure, function, regulation, and role in disease. J Mol Med (Berl) 2002;80(12):753–769. doi: 10.1007/s00109-002-0384-9. [DOI] [PubMed] [Google Scholar]
- 40.Nordestgaard BG, Tybjaerg-Hansen IDL, VLDL, chylomicrons and atherosclerosis. Eur J Epidemiol. 1992;8(Suppl 1):92–98. doi: 10.1007/BF00145358. [DOI] [PubMed] [Google Scholar]
- 41.Loscalzo J. Lipoprotein(a): a unique risk factor for atherothrombotic disease. Arteriosclerosis. 1990;10(5):672–679. doi: 10.1161/01.atv.10.5.672. [DOI] [PubMed] [Google Scholar]
- 42.Milionis HJ, Elisaf MS, Tselepis A, Bairaktari E, Karabina SA, Siamopoulos KC. Apolipoprotein(a) phenotypes and lipoprotein(a) concentrations in patients with renal failure. Am J Kidney Dis. 1999;33(6):1100–1106. doi: 10.1016/S0272-6386(99)70147-2. [DOI] [PubMed] [Google Scholar]
- 43.Gaw A, Boerwinkle E, Cohen JC, Hobbs HH. Comparative analysis of the apo(a) gene, apo(a) glycoprotein, and plasma concentrations of Lp(a) in three ethnic groups. Evidence for no common “null” allele at the apo(a) locus. J Clin Invest. 1994;93(6):2526–2534. doi: 10.1172/JCI117263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kronenberg F, Kuen E, Ritz E, et al. Lipoprotein(a) serum concentrations and apolipoprotein(a) phenotypes in mild and moderate renal failure. J Am Soc Nephrol. 2000;11(1):105–115. doi: 10.1681/ASN.V111105. [DOI] [PubMed] [Google Scholar]
- 45.Palmer SC, Navaneethan SD, Craig JC, et al. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev. 2014;5:CD007784. doi: 10.1002/14651858.CD007784.pub2. [DOI] [PubMed] [Google Scholar]
- 46.Rysz J, Gluba-Brzózka A, Banach M, Wiêcek A. Should we use statins in all patients with chronic kidney disease without dialysis therapy? The current state of knowledge. Int Urol Nephrol. 2015;47(5):805–813. doi: 10.1007/s11255-015-0937-9. [DOI] [PubMed] [Google Scholar]
- 47.Chawla V, Greene T, Beck GJ, et al. Hyperlipidemia and long-term outcomes in nondiabetic chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(9):1582–1587. doi: 10.2215/CJN.01450210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293(14):1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 49.Vaziri ND, Liang KH. Hepatic HMG-CoA reductase gene expression during the course of puromycin-induced nephrosis. Kidney Int. 1995;48(6):1979–1985. doi: 10.1038/ki.1995.500. [DOI] [PubMed] [Google Scholar]
- 50.Vaziri ND. Molecular mechanisms of lipid disorders in nephrotic syndrome. Kidney Int. 2003;63(5):1964–1976. doi: 10.1046/j.1523-1755.2003.00941.x. [DOI] [PubMed] [Google Scholar]
- 51.Rudel L, Shelness G. Cholesterol esters and atherosclerosis–a game of ACAT and mouse. Nature Medicine. 2000;6(12):1313–1314. doi: 10.1038/82110. [DOI] [PubMed] [Google Scholar]
- 52.Vaziri ND, Liang KH. Upregulation of Acyl-Coenzyme A: Cholesterol acyltransferase (ACAT) in nephrotic syndrome. Kidney Int. 2002;61(5):1769–1775. doi: 10.1046/j.1523-1755.2002.00319.x. [DOI] [PubMed] [Google Scholar]
- 53.Vaziri ND, Liang K, Parks JS. Down-regulation of hepatic lecithin:cholesterol acyltransferase gene expression in chronic renal failure. Kidney Int. 2001;59(6):2192–2196. doi: 10.1046/j.1523-1755.2001.00734.x. [DOI] [PubMed] [Google Scholar]
- 54.Kunnen S, Van Eck M. Lecithin:cholesterol acyltransferase: old friend or foe in atherosclerosis? J Lipid Res. 2012;53(9):1783–1799. doi: 10.1194/jlr.R024513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guarnieri GF, Moracchiello M, Campanacci L, et al. Lecithin-cholesterol acyltransferase (LCAT) activity in chronic uremia. Kidney Int. 1978;(8 Suppl):S26–30. [PubMed] [Google Scholar]
- 56.Calabresi L, Simonelli S, Conca P, et al. Acquired lecithin:cholesterol acyltransferase deficiency as a major factor in lowering plasma HDL levels in chronic kidney disease. J Intern Med. 2015;277(5):552–561. doi: 10.1111/joim.12290. [DOI] [PubMed] [Google Scholar]
- 57.Beddhu S, Kimmel PL, Ramkumar N, Cheung AK. Associations of metabolic syndrome with inflammation in CKD: results From the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2005;46(4):577–586. doi: 10.1053/j.ajkd.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 58.Navab KD, Elboudwarej O, Gharif M, et al. Chronic inflammatory disorders and accelerated atherosclerosis: chronic kidney disease. Curr Pharm Des. 2011;7(1):17–20. doi: 10.2174/138161211795049787. [DOI] [PubMed] [Google Scholar]
- 59.Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J Lipid Res. 1995;36(2):211–228. [PubMed] [Google Scholar]
- 60.Voloshyna I, Reiss AB. The ABC transporters in lipid flux and atherosclerosis. Prog Lipid Res. 2011;50(3):213–224. doi: 10.1016/j.plipres.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 61.Van Eck M, Pennings M, Hoekstra M, Out R, Van Berkel TJ. Scavenger receptor BI and ATP-binding cassette transporter A1 in reverse cholesterol transport and atherosclerosis. Curr Opin Lipidol. 2005;16(3):307–315. doi: 10.1097/01.mol.0000169351.28019.04. [DOI] [PubMed] [Google Scholar]
- 62.Bodzioch M, Orsó E, Klucken J, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22(4):347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 63.Gelissen IC, Harris M, Rye KA, et al. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler Thromb Vasc Biol. 2006;26(3):534–540. doi: 10.1161/01.ATV.0000200082.58536.e1. [DOI] [PubMed] [Google Scholar]
- 64.Wang X, Collins HL, Ranalletta M, Rader DJ, et al. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117(8):2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mineo C, Shaul PW. Functions of scavenger receptor class B, type I in atherosclerosis. Curr Opin Lipidol. 2012;23(5):487–493. doi: 10.1097/MOL.0b013e328357ba61. [DOI] [PubMed] [Google Scholar]
- 66.Barter PJ, Brewer HB, Jr, Chapman MJ, Hennekens CH, Rader DJ, Tall AR. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23(2):160–167. doi: 10.1161/01.atv.0000054658.91146.64. [DOI] [PubMed] [Google Scholar]
- 67.Kimura H, Miyazaki R, Imura T, et al. Hepatic lipase mutation may reduce vascular disease prevalence in hemodialysis patients with high CETP levels. Kidney Int. 2003;64(5):1829–1837. doi: 10.1046/j.1523-1755.2003.00285.x. [DOI] [PubMed] [Google Scholar]
- 68.Cardinal H, Raymond MA, Hebert MJ, Madore F. Uraemic plasma decreases the expression of ABCA1, ABCG1 and cell-cycle genes in human coronary arterial endothelial cells. Nephrol Dial Transplant. 2007;22(2):409–416. doi: 10.1093/ndt/gfl619. [DOI] [PubMed] [Google Scholar]
- 69.Attman PO, Alaupovic P, Gustafson A. Serum apolipoprotein profile of patients with chronic renal failure. Kidney Int. 1987;32(3):368–375. doi: 10.1038/ki.1987.219. [DOI] [PubMed] [Google Scholar]
- 70.Lo JC, Go AS, Chandra M, Fan D, Kaysen GA. GFR, body mass index, and low high-density lipoprotein concentration in adults with and without CKD. Am J Kidney Dis. 2007;50(4):552–558. doi: 10.1053/j.ajkd.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 71.Rogacev KS, Zawada AM, Emrich I, et al. Lower Apo A-I and Lower HDL-C Levels Are Associated With Higher Intermediate CD14++CD16+ Monocyte Counts That Predict Cardiovascular Events in Chronic Kidney Disease. Arterioscler Thromb Vasc Biol. 2014;34(9):2120–2127. doi: 10.1161/ATVBAHA.114.304172. [DOI] [PubMed] [Google Scholar]
- 72.Feig JE, Shamir R, Fisher EA. Atheroprotective effects of HDL: beyond reverse cholesterol transport. Curr Drug Targets. 2008;9(3):196–203. doi: 10.2174/138945008783755557. [DOI] [PubMed] [Google Scholar]
- 73.Barter PJ, Nicholls S, Rye K-A, Anantharamaiah G, Navab M, Fogelman AM. Anti-inflammatory properties of HDL. Circ Res. 2004;95(8):764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 74.Rader DJ. Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest. 2006;116(12):3090–3100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.AIM-HIGH Investigators. Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 76.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dodani S, Grice DG, Joshi S. Is HDL function as important as HDL quantity in the coronary artery disease risk assessment? J Clin Lipidol. 2009;3(2):70–77. doi: 10.1016/j.jacl.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 78.Kon V, Ikizler TA, Fazio S. Importance of high-density lipoprotein quality: Evidence from chronic kidney disease. Curr Opin Nephrol Hypertens. 2013;22(3):259–265. doi: 10.1097/MNH.0b013e32835fe47f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alabakovska SB, Todorova BB, Labudovic DD, Tosheska KN. Related LDL and HDL subclass distribution in patients with end-stage renal diseases. Clin Biochem. 2002;35(3):211–216. doi: 10.1016/s0009-9120(02)00300-4. [DOI] [PubMed] [Google Scholar]
- 80.Holzer M, Birner-Gruenberger R, Stojakovic T, et al. Uremia alters HDL composition and function. J Am Soc Nephrol. 2011;22(9):1631–1641. doi: 10.1681/ASN.2010111144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dirican M, Akca R, Sarandol E, Dilek K. Serum paraoxonase activity in uremic predialysis and hemodialysis patients. J Nephrol. 2004;17(6):813–818. [PubMed] [Google Scholar]
- 82.Dantoine TF, Debord J, Charmes JP, et al. Decrease of serum paraoxonase activity in chronic renal failure. J Am Soc Nephrol. 1998;9(11):2082–2088. doi: 10.1681/ASN.V9112082. [DOI] [PubMed] [Google Scholar]
- 83.Weichhart T, Kopecky C, Kubicek M, et al. Serum amyloid A in uremic HDL promotes inflammation. J Am Soc Nephrol. 2012;23(5):934–947. doi: 10.1681/ASN.2011070668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Malle E, Marsche G, Panzenboeck U, Sattler W. Myeloperoxidase-mediated oxidation of high-density lipoproteins: Fingerprints of newly recognized potential proatherogenic lipoproteins. Arch Biochem Biophys. 2006;445(2):245–255. doi: 10.1016/j.abb.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 85.Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN. Paraoxonase inhibits high density lipoprotein (HDL) oxidation and preserves its functions: A possible peroxidative role for paraoxonase. J Clin Invest. 1998;101(8):1581–1590. doi: 10.1172/JCI1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Y, Mackness B, Mackness M. Comparison of the ability of paraoxonases 1 and 3 to attenuate the in vitro oxidation of low-density lipoprotein and reduce macrophage oxidative stress. Free Radic Biol Med. 2008;45(6):743–748. doi: 10.1016/j.freeradbiomed.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 87.Rosenblat M, Vaya J, Shih D, Aviram M. Paraoxonase 1 (PON1) enhances HDL-mediated macrophage cholesterol efflux via the ABCA1 transporter in association with increased HDL binding to the cells: a possible role for lysophosphatidylcholine. Atherosclerosis. 2005;179(1):69–77. doi: 10.1016/j.atherosclerosis.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 88.Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371(25):2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yamamoto S, Yancey PG, Ikizler TA, et al. Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol. 2012;60(23):2372–2379. doi: 10.1016/j.jacc.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115(10):1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 91.Besler C, Heinrich K, Rohrer L, et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest. 2011;121(7):2693–2708. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Speer T, Rohrer L, Blyszczuk P, et al. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity. 2013;38(4):754–768. doi: 10.1016/j.immuni.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 93.Zewinger S, Speer T, Kleber ME, et al. HDL Cholesterol is not associated with lower mortality in patients with kidney dysfunction. J Am Soc Nephrol. 2014;25(5):1073–1082. doi: 10.1681/ASN.2013050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bonomini M, Reale M, Santarelli P, et al. Serum levels of soluble adhesion molecules in chronic renal failure and dialysis patients. Nephron. 1998;79(4):399–407. doi: 10.1159/000045084. [DOI] [PubMed] [Google Scholar]
- 95.Annuk M, Fellström B, Akerblom O, Zilmer K, Vihalemm T, Zilmer M. Oxidative stress markers in pre-uremic patients. Clin Nephrol. 2001;56(4):308–14. [PubMed] [Google Scholar]
- 96.Niwa T. Indoxyl sulfate is a nephro-vascular toxin. J Ren Nutr. 2010;20(Suppl 5):S2–S6. doi: 10.1053/j.jrn.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 97.Barreto FC, Barreto DV, Liabeuf S, et al. European Uremic Toxin Work Group (EUTox) Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4(10):1551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tumur Z, Shimizu H, Enomoto A, Miyazaki H, Niwa T. Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-kappaB activation. Am J Nephrol. 2010;31(5):435–441. doi: 10.1159/000299798. [DOI] [PubMed] [Google Scholar]
- 99.Dou L, Bertrand E, Cerini C, et al. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004;65(2):442–451. doi: 10.1111/j.1523-1755.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 100.Yamamoto H, Tsuruoka S, Ioka T, et al. Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells. Kidney Int. 2006;69(10):1780–1785. doi: 10.1038/sj.ki.5000340. [DOI] [PubMed] [Google Scholar]
- 101.Muteliefu G, Enomoto A, Niwa T. Indoxyl sulfate promotes proliferation of human aortic smooth muscle cells by inducing oxidative stress. J Ren Nutr. 2009;19(1):29–32. doi: 10.1053/j.jrn.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 102.Atamer A, Kocyigit Y, Ecder SA, et al. Effect of oxidative stress on antioxidant enzyme activities, homocysteine and lipoproteins in chronic kidney disease. J Nephrol. 2008;21(6):924–930. [PubMed] [Google Scholar]
- 103.Amaki T, Suzuki T, Nakamura F, et al. Circulating malondialdehyde modified LDL is a biochemical risk marker for coronary artery disease. Heart. 2004;90(10):1211–1213. doi: 10.1136/hrt.2003.018226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ok E, Basnakian AG, Apostolov EO, Barri YM, Shah SV. Carbamylated low-density lipoprotein induces death of endothelial cells: a link to atherosclerosis in patients with kidney disease. Kidney Int. 2005;68(1):173–178. doi: 10.1111/j.1523-1755.2005.00391.x. [DOI] [PubMed] [Google Scholar]
- 105.Apostolov EO, Ray D, Savenka AV, Shah SV, Basnakian AG. Chronic uremia stimulates LDL carbamylation and atherosclerosis. J Am Soc Nephrol. 2010;21(11):1852–1857. doi: 10.1681/ASN.2010040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Speer T, Owala FO, Holy EW, et al. Carbamylated low-density lipoprotein induces endothelial dysfunction. Eur Heart J. 2014;35(43):3021–3032. doi: 10.1093/eurheartj/ehu111. [DOI] [PubMed] [Google Scholar]
- 107.Apostolov EO, Ray D, Alobuia WM, et al. Endonuclease G mediates endothelial cell death induced by carbamylated LDL. Am J Physiol Heart Circ Physiol. 2011;300(6):H1997–2004. doi: 10.1152/ajpheart.01311.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Asci G, Basci A, Shah SV, et al. Carbamylated low-density lipoprotein induces proliferation and increases adhesion molecule expression of human coronary artery smooth muscle cells. Nephrology (Carlton) 2008;13(6):480–486. doi: 10.1111/j.1440-1797.2008.00948.x. [DOI] [PubMed] [Google Scholar]
- 109.Apostolov EO, Shah SV, Ok E, Basnakian AG. Carbamylated low-density lipoprotein induces monocyte adhesion to endothelial cells through intercellular adhesion molecule-1 and vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol. 2007;27(4):826–832. doi: 10.1161/01.ATV.0000258795.75121.8a. [DOI] [PubMed] [Google Scholar]
- 110.Hartmann B, Czock D, Keller F. Drug therapy in patients with chronic renal failure. Dtsch Arztebl Int. 2010;107(37):647–655. doi: 10.3238/arztebl.2010.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wanner C, Tonelli M. Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85(6):1303–1309. doi: 10.1038/ki.2014.31. [DOI] [PubMed] [Google Scholar]
- 112.Sarnak MJ, Bloom R, Muntner P, et al. KDOQI US Commentary on the 2013 KDIGO Clinical Practice Guideline for Lipid Management in CKD. Am J Kidney Dis. 2015;65(3):354–366. doi: 10.1053/j.ajkd.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 113.Hou W, Lv J, Perkovic V, et al. Effect of statin therapy on cardiovascular and renal outcomes in patients with chronic kidney disease: a systematic review and meta-analysis. Eur Heart J. 2013;34(24):1807–1817. doi: 10.1093/eurheartj/eht065. [DOI] [PubMed] [Google Scholar]
- 114.Strippoli GF, Navaneethan SD, Johnson DW, et al. Effects of statins in patients with chronic kidney disease: meta-analysis and meta-regression of randomised controlled trials. BMJ. 2008;336(7645):645–651. doi: 10.1136/bmj.39472.580984.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McCullough PA, Di Loreto MJ. Fibrates and cardiorenal Coll outcomes. J Am Cardiol. 2012;60(20):2072–2073. doi: 10.1016/j.jacc.2012.06.058. [DOI] [PubMed] [Google Scholar]
- 116.Wu J, Song Y, Li H, Chen J. Rhabdomyolysis associated with fibrate therapy: review of 76 published cases and a new case report. Eur J Clin Pharmacol. 2009;65(12):1169–1174. doi: 10.1007/s00228-009-0723-7. [DOI] [PubMed] [Google Scholar]
- 117.Ahmed MH. Niacin as potential treatment for dyslipidemia and hyperphosphatemia associated with chronic renal failure: the need for clinical trials. Ren Fail. 2010;32(5):642–646. doi: 10.3109/08860221003753323. [DOI] [PubMed] [Google Scholar]
- 118.Kalil RS, Wang JH, de Boer IH, et al. Effect of extended-release niacin on cardiovascular events and kidney function in chronic kidney disease: a post hoc analysis of the AIM-HIGH trial. Kidney Int. 2015 doi: 10.1038/ki.2014.383. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ricardo AC, Anderson CA, Yang W, et al. CRIC Study Investigators; CRIC Study Investigators. Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2015;65(3):412–424. doi: 10.1053/j.ajkd.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Salmean YA, Zello GA, Dahl WJ. Foods with added fiber improve stool frequency in individuals with chronic kidney disease with no impact on appetite or overall quality of life. BMC Res Notes. 2013;6:510. doi: 10.1186/1756-0500-6-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Voloshyna I, Hai O, Littlefield MJ, Carsons S, Reiss AB. Resveratrol mediates anti-atherogenic effects on cholesterol flux in human macrophages and endothelium via PPARγ and adenosine. Eur J Pharmacol. 2013;698(1–3):299–309. doi: 10.1016/j.ejphar.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 122.Toklu HZ, Sehirli O, Erşahin M, et al. Resveratrol improves cardiovascular function and reduces oxidative organ damage in the renal, cardiovascular and cerebral tissues of two-kidney, one-clip hypertensive rats. J Pharm Pharmacol. 2010;62(12):1784–1793. doi: 10.1111/j.2042-7158.2010.01197.x. [DOI] [PubMed] [Google Scholar]
- 123.Cottart CH, Nivet-Antoine V, Laguillier-Morizot C, Beaudeux JL. Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res. 2010;54(1):7–16. doi: 10.1002/mnfr.200900437. [DOI] [PubMed] [Google Scholar]
- 124.Zuo Y, Yancey P, Castro I, et al. Renal dysfunction potentiates foam cell formation by repressing ABCA1. Arterioscler Thromb Vasc Biol. 2009;29(9):1277–1282. doi: 10.1161/ATVBAHA.109.188995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ayodele OE, Alebiosu CO. Burden of chronic kidney disease: an international perspective. Adv Chronic Kidney Dis. 2010;17(3):215–224. doi: 10.1053/j.ackd.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 126.Tonelli M, Muntner P, Lloyd A, et al. Alberta Kidney Disease Network. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: A population-level cohort study. Lancet. 2012;380(9844):807–814. doi: 10.1016/S0140-6736(12)60572-8. [DOI] [PubMed] [Google Scholar]
- 127.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 128.Stenvinkel P, Pecoits-Filho R, Lindholm B. Coronary artery disease in end-stage renal disease: no longer a simple plumbing problem. J Am Soc Nephrol. 2003;14(7):1927–1939. doi: 10.1097/01.asn.0000069165.79509.42. [DOI] [PubMed] [Google Scholar]
- 129.Bansal N. Clinically silent myocardial infarctions in the CKD community. Nephrol Dial Transplant. 2012;27(9):3387–3391. doi: 10.1093/ndt/gfs171. [DOI] [PubMed] [Google Scholar]