Abstract
Methamphetamine use has increased significantly and become a global health concern. Craving is known to predict methamphetamine use and relapse following abstinence. Some have suggested that cravings are automatic, generalized, and uncontrollable, but experimental work addressing these claims is lacking. In two exploratory studies we tested the boundary conditions of methamphetamine craving by asking: (1) is craving specific to users’ preferred route of administration? and (2) can craving be regulated by cognitive strategies? Two groups of methamphetamine users were recruited. In Study 1, participants were grouped by their preferred route of administration (intranasal vs. smoking), and rated their craving in response to photographs and movies depicting methamphetamine use (via the intranasal vs. smoking route). In Study 2, methamphetamine smokers implemented cognitive regulation strategies while viewing photographs depicting methamphetamine smoking. Strategies involved either focusing on the positive aspects of smoking methamphetamine or the negative consequences of doing so – the latter strategy based on treatment protocols for addiction. In Study 1, we found a significant interaction between group and route of administration, such that participants who preferred to smoke methamphetamine reported significantly stronger craving for smoking stimuli, whereas those who preferred the intranasal route reported stronger craving for intranasal stimuli. In Study 2, participants reported significantly lower craving when focusing on the negative consequences associated with methamphetamine use. Taken together, these findings suggest that strength of craving for methamphetamine is moderated by users’ route of administration and can be reduced by cognitive strategies. This has important theoretical, methodological, and clinical implications.
Keywords: Craving, methamphetamine, regulation, route of administration
Over the past two decades, illicit methamphetamine use has become a global concern, with amphetamines being second only to marijuana in prevalence of use worldwide (United Nations, 2011). In the United States in 2012, approximately 439,000 individuals aged 12 and over reported current use of illicit methamphetamine (Substance Abuse and Mental Health Services Administration, 2012). In addition, there were 110,000 admissions for methamphetamine use-related treatment (Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, 2013). These figures underscore the need to understand factors that may increase or decrease methamphetamine use.
One such factor is craving, which has long been considered a key feature in addiction disorders (Kavanagh & Connor, 2012; O’Brien, Childress, Ehrman, & Robbins, 1998; Sinha & Li, 2007; Volkow et al., 2006; World Health Organization, 1955) and has been repeatedly linked to both drug use and relapse (e.g., cocaine, nicotine, alcohol; Allen, Bade, Hatsukami, & Center, 2008; Crits-Christoph et al., 2007; Heinz, 2005; Shiffman et al., 2013; see Kober, 2014 for a review). Methamphetamine craving has been shown to rise in response to methamphetamine-related cues (Culbertson et al., 2010; Ekhtiari, Alam-Mehrjerdi, Nouri, George, & Mokri, 2010; Newton et al., 2006), and to predict methamphetamine use and relapse after treatment (Galloway, Singleton, & The Methamphetamine Treatment Project Corporate Authors, 2008; Hartz, Frederick-Osborne, & Galloway, 2001). Recently, the accumulated evidence resulted in the addition of craving as a diagnostic criterion to Addiction Disorders in DSM-5, where craving is defined as “a strong desire or urge to use drugs” (American Psychiatric Association, 2013). Other definitions include “obsessional thoughts about drugs, triggering compulsive drug-seeking and drug taking behavior” (Ciccocioppo, 1999), “irresistible desire” (World Health Organization, 1955) and “so irresistible that it almost inevitably leads to drug seeking and drug taking” (Robinson & Berridge, 1993). This notion of craving as not only a strong desire, but an uncontrollable urge, is echoed in much of the popular media’s approach to drugs in general, and methamphetamine in particular, including the campaign “Not Even Once” (Hart, 2013; The Partnership at Drugfree.org, 2013). But is craving, in fact, so uncontrollable?
Indeed, the suggestion that craving is uncontrollable is inconsistent with clinical findings that cognitive-behavioral treatments (CBT) - that include key modules on regulation of craving - are at least somewhat effective in reducing craving and use for various drugs (e.g., Carroll, 1996; Dutra et al., 2008) including methamphetamine (Lee & Rawson, 2008; Smout et al., 2010). Nevertheless, few studies to date have experimentally investigated boundary conditions that influence or modulate craving in methamphetamine users. Understanding such factors is critically important in our efforts to develop better treatments for methamphetamine addiction.
To investigate the boundary conditions of methamphetamine craving directly, we recruited methamphetamine users to participate in two exploratory laboratory studies. In the first study, we tested the effect of methamphetamine-related cues on craving. Because illicit methamphetamine users in the US vary with regards to preferred route of administration (e.g., intranasal, smoking, or intravenous; see Substance Abuse and Mental Health Services Administration, Office of Applied Studies, 2009), we specifically tested whether route of administration is a moderator of cue-induced methamphetamine craving. Because cue-induced cravings are conditioned responses (based on repeated pairing of methamphetamine cues with methamphetamine administration; Kober, Turza, & Hart, 2009; Martin-Soelch, Linthicum, & Ernst, 2007; Tiffany & Conklin, 2000; Weiss, 2005), we hypothesized that strength of cue-induced cravings depends on individual use history and therefore differ by route of administration. Therefore, we grouped participants according to their self-reported “most common” and “preferred” route of administration: intranasal administration or smoking (we were not able to recruit intravenous users, due to their relatively low rate in NYC population). Participants completed a cue-induced craving task that consisted of viewing photographs and movies depicting methamphetamine smoking or intranasal administration of methamphetamine. We predicted that methamphetamine craving would be elicited in a cue-specific manner, such that methamphetamine users would experience stronger craving in response to stimuli that depict their preferred route of administration (e.g., those who most commonly smoke methamphetamine would experience stronger craving for smoking stimuli).
In this first study, as predicted, we observed higher cue-induced craving towards stimuli that matched users’ route of administration. Given these findings, we recruited a new cohort of methamphetamine smokers in a second study in which we induced strong craving by presenting stimuli that depicted the group’s preferred route of administration (smoking), and then tested whether these participants could modulate their craving using cognitive strategies drawn from CBT treatments for addiction. Such strategies direct users to think differently about their craving by making reappraisals about the meaning of drug cues. Participants in this study completed the Regulation of Craving (ROC) task, following our prior work with cigarette smokers (Kober, Mende-Siedlecki, et al., 2010; Kober, Kross, Mischel, Hart, & Ochsner, 2010; Westbrook et al., 2013). In this task, participants viewed photographs of methamphetamine, methamphetamine-smoking, and food, and were instructed to implement cognitive regulation strategies to increase or decrease their craving. After each trial, participants reported their craving on a continuous rating scale. Importantly, it has not been previously shown whether methamphetamine users can exercise cognitive control over their methamphetamine craving in experimental settings. Rather, it has been suggested that methamphetamine users are cognitively impaired (Scott et al., 2007), and that craving may be uncontrollable (Robinson & Berridge, 1993; The Partnership at Drugfree.org, 2013). Nevertheless, we predicted that methamphetamine users would be able to regulate their craving for methamphetamine as well as food.
Study 1 – Route of Administration and Methamphetamine Craving
Method
Participants
Twenty-one current methamphetamine users were recruited to participate in the study (see Table 1 for demographic information). Participants were recruited via posters on bulletin boards, online ads, and newspaper advertisements in New York City, and by re-contacting individuals who participated in prior studies in this population (e.g., Hart et al., 2008). Participants were initially screened by phone and then were screened in person during their first visit at the New York State Psychiatric Institute, following our prior work (e.g., Kirkpatrick, Gunderson, Johanson, et al., 2012). Specifically, all participants passed medical and psychiatric evaluations prior to study enrollment, and were deemed healthy by a physician. They were also within normal weight ranges according to the Metropolitan Life Insurance Company height/weight table. Following our prior work with this population (Kirkpatrick, Gunderson, Levin, Foltin, & Hart, 2012), participants were eligible if they reported regular illicit methamphetamine use, fulfilled criteria for methamphetamine abuse or dependence as assessed by the Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 2002), tested positive for methamphetamine on a urine toxicology screening, and did not report any neurological, psychiatric, or movement disorders, seizures, use of mood-altering medications in the past 6 months, or any medical condition that might impair cognitive function (including abuse/dependence on any other drug except methamphetamine and nicotine).
Table 1. Summary demographics for Studies 1 and 2.
Between group comparisons for Study 1 indicated no significant differences in age, education, or race (all ps > .1). Due to missing data, frequencies for ethnicity and race are incomplete, with only 15 of 19 participants’ data available for Study 1, and 10 of 13 available for Study 2.
| Study 1 | Study 2 | ||
|---|---|---|---|
| Group (N) | Intranasal (6) | Smoking (13) | Smoking (13) |
| Mean Age ± SD | 40 ± 4.6 | 36 ± 8.8 | 40 ± 5.8 |
| Mean Education ± SD | 15.3 ± 1.15 | 15.3 ± 1.95 | 14.8 ± 1.40 |
| Male | 6 | 12 | 13 |
| Female | 0 | 1 | 0 |
| Ethnicity | |||
| Hispanic | 1 | 3 | 5 |
| Non-Hispanic | 2 | 9 | 5 |
| Race | |||
| White | 1 | 3 | 3 |
| Black | 1 | 4 | 5 |
| Asian | 0 | 1 | 0 |
| Native American | 0 | 1 | 0 |
| Other (non-specified) | 1 | 3 | 2 |
Two participants were excluded prior to data analysis due to self-reported non-compliance on the task, and 19 participants (1 female) were therefore included in the final analysis. Their age ranged from 26 to 47 years (M = 37.52, SD = 7.65). Education level ranged from 12 to 17 years (M = 15.3 years, SD = 2.04). Overall, participants reported using methamphetamine an average of 4.81 times in the month prior to their second laboratory visit (SD = 4.38), between 0.5 to 4 grams per use (M = 1.05 grams per use, SD = 1.07; which indicates several days of use per “time”). In the final sample, 6 participants reported intranasal methamphetamine use and 13 participants reported smoking of methamphetamine as their preferred (and most common) route of administration. Intranasal users reported using an average of 6.33 times per month (Range: 1 – 17.5; SD = 5.95) and 1.3 grams per use (Range: 0.5 – 4; SD = 1.53). Smokers reported using 4.45 times per month (Range: 1 – 14; SD = 3.91) and 0.875 grams per use (Range: 0.5 – 1.25; SD = 0.323). Differences in frequency and amount between groups were not significant (p = .45 and p = .61, respectively). Additionally, there were no differences in age, race, or education between groups (see Table 1). All participants gave informed consent in accordance with the Institutional Review Boards of Columbia University and the New York State Psychiatric Institute. Testing sessions were typically conducted in the afternoon, and participants were instructed ahead of time to abstain from caffeine, food, cigarettes, and drugs for at least 2 hours prior to the laboratory session, which lasted one hour. This 2-hour abstinence period followed that in our prior work (Kober, et al., 2010a; Kober, et al., 2010b).
Stimuli
During the task, participants were shown 75 images of food, 84 images of methamphetamine, and 42 short videos depicting methamphetamine use. All 75 food stimuli were previously shown to elicit food craving (Kober, Mende-Siedlecki, et al., 2010; Kober, Kross, et al., 2010; Simmons, Martin, & Barsalou, 2005; Sobik, Hutchison, & Craighead, 2005). These stimuli were included to serve as a control and to differentiate craving for methamphetamine from food craving. Stimuli depicting methamphetamine (both pictures and videos) were aggregated from online sources, documentaries, and feature films depicting methamphetamine use, and were divided into three categories: 26 pictures of people smoking methamphetamine (“Smoking”), 27 pictures of people snorting methamphetamine (“Intranasal”), and 31 pictures with no specified route of administration present (“Substance Only”). The video stimuli consisted of 42 videos with a similar breakdown: 21 videos of people smoking methamphetamine, 14 videos of people snorting methamphetamine, and 7 videos of the substance only without any route of administration.
Methamphetamine cue task
On each of 200 trials, participants first saw a fixation cross for 2 seconds, and then a randomly selected methamphetamine or food picture was presented for 6 seconds. Next, participants reported the extent of their cue-induced craving using a 1 (not at all) to 5 (very much) rating scale (Figure 1, top). This single-item scale has been used previously to measure craving (e.g., Hogarth, Dickinson, & Duka, 2010; Preston et al., 2009; Waters et al., 2004) including methamphetamine craving (e.g., Dean et al., 2009; Galloway et al., 2008; Hartz et al., 2001; Nakama et al., 2008), and has both construct and predictive validity (Berlin, Singleton, & Heishman, 2013; Hartz et al., 2001; Galloway et al., 2008, 2010). Upon successful completion of the task, participants were debriefed and paid $50 for their time.
Figure 1. Sequence of events on each trial in Studies 1 (top) and 2 (bottom).
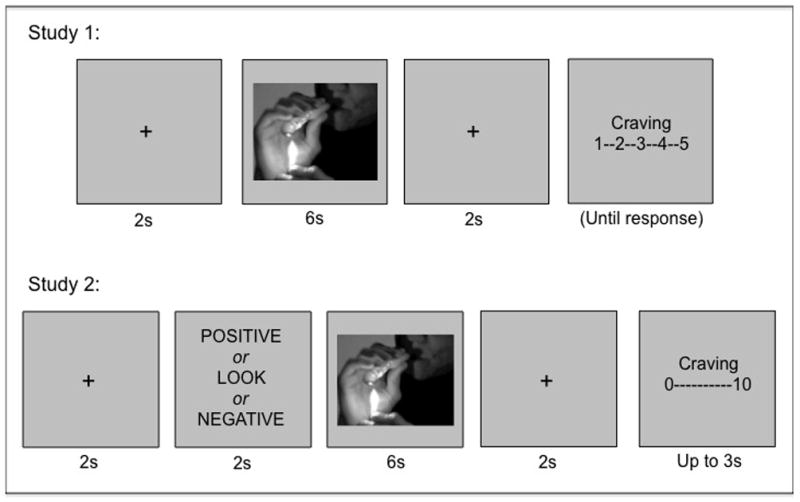
In Study 1, participants first saw a fixation cross for 2 seconds, followed by an image (methamphetamine, methamphetamine smoking, intranasal use, or food) for 6 seconds, followed by another fixation cross. At the end of each trial, they were asked to report their craving for methamphetamine or food on a 1–5 scale (1= not at all and 5 = very much). In Study 2, participants first saw a fixation cross for 2 seconds, then received instructions for 2 seconds: LOOK, POSITIVE, or NEGATIVE, followed by an image (of methamphetamine, methamphetamine smoking, or food) for 6 seconds, followed by a fixation cross. At the end of each trial, they were asked to report their craving for methamphetamine or food on a 0–10 scale (0= not at all and 10 = very much).
Results
Focusing on methamphetamine stimuli to test our hypotheses, we performed a 2 (Group: Intranasal or Smoking) × 3 (Stimulus type: Intranasal, Smoking, or Substance only) × 2 (Stimulus Modality: Picture or Video) mixed ANOVA, with group as a between-subjects factor, stimulus type and modality as within-subjects factors, and reported craving for methamphetamine as the dependent variable. There were no significant main effects. However, we found a significant crossover interaction between Group and Stimulus type (F(2, 34) = 15.74, p < .001), indicating that participants’ preferred route of administration differentially affected their reported craving for different stimulus categories (See Figure 2). Post hoc paired t-tests showed that, in line with our hypotheses, methamphetamine smokers reported more craving for Smoking and for Substance Only stimuli compared to Intranasal stimuli (t(12) = 3.45, p = .005; t(12) = 3.11, p < .01). Conversely, intranasal users reported more craving for Intranasal and Substance Only stimuli than Smoking stimuli (t(6) = 3.06, p < .05; t(6) = 2.72, p < .05). Independent sample t-tests showed that methamphetamine smokers also reported significantly higher craving to smoking stimuli compared to the intranasal users (t(17) = 2.24, p < .05). Paired t-tests also revealed that overall, methamphetamine users reported significantly higher craving for all methamphetamine stimuli compared to food stimuli (t(18) = 3.09, p < .01; Figure 3). Lastly, an independent sample t-test revealed that methamphetamine smokers and intranasal users did not differ in reported craving for food (t(17) = 1.00, p = .33).
Figure 2. Reported craving in Study 1.
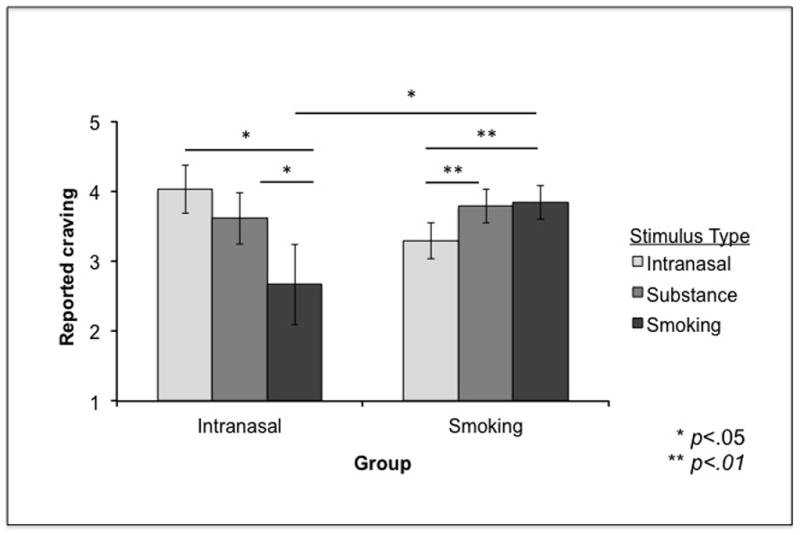
There was a crossover interaction between Stimulus Type and Group, indicating that participants’ preferred route of administration differentially affected their reported craving for different stimulus categories (intranasal, substance-only, or smoking). Error bars represent standard errors.
Figure 3. Reported craving in Study 1.
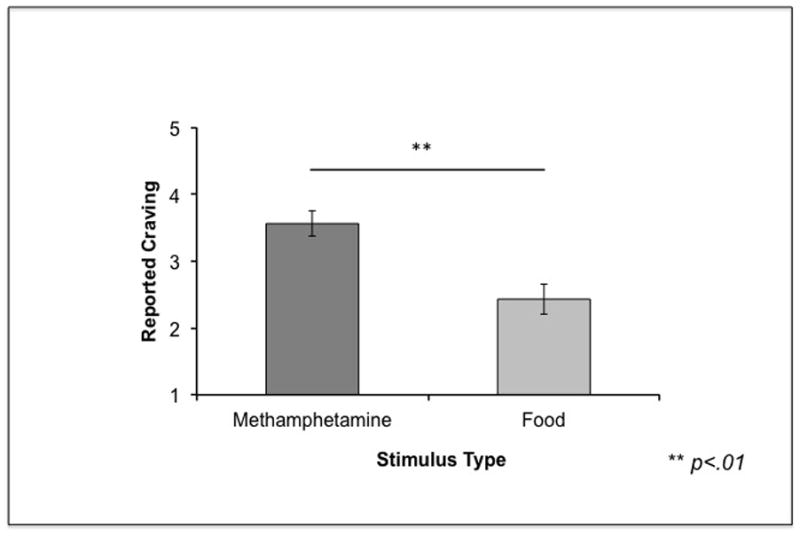
Participants reported greater craving for methamphetamine compared to food stimuli. Error bars represent standard errors.
Study 2 – Regulation of Methamphetamine Craving
Method
The results of Study 1 suggested that methamphetamine users report stronger cue-induced craving in a manner that is consistent with their preferred route of administration, such that smokers report greater craving to smoking stimuli, and intranasal users report greater craving to stimuli depicting intranasal use. In Study 2, we wanted to induce strong cue-induced craving to best test the efficacy of regulation strategies. We used the data from Study 1 to restrict the sample – and our stimuli – to a single route of administration, so that we could use the same stimuli across all participants. We specifically recruited smokers rather than intranasal users because in recruitment across both studies we found markedly higher response rates of methamphetamine smokers (vs. intranasal users), which may reflect different base rates in the greater population (see Rawson, Gonzales, Marinelli-Casey, & Ang, 2007).
Participants
A different group of thirteen methamphetamine-smoking participants was recruited for this second study (Table 1). Participants were recruited and screened for eligibility using the same procedures as Study 1, at the New York State Psychiatric Institute. Participants’ age and level of education were comparable to those observed in Study 1 (See Table 1). All participants reported smoking methamphetamine as their primary route of administration, with average use of 7.86 times a month (0.75 to 12 grams per use, M = 2.18, SD = 2.68). As in Study 1, participants were excluded if they reported any psychiatric or neurological disorders, movement disorders, current or past history of mood altering medication (within the past 6 months), or any medical condition that might impair cognitive function. All participants gave informed consent in accordance with Columbia University and New York State Psychiatric Institute Review Boards, and again were asked to abstain from caffeine, food, cigarettes, and drugs for at least 2 hours prior to scheduled laboratory appointment. The laboratory session in this study lasted 1.5–2 hours, due to the time needed to train subjects on the various strategies and have them practice before they completed the ROC task.
Stimuli
As this study included only methamphetamine-smoking participants, methamphetamine stimuli were selected from the Smoking and Substance Only picture categories in Study 1, based on their effectiveness in eliciting craving (i.e. the top 54 from those picture sets). All selected stimuli were rated at least 3.5 on a 1 to 5 scale. The final stimulus set consisted of 108 pictures: 54 food stimuli and 54 methamphetamine stimuli.
ROC task strategy training
Participants were first trained to perform the task for 25–30 minutes. Initially, they were introduced to three instructions (POSITIVE, LOOK, and NEGATIVE) that would direct them to think about methamphetamine and food picture cues in different ways (i.e., “regulation strategies”). Then, following our prior work (Kober, Mende-Siedlecki, et al., 2010; Kober, Kross, et al., 2010), they were trained to implement these regulation strategies based on the following instructions: The POSITIVE instruction directed participants to focus on any subjective (person-specific) positive effects (e.g. psychological, physiological, sexual) of consuming the depicted item (methamphetamine or food). For example, if the instruction POSITIVE was followed by a methamphetamine cue, participants might choose to focus on how smoking methamphetamine might cause pleasant physical sensations, increase their energy, and make them feel good (Hart, Ward, Haney, Foltin, & Fischman, 2001). The NEGATIVE instruction directed participants to focus on any subjective negative consequences (e.g. psychological, physiological, economic, interpersonal) of consuming the item. For example, if NEGATIVE was followed by a methamphetamine cue, participants might choose to think about how tired or sad they might feel the next day, how much money they spend on methamphetamine use, and any damage to their relationships resulting from use. Importantly, this NEGATIVE instruction is a cognitive strategy drawn from CBT manuals for stimulant addiction (Carroll, 1996) and is used in treatment contexts to reduce craving. For the LOOK cue, which served as a baseline, participants were instructed to respond naturally to the stimulus, without altering their response in any way. Participants were also instructed to honestly report their level of craving at the end of each trial. Following feedback from participants in Study 1, we adapted the scale in Study 2 to be used with a computer mouse, on a continuous scale ranging from 0 (not at all) to 10 (very much). And, although the numerical anchors differed from the 1 – 5 scale used in Study 1, we used the same craving question and anchor labeling, so we believe response data are comparable and mathematically equivalent across scales.
Once participants indicated that they understood and could follow the instructions, they completed 12 practice trials with 12 sample stimuli that were not used in the subsequent ROC task (6 food, 6 methamphetamine). The experimenters asked participants to verbally implement the instructions during the first several practice trials to ensure that they were properly followed. Participants continued to the main task only after they confirmed that they comprehended the task and the associations between instructions and strategies.
ROC task
Each trial began with a 2-second instruction (POSITIVE, NEGATIVE or LOOK), followed by a 6-second presentation of either a methamphetamine or food picture (Figure 1, bottom). The picture was followed by a 2-second delay, after which participants rated the extent of their craving. Instructional cues and stimulus combinations were counterbalanced across participants. After the task, participants were debriefed and paid $75 for their time.
Results
We performed a 3 (Instructions: POSITIVE, NEGATIVE or LOOK) × 2 (Stimulus type: Food or Methamphetamine) repeated measures ANOVA with participants’ reported craving as the dependent variable; both Instruction and Stimulus were within-subject factors. There was a significant main effect of Instruction on craving (F(2,24) = 17.84, p < .01; Figure 4) as well as a significant linear contrast effect of instruction on reported craving (F(1,12) = 18.07, p < .01). However, there was no effect of stimulus type, and no interaction of instruction and stimulus type on reported craving. Post hoc paired t-tests revealed significantly lower craving following the NEGATIVE vs. LOOK instruction (t(12) = −4.28, p < .005). We observed a similar pattern of reduction when comparing the effect of the NEGATIVE vs. POSITIVE instruction on craving (t(12)= −4.12, p < .005; See Figure 4). Craving was marginally different between POSITIVE and LOOK instructions (t(12)=2.51, p = .03); this trend was driven by the difference observed in food stimuli only (t(12)=2.84, p < .02) but not for methamphetamine (p > .05). Finally, as in Study 1, we tested craving level separately in each condition via one-sample t-tests and found that craving levels were significantly greater than the low point of the scale (0-Not at all) in all instruction conditions (including NEGATIVE; all p’s < .05).
Figure 4. Reported craving in Study 2.
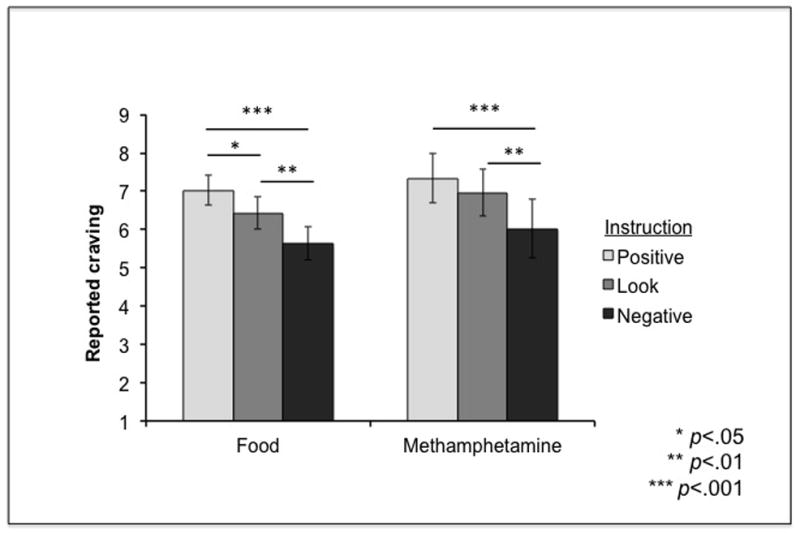
There was a main effect of Instruction such that regulation on NEGATIVE instruction trials (modeled after CBT treatment protocols) was associated with significantly lower craving compared to LOOK and POSTIVE instruction trials, across stimuli. Error bars represent standard errors.
Discussion
In the current studies, we sought to investigate the boundary conditions of methamphetamine craving, and experimentally investigate factors that may influence it. In Study 1, we asked whether methamphetamine craving is cue-specific and depends on one’s preferred route of administration. We found that intranasal users reported greater craving in response to stimuli depicting intranasal use compared to smoking, whereas methamphetamine smokers reported greater craving in response to stimuli depicting smoking. Although craving in all conditions was significantly different from 0, craving was much stronger when the stimulus type “matched” participants’ preferred route of administration (e.g., smoking stimuli for methamphetamine smokers). These results are broadly consistent with prior work suggesting cue-induced craving in methamphetamine users (Lee & Rawson, 2008; Smout et al., 2010) but suggest a moderating effect for methamphetamine user’s preferred route of administration.
To our knowledge, this is the first demonstration of stimulus-specificity in methamphetamine craving that depends on route of administration (Figure 2). Such specificity is consistent with the conceptualization of craving as an experienced-based, conditioned response resulting from the pairing of cues with drug effects in instances of prior drug use (Martin-Soelch et al., 2007; Tiffany & Conklin, 2000; Weiss, 2005) rather than a generalized response to drug cues. For example, for an individual who has past experience with (most commonly uses, and currently prefers) intranasal administration of methamphetamine, intranasal cues were repeatedly paired with the accompanying effects of methamphetamine, and therefore became meaningful drivers of craving and methamphetamine-seeking behavior. The opposite would be true for individuals who smoke methamphetamine, as only smoking-specific methamphetamine cues serve as conditioned stimuli that elicit craving. Although the patterns of findings from Study 1 might be predicted by learning theory, this has not been demonstrated empirically in methamphetamine users. The reporting of such effects is critical for informing subsequent clinical work aimed to correctly (and strongly) modeling cue-induced craving, and ultimately individualizing treatments for addiction (e.g., Robinson, Yager, Cogan, & Saunders, 2014) These findings also highlight the importance of considering users’ preferred route of administration when selecting stimuli and measuring other boundary conditions of craving.
We did exactly that in Study 2. Findings from Study 1 allowed us to select a subset of highly appetitive methamphetamine cues that were route-of-administration-specific, to test the question of regulation of craving. In this second study, we asked whether methamphetamine craving can be modulated with cognitive strategies, and specifically whether CBT-treatment-based strategies would experimentally reduce craving for methamphetamine. We reported that methamphetamine smokers were able to use a CBT-based strategy (indicated by the NEGATIVE instruction) that directed them to focus on the negative consequences of smoking methamphetamine. Although this strategy did not eliminate craving completely, it significantly reduced their craving for methamphetamine. This finding argues against the view of craving for methamphetamine as uncontrollable, and is consistent with our prior work with cigarette smokers (Kober, Mende-Siedlecki, et al., 2010; Kober, Kross, et al., 2010), in which we showed that cigarette smokers can regulate craving for cigarettes using similar cognitive strategies. Further, our findings dovetail with a qualitative analysis of methamphetamine users’ accounts of craving, suggesting that craving may be overcome using personalized strategies (Bruehl, Lende, Schwartz, Sterk, & Elifson, 2006), and with the work of Volkow and colleagues showing similar effects with cocaine users (Volkow et al., 2010).
Our findings have several additional implications for research on methamphetamine users, for the media’s treatment of methamphetamine use, and for our understanding of treatment for methamphetamine addictions and addiction more generally. For example, some research suggests that chronic methamphetamine use is marked by cognitive deficits across domains, including executive functions and memory processes (Scott et al., 2007). But others have been critical of this perspective (Hart, Marvin, Silver, & Smith, 2012), highlighting methodological and interpretive issues that overstate these deficits. These issues include inferring causal links between methamphetamine use and cognitive abilities from correlational data (e.g., Thompson et al., 2004), and poorly-matched control groups (e.g., Chang et al., 2002; Thompson et al., 2004). Our experimental findings, although preliminary, suggest that methamphetamine users are in fact able to exercise control over craving by implementing cognitive strategies – an indication that at least some executive functioning is preserved.
From a clinical perspective, the results provide experimental evidence for the efficacy of an important component of CBT – learning of strategies for the regulation of craving. The craving reduction we observed when participants implemented the NEGATIVE strategy suggests that methamphetamine users can effectively use this CBT-based approach in experimental settings. We also reported a marginal difference in methamphetamine craving between the LOOK and POSITIVE strategies. Although speculative, we interpret this pattern as indicative of participants’ spontaneous amplification of rewarding properties of the presented methamphetamine stimuli in the LOOK condition. Indeed, it has been suggested that people cognitively elaborate on the rewarding and motivational features of a stimulus, which results in increased likelihood of behavior to acquire it (Hofmann & Van Dillen, 2012; Kavanagh, Andrade, & May, 2005). Whether such elaboration occurs consciously or outside of awareness, it seems that the NEGATIVE strategy was nonetheless effective in diminishing craving relative to both LOOK and POSITIVE instruction conditions. The difference between the LOOK and POSITIVE strategies for food stimuli could suggest that methamphetamine users do not engage in amplification or elaboration for food, which might indicate differential reward value for food versus methamphetamine in methamphetamine users.
The current studies were exploratory in nature, and suffer from several limitations. First, the sample sizes are small, due to the difficulty of recruiting individuals with methamphetamine use disorders. Nevertheless, the sample sizes are comparable to prior studies of the same population (e.g., Kirkpatrick, Gunderson, Johanson, et al., 2012; Kirkpatrick, Gunderson, Perez, et al., 2012; Kirkpatrick, Gunderson, Levin, et al., 2012), and the findings were statistically robust despite the low power. Second, the inclusion of only a single female participant may limit the generalizability of results. Notably, we made great efforts to recruit females as well as males, however, most recruited participants happened to be male, which may reflect the fact that the New York City population of methamphetamine users seems to skew heavily male (Dombrowski et al., 2012). Third, in Study 1, there were unequal numbers of stimuli in each category. However, the results did not change when we restricted the number of analyzed stimuli to evenly-numbered, random subsets. Therefore, we are confident that this unequal number of stimuli does not account for our results, and we corrected for this in Study 2 by using equal numbers of stimuli. Finally, additional data on duration of methamphetamine use, and time since last use could have been helpful for data interpretation, but unfortunately were not available.
Future studies should replicate and extend these findings by testing for double dissociation effects (as we observed in Study 1) and regulation effects (as those found in Study 2) in other user groups (e.g., intranasal and intravenous users) as well as with heavier users of methamphetamine, and with longer periods of abstinence. Based on previous work in other drugs (e.g., cocaine; see Volkow et al., 2010), one might expect the data here to generalize to more frequent users. Additionally, investigators should test whether performance on these tasks in a single session is predictive of successful regulation of craving for methamphetamine over time. Critically, the strategies implemented in Study 2 were drawn out of CBT manuals for stimulant addiction (Carroll, 1998), which have shown some efficacy in treating stimulant use disorders. Indeed, cocaine users who demonstrated better retention of such regulation strategies post-treatment are most likely to maintain abstinence over time (Carroll, Nich, Frankforter, & Bisighini, 1999; Kiluk, Nich, Babuscio, & Carroll, 2010).
Despite the limitations, our findings suggest that methamphetamine craving has multiple boundary conditions, one of which is individualized cue specificity (i.e., matching methamphetamine stimulus type with methamphetamine users’ preferred route of administration; Study 1), and the other characterized by reduced craving following implementation of cognitive strategies (i.e., those adapted from CBT; Study 2). These findings are encouraging, especially as they challenge the notion that compulsive methamphetamine use is inevitable due to chronic, unruly cravings for the drug (e.g., the media campaign stating, “Meth: Not Even Once”). We hope that the findings from Study 1 will encourage researchers to use the best-suited and “matched” stimuli in cue-induced craving studies, to induce strong craving which are more ecologically-valid in modeling real-life craving. We also hope that findings from Study 2 will inform future clinical work that will incorporate individualized cognitive strategies into methamphetamine treatment programs, test these strategies’ effectiveness, and examine how they may be enhanced via training, with the goal of reducing craving and relapse in the short and long term. For example, one might imagine a treatment protocol consisting of repeated training sessions of using the NEGATIVE strategy when viewing route-of-administration-specific methamphetamine cues. Similarly, other “cool” cognitive strategies (such as mindful acceptance; Westbrook et al., 2013) should be developed and tested against each other to determine which strategies might be effective in reducing not only methamphetamine craving, but also methamphetamine use over time. Indeed, we believe that such investigations are important even if such strategies hold therapeutic promise for just a subset of methamphetamine users.
Contributor Information
Richard B. Lopez, Department of Psychological & Brain Sciences, Dartmouth College
Chukwudi Onyemekwu, Department of Psychology, Columbia University
Carl L. Hart, Department of Psychology, Columbia University
Kevin N. Ochsner, Department of Psychology, Columbia University
Hedy Kober, Departments of Psychiatry & Psychology, Yale University.
References
- Allen SS, Bade T, Hatsukami D, Center B. Craving, withdrawal, and smoking urges on days immediately prior to smoking relapse. Nicotine & Tobacco Research. 2008;10:35–45. doi: 10.1080/14622200701705076. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Berlin I, Singleton EG, Heishman SJ. Predicting smoking relapse with a multidimensional versus a single-item tobacco craving measure. Drug and Alcohol Dependence. 2013;132(3):513–520. doi: 10.1016/j.drugalcdep.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Bruehl AM, Lende DH, Schwartz M, Sterk CE, Elifson K. Craving and control: Methamphetamine users’ narratives. Journal of Psychoactive Drugs. 2006;38(3):385–392. doi: 10.1080/02791072.2006.10400602. [DOI] [PubMed] [Google Scholar]
- Carroll KM. Relapse prevention as a psychosocial treatment: A review of controlled clinical trials. Experimental and Clinical Psychopharmacology. 1996;4(1):46–54. [Google Scholar]
- Carroll KM. Therapy Manuals for Drug Addiction. Bethesda, MD: National Institute on Drug Abuse; 1998. A Cognitive-Behavioral Approach: Treating Cocaine Addiction. [Google Scholar]
- Carroll KM, Nich C, Frankforter TL, Bisighini RM. Do patients change in the ways we intend? Assessing acquisition of coping skills among cocaine-dependent patients. Psychological Assessment. 1999;11(1):77–85. [Google Scholar]
- Chang L, Ernst T, Speck O, Patel H, DeSilva M, Leonido-Yee M, Miller EN. Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry Research: Neuroimaging. 2002;114:65–79. doi: 10.1016/s0925-4927(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R. The role of serotonin in craving: from basic research to human studies. Alcohol and Alcoholism. 1999;34:244–253. doi: 10.1093/alcalc/34.2.244. [DOI] [PubMed] [Google Scholar]
- Crits-Christoph P, Beth Connolly Gibbons M, Barber JP, Hu B, Hearon B, Worley M, Gallop R. Predictors of sustained abstinence during psychosocial treatments for cocaine dependence. Psychotherapy Research. 2007;17(2):240–252. [Google Scholar]
- Culbertson C, Nicolas S, Zaharovits I, London ED, La Garza RD, Brody AL, Newton TF. Methamphetamine craving induced in an online virtual reality environment. Pharmacology Biochemistry and Behavior. 2010;96:454–460. doi: 10.1016/j.pbb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AC, London ED, Sugar CA, Kitchen CMR, Swanson AN, Heinzerling KG, Shoptaw S. Predicting adherence to treatment for methamphetamine dependence from neuropsychological and drug use variables. Drug and Alcohol Dependence. 2009;105(1–2):48–55. doi: 10.1016/j.drugalcdep.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski K, Khan B, Wendel T, McLean K, Misshula E, Curtis R. Estimating the Size of the Methamphetamine-Using Population in New York City Using Network Sampling Techniques. Advances in Applied Sociology. 2012;2(4):245–252. doi: 10.4236/aasoci.2012.24032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Ekhtiari H, Alam-Mehrjerdi Z, Nouri M, George S, Mokri A. Designing and evaluation of reliability and validity of visual cue-induced craving assessment task for methamphetamine smokers. Basic and Clinical Neuroscience. 2010;1:34–37. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Galloway GP, Singleton EG, Buscemi R, Baggott MJ, Dickerhoof RM, Mendelson JE. An examination of drug craving over time in abstinent methamphetamine users. The American Journal on Addictions. 2010;19(6):510–514. doi: 10.1111/j.1521-0391.2010.00082.x. [DOI] [PubMed] [Google Scholar]
- Galloway GP, Singleton EG The Methamphetamine Treatment Project Corporate Authors. How long does craving predict use of methamphetamine? Assessment of use one to seven weeks after the assessment of craving. Substance Abuse: Research and Treatment. 2008;1:63–79. [PMC free article] [PubMed] [Google Scholar]
- Hart CL. High Price: A Neuroscientist’s Journey of Self-Discovery That Challenges Everything You Know About Drugs and Society. New York: Harper; 2013. [Google Scholar]
- Hart CL, Gunderson EW, Perez A, Kirkpatrick MG, Thurmond A, Comer SD, Foltin RW. Acute Physiological and Behavioral Effects of Intranasal Methamphetamine in Humans. Neuropsychopharmacology. 2008;33(8):1847–1855. doi: 10.1038/sj.npp.1301578. http://doi.org/10.1038/sj.npp.1301578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Marvin CB, Silver R, Smith EE. Is cognitive functioning impaired in methamphetamine users? A critical review. Neuropsychopharmacology. 2012;37:586–608. doi: 10.1038/npp.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Foltin RW, Fischman MW. Methamphetamine self-administration by humans. Psychopharmacology. 2001;157:75–81. doi: 10.1007/s002130100738. [DOI] [PubMed] [Google Scholar]
- Hartz DT, Frederick-Osborne SL, Galloway GP. Craving predicts use during treatment for methamphetamine dependence: a prospective, repeated-measures, within-subject analysis. Drug and Alcohol Dependence. 2001;63(3):269–276. doi: 10.1016/s0376-8716(00)00217-9. [DOI] [PubMed] [Google Scholar]
- Heinz A. Correlation of Alcohol Craving With Striatal Dopamine Synthesis Capacity and D2/3 Receptor Availability: A Combined [18F]DOPA and [18F]DMFP PET Study in Detoxified Alcoholic Patients. American Journal of Psychiatry. 2005;162(8):1515–1520. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Van Dillen L. Desire: the new hot spot in self-control research. Current Directions in Psychological Science. 2012;21:317–322. [Google Scholar]
- Hogarth L, Dickinson A, Duka T. The associative basis of cue-elicited drug taking in humans. Psychopharmacology. 2010;208(3):337–351. doi: 10.1007/s00213-009-1735-9. [DOI] [PubMed] [Google Scholar]
- Kavanagh DJ, Andrade J, May J. Imaginary relish and exquisite torture: the elaborated intrusion theory of desire. Psychological Review. 2005;112:446. doi: 10.1037/0033-295X.112.2.446. [DOI] [PubMed] [Google Scholar]
- Kavanagh DJ, Connor JP. Craving: A research update. Addictive Behaviors. 2012;38(2):1499–1500. doi: 10.1016/j.addbeh.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Kiluk BD, Nich C, Babuscio T, Carroll KM. Quality versus quantity: acquisition of coping skills following computerized cognitive–behavioral therapy for substance use disorders. Addiction. 2010;105(12):2120–2127. doi: 10.1111/j.1360-0443.2010.03076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Johanson C, Levin FR, Foltin RW, Hart CL. Comparison of intranasal methamphetamine and d-amphetamine self-administration by humans. Addiction. 2012;107:783–791. doi: 10.1111/j.1360-0443.2011.03706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Levin FR, Foltin RW, Hart CL. Acute and residual interactive effects of repeated administrations of oral methamphetamine and alcohol in humans. Psychopharmacology. 2012;219(1):191–204. doi: 10.1007/s00213-011-2390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Perez AY, Haney M, Foltin RW, Hart CL. A direct comparison of the behavioral and physiological effects of methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2012;219(1):109–122. doi: 10.1007/s00213-011-2383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H. Emotion regulation in substance use disorders. In: Gross J, editor. Handbook of Emotion Regulation. 2. New York, NY: Guilford; 2014. [Google Scholar]
- Kober H, Kross EF, Mischel W, Hart CL, Ochsner KN. Regulation of craving by cognitive strategies in cigarette smokers. Drug and Alcohol Dependence. 2010;106:52–55. doi: 10.1016/j.drugalcdep.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Turza AC, Hart CL. Risk factors for substance use, abuse, and dependence: Learning. In: Kranzler HR, Korsmeyer P, editors. Encyclopedia of Drugs, Alcohol, and Addictive Behavior. 3. Macmillan Reference USA; 2009. [Google Scholar]
- Lee NK, Rawson RA. A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug and Alcohol Review. 2008;27:309–317. doi: 10.1080/09595230801919494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Soelch C, Linthicum J, Ernst M. Appetitive conditioning: Neural bases and implications for psychopathology. Neuroscience & Biobehavioral Reviews. 2007;31(3):426–440. doi: 10.1016/j.neubiorev.2006.11.002. http://doi.org/10.1016/j.neubiorev.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychological Review. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- Nakama H, Chang L, Cloak C, Jiang C, Alicata D, Haning W. Association between Psychiatric Symptoms and Craving in Methamphetamine Users. The American Journal on Addictions. 2008;17(5):441–446. doi: 10.1080/10550490802268462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, Fong T, Wallace CL, Li SH, Kahn R. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31:1537–1544. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: Can they explain compulsion? Journal of Psychopharmacology. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Preston KL, Vahabzadeh M, Schmittner J, Lin JL, Gorelick DA, Epstein DH. Cocaine craving and use during daily life. Psychopharmacology. 2009;207(2):291–301. doi: 10.1007/s00213-009-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RA, Gonzales R, Marinelli-Casey P, Ang A. Methamphetamine Dependence: A Closer Look at Treatment Response and Clinical Characteristics Associated with Route of Administration in Outpatient Treatment. The American Journal on Addictions. 2007;16(4):291–299. doi: 10.1080/10550490701389864. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: Individual differences. Neuropharmacology. 2014;76(Part B):450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychology Review. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Dunbar M, Kirchner T, Li X, Tindle H, Anderson S, Scholl S. Smoker reactivity to cues: Effects on craving and on smoking behavior. Journal of Abnormal Psychology. 2013;122(1):264–280. doi: 10.1037/a0028339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Martin A, Barsalou LW. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cerebral Cortex. 2005;15:1602–1608. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CSR. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug and Alcohol Review. 2007;26(1):25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Smout MF, Longo M, Harrison S, Minniti R, Wickes W, White JM. Psychosocial treatment for methamphetamine use disorders: a preliminary randomized controlled trial of cognitive behavior therapy and acceptance and commitment therapy. Substance Abuse. 2010;31:98–107. doi: 10.1080/08897071003641578. [DOI] [PubMed] [Google Scholar]
- Sobik L, Hutchison K, Craighead L. Cue-elicited craving for food: a fresh approach to the study of binge eating. Appetite. 2005;44:253–261. doi: 10.1016/j.appet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Treatment Episode Data Set (TEDS): 2001–2011. State Admissions to Substance Abuse Treatment Services. Rockville, MD: 2013. [Google Scholar]
- The Partnership at Drugfree.org. Meth Project. New York, NY: 2013. [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. Journal of Neuroscience. 2004;24:6028–36. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95(8s2):145–153. doi: 10.1080/09652140050111717. http://doi.org/10.1046/j.1360-0443.95.8s2.3.x. [DOI] [PubMed] [Google Scholar]
- United Nations. Amphetamines and Ecstasy - 2011 Global ATS Assessment. Vienna, Austria: United Nations Office on Drugs and Crime; 2011. [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. NeuroImage. 2010;49(3):2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Wong C. Cocaine Cues and Dopamine in Dorsal Striatum: Mechanism of Craving in Cocaine Addiction. The Journal of Neuroscience. 2006;26(24):6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. Journal of Consulting and Clinical Psychology. 2004;72(6):1136–1143. doi: 10.1037/0022-006X.72.6.1136. http://doi.org/10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Current Opinion in Pharmacology. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Westbrook C, Creswell JD, Tabibnia G, Julson E, Kober H, Tindle HA. Mindful attention reduces neural and self-reported cue-induced craving in smokers. Social Cognitive and Affective Neuroscience. 2013;8(1):73–84. doi: 10.1093/scan/nsr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. The “craving” for alcohol. Quarterly Journal of Studies on Alcohol. 1955;16:33–66. [Google Scholar]


