Abstract
Background
Membrane-associated guanylate kinases (MAGUKs) assemble ion channels, cell-adhesion molecules and components of second messenger cascades into synapses, and are therefore potentially important for co-ordinating synaptic strength and structure. Here, we have examined the targeting of the Drosophila MAGUK Discs-large (DLG) to larval neuromuscular junctions.
Results
During development, DLG was first found associated with the muscle subcortical compartment and plasma membrane, and later was recruited to the postsynaptic membrane. Using a transgenic approach, we studied how mutations in various domains of the DLG protein affect DLG targeting. Deletion of the HOOK region — the region between the Src homology 3 (SH3) domain and the guanylate-kinase-like (GUK) domain — prevented association of DLG with the subcortical network and rendered the protein largely diffuse. Loss of the first two PDZ domains led to the formation of large clusters throughout the plasma membrane, with scant targeting to the neuromuscular junction. Proper trafficking of DLG missing the GUK domain depended on the presence of endogenous DLG.
Conclusions
Postsynaptic targeting of DLG requires a HOOK-dependent association with extrasynaptic compartments, and interactions mediated by the first two PDZ domains. The GUK domain routes DLG between compartments, possibly by interacting with recently identified cytoskeletal-binding partners.
Background
Membrane-associated guanylate kinases (MAGUKs) cluster ion channels, cell-adhesion molecules (CAMs), cytoskeletal proteins and components of second messenger cascades, and are therefore central elements in synaptic architecture [1,2]. Their ability to bind several proteins is reflected by their modular structure. The Drosophila MAGUK Discs-large (DLG) and its mammalian homologs PSD-95, SAP97, PSD-93 and SAP102 share a modular organization composed of three protein-interaction motifs known as PDZ domains, followed by a Src homology 3 (SH3) domain and a guanylate-kinase-like domain (GUK) [2].
The PDZ domains bind carboxy-terminal S/TXV motifs (tS/TXV) [2]. This type of interaction underlies the binding of Shaker-type potassium channels, N-methyl-D-aspartate (NMDA) receptor subunits or the Drosophila CAM Fasciclin II (FasII) to the first and second PDZ domains (PDZ1 and 2) [3–7]. It also mediates binding of the neurexin ligand neuroligin, the Rho effector Citron, the Ras-GTPase-activating protein SynGAP, and the cytoskeleton-associated protein CRIPT to the third PDZ (PDZ3) domain [8–13]. The SH3 domain of PSD-95 has been implicated in binding kainate receptors [14]. The GUK domain, which is enzymatically inactive [15], can bind members of the GKAP/SAPAP family of synaptic proteins [16,17]. This interaction may link MAGUKs to other synaptic scaffolding proteins, such as ProSAP (also known as Shank) and Homer [18–21]. In addition, an interaction of the GUK domain with microtubule-associated protein-1a (MAP-1a) suggests a role for this domain in trafficking of MAGUKs [22]. Different modes of MAGUK oligomerization have been proposed, including head-to-head dimerization and a calmodulin-supported interaction between the GUK domain and a region carboxy-terminal to the SH3 domain [23,24].
The pivotal role of MAGUKs in synaptic assembly raises the question of whether they are regulated during synaptic plasticity. Such regulation might take place at different levels, including targeting and localization. Indeed, we have found that synaptic localization of DLG at larval neuromuscular junctions (NMJs) is dramatically decreased by phosphorylation through Ca++/calmodulin-dependent protein kinase II (CaMKII) [25]. This regulation of DLG localization may contribute to activity-dependent structural plasticity. Though CaMKII-dependent DLG phosphorylation is important for the local and temporary regulation of DLG at the synapse, the mechanisms by which DLG is first targeted to synapses are still unclear. Previous studies of the targeting of DLG or SAP97 in epithelial cells, or targeting of PSD-95 in cultured neurons, yielded surprisingly disparate results [26–29], suggesting that cell-type- and/or isoform-specific features are important for targeting MAGUKs to cellular junctions. In line with this idea, some of the regions that were found critical for targeting — notably the amino termini and the region between the SH3 and GUK domains (the HOOK region) [26] — are barely conserved among MAGUKs. Most of these studies, however, were done in the presence of endogenous MAGUK, which might affect the targeting behavior of transgenically expressed isoforms by direct or indirect interactions.
To determine which DLG domains are required for post-synaptic targeting, we expressed various DLG deletion constructs in muscle and determined their subcellular localization both in the presence and absence of endogenous DLG. We found that DLG was first directed to a subcortical network and the muscle membrane, from where it was targeted to the postsynaptic membrane. In this two-step process, the HOOK region was required for the first step, whereas the PDZ1 and 2 domains were involved in the second. Strikingly, synaptic localization of a DLG mutant missing the GUK domain depended on endogenous DLG, providing evidence that the GUK domain is involved in DLG transport and co-targeting of DLG molecules in vivo.
Results
DLG localization at synaptic and extrasynaptic regions during development
In third instar larval muscles, DLG is concentrated primarily at type I NMJs [30]. Closer examination revealed evenly distributed, weak immunoreactive spots at the muscle surface (Figure 1a). In addition, a subcortical immunoreactive network, localized to the same range of optical sections as the muscle nuclei, was observed (see below). Both types of extrasynaptic staining were specific to DLG as they were not observed in mutant flies that express extremely low levels of a truncated form of DLG (dlgX1-2 mutants) [5] (data not shown).
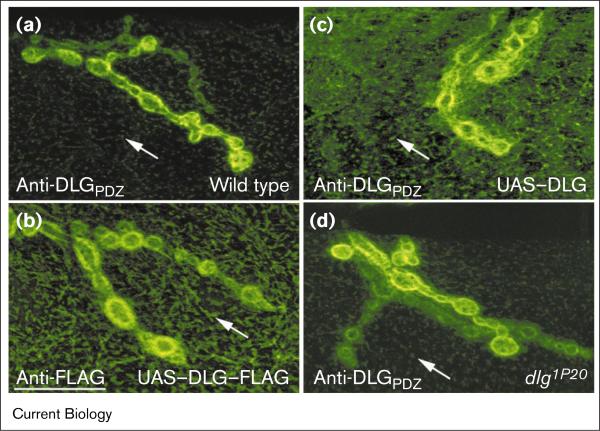
Figure 1.
DLG expression at body wall muscles of wild-type and UAS–DLG-expressing flies, and dlg1P20 mutants. The third instar body wall muscles shown were stained with antibodies directed against (a,c,d) DLG (anti-DLGPDZ antibody) or (b) the FLAG epitope tag (anti-FLAG antibody). (a) In wild-type flies, endogenous DLG immunoreactivity was concentrated at the NMJ, with weak expression at extrasynaptic regions (arrow). In flies expressing (c) UAS–DLG and (b) UAS–DLG–FLAG, DLG was concentrated at the NMJ, but was also prominent at extrasynaptic regions. (d) In dlg1P20 mutants, in which the last 40 carboxy-terminal amino acids of DLG have been deleted, expression of endogenous and extrasynaptic DLG was similar to that in wild-type flies. All the confocal images in this figure were acquired using the same confocal parameters. The scale bar represents 17 μm.
To determine whether DLG localization at extrasynaptic regions might represent intermediate trafficking steps, we analyzed DLG expression in muscles during development. Previously, we first observed DLG at presynaptic termini in stage 17 embryos, whereas DLG localization at the postsynaptic membrane was not detected before the first larval instar stage [31]. Here, we used a highly sensitive anti-DLG antibody (anti-DLGPDZ antibody [25]) to determine the time course of DLG expression at extra-synaptic regions. In stage 16 embryos, DLG immunoreactivity was apparent in the ventral nerve cord (Figure 2a), but virtually absent in both presynaptic termini and body wall muscles (Figure 2b,c). At stage 17, after the initial formation of synaptic boutons, DLG was detected at sites of contact between nerves and muscles (Figure 2d,e). Double labeling with the neuron-specific anti-HRP antibody confirmed that this immunoreactivity was presynaptic and that DLG was still absent from the postsynaptic junctional region (Figure 2f). At the same stage, however, extrasynaptic DLG immunoreactivity became detectable, both as spots distributed throughout the muscle surface (Figure 2d) and as a subcortical network (Figure 2e). Thus, expression of DLG at distinct extrasynaptic sites clearly precedes its concentration at the postsynaptic membrane.
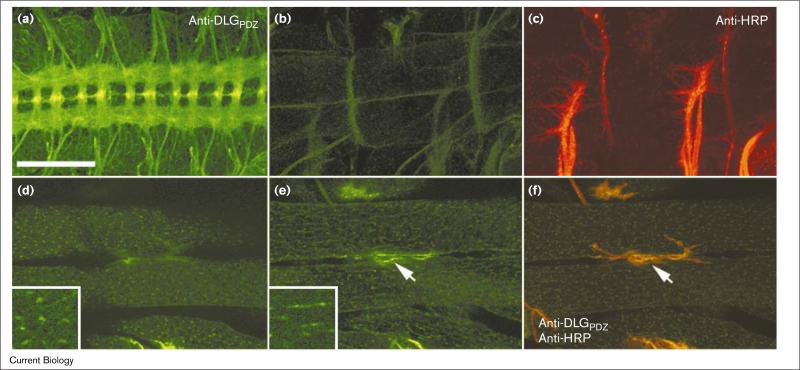
Figure 2.
Developmental expression of synaptic and extrasynaptic DLG at body wall muscles of embryos. (a–c) Stage 16 (a) embryonic nervous system and (b,c) body wall muscles labeled with (a,b) anti-DLGPDZ or (c) anti-horseradish peroxidase (HRP) antibodies. (b,c) Same views of a preparation that was double-labeled. At this stage, DLG was concentrated in the central nervous system, and absent from muscles and synaptic terminals. (d–f) Stage 17 embryonic body wall muscles stained with anti-DLGPDZ antibody. (d,e) Single confocal slices taken (d) at the surface of the muscles and (e) at the level of the muscle nuclei. (f) Same view as (e), but double-stained with anti-HRP antibody to visualize the presynaptic aspect of the NMJ (arrow). Insets in (d,e) are high-magnification views of the extrasynaptic signal. At this stage of development, DLG appeared (f) at the presynaptic region, as well as (d) in a punctate pattern at the muscle surface and (e) in an intracellular network. The scale bar represents 28 μm, except in the insets where it represents 7 μm.
A continuous shift of DLG immunoreactivity from extrasynaptic to synaptic sites was observed during larval development. By the first instar stage, when DLG began to accumulate at the postsynaptic junctional membrane, extrasynaptic DLG immunoreactivity at both the muscle membrane and the subcortical network was strong (Figure 3a,b). By the second instar stage, synaptic DLG localization was very strong, whereas extrasynaptic DLG localization was significantly reduced (Figure 3c,d). In third instar larvae, DLG was almost exclusively localized at synapses and only little DLG could still be detected at the surface (Figure 3e) and subcortical region (Figure 3f).
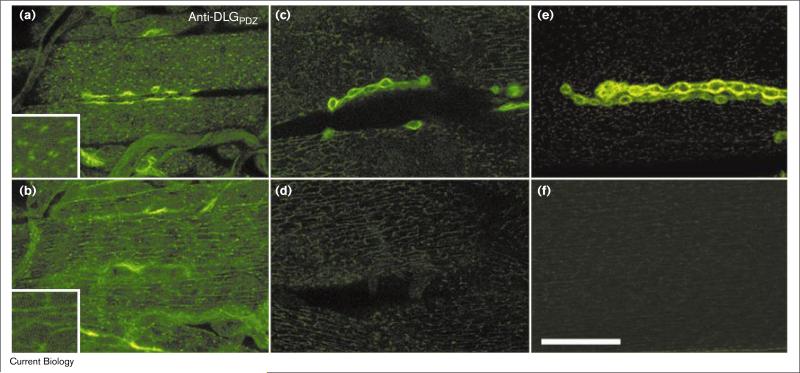
Figure 3.
Developmental expression of synaptic and extrasynaptic DLG localization at body wall muscles of larvae. Anti-DLGPDZ antibody immunoreactivity at body wall muscles of (a,b) first, (c,d) second, and (e,f) third instar larvae. (a,c,e) Single confocal slices at the surface of the muscles. (b,d,f) Single confocal slices at the level of the muscle nuclei. Insets in (a,b) are high-magnification views of extrasynaptic DLG. In first instar larvae, extrasynaptic DLG was still very prominent both at the muscle surface and intracellular network. Extrasynaptic immunoreactivity was very weak in the third instar stage. All confocal images were acquired using the same confocal parameters. The scale bar represents 28 μm, except in the insets where it represents 7 μm.
Targeted expression of epitope-tagged DLG variants in body wall muscles
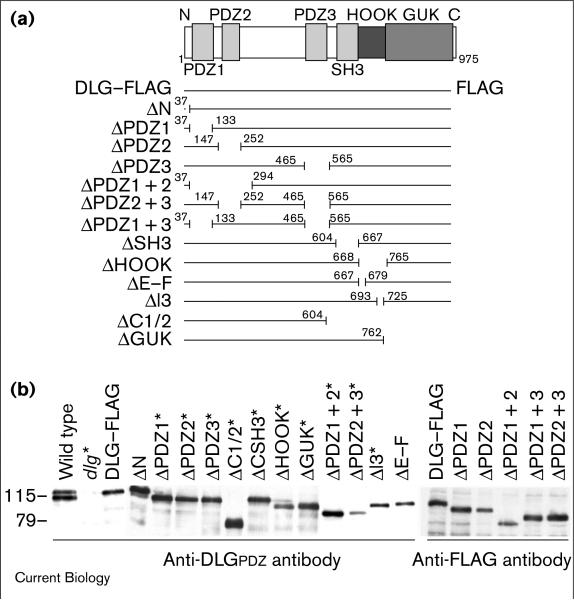
The developmental analysis suggested a stepwise post-synaptic targeting of DLG. To determine which domains of DLG are required for distinct targeting steps, we studied the subcellular distribution of FLAG-epitope-tagged DLG deletion variants upon targeted expression in body wall muscles. For this, we took advantage of the GAL4-UAS expression system [32], using the GAL4 strain C57 as a muscle-specific activator. Immunoblot analyses confirmed that the dlg–FLAG deletion constructs depicted in Figure 4a were expressed at comparable levels and gave rise to proteins of the appropriate molecular weight (Figure 4b). To evaluate the possible influence of endogenous DLG on the localization of transgenic DLG variants, we also targeted expression of each construct in dlgX1-2 mutants [5,33].
Figure 4.
Expression of FLAG-tagged DLG constructs. (a) Diagrammatic representation of the control (DLG–FLAG) and deletion constructs. The top diagram is a representation of the modular structure of DLG. The numbers in each line represent the amino acids immediately adjacent to the deletions. N, amino terminus; C, carboxyl terminus. (b) Western blot analyses of body wall muscle extracts from larvae expressing DLG constructs. Left, anti-DLGPDZ antibodies were used to detect the transgenic proteins in a dlgX1-2 mutant background. Right, anti-FLAG antibody was used to detect those variants that lacked partially or completely the epitopes for anti-DLGPDZ antibody, that is, the PDZ1 and 2 domains. Asterisks represent extracts of dlgX1-2 body wall muscles expressing the deletion constructs. All other lanes represent wild-type body wall muscle extracts expressing the deletion constructs. Numbers on the left represent molecular weight in kDa.
In all constructs, the last carboxy-terminal 40 amino acids of DLG were replaced by the FLAG epitope (Figure 4a; [26]). The importance of the carboxyl terminus for the synaptic localization of another MAGUK, PSD-95, is controversial [28,29]. Several observations demonstrated, however, that carboxy-terminal FLAG tagging has no detectable effect on the subcellular localization of DLG (Figure 1). The hypomorphic allele dlg1P20 gives rise to a truncated protein lacking the same carboxy-terminal amino aids [33]. The distribution of this gene product appeared indistinguishable from the wild type (Figure 1a,d). Upon targeted expression in muscles, DLG–FLAG became enriched postsynaptically around type I boutons (Figure 1b). Prominent FLAG immunoreactivity was also detected extrasynaptically at both the surface and the subcortical network (Figure 1b; a three-dimensional projection of confocal slices can be found at http://www.bio.umass.edu/biology/budnik [DLGFLAG.tif]). The extrasynaptic immunoreactivity in DLG–FLAG-expressing flies was clearly stronger than in the wild type (compare Figure 1a and b). However, a very similar increase in extrasynaptic immunoreactivity was observed upon expression of transgenic, non-tagged DLG with an intact carboxyl terminus (Figure 1c, [34]). This suggests that overexpression per se rather than the carboxy-terminal truncation is responsible for increased extrasynaptic localization of DLG–FLAG.
The amino terminus of DLG is not required for synaptic localization
The sequence preceding PDZ1 exhibits only weak or no homology between various MAGUKs. Several studies have implicated the amino terminus of both PSD-95 and SAP97 in junctional targeting [27,28,35,36]. To determine whether the amino terminus of DLG is of similar importance, we expressed transgenic DLG missing the amino terminus (ΔN). This construct exhibited a synaptic localization (Figure 5a) indistinguishable from the control (DLG–FLAG, Figure 1a). This result was found both when ΔN was expressed in the presence (Figure 5a) or absence (in dlgX1-2 mutants, data not shown) of endogenous DLG.
Figure 5.
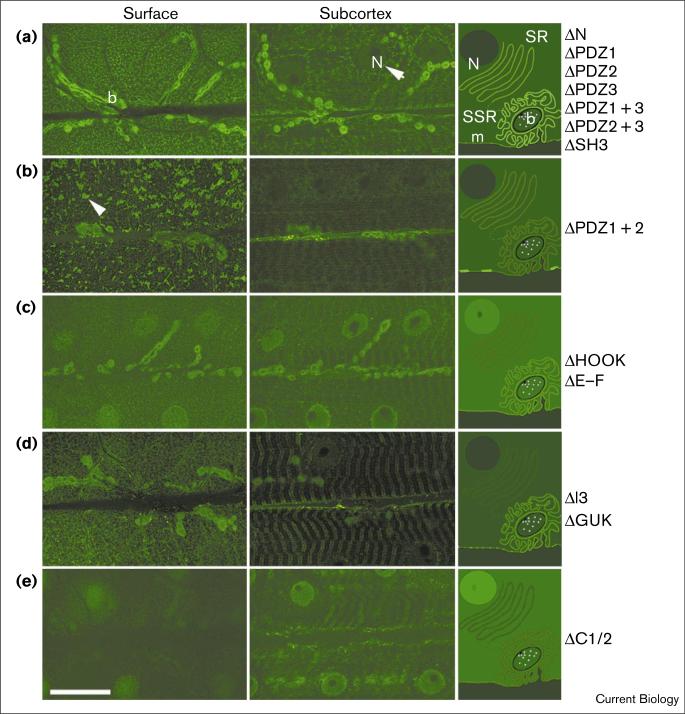
Localization of DLG–FLAG deletion constructs in body wall muscles when expressed in a wild-type background. Left and middle columns are single confocal slices at the surface (left) and at the level of muscle nuclei (middle) of third instar body wall muscles stained with anti-FLAG antibodies. The right column is a diagrammatic representation of the distribution of FLAG immunoreactivity at different muscle sites, depicted at right angles to the plane of section of the micrographs. N, nucleus; SR, sarcoplasmic reticulum; SSR, subsynaptic reticulum; b, bouton; m, membrane. Listed on the right of the column are the names of constructs with similar patterns of FLAG distribution. (a) FLAG immunoreactivity in the DLG–FLAG control, which was similar to the immunoreactivity pattern observed for ΔN, ΔPDZ1, ΔPDZ2, ΔPDZ3, ΔPDZ1 + 3, ΔPDZ2 + 3, and ΔSH3. The arrowhead points to a segment of the immunoreactive subcortical network. (b) Localization of ΔPDZ1 + 2 to membrane-associated ectopic clusters (arrowhead). Note the severe reduction of immunoreactivity in boutons and subcortical area. (c) Nuclear, synaptic, cytoplasmic and membrane localization of ΔHOOK, which was similar to the localization of ΔE–F. (d) Weak synaptic and membrane localization of ΔGUK, which was similar to ΔI3 localization. (e) Nuclear and cytoplasmic localization of ΔC1/2. All confocal images were acquired using the same parameters. The scale bar represents 40 μm.
PDZ1 or 2 are required for synaptic but not plasma membrane targeting
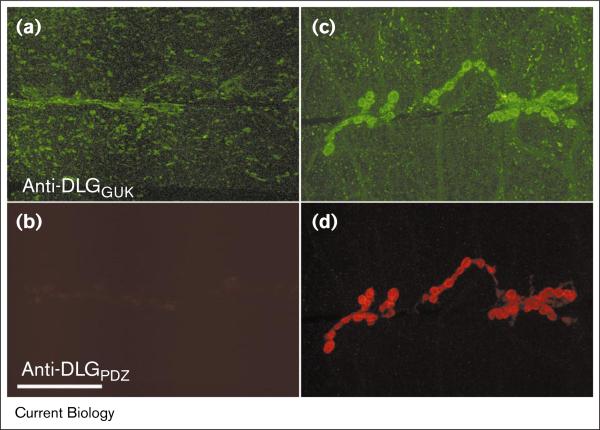
In both the wild type and dlg mutant background, deletion of any single PDZ domain did not affect the synaptic localization of DLG (Figure 5a). Deletion of PDZ3 in combination with either PDZ1 or PDZ2 also did not affect localization. In contrast, deletion of both PDZ1 and PDZ2 (ΔPDZ1 + 2) had dramatic effects on localization. This variant became localized at the surface of the muscle, with little synaptic localization (Figure 5b; see also http://www.bio.umass.edu/biology/budnik [deltaPDZ1-2.tif]). The immunoreactivity at the plasma membrane appeared as large spots or clusters distributed throughout the muscle membrane. The ΔPDZ1 + 2 variant could also be detected at the subcortical network, although the intensity of FLAG immunoreactivity was significantly weaker than in DLG–FLAG-expressing muscles (Figure 5b; middle panel). Thus, in the absence of PDZ1 and 2, DLG becomes transported to the muscle membrane, but fails to be directed to synaptic sites, and instead accumulates in ectopic clusters. This conclusion was reached by expressing ΔPDZ1 + 2 in both the wild type and in dlgX1-2 mutants. To determine whether the distribution of endogenous DLG was altered by the presence of these ectopic clusters, we performed double labeling experiments in which ΔPDZ1 + 2 and endogenous DLG proteins were discriminated using anti-DLGGUK (Figure 6a,c) or anti-DLGPDZ (Figure 6b,d) antibodies. The anti-DLGGUK antibody recognizes both endogenous and transgenic DLG, but the anti-DLGPDZ antibody does not recognize ΔPDZ1 + 2 (Figure 6b). Notably, endogenous DLG did not become trapped in clusters containing the ΔPDZ1 + 2 variant, but remained synaptically localized (Figure 6d).
Figure 6.
Distribution of endogenous DLG in flies expressing the ΔPDZ1 + 2 variant. (a) Anti-DLGGUK antibody immunoreactivity in dlgX1-2 body wall muscles expressing ΔPDZ1 + 2. (b) Anti-DLGPDZ antibody immunoreactivity in the same preparation as in (a). Note that the anti-DLGPDZ antibody used (which was directed against the PDZ1 and 2 domains) does not recognize the ΔPDZ1 + 2 protein. (c) Anti-DLGGUK antibody immunoreactivity in wild-type flies expressing ΔPDZ1 + 2. Both endogenous and transgenic protein were labeled by this antibody. (d) Anti-DLGPDZ antibody immunoreactivity in the same preparation as in (c), showing that the presence of extrasynaptic clusters to which ΔPDZ1 + 2 was localized did not affect the distribution of endogenous DLG. The scale bar represents 42 μm.
Interestingly a transgenic protein comprising the amino terminus and all three PDZ domains (ΔC1/2, Figure 4a) failed to localize to the plasma membrane or synapses, and was instead found highly enriched in nuclei and cytoplasm (Figure 5e and http://www.bio.umass.edu/biology/budnik [deltaC12.tif]). This result indicates that the PDZ1 and 2 domains are necessary but not sufficient for synaptic targeting.
So far, the Shaker potassium channel and FasII are the only synaptic proteins that have been reported to bind PDZ1 and 2 domains of DLG. Synaptic DLG localization remains largely unaffected by mutations that abolish the PDZ-binding motif of Shaker or which dramatically reduce the level of FasII [5–7]. We therefore generated fasIIe76 Sh102 double mutants to test whether simultaneous loss of both binding partners affects DLG localization at NMJs or even mimics the effect of deleting the PDZ1 and 2 domains. DLG-specific immunofluorescence appeared largely normal in these double mutants (data not shown).
The SH3 domain is not involved in synaptic targeting
The involvement of the SH3 domain in synaptic targeting of MAGUKs has been controversial [28,29]. To determine the role of the SH3 domain in DLG targeting, we tested both the allele dlgm30, in which the SH3 domain is affected by a point mutation [33], and flies expressing a DLG variant in which the SH3 domain was deleted (ΔSH3). In both dlgm30 and ΔSH3 flies, DLG was normally targeted to synaptic sites (Figure 5a). In the case of the ΔSH3 line, similar results were obtained in the presence and absence of endogenous DLG.
The HOOK region is required for plasma membrane targeting but not association to the membrane
The HOOK region (Figure 4) has been implicated in the association of DLG with septate junctions in epithelial cells [26]. This domain is among the least conserved regions of MAGUKs, but two sub-regions are moderately conserved among specific subsets of MAGUKs. A band 4.1-binding motif known as I3 is also found in SAP97, PSD-93 and p55 and may link these MAGUKs to the actin/spectrin cytoskeleton [37,38]. Another short stretch (E–F region), presumed to form an α-helix at the amino terminus of the HOOK region, is found in all DLG-like MAGUKs, and has been implicated in calmodulin-dependent dimerization of PSD-95 and SAP102 [24].
Deletion of the entire HOOK region (ΔHOOK) dramatically affected the synaptic localization of DLG both in the presence and absence of endogenous DLG (Figure 5c; see also http://www.bio.umass.edu/biology/budnik [delta-HOOK.tif]). In general, some synaptic localization was observed, although weaker than in DLG–FLAG controls. In addition, the FLAG signal appeared in the muscle nuclei and throughout the cytoplasm. Extrasynaptic localization at the plasma membrane was weak compared with DLG–FLAG controls, and virtually no specific immunoreactivity was detected at the subcortical network (Figure 5c). The relative intensity of the signals at synapses versus nuclei varied, even at the muscles within the same sample. Strikingly, very similar results were obtained when the amino-terminal helix within the HOOK region was disrupted by deleting only 13 amino acids (ΔE–F, Figure 4c). Deletion of the I3 region (ΔI3) also resulted in weak synaptic localization of the transgenic protein when expressed in wild type (Figure 5d). Unlike ΔHOOK and ΔE-F, however, deletion of I3 did not result in nuclear localization. Surprisingly, ΔI3 exhibited strong synaptic localization when expressed in dlgX1-2 mutants (Figure 7c).
Figure 7.
FLAG localization in dlgX1-2 mutants expressing (a) DLG–FLAG, (b) ΔGUK, and (c) ΔI3. Unlike the situation in the wild type, the ΔGUK protein failed to localize at synapses in the mutant background, whereas the ΔI3 protein showed an increased localization to synapses. The scale bar represents 35 μm.
The GUK domain is required for synaptic targeting
The role of the GUK domain in MAGUKs has remained obscure. In the case of fly epithelial cells, deletion of the GUK domain does not alter localization of DLG to septate junctions [26]. Various cytoskeletal and synapse-associated proteins have been reported to bind to the GUK domain of mammalian MAGUKs, suggesting that this domain may be important for synaptic localization [16,17,22,39]. To ascertain the role of GUK in synaptic targeting, we examined transgenic flies expressing a DLG variant lacking the GUK domain (ΔGUK). In the presence of endogenous DLG, ΔGUK became localized to synaptic sites, although this synaptic expression appeared consistently weaker than in DLG–FLAG-expressing controls (Figure 5d and http://www.bio.umass.edu/biology/budnik [deltaGUK.tif]). Notably, however, synaptic localization of ΔGUK was barely or not detectable upon expression in dlgX1-2 mutants (Figure 7b). We therefore conclude that endogenous DLG mediates synaptic targeting of the ΔGUK variant.
Discussion
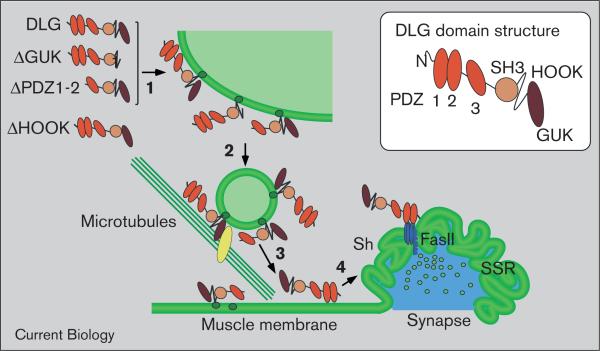
Postsynaptic DLG trafficking to NMJs comprises at least three locations: an intracellular subcortical network, the plasma membrane, and the synaptic membrane (Figure 8). In late embryos, DLG was clearly detected at the subcortical network and extrasynaptically at the plasma membrane before becoming localized postsynaptically. The identity of the subcortical compartment remains elusive but its network-like appearance suggests that it represents an extensive membranous structure such as the sarcoplasmic reticulum. Interestingly, El-Husseini et al. [40] have documented that the membrane localization of PSD-95 is preceded by transient association of the protein with intra-cellular membranes, for example, a perinuclear endosomal compartment or the smooth endoplasmic reticulum in dendritic spines. Similarly, GRIP1, another synaptic scaffolding molecule, is also found at intracellular vesicular structures [41].
Figure 8.
DLG targeting to synapses is a stepwise process that requires multiple domains. The deletion analysis supports a model in which the HOOK region mediates direct or indirect association of DLG with an intracellular membrane compartment (1), presumably the sarcoplasmic reticulum, from which DLG is transported to the muscle plasma membrane (2 and 3). This transport may involve the association of vesicle-bound DLG with microtubules and/or a motor protein, as has been suggested for other MAGUKs [22,40]. In a process that requires the PDZ1 and 2 domains, DLG is then recruited to the subsynaptic reticulum (SSR).
Deletions affecting DLG localization at extrasynaptic sites (for example, ΔHOOK, ΔPDZ1 + 2) also decreased synaptic DLG. This observation argues against independent targeting to different subcellular compartments and led us to propose that the stepwise trafficking observed in early development also occurs at later stages. The DLG targeting route described here is reminiscent of that of gluta-mate receptors and a CD8–Shaker fusion protein in Drosophila, which also become distributed throughout the muscle membrane before synaptic enrichment [7,42,43].
Our deletion analysis allowed us to relate individual domains of DLG to particular targeting steps (Figure 8). We found that the first two PDZ domains, the HOOK region and the GUK domain contributed to synaptic targeting of DLG differently. The HOOK region was required for entry into the normal targeting route through the subcortical network. In contrast to observations on epithelia, where ΔHOOK was found to be exclusively nuclear [26], synaptic localization of ΔHOOK was not completely impaired. This suggests that the protein can still reach the synapse, perhaps by diffusion, and that other domains then mediate its localization. The importance of the HOOK region for targeting to both septate and synaptic junctions appears surprising, because it is not well conserved among MAGUKs. Nevertheless, a short stretch of amino acids at the amino terminus of the HOOK region of all DLG-like MAGUKs is predicted to form an α-helix with a cluster of basic residues on one side. This helix has been reported to mediate a calmodulin-dependent dimerization of PSD-95 and SAP102 [24]. Our finding, that disruption of this helix mimics the effect of the entire ΔHOOK deletion, confirms that it is of particular importance in vivo. It remains to be determined whether it functions in synaptic targeting by promoting dimerization of DLG, by contributing to an intramolecular interaction [44], and/or by mediating heterophilic interactions.
The I3 region within the HOOK domain has been suggested to link MAGUKs to the actin/spectrin cytoskeleton through binding to protein 4.1 [37,38]. We found that the ΔI3 protein had a stronger synaptic localization when expressed in dlgX1-2 mutants. An interpretation of this result is that endogenous DLG competes out ΔI3 protein in its binding to the synaptic cytoskeleton.
None of our deletions resulted in an obvious accumulation solely at the subcortical network. This suggests that no additional domains are required to leave this compartment. Our findings support a role for the GUK domain to direct subsequent transport. Recent evidence suggests that targeting of PSD-95 and PSD-93 involves a micro-tubule-dependent transport of vesiculo-tubular structures [22,40]. These MAGUKs can be linked to microtubules by binding of the PDZ3 and GUK domains to the micro-tubule-associated proteins CRIPT or MAP1A, respectively [11,22,45]. Although these binding partners are suggestive for a role of these domains in MAGUK trafficking, no such function could be unraveled previously [26–29]. Removal of PDZ3 had no obvious effect on synaptic localization of DLG. Synaptic localization of the ΔGUK protein was, however, diminished in wild-type flies and virtually abolished in dlgX1-2 mutants. This dependency on endogenous DLG might be explained by dimerization, in agreement with the studies of Masuko et al. [24]. Alternatively, endogenous DLG could promote vesiculo-tubular trafficking (Figure 8) [40] and thus allow the truncated version to hitchhike on the same vesicles. This explanation would also apply to the finding that the GUK domain was dispensable for targeting of PSD-95 in cultured neurons or slices, in which the endogenous MAGUK is expressed [28,29].
Consistent with other studies of MAGUK targeting [26–29], we found that the PDZ domains were neither sufficient to target DLG to specific extrasynaptic sites nor to dock the protein at the synapse. Nonetheless, the PDZ1 and 2 domains were found to contribute to synaptic targeting. We suggest that the PDZ1 or PDZ2 domain is required for the final step in DLG targeting, from plasma membrane to synapses, but is not necessary to direct DLG to the plasma membrane (Figure 8). Thus, it may be assumed that the interaction with at least one PDZ binding protein is required to transport the protein to the synapse. A double mutant, in which the only known binding partners for the PDZ1 and 2 domains of DLG, Shaker and FasII were abolished or dramatically reduced, had no obvious effect on synaptic targeting of DLG. Interestingly, a non-synaptic PDZ-binding protein, Cypin, may regulate synaptic targeting of PSD-95 and SAP102 at extrasynaptic sites [46].
It remains to be determined whether the association of DLG with intracellular membrane compartments serves solely to target DLG itself. As discussed for GRIP1 and PSD-95, this step could also contribute to the sorting and co-transport of other synaptic molecules such as ion channels [40,41]. The pre-formation of a junctional protein complex at intracellular compartments has been exemplified by the interaction of E-cadherin and β-catenin at the endoplasmic reticulum [47]. The clustering of glycine receptors by gephyrin provides a contrary example, however, as gephyrin traps receptor molecules only at developing synapses [48]. Unfortunately, the limited sensitivity of available Shaker-specific antibodies has impaired our attempts to distinguish between intracellular and synaptic assembly of the DLG–Shaker complex. It appears obvious, however, that each targeting step represents an additional site at which the molecular composition of synaptic junctions could be regulated.
Conclusions
DLG-like MAGUKs are highly enriched at glutamatergic synapses in mammals and insects. The postsynaptic accumulation of DLG at the fly NMJ is based on a targeting mechanism that includes the temporary association of the protein with extrasynaptic compartments before its recruitment to the synapse. The HOOK region mediates the association of DLG with an extensive subcortical network, presumably the sarcoplasmic reticulum. DLG may reach the plasma membrane at any point. The first and second PDZ domains promote the subsequent transport to the synapse. Despite the identification of various binding partners, the role of the GUK domain of DLG-like MAGUKs in vivo has remained obscure. We have now unraveled an essential role for the GUK domain in synaptic targeting, which is concealed in the presence of endogenous DLG.
Materials and methods
Flies
Flies were kept at 25°C on standard medium. The following dlg mutants were used: y w sn dlgXI-2/Basc, y w f dlg1P20/Basc and y w dlgm30/Basc. UAS strains were described in [26] or generated as described below. For expression in a dlg mutant background, females from y w sn dlgXI-2/Basc; C57/C57 were crossed to UAS target males. The dlgXI-2 mutant progeny were identified using the y marker and tumorous imaginal discs. The fasIIe76 Sh102 flies were generated by meiotic crossover. The presence of Sh102 was confirmed by ether-induced leg shaking, whereas fasIIe76 was identified by allele-specific restriction fragment length polymorphisms.
Constructs
The ExSite mutagenesis kit (Stratagene) was employed to generate the deletions ΔN, ΔI3 and ΔE–F on a dlg cDNA [26]. Mutated subclones were verified by sequencing. A sequence encoding the FLAG epitope followed by a stop codon was cloned in-frame between the EcoRI and BglII sites at base pairs 2755 and 2804, respectively [26,49]. For germ-line transformation, the cDNAs were cloned into the pUAST vector [32,50].
Body wall preparations, antibodies, and immunofluorescence
Dissections were performed as described [51]. For detection of FLAG-tagged DLG variants, body walls were fixed for 25 min in 4% paraformaldehyde, washed 3 × 15 min in PBS containing 0.2% triton X-100 (PBST). Monoclonal anti-FLAG antibody (M2; Sigma, 1:1000 in PBST) was applied for 2 h at room temperature. Samples were washed, incubated with FITC-conjugated donkey anti-mouse IgG (Jackson Laboratories) at 1:160 (2 h, room temperature), washed and mounted in Vectashield medium (Vector). A GUK-domain-specific polyclonal serum (anti-DLGGUK) was obtained from a rat immunized with an affinity-purified Histidine-tagged fusion protein comprising amino acids 849–960 of DLG. Specificity of this serum was confirmed by the lack of immunoreactivity in dlgv59 mutants. This serum was applied overnight at 4°C at 1:100 on body walls fixed in Bouin's fixative for 30 min. Anti-DLGPDZ antibody was used as in [25]. For double labeling, Texas-Redconjugated goat anti-HRP antibodies (Jackson Laboratories) were used at 1:100. Confocal imaging was performed using a BioRad MRC 600 Laser attached to a Nikon microscope. Z-series were taken at 0.1 μm steps for Figures 1,2,5,7 and at 0.3 μm for all others. Samples selected for direct comparison were scanned with identical confocal settings. Images were manipulated (Z-series projection, cropping, analysis of confocal slices) using the NIH Image program (version 1.62).
Developmental analysis
Late embryos were mechanically dechorionated, devitellinized and filleted using sharpened tungsten needles. Initial staging of embryos was based on the degree of midgut development [52]. First instar larvae were dissected shortly after hatching; second instar larvae were selected based on defined egg-laying intervals, size, and appearance of anterior spiracles.
Immunoblot analyses
For each genotype, body walls from 10 third instar larvae were transferred to pre-cooled homogenizers, frozen at −70°C and homogenized upon addition of 52.5 μl RIPA buffer containing 2 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin; 7.5 μl 1 M DTT was added and the homogenate centrifuged for 5 min at 3,000 rpm at 4°C. SDS-protein sample buffer (15 μl of 5× stock) was added to the supernatants and boiled for 7 min; 15 μl of the protein samples was separated on 8% polyacrylamide gels and blotted onto Immobilon-P transfer membrane (Millipore). The membrane was blocked for 1 h at room temperature in PBS-containing 0.05% Tween, 10% horse serum, 1% goat serum, 3% BSA and 2mM NaN3. Primary antibodies were used at 1:1,000 (anti-FLAG) or 1:10,000 (anti-DLGPDZ). HRP-conjugated secondary antibodies were used at 1:3,000. Immunoreactive bands were detected using the ECL chemoluminescence kit (Amersham).
Acknowledgements
This work was supported by grants from the National Institutes of Health (RO1 NS37061 and RO1 NS30072) to V.B., Land Sachsen-Anhalt, the Max-Kade Foundation and the Fondsder Chemischen Industrie to S.E., U.T., and E.D.G., and from the Human Frontiers Science Program to V.B. and E.D.G.
References
- 1.Koh Y-H, Gramates LS, Budnik V. The Drosophila larval neuromuscular junction: molecular components and mechanisms underlying synaptic plasticity. Microscopy Res Tech. 2000;49:14–25. doi: 10.1002/(SICI)1097-0029(20000401)49:1<14::AID-JEMT3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 2.Garner CC, Nash J, Huganir RL. PDZ domains in synapse assembly and signalling. Trends Cell Biol. 2000;10:274–280. doi: 10.1016/s0962-8924(00)01783-9. [DOI] [PubMed] [Google Scholar]
- 3.Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- 4.Kornau H-C, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 5.Tejedor FJ, Bokhari A, Rogero O, Zhang J, Gorczyca M, Kim E, et al. Essential role for dlg in synaptic clustering of shaker K+ channels in vivo. J Neurosci. 1997;17:152–159. doi: 10.1523/JNEUROSCI.17-01-00152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas U, Kim E, Kuhlendahl S, Koh Y-H, Gundelfinger ED, Sheng M, et al. Synaptic clustering of the cell adhesion molecule Fasciclin II by discs-large and its role in the regulation of presynaptic structure. Neuron. 1997;19:787–799. doi: 10.1016/s0896-6273(00)80961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zito K, Fetter RD, Goodman CS, Isacoff EY. Synaptic clustering of Fasciclin II and Shaker: essential targeting sequences and role of Dlg. Neuron. 1997;19:1007–1016. doi: 10.1016/s0896-6273(00)80393-1. [DOI] [PubMed] [Google Scholar]
- 8.Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, et al. Binding of neuroligins to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Liao D, Lau LF, Huganir RF. SynGAP: a synaptic RasGAP that associates with the PSD-95/SAP90 protein family. Neuron. 1998;20:683–691. doi: 10.1016/s0896-6273(00)81008-9. [DOI] [PubMed] [Google Scholar]
- 10.Chen H-J, Rojas-Soto M, Oguni A, Kennedy MB. A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron. 1998;20:895–904. doi: 10.1016/s0896-6273(00)80471-7. [DOI] [PubMed] [Google Scholar]
- 11.Niethammer M, Valtschanoff JG, Kapoor TM, Allison DW, Weinberg RJ, Craig AM, Sheng M. CRIPT, a novel postsynaptic protein that binds to the third PDZ domain of PSD-95/SAP90. Neuron. 1998;20:693–707. doi: 10.1016/s0896-6273(00)81009-0. [DOI] [PubMed] [Google Scholar]
- 12.Furuyashiki T, Fujisawa K, Fujita A, Madaule P, Uchino S, Mishina M, et al. Citron, a Rho-target, interacts with PSD-95/SAP-90 at glutamatergic synapses in the thalamus. J Neurosci. 1999;19:109–118. doi: 10.1523/JNEUROSCI.19-01-00109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W, Vazquez L, Apperson M, Kennedy MB. Citron binds to PSD-95 at glutamatergic synapses on inhibitory neurons in the hippocampus. J Neurosci. 1999;19:96–108. doi: 10.1523/JNEUROSCI.19-01-00096.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia EP, Mehta S, Blair LAC, Wells DG, Shang J, Fukushima T, et al. SAP90 binds and clusters Kainate receptors causing incomplete desensitization. Neuron. 1998;21:727–739. doi: 10.1016/s0896-6273(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 15.Kuhlendahl S, Spangenberg O, Konrad M, Kim E, Garner CC. Functional analysis of the guanylate kinase-like domain in the synapse-associated protein SAP97. Eur J Biochem. 1998;252:305–313. doi: 10.1046/j.1432-1327.1998.2520305.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim E, Naisbitt S, Hsueh YP, Rao A, Rothschild A, Craig AM, Sheng M. GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules. J Cell Biol. 1997;136:669–678. doi: 10.1083/jcb.136.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeuchi M, Hata Y, Hirao K, Toyoda A, Irie M, Takai Y. SAPAPs. A family of PSD-95/SAP90-associated proteins localized at postsynaptic density. J Biol Chem. 1997;272:11943–11951. doi: 10.1074/jbc.272.18.11943. [DOI] [PubMed] [Google Scholar]
- 18.Boeckers TM, Kreutz MR, Winter C, Zuschratter W, Smalla K-H, Sanmarti-Vila L, et al. Proline-rich synapse-associated protein-1/Cortactin binding protein 1 (ProSAP1/CortBP1) is a PDZ-domain protein highly enriched in the postsynaptic density. J Neurosci. 1999;19:6506–6518. doi: 10.1523/JNEUROSCI.19-15-06506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boeckers TM, Winter C, Smalla K-H, Kreutz MR, Bockmann J, Seidenbecher C, et al. Proline-rich synapse-associated proteins ProSAP1 and ProSAP2 interact with synaptic proteins of the SAPAP/GKAP family. Biochem Biophys Res Commun. 1999;264:247–252. doi: 10.1006/bbrc.1999.1489. [DOI] [PubMed] [Google Scholar]
- 20.Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, et al. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- 21.Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, et al. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- 22.Brenman JE, Topinka JR, Cooper EC, McGee AW, Rosen J, Milroy T, et al. Localization of postsynaptic density-93 to dendritic microtubules and interaction with microtubule-associated protein 1A. J Neurosci. 1998;18:8805–8813. doi: 10.1523/JNEUROSCI.18-21-08805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsueh Y-P, Sheng M. Requirement of N-terminal cysteines of PSD-95 for PSD-95 multimerization and ternary complex formation, but not for binding to potassium channel Kv1.4. J Biol Chem. 1999;274:532–536. doi: 10.1074/jbc.274.1.532. [DOI] [PubMed] [Google Scholar]
- 24.Masuko N, Makino K, Kuwahara H, Fukunaga K, Sudo T, Araki N, et al. Interaction of NE-dlg/SAP102, a neuronal and endocrine tissue-specific membrane-associated guanylate kinase protein, with calmodulin and PSD-95/SAP90. A possible regulatory role in molecular clustering at synaptic sites. J Biol Chem. 1999;274:5782–5790. doi: 10.1074/jbc.274.9.5782. [DOI] [PubMed] [Google Scholar]
- 25.Koh Y-H, Popova E, Thomas U, Griffith LC, Budnik V. Regulation of DLG localization at synapses by CaMKII-dependent phosphorylation. Cell. 1999;98:353–363. doi: 10.1016/s0092-8674(00)81964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hough CD, Woods DF, Park S, Bryant PJ. Organizing a functional junctional complex requires specific domains of the Drosophila MAGUK Discs large. Genes Dev. 1997;11:3242–3253. doi: 10.1101/gad.11.23.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu H, Reuver SM, Kuhlendahl S, Chung WJ, Garner CC. Subcellular targeting and cytoskeletal attachment of SAP97 to the epithelial lateral membrane. J Cell Sci. 1998;111:2365–2376. doi: 10.1242/jcs.111.16.2365. [DOI] [PubMed] [Google Scholar]
- 28.Craven SE, El-Husseini AE, Bredt DS. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron. 1999;22:497–509. doi: 10.1016/s0896-6273(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 29.Arnold DB, Clapham DE. Molecular determinants for subcellular localization of PSD-95 with an interacting K+ channel. Neuron. 1999;23:149–157. doi: 10.1016/s0896-6273(00)80761-8. [DOI] [PubMed] [Google Scholar]
- 30.Lahey T, Gorczyca M, Jia XX, Budnik V. The Drosophila tumor suppressor gene dlg is required for normal synaptic bouton structure. Neuron. 1994;13:823–835. doi: 10.1016/0896-6273(94)90249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guan B, Hartmann B, Koh Y-H, Gorczyca M, Budnik V. The Drosophila tumor suppressor gene, dlg, is involved in structural plasticity at a glutamatergic synapse. Curr Biol. 1996;6:695–706. doi: 10.1016/s0960-9822(09)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 33.Woods DF, Hough C, Peel D, Callaini G, Bryant PJ. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J Cell Biol. 1996;134:1469–1482. doi: 10.1083/jcb.134.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Budnik V, Koh Y-H, Guan B, Hough C, Woods DF, Gorczyca M. Regulation of synapse structure and function by the Drosophila tumor suppressor gene, dlg. Neuron. 1996;17:627–640. doi: 10.1016/s0896-6273(00)80196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim E, Cho KO, Rothschild A, Sheng M. Heteromultimerization and NMDA receptor-clustering activity of Chapsyn-110, a member of the PSD-95 family of proteins. Neuron. 1996;17:103–113. doi: 10.1016/s0896-6273(00)80284-6. [DOI] [PubMed] [Google Scholar]
- 36.Topinka JR, Bredt DS. N-terminal palmitoylation of PSD-95 regulates association with cell membranes and interaction with K+ channel Kv1.4. Neuron. 1998;20:125–134. doi: 10.1016/s0896-6273(00)80440-7. [DOI] [PubMed] [Google Scholar]
- 37.Lue RA, Marfatia SM, Branton D, Chishti AH. Cloning and characterization of hdlg: the human homologue of the Drosophila discs large tumor suppressor binds to protein 4.1. Proc Natl Acad Sci USA. 1994;91:9818–9822. doi: 10.1073/pnas.91.21.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lue RA, Brandin E, Chan EP, Branton D. Two independent domains of hDlg are sufficient for subcellular targeting: the PDZ1-2 conformational unit and an alternatively spliced domain. J Cell Biol. 1996;135:1125–1137. doi: 10.1083/jcb.135.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deguchi M, Hata Y, Takeuchi M, Ide N, Hirao K, Yao I, et al. BEGAIN (brain-enriched guanylate kinase-associated protein), a novel neuronal PSD-95/SAP90-binding protein. J Biol Chem. 1998;273:26269–26272. doi: 10.1074/jbc.273.41.26269. [DOI] [PubMed] [Google Scholar]
- 40.El-Husseini AE, Craven SE, Chetkovich DM, Firestein BL, Schnell E, Aoki C, Bredt DS. Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering. J Cell Biol. 2000;148:159–172. doi: 10.1083/jcb.148.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong H, Zhang P, Song I, Petralia RS, Liao D, Huganir RL. Characterization of the glutamate receptor-interacting proteins GRIP1 and GRIP2. J Neurosci. 1999;19:6930–6941. doi: 10.1523/JNEUROSCI.19-16-06930.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Broadie K, Bate M. Innervation directs receptor synthesis and localization in Drosophila embryo synaptogenesis. Nature. 1993;361:350–353. doi: 10.1038/361350a0. [DOI] [PubMed] [Google Scholar]
- 43.Saitoe M, Tanaka S, Takata K, Kidokoro Y. Neural activity affects distribution of glutamate receptors during neuromuscular junction formation in Drosophila embryos. Dev Biol. 1997;184:48–60. doi: 10.1006/dbio.1996.8480. [DOI] [PubMed] [Google Scholar]
- 44.McGee AW, Bredt DS. Identification of an intramolecular interaction between the SH3 and guanylate kinase domains of PSD-95. J Biol Chem. 1999;274:17431–17436. doi: 10.1074/jbc.274.25.17431. [DOI] [PubMed] [Google Scholar]
- 45.Passafaro M, Sala C, Niethammer M, Sheng M. Microtubule binding by CRIPT and its potential role in the synaptic clustering of PSD-95. Nat Neurosci. 1999;2:1063–1069. doi: 10.1038/15990. [DOI] [PubMed] [Google Scholar]
- 46.Firestein BL, Brenman JE, Aoki C, Sanchez-Perez AM, El-Husseini AE, Bredt DS. Cypin: a cytosolic regulator of PSD-95 postsynaptic targeting. Neuron. 1999;24:659–672. doi: 10.1016/s0896-6273(00)81120-4. [DOI] [PubMed] [Google Scholar]
- 47.Chen YT, Stewart DB, Nelson WJ. Coupling assembly of the E-cadherin/beta-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. J Cell Biol. 1999;144:687–699. doi: 10.1083/jcb.144.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirsch J, Betz H. Glycine-receptor activation is required for receptor clustering in spinal neurons. Nature. 1998;392:717–720. doi: 10.1038/33694. [DOI] [PubMed] [Google Scholar]
- 49.Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- 50.Spradling AC. P-element-mediated transformation. In: Roberts DB, editor. Drosophila — a Practical Approach. IRL Press; Oxford: 1986. pp. 175–198. [Google Scholar]
- 51.Budnik V, Zhong Y, Wu C-F. Morphological plasticity of motor axon terminals in Drosophila mutants with altered excitability. J Neurosci. 1990;10:3754–3768. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gorczyca M, Phillis RW, Budnik V. The role of tinman, a mesodermal cell fate gene, in axon pathfinding during the development of the transverse nerve in Drosophila. Development. 1994;120:2143–2152. doi: 10.1242/dev.120.8.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]