Abstract
N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) is a natural tetrapeptide with anti-inflammatory and anti-fibrotic properties. Its effect on salt-sensitive (SS) hypertension is unknown. We hypothesized that in Dahl SS rats on high salt diet, Ac-SDKP prevents loss of nephrin expression and renal immune cell infiltration, leading to a decrease in albuminuria, renal inflammation, fibrosis and glomerulosclerosis. To test this, Dahl SS rats and consomic SS13BN controls were fed either a low salt (LS=0.23%NaCl) or high salt (HS=4%NaCl) diet and treated for 6 weeks with vehicle (veh) or Ac-SDKP at either low or high dose (800 or 1600 µg/kg/day, respectively). High salt increased systolic blood pressure in SS rats (HS+veh 186±5 vs. LS+veh 141±3 mmHg; p<0.005) but not in SS13BN rats. Ac-SDKP did not affect blood pressure. Compared to low salt, high salt induced albuminuria, renal inflammation, fibrosis and glomerulosclerosis in both strains, but the damages were higher in SS compared to SS13BN. Interestingly, in SS13BN rats, Ac-SDKP prevented albuminuria induced by high salt (HS+veh 44±8 vs. HS+low Ac-SDKP 24±3 or HS+high Ac-SDKP 8±1 mg/24h; p<0.05); whereas in SS rats only high Ac-SDKP dose significantly attenuated albuminuria (HS+veh 94±10 vs. HS+high Ac-SDKP 57±7 mg/24h; p<0.05). In both strains, Ac-SDKP prevented high salt-induced inflammation, interstitial fibrosis and glomerulosclerosis. In summary, in SS rats on high salt diet, at low and high doses, Ac-SDKP prevented renal damage without affecting the blood pressure. Only the high dose of Ac-SDKP attenuated high salt-induced albuminuria. Conversely, in SS13BN rats, both doses of Ac-SDKP prevented high salt-induced renal damage and albuminuria.
Keywords: Ac-SDKP, salt-sensitive hypertension, albuminuria, inflammation, renal damage
INTRODUCTION
Salt-sensitivity is associated with severe progression of hypertensive target-organ damage, including end-stage renal disease1, 2. The underlying mechanism(s) of SS hypertension and associated renal injury are still not clear, but it is thought that inflammation plays an important role3, 4. Previous studies showed that high salt (HS) induced hypertension and renal injury is associated with increased albuminuria, immune cell infiltration, tubulointerstitial injury and glomerulosclerosis4–6.
The Dahl SS rat is a model of hypertension and renal disease that exhibits phenotypic traits common for sodium-sensitive hypertension and renal dysfunction observed in the human population. When studying the Dahl SS model, it is valuable to use the consomic SS13BN rat as a control strain. It is generated by substituting chromosome 13 from a normotensive Brown Norway (BN) rat into the SS genome, which makes it 98% genetically identical to the SS rat. Salt-induced hypertension, albuminuria and renal injury is attenuated in SS13BN rats7, 8.
Glomerular filtration barrier integrity and function strongly depend on nephrin, an important transmembrane protein fundamental for the podocyte slit diaphragm function; regulating renal filtration and selectively allowing small molecules like ions to pass through, while excluding the passage of large molecules like proteins9, 10. A decrease in nephrin expression or its function may cause massive proteinuria11, 12.
N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) is a natural tetrapeptide released from its precursor thymosin β4, by prolyl oligopeptidase13. Ac-SDKP is found in human plasma and circulating mononuclear cells14 and various organs15, and is hydrolyzed mainly by angiotensin-converting enzyme (ACE)16. We previously showed that the anti-inflammatory and anti-fibrotic effects of ACEi are mediated by an increase in endogenous Ac-SDKP17, 18. Animal studies have demonstrated anti-inflammatory and anti-fibrotic properties of Ac-SDKP19–21, and that a decrease in endogenous Ac-SDKP levels promotes heart and kidney fibrosis22. However, the effect of Ac-SDKP on SS hypertension is still unknown. We therefore designed a new study to test the novel hypothesis that the administration of Ac-SDKP to Dahl SS hypertensive or consomic non-hypertensive rats on a HS diet prevents: 1) loss of nephrin expression, 2) renal macrophage infiltration and 3) T cell infiltration, and that these changes lead to decreases in albuminuria, renal inflammation, fibrosis and glomerulosclerosis, without affecting blood pressure.
METHODS
Male Dahl SS and consomic SS13BN rats at 4 weeks of age (Charles River Laboratories, Wilmington, MA) were fed either a low salt (LS=0.23%NaCl) or high salt (HS=4%NaCl) diet and treated for 6 weeks with vehicle (veh) or Ac-SDKP at either low or high dose (800 or 1600 µg/kg/day, respectively). Measurements of blood pressure and albuminuria and histological studies were performed. This study was approved by the Henry Ford Hospital Institutional Animal Care and Use Committee.
An expanded Methods section is available in the online Data Supplement.
RESULTS
Systolic Blood Pressure, Body Weight and Organ Weight
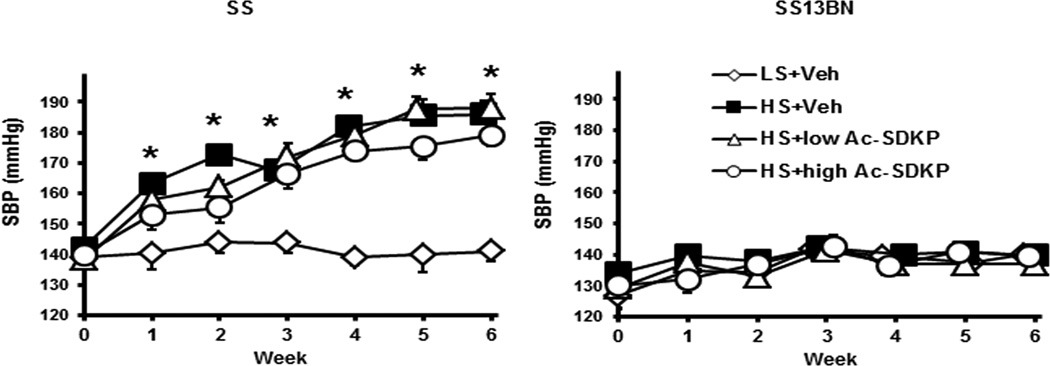
In Dahl SS rats, 6 weeks of HS diet significantly increased SBP compared to LS diet (HS+Veh 186±5 vs. LS+Veh 141±3 mmHg, P<0.005). In contrast, in SS13BN rats, HS did not affect SBP (HS+Veh 140±3 vs. LS+Veh 140±2 mmHg). Ac-SDKP did not attenuate the increased blood pressure induced by HS diet in SS rats (Figure 1). HS diet significantly increased kidney weight (KW) to body weight (BW) ratio and heart weight (HW) to BW ratio in both Dahl SS and SS13BN rats. Ac-SDKP did not prevent renal and cardiac hypertrophy induced by HS diet in either strain. (Supplement Figure S1).
Figure 1. Effect of Ac-SDKP on systolic blood pressure (SBP).
SBP was measured weekly by tail cuff. In Dahl salt-sensitive (SS) rats but not in consomic SS13BN rats, HS diet increased significantly SBP compared to LS diet. Ac-SDKP had no effect on SBP. Data are expressed as mean ± SEM. N=6–7 in each group. *P<0.005 HS+Veh vs LS+Veh
Urinary Ac-SDKP Excretion
In Dahl SS and SS13BN rats, 24-hour urinary Ac-SDKP excretion was significantly higher (5 to 11 fold) in Ac-SDKP treated, compared to vehicle treated groups (Supplement Figure S2). In the vehicle treated animals, the urinary Ac-SDKP excretion in SS13BN rats was significantly elevated compared to Dahl SS rats (Supplement Figure S3).
Albumin and Protein Excretion
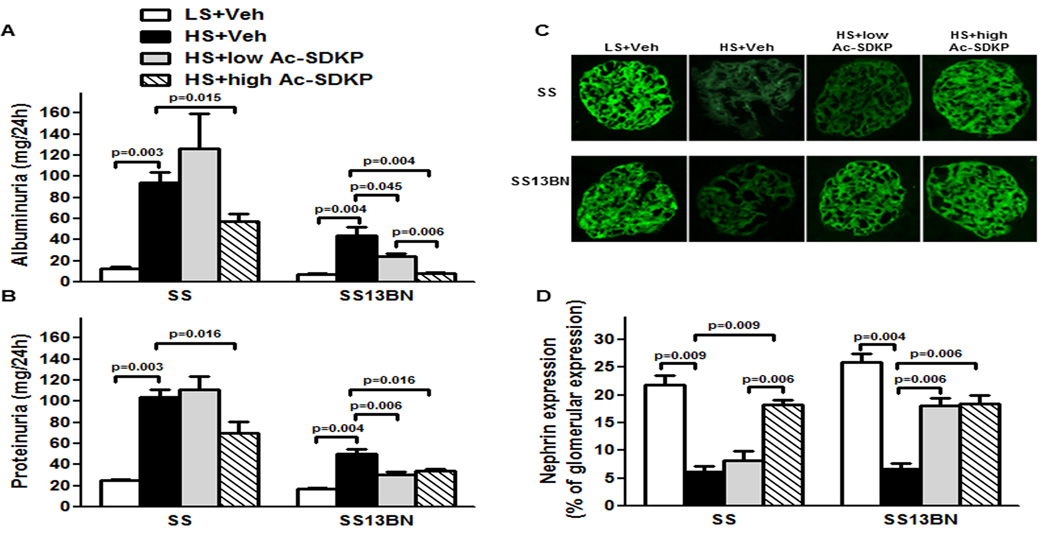
Compared to LS, HS diet increased albuminuria in Dahl SS and in SS13BN rats (Figure 2A). Similarly to albuminuria, HS diet increased proteinuria in both strains (Figure 2B). Greater increases were observed in Dahl SS compared to SS13BN rats. In SS13BN rats, low dose and high dose of Ac-SDKP significantly reduced HS-induced albuminuria and proteinuria (Figure 2A and B). In Dahl SS rats only high Ac-SDKP dose significantly reduced albuminuria and proteinuria (Figure 2A and B) induced by HS diet.
Figure 2. Effect of Ac-SDKP on albuminuria, proteinuria and glomerular nephrin expression.
(A) 24h urinary albumin and (B) 24h urinary protein at the end of 6 weeks treatment in Dahl salt-sensitive (SS) and consomic SS13BN rats. In both SS and SS13BN, HS diet significantly increased albuminuria and proteinuria compared to LS diet. Greater increases were observed in SS rats compared to SS13BN rats. In SS rats only at high dose, Ac-SDKP attenuated HS-induced albuminuria and proteinuria. However, low and high doses of Ac-SDKP prevented HS-induced albuminuria in SS13BN. Data are expressed as mean ± SEM. N=6–7 in each group. (C) Representative images of glomerular nephrin expression captured using ×20 microscope objective. Green indicates positive staining for nephrin. (D) Quantitative data analysis of nephrin expression. In SS and consomic SS13BN rats, HS diet significantly decreased nephrin expression compared to LS diet. In SS rats only high dose of Ac-SDKP significantly attenuated HS-induced decrease of nephrin expression. However in SS13BN, low and high doses of Ac-SDKP significantly prevented HS-induced decrease of nephrin expression. Data are expressed as percentage of the glomerular area and expressed as mean ± SEM. N=6–7 in each group.
Glomerular Nephrin Expression
In Dahl SS and SS13BN rats, HS diet significantly decreased nephrin expression. In Dahl SS rats, only the high dose of Ac-SDKP significantly attenuated HS-induced decrease in nephrin expression, while in SS13BN rats on HS diet, both low and high doses of Ac-SDKP significantly prevented decrease in nephrin expression (Figure 2C and D).
Renal Macrophage and T Cell Infiltration
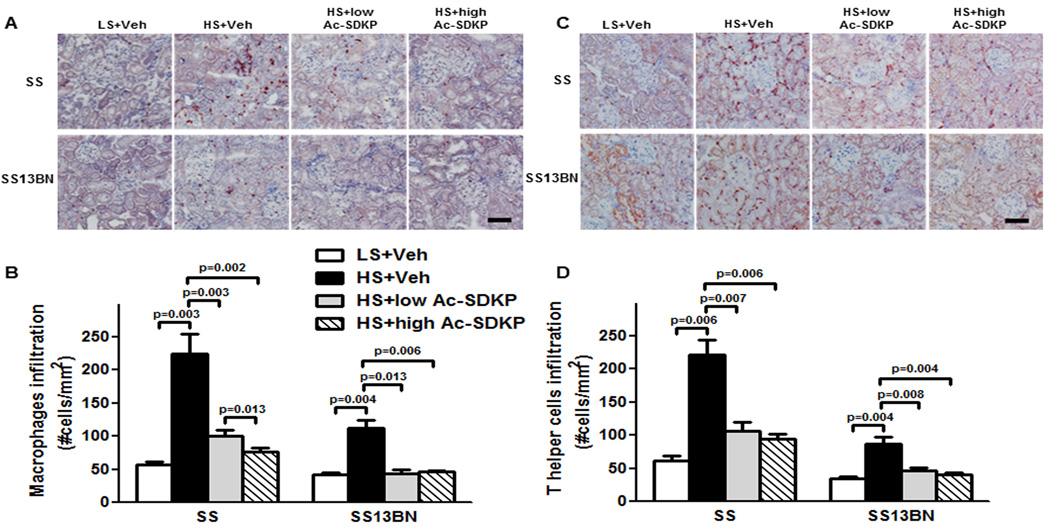
Macrophage and T helper cell infiltration was examined by immunohistochemistry. In Dahl SS and SS13BN, HS diet markedly increased the number of infiltrating macrophages as detected by CD68+ positivity (Figure 3A and B), and T helper cells detected by CD4+ positivity (Figure 3C and D). The number of infiltrating renal macrophages and T cells induced by HS were higher in Dahl SS, compared to SS13BN rats. The infiltrated cells are mainly located in the glomerular and interstitial area (Figure S4A and B). Treatments with low and high dose of Ac-SDKP markedly reduced the numbers of infiltrating cells in both strains.
Figure 3. Effect of Ac-SDKP on renal macrophage and T cell infiltration.
(A and C) Representative images of renal macrophage and T helper cell infiltration. Red staining in the cytoplasm indicates a positive immunohistochemistry staining for macrophages (anti-CD68 antibody) and T helper cells (anti-CD4 antibody). Shown are images captured using ×20 microscope objective. Scale bar = 100 µm. (B and D) Quantitative data analysis. In Dahl salt-sensitive (SS) and consomic SS13BN rats, HS diet markedly increased macrophages and T helper cells in the kidney. Low or high dose of Ac-SDKP significantly reduced HS-induced renal macrophage and T helper cell infiltration. Data are calculated as number of cells per mm2 and expressed as mean ± SEM. N=6–7 in each group.
Renal Interstitial Fibrosis and Collagen Content
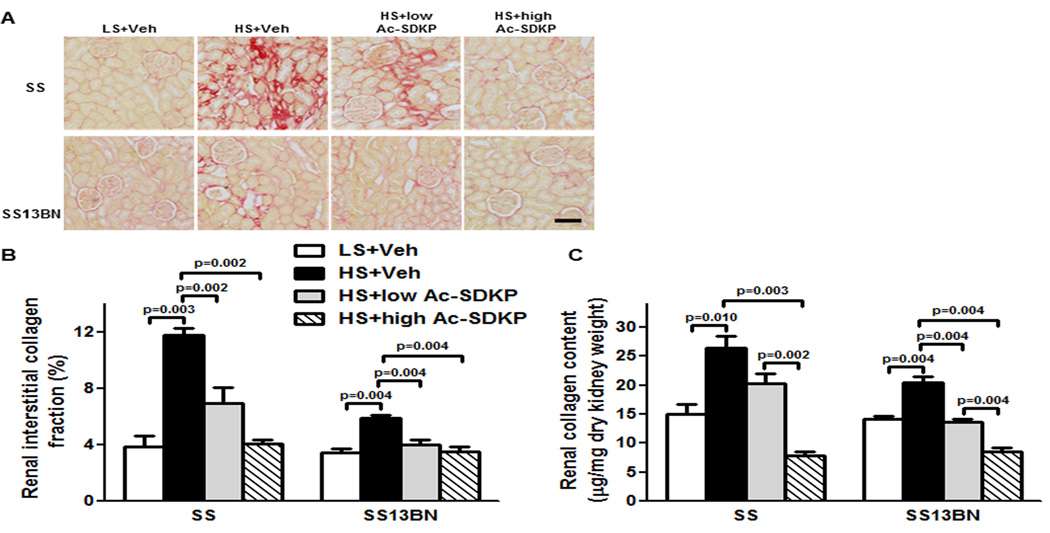
In both Dahl SS and SS13BN rats, the renal interstitial collagen fraction was higher with HS diet compared to LS diet. Treatment with either low or high doses of Ac-SDKP reduced the interstitial collagen fraction compared to animals on HS treated with vehicle (Figure 4A and B). In addition, analysis of renal collagen content by the hydroxyproline assay showed that the increases caused by HS diet could be dose-dependently prevented by Ac-SDKP in both Dahl SS and SS13BN rats (Figure 4C).
Figure 4. Effect of Ac-SDKP on renal interstitial fibrosis and collagen content.
(A) Representative images of renal interstitial fibrosis. Red color indicates collagen deposition revealed by picrosirius staining. Shown are images captured using ×20 microscope objective. Scale bar = 100 µm. (B) Quantitative data analysis. In Dahl salt-sensitive (SS) and consomic SS13BN rats, low or high dose of Ac-SDKP significantly prevented HS-induced renal interstitial collagen deposition. Data are calculated as a percentage of the fibrotic area and expressed as mean ± SEM. N=6–7 in each group. (C) Quantitative analysis of total renal collagen content determined by hydroxyproline assay. In SS and consomic SS13BN rats, HS diet significantly increased renal collagen content compared to LS diet. In SS and SS13BN rats, Ac-SDKP significantly decreased HS-induced renal collagen content. Greater decrease was observed with high Ac-SDKP compared to low Ac-SDKP in both strains. Data are expressed as a microgram of collagen per milligram of dry kidney weight and expressed as mean ± SEM. N=6–7 in each group.
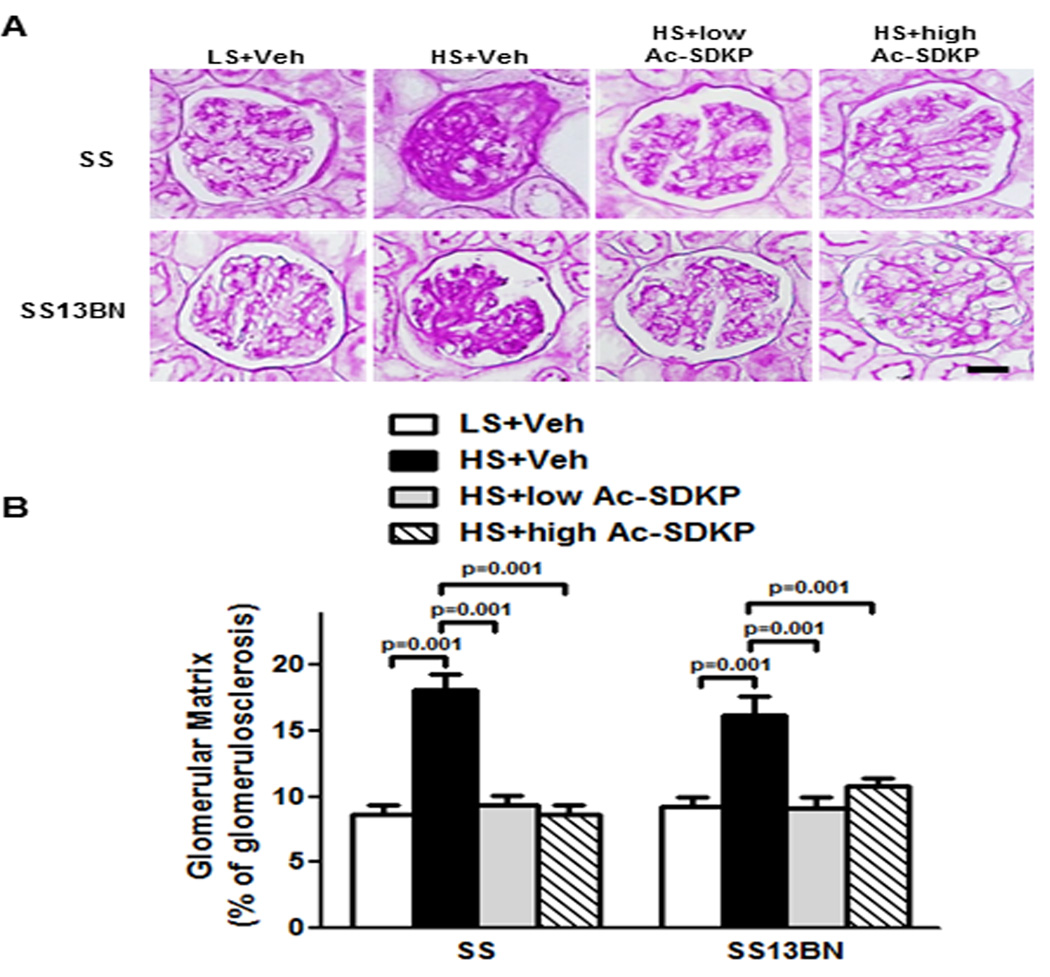
Glomerulosclerosis
The effect of Ac-SDKP on glomerulosclerosis was assessed by periodic acid Schiff staining (PAS). Dahl SS and SS13BN rats on HS exhibited glomerulosclerosis, detected as dark purple regions of extracellular matrix deposition within the glomerular tufts (Figure 5). The glomerulosclerosis noted in response to a HS diet in both strains was significantly attenuated by treatment with either dose of Ac-SDKP.
Figure 5. Effect of Ac-SDKP on glomerular matrix deposition.
(A) Representative images of the glomerular matrix. Dark-purple regions indicate extracellular matrix stained within the glomerular tufts by periodic acid-Schiff staining. Shown are images captured using ×40 microscope objective. Scale bar = 25 µm. (B) Quantitative data analysis. In Dahl salt-sensitive (SS) and consomic SS13BN, glomerulosclerosis is significantly increased by HS diet compared to LS diet. Ac-SDKP, low or high dose significantly prevented HS-induced glomerulosclerosis in both strains. Data are as expressed as mean ± SEM. N=6–7 in each group.
DISCUSSION
In this study, we examined the protective effects of Ac-SDKP on renal damage in SS hypertension. We assessed the effects of either low or high Ac-SDKP dose on renal injury in Dahl SS and consomic SS13BN rats fed a HS diet. Consistent with previous studies, our results showed that in Dahl SS rats, HS diet caused hypertension, accompanied by a decrease in nephrin expression and an increase in albuminuria and proteinuria. This was coupled with renal inflammation (macrophage and T helper cell infiltration in the glomerular and interstitial area), tubulointerstitial injury (interstitial fibrosis and collagen deposition) and glomerulosclerosis. Interestingly, in consomic SS13BN controls, even without an increase in blood pressure, HS diet also induced a decrease in nephrin expression, an increase in albuminuria and proteinuria, renal inflammation, tubulointerstitial injury and glomerulosclerosis, but to a lesser degree than in Dahl SS rats.
Our results suggest that HS diet per se, even without further increases in blood pressure, can induce renal injury in a non-salt-sensitive hypertensive model. The underlying mechanisms by which HS diet exerts deleterious effects, even when the blood pressure does not increase, are still not clear. There is new evidence suggesting that NaCl initiates pro-inflammatory and pro-fibrotic cascades without affecting blood pressure. For example, naïve T cell differentiation to pro-inflammatory Th17 cells is exacerbated by NaCl23. Also, dietary salt increases the expression of the fibrogenic growth factor, transforming growth factor-β1 (TGF-β1), and the production of endothelial nitrite and nitrate (NOx) in rat aortic endothelium through both p38 and p42/44 MAPK pathways24. DuPont et al25 demonstrated that excess high dietary sodium intake impairs endothelium-dependent dilation in healthy, normotensive, salt-resistant humans independently of changes in blood pressure.
Since the consomic SS13BN controls share the same genetic background with Dahl SS rats (98% genetically identical), it is likely that chromosome 13 plays an important role in the development of salt-induced hypertension. However, the genetic pre-disposition factors may play a key role in the susceptibility to renal disease independent of increased blood pressure. The higher degree of renal damage observed in Dahl SS rats could be due to a combined effect of hypertension and HS diet in inducing the renal damage; whereas in consomic SS13BN controls, HS was the only factor inducing the renal damage. Due to its anti-inflammatory and anti-fibrotic properties, the higher endogenous Ac-SDKP levels may be responsible for less renal damage observed in the consomic controls. Clinical and animal studies have established proteinuria as a marker of glomerular damage and a promoter of tubulointerstitial inflammation and fibrosis leading to renal failure26–28. The mechanisms by which urinary proteins induce tubulointerstitial damage are not fully understood and are still under investigation. It is generally accepted that during progression of chronic renal disease, proximal tubular cells are activated after reabsorbing an excessive amount of filtered proteins. This leads to a tubular injury and interstitial inflammation characterized by macrophage and T cell interstitial infiltration, which appear to play a major role in the up-regulation of pro-fibrotic molecules including TGF-β27.
Here we showed that in both Dahl SS and consomic SS13BN rats, HS diet significantly increased albumin and protein excretion. Greater increases were observed in Dahl SS, compared to SS13BN rats. Interestingly, in consomic SS13BN controls, treatment with either low or high dose of Ac-SDKP significantly prevented HS-induced albuminuria and proteinuria, while in Dahl SS rats, the same effect was achieved only with the high dose of Ac-SDKP. These data indicate that the effective Ac-SDKP dose for the prevention of HS-induced albuminuria and/or proteinuria depends on the strain and the stage of renal disease. To further analyze the mechanism of HS-induced albuminuria and proteinuria, we investigated the expression of nephrin. We showed that in both strains on HS diet, the increase in albuminuria and proteinuria was accompanied with a decrease of nephrin expression. In consomic SS13BN controls on HS diet, treatment with either low or high Ac-SDKP dose significantly prevented the decrease in nephrin expression, while in Dahl SS rats on HS diet only a high Ac-SDKP dose had this effect, indicating that the protective effect of Ac-SDKP on proteinuria is achieved by preventing the decrease in nephrin expression.
This study also showed that the high levels of albuminuria and proteinuria are accompanied by renal macrophage and T helper cell infiltration, fibrosis and glomerulosclerosis. Infiltrating immune cells play an important role in mediating SS hypertension and renal disease29, 30. Animal studies using immunomodulatory treatments that decreased T cell infiltration showed amelioration of SS hypertension and renal disease3, 31. However, it is still not clear if the inflammation is a cause or a consequence of the SS hypertension. Our data showed that in Dahl SS and in consomic SS13BN control rats, HS diet markedly increased renal macrophage and T helper cell infiltration. The number of infiltrating renal macrophages and T cells in rats on HS diet was higher in Dahl SS rats compared to consomic SS13BN rats, indicating a correlation between the degree of inflammation and proteinuria. In both strains, low and high doses of Ac-SDKP prevented renal macrophage and T helper cell infiltration induced by HS diet without a decrease in blood pressure.
Parallel to the macrophage and T cell infiltration, in Dahl SS and in consomic SS13BN controls, HS-induced renal interstitial fibrosis was accompanied with an increase in renal collagen content and glomerulosclerosis. In both strains, low and high doses of Ac-SDKP significantly prevented HS-induced renal interstitial fibrosis. Ac-SDKP significantly decreased HS-induced renal collagen content, but a greater decrease was observed with the dose of high Ac-SDKP. At low and high doses, Ac-SDKP also significantly abrogated HS-induced glomerulosclerosis in both strains.
We and others have already shown protective effects of Ac-SDKP in the heart, kidney and brain32–34. We have previously reported possible mechanism(s) by which Ac-SDKP protects organ damage. These effects are related to a decrease in pro-inflammatory pathways such as NF-kB activation, cytokine release, and ICAM-1 expression35, 36. Additionally, we also showed that in-vitro, Ac-SDKP exerts anti-inflammatory effects by inhibiting: 1) differentiation of bone marrow stem cells (BMSC) to macrophages, 2) activation and migration of macrophages, and 3) release of the proinflammatory cytokine TNF-alpha by activated macrophages37. The anti-inflammatory and anti-fibrotic effects of Ac-SDKP are observed not only in Ang II-induced hypertension but also in Ang II-independent models20, 32. Thus, more studies are needed to delineate the exact mechanism of the anti-inflammatory and anti-fibrotic effects of Ac-SDKP in the kidney.
We conclude that, in Dahl SS rats on HS diet, a low dose of Ac-SDKP prevented renal macrophage and T cell infiltration, renal fibrosis and glomerulosclerosis, but failed to prevent albuminuria and proteinuria, indicating that inflammation and fibrosis may not be the only factors contributing to high albuminuria. In this strain, a higher dose of Ac-SDKP was needed to prevent not only renal inflammation, fibrosis, and glomerulosclerosis, but also albuminuria and proteinuria induced by HS diet. We speculate that in Dahl SS rats, high dose of Ac-SDKP reduced albuminuria and proteinuria due to a complete inhibition of macrophage infiltration and renal fibrosis. These Ac-SDKP effects were independent of changes in blood pressure. On the other hand, in consomic SS13BN controls, both low and high Ac-SDKP doses prevented albuminuria and proteinuria, macrophage and T cell infiltration, renal fibrosis and glomerulosclerosis induced by HS diet.
PERSPECTIVES
Our pre-clinical study demonstrates that high salt diet could exert harmful effects not only in salt-sensitive individuals, but also in non-sensitive individuals and that Ac-SDKP exerts strong renal protective effects without affecting the blood pressure. On the other hand, ACEi is widely used to treat hypertension and related cardiac and renal diseases and dysfunction. However, some patients can not tolerate ACEi-associated side effects such as hypotension, hyperkalemia and angioedema38. Moreover, ACEi increases circulating and tissue Ac-SDKP. Ac-SDKP was shown to mediate part of the protective effects of ACEi18. Thus, Ac-SDKP or its analog, resistant to enzymatic degradation, could be a novel and useful therapeutic strategy for treating high salt-induced renal damages and dysfunction in either salt-sensitive or non-salt-sensitive subjects.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
This is the first study providing the evidence of the protective effects of Ac-SDKP on renal damage not only in salt-sensitive hypertensive but also in consomic non-hypertensive animals.
What is relevant?
Fifty percent of hypertensive and 20 percent of normotensive patients are salt-sensitive. Salt-sensitivity is associated with severe progression of hypertensive target-organ damage, including end-stage renal disease.
Summary
Our results showed that in Dahl SS rats on HS diet, at low dose, Ac-SDKP prevented renal damage without lowering the blood pressure, but failed to correct albuminuria. Interestingly, treatment with a high dose of Ac-SDKP was not only able to achieve the anti-inflammatory and anti-fibrotic effects as seen with low dose, but also improved renal function by decreasing HS-induced albuminuria. On the other hand, in consomic SS13BN rats, both doses of Ac-SDKP prevented HS-induced renal damage and albuminuria.
Acknowledgments
SOURCE OF FUNDING
This work was supported by National Institutes of Health Grant P01HL028982.
Footnotes
DISCLOSURE
None
REFERENCES
- 1.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 2.Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications. Hypertension. 1994;23:531–550. doi: 10.1161/01.hyp.23.4.531. [DOI] [PubMed] [Google Scholar]
- 3.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the dahl salt-sensitive rat. Hypertension. 2006;48:149–156. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 4.Mattson DL. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. American journal of physiology. Renal physiology. 2014;307:F499–F508. doi: 10.1152/ajprenal.00258.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rafiq K, Nishiyama A, Konishi Y, Morikawa T, Kitabayashi C, Kohno M, Masaki T, Mori H, Kobori H, Imanishi M. Regression of glomerular and tubulointerstitial injuries by dietary salt reduction with combination therapy of angiotensin ii receptor blocker and calcium channel blocker in dahl salt-sensitive rats. PloS one. 2014;9:e107853. doi: 10.1371/journal.pone.0107853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen B, Hagiwara M, Yao YY, Chao L, Chao J. Salutary effect of kallistatin in salt-induced renal injury, inflammation, and fibrosis via antioxidative stress. Hypertension. 2008;51:1358–1365. doi: 10.1161/HYPERTENSIONAHA.107.108514. [DOI] [PubMed] [Google Scholar]
- 7.Cowley AW, Jr, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown norway chromosome 13 confers protection from high salt to consomic dahl s rat. Hypertension. 2001;37:456–461. doi: 10.1161/01.hyp.37.2.456. [DOI] [PubMed] [Google Scholar]
- 8.Liang M, Lee NH, Wang H, Greene AS, Kwitek AE, Kaldunski ML, Luu TV, Frank BC, Bugenhagen S, Jacob HJ, Cowley AW., Jr Molecular networks in dahl salt-sensitive hypertension based on transcriptome analysis of a panel of consomic rats. Physiological genomics. 2008;34:54–64. doi: 10.1152/physiolgenomics.00031.2008. [DOI] [PubMed] [Google Scholar]
- 9.Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestil M, Jalanko H, Holmberg C, Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7962–7967. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawachi H, Koike H, Shimizu F. Molecular structure and function of the slit diaphragm: Expression of nephrin in proteinuric states and in developing glomeruli. Nephrol. Dial. Transplant. 2002;17(Suppl 9):20–22. doi: 10.1093/ndt/17.suppl_9.20. [DOI] [PubMed] [Google Scholar]
- 11.Ma R, Liu L, Liu X, Wang Y, Jiang W, Xu L. Triptolide markedly attenuates albuminuria and podocyte injury in an animal model of diabetic nephropathy. Experimental and therapeutic medicine. 2013;6:649–656. doi: 10.3892/etm.2013.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 13.Cavasin MA, Rhaleb NE, Yang XP, Carretero OA. Prolyl oligopeptidase is involved in release of the antifibrotic peptide ac-sdkp. Hypertension. 2004;43:1140–1145. doi: 10.1161/01.HYP.0000126172.01673.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pradelles P, Frobert Y, Creminon C, Liozon E, Masse A, Frindel E. Negative regulator of pluripotent hematopoietic stem cell proliferation in human white blood cells and plasma as analysed by enzyme immunoassay. Biochemical and biophysical research communications. 1990;170:986–993. doi: 10.1016/0006-291x(90)90489-a. [DOI] [PubMed] [Google Scholar]
- 15.Junot C, Nicolet L, Ezan E, Gonzales MF, Menard J, Azizi M. Effects of angiotensin-converting enzyme inhibition on plasma, urine, and tissue concentrations of hemoregulatory peptide acetyl-ser-asp-lys-pro in rats. J. Cardiovasc. Pharmacol. 1999;291:982–987. [PubMed] [Google Scholar]
- 16.Azizi M, Rousseau A, Ezan E, Guyene TT, Michelet S, Grognet JM, Lenfant M, Corvol P, Menard J. Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator n-acetyl-seryl-aspartyl-lysyl-proline. Journal of Clinical Investigation. 1996;97:839–844. doi: 10.1172/JCI118484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng H, Carretero OA, Vuljaj N, Liao TD, Motivala A, Peterson EL, Rhaleb NE. Angiotensin-converting enzyme inhibitors: A new mechanism of action. Circulation. 2005;112:2436–2445. doi: 10.1161/CIRCULATIONAHA.104.528695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng H, Carretero OA, Liao TD, Peterson EL, Rhaleb NE. Role of n-acetyl-seryl-aspartyl-lysyl-proline in the antifibrotic and anti-inflammatory effects of the angiotensin-converting enzyme inhibitor captopril in hypertension. Hypertension. 2007;49:695–703. doi: 10.1161/01.HYP.0000258406.66954.4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang F, Yang XP, Liu YH, Xu J, Cingolani O, Rhaleb NE, Carretero OA. Ac-sdkp reverses inflammation and fibrosis in rats with heart failure after myocardial infarction. Hypertension. 2004;43:229–236. doi: 10.1161/01.HYP.0000107777.91185.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa P, Liu Y, Liao TD, Chen X, Gonzalez GE, Bobbitt KR, Smolarek D, Peterson EL, Kedl R, Yang XP, Rhaleb NE, Carretero OA. Treatment with n-acetyl-seryl-aspartyl-lysyl-proline prevents experimental autoimmune myocarditis in rats. American Journal of Physiology. Heart and Circulatory Physiology. 2012;303:H1114–H1127. doi: 10.1152/ajpheart.00300.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhaleb NE, Peng H, Yang XP, Liu YH, Mehta D, Ezan E, Carretero OA. Long-term effect of n-acetyl-seryl-aspartyl-lysyl-proline on left ventricular collagen deposition in rats with 2-kidney, 1-clip hypertension. Circulation. 2001;103:3136–3141. doi: 10.1161/01.cir.103.25.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavasin MA, Liao TD, Yang XP, Yang JJ, Carretero OA. Decreased endogenous levels of ac-sdkp promote organ fibrosis. Hypertension. 2007;50:130–136. doi: 10.1161/HYPERTENSIONAHA.106.084103. [DOI] [PubMed] [Google Scholar]
- 23.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic th17 cells. Nature. 2013;496:518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ying WZ, Sanders PW. Increased dietary salt activates rat aortic endothelium. Hypertension. 2002;39:239–244. doi: 10.1161/hy0202.104142. [DOI] [PubMed] [Google Scholar]
- 25.DuPont JJ, Greaney JL, Wenner MM, Lennon-Edwards SL, Sanders PW, Farquhar WB, Edwards DG. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. Journal of hypertension. 2013;31:530–536. doi: 10.1097/HJH.0b013e32835c6ca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eddy AA. Proteinuria and interstitial injury. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2004;19:277–281. doi: 10.1093/ndt/gfg533. [DOI] [PubMed] [Google Scholar]
- 27.Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? Journal of the American Society of Nephrology : JASN. 2006;17:2974–2984. doi: 10.1681/ASN.2006040377. [DOI] [PubMed] [Google Scholar]
- 28.Ruggenenti P, Remuzzi G. The role of protein traffic in the progression of renal diseases. Annual review of medicine. 2000;51:315–327. doi: 10.1146/annurev.med.51.1.315. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: All for one and one for all. American Journal of Physiology. Renal Physiology. 2004;286:F606–F616. doi: 10.1152/ajprenal.00269.2003. [DOI] [PubMed] [Google Scholar]
- 30.De MC, Lund H, Mattson DL. High dietary protein exacerbates hypertension and renal damage in dahl ss rats by increasing infiltrating immune cells in the kidney. Hypertension. 2011;57:269–274. doi: 10.1161/HYPERTENSIONAHA.110.154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodríguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin ii exposure. Kidney international. 2001;59:2222–2232. doi: 10.1046/j.1523-1755.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 32.Liu YH, D'Ambrosio M, Liao TD, Peng H, Rhaleb NE, Sharma U, Andre S, Gabius HJ, Carretero OA. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. American Journal of Physiology. Heart and Circulatory Physiology. 2009;296:H404–H412. doi: 10.1152/ajpheart.00747.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao TD, Yang XP, D'Ambrosio M, Zhang Y, Rhaleb NE, Carretero OA. N-acetyl-seryl-aspartyl-lysyl-proline attenuates renal injury and dysfunction in hypertensive rats with reduced renal mass: Council for high blood pressure research. Hypertension. 2010;55:459–467. doi: 10.1161/HYPERTENSIONAHA.109.144568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L, Chopp M, Teng H, Ding G, Jiang Q, Yang XP, Rhaleb NE, Zhang ZG. Combination treatment with n-acetyl-seryl-aspartyl-lysyl-proline and tissue plasminogen activator provides potent neuroprotection in rats after stroke. Stroke. 2014;45:1108–1114. doi: 10.1161/STROKEAHA.113.004399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez GE, Rhaleb NE, Nakagawa P, Liao TD, Liu Y, Leung P, Dai X, Yang XP, Carretero OA. N-acetyl-seryl-aspartyl-lysyl-proline reduces cardiac collagen cross-linking and inflammation in angiotensin ii-induced hypertensive rats. Clinical science. 2014;126:85–94. doi: 10.1042/CS20120619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin CX, Rhaleb NE, Yang XP, Liao TD, D'Ambrosio MA, Carretero OA. Prevention of aortic fibrosis by n-acetyl-seryl-aspartyl-lysyl-proline in angiotensin ii-induced hypertension. American Journal of Physiology. Heart and Circulatory Physiology. 2008;295:H1253–H1261. doi: 10.1152/ajpheart.00481.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma U, Rhaleb NE, Pokharel S, Harding P, Rasoul S, Peng H, Carretero OA. Novel anti-inflammatory mechanisms of n-acetyl-ser-asp-lys-pro in hypertension-induced target organ damage. American Journal of Physiology. Heart and Circulatory Physiology. 2008;294:H1226–H1232. doi: 10.1152/ajpheart.00305.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.C EG, de Almeida Zia VA, Vilela-Martin JF. Blockade of renin angiotensin system in heart failure post-myocardial infarction: What is the best therapy? Recent patents on cardiovascular drug discovery. 2014;9:28–37. doi: 10.2174/1574892809666140702111311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.