Abstract
The assemblage of specific ion channels and receptors at synaptic sites is crucial for signaling between pre- and postsynaptic cells. However, the mechanisms by which proteins are targeted to and clustered at synapses are poorly understood. Here we show that the product of theDrosophila discs-large gene, DLG, is colocalized with Shaker K+ channels, which are clustered at glutamatergic synapses at the larval neuromuscular junction. In heterologous cells, DLG can cluster Shaker-type K+ channels, and, in the yeast two-hybrid system, the DLG PDZ1–2 domains bind directly to the C-terminal tail of Shaker proteins. We also demonstrate that DLG-Shaker interactions are required in vivo for Shaker clustering at the neuromuscular junction. Synaptic clustering of Shaker channels is abolished not only by mutations in dlg but also by a mutation in Shaker that deletes its C-terminal DLG binding motif. Analyses of various dlg mutant alleles suggest that channel clustering and synaptic targeting functions depend on distinct DLG domains. These studies demonstrate for the first time that DLG plays an important role in synaptic organization in vivo that correlates with its ability to bind directly to specific membrane proteins of the synapse.
Keywords: discs-large, Drosophila, glutamatergic synapse, IA, ion channel clustering, MAGUK, neuromuscular junction, PDZ, potassium channels, PSD-95/SAP90, Shaker, synapse, synapse targeting
Signal transmission between neurons and their targets depends on the appropriate organization of proteins involved in neurotransmitter release and postsynaptic signal transduction (for review, see Froehner, 1993; Hall and Sanes, 1993). For example, localization of presynaptic Ca2+ channels in close proximity to active zones allows rapid vesicle exocytosis after Ca2+ influx. At the postsynaptic membrane, clustering of ionotropic neurotransmitter receptors in regions apposed to the presynaptic terminal allows the generation of postsynaptic depolarizations sufficient to trigger action potentials (Froehner, 1993). The mechanisms by which proteins become so precisely organized in synapses, however, are mainly unknown. At the vertebrate neuromuscular junction (NMJ), clustering of postsynaptic acetylcholine receptors (AChR) seems to be mediated by direct interactions with the protein rapsyn (Apel and Merlie, 1995). In the vertebrate CNS a clustering protein, gephyrin, which is required for aggregation of glycine receptors at inhibitory synapses, also has been identified (Khuse et al., 1995).
The diversity of synaptic proteins and the heterogeneity of neuronal synapses, however, predict that components in addition to rapsyn and gephyrin are required to assemble a functional synapse (Hall and Sanes, 1993). Recent studies suggest that a family of mammalian membrane-associated guanylate kinases (MAGUKs), including PSD95/SAP90, also may subserve such a role (Cho et al., 1992; Kistner et al., 1993) (for review, see Budnik, 1996; Garner and Kindler, 1996; Gomperts, 1996). PSD95/SAP90 is localized at synapses, and in vitro it interacts directly with NMDA receptors and Shaker-type K+channels (Kim et al., 1995, 1996; Kornau et al., 1995; Kim and Sheng, 1996; Niethammer et al., 1996).
MAGUKs are multidomain proteins that contain three PDZ domains, asrc homology 3 (SH3) domain, and a guanylate kinase-like domain (Woods and Bryant, 1991; Doyle et al., 1996) (for review, seeWoods and Bryant, 1993; Budnik, 1996). Direct binding occurs between specific PDZ domains of PSD95/SAP90 and the amino acid motif ET/SXV (in which E denotes glutamate, T/S threonine or serine, X any amino acid, and V, valine) at the C terminus of NMDA receptors (Kornau et al., 1995; Niethammer et al., 1996) and mammalian Shaker-type channel subunits (Kim et al., 1995). Coexpression of Shaker-type K+channels or NMDA receptors with PSD-95 in cultured heterologous cells results in the formation of K+ channel or NMDA receptor clusters (Kim et al., 1995, 1996; Kim and Sheng, 1996). These results have led to the speculation that PSD95 mediates the aggregation of specific membrane proteins at synaptic sites. However, in vivo studies are, so far, lacking that would prove this hypothesis and demonstrate the specificity of such a role.
discs-large (dlg) is a Drosophila homolog of the PSD-95 MAGUK. It also contains three PDZ domains and is localized in the fly CNS and in glutamatergic synapses at the larval NMJ (Woods and Bryant, 1991; Lahey et al., 1994; Budnik et al., 1996). Moreover,Drosophila Shaker channel subunits also contain a C-terminal ETDV sequence like their mammalian Shaker counterparts (Pongs et al., 1988; Schwarz et al., 1988). Because a variety of mutations in both genes exist, the Drosophila system offered an opportunity to determine whether DLG actually is involved in the synaptic localization of Shaker K+ channels in the intact organism.
MATERIALS AND METHODS
Fly stocks. Flies were raised at 22–25°C in standard cornmeal/molasses media. The following stocks were used for these studies: y f dlgX1-2/Basc, y w dlgm52/Bnsn, y f dlgv59/FM7/Y y+dlg+, y f dlg1P20/FM7, y f dlgsw/FM7, and Df(1)N71/FM7; Dp (1,2) 65v/+ is a deficiency that uncovers the dlg locus (referred to as Df in the text). These dlg mutant stocks are described in Perrimon (1989), Woods and Bryant (1991), and Woods et al. (1996). All anatomical experiments were done in dlg mutants over Df. Males from the stockB55D/W32P/C(1)M3 are deficient in the Sh genomic region and were used as controls for the specificity of the anti-Shaker antibody (Ferrús et al., 1990;Rogero and Tejedor, 1995). Sh102 is a mutant that produces a truncated form of Shaker protein (Gisselman et al., 1989). BG487 is a Gal-4 P-element insertion strain isolated in a previous enhancer trap screen, and UAS-dlgtransformants are described elsewhere (Budnik et al., 1996). As wild-type control, the strain Canton-S was used. Genetic markers and balancer chromosomes are described in Lindsley and Zimm (1992).
Immunocytochemistry and Western blot analysis. For anti-Shaker immunocytochemistry, body wall muscles were dissected on ice and fixed at 4°C for 15 min in fresh Bouin’s and for 15 min in Bouin’s containing 0.2% Triton X-100. After being washed [0.1m phosphate buffer, pH 7.2, and 0.1% Triton X-100 (PBT)], samples were incubated overnight at 4°C and then for 1 hr at room temperature with 1:20 affinity-purified rabbit anti-Sh antiserum (Rogero and Tejedor, 1995) diluted in PBT containing 0.2% Triton X-100 and 5 mg/ml BSA (PBTS). Samples were washed and then incubated with 1:200 FITC-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA), washed, and incubated overnight at 4°C with a mouse anti-FITC monoclonal antibody (Sigma, St. Louis, MO) at 1:500 dilution. After washes, samples finally were incubated in 1:200 FITC-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch), washed again, mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA), and visualized under epifluorescence or confocal microscopy as in Lahey et al. (1994). For double-labeling experiments, anti-DLG antibody (Woods and Bryant, 1991; Lahey et al., 1994) was applied simultaneously with anti-FITC monoclonal at 1:250 dilution, followed by simultaneous incubation with Texas Red-conjugated donkey anti-rabbit and FITC-conjugated donkey anti-mouse. No specific staining was observed in controls in which one or both primary antibodies were omitted.
The procedures for the isolation of membrane and cytosolic fractions ofDrosophila CNS and Western blot analysis of these proteins using anti-Shaker antiserum were described previously (Rogero and Tejedor, 1995). Isolation and Western blot analysis of body-wall muscle proteins with anti-DLG are described in Lahey et al. (1994), using anti-DLG antibody at a 1:10,000 dilution and HRP-conjugated anti-rabbit IgG at 1:2000 dilution.
Yeast two-hybrid assay. The coding sequence for the last 10 amino acids of Shaker B1 (Schwarz et al., 1988) was synthesized as complementary oligonucleotides and fused to the LexA DNA binding domain [13 of the last 14 amino acids are identical in allDrosophila Shaker splice variants, including the C-terminal four amino acids–ETDV (Schwarz et al., 1988)]. The LexA–Kv1.4 C-terminal tail constructs (wild-type–ETDV, and mutant–ETDE) have been described previously (Kim et al., 1995). PDZ domains of DLG [PDZ1–2 (amino acids 20–265); or PDZ3 (amino acids 483–570)] were fused to the GAL-4 activation domain of the vector pGAD10. Analogous constructs of PDZ domains from PSD95 have been described (Kim et al., 1995). Various combinations of these were transformed into the L40 yeast strain harboring the reporter genes HIS3 and β-galactosidase (β-gal) (Bartel et al., 1993; Kim et al., 1995). HIS3 activity was determined by the percentage of colonies growing on histidine-lacking medium, and β-gal activity was determined by the time required for colonies to turn blue in X-gal filter lift assay at room temperature.
Transfection experiments. Kv1.4, Shaker B1, anddlg cDNAs were subcloned into the mammalian expression vector GW1-CMV (British Biotechnology, Oxford, UK) and transfected into COS7 cells by the lipofectamine method (Life Technologies, Gaithersburg, MD). Cells were fixed with 2% formaldehyde 2 d after transfection and visualized by immunocytochemistry using anti-Kv1.4 (Sheng et al., 1992) or anti-DLG antibodies (Lahey et al., 1991; Woods and Bryant, 1991).
RESULTS
Shaker and DLG are colocalized at Type I synaptic boutons
Colocalization of Shaker and DLG proteins is a prerequisite for their direct interaction in vivo. To determine whether there was spatial overlap between DLG and Shaker, we examined the distribution of these proteins in Drosophila larval body wall muscles using anti-DLG (Woods and Bryant, 1991; Lahey et al., 1994) and anti-Drosophila Shaker antibodies (Rogero and Tejedor, 1995). The Shaker antibodies equally recognize all Shaker channel splice isoforms (Rogero and Tejedor, 1995). Electrophysiological and genetic studies have demonstrated previously that a Shaker-mediated K+ current (IA) is expressed in the larval body wall muscles (Wu and Haugland, 1985; Haugland and Wu, 1990).
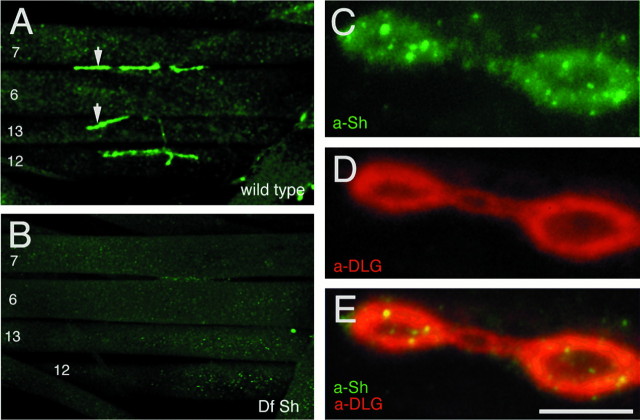
We found that Shaker immunoreactivity was concentrated around Type I synaptic boutons at the larval NMJ (Fig.1A). This immunoreactive pattern is highly reminiscent of anti-DLG immunoreactivity (Lahey et al., 1994). Four types of potassium currents, including a delayed rectifier (IK), two Ca2+-dependent potassium currents with different kinetic properties, andIA, which are mediated by channels coded in different genes, are found in the body wall muscles (Singh and Wu, 1989). Therefore, it was important to determine the specificity of the anti-Shaker staining, although the antibody used was generated against a region of the Sh sequence with low homology to the other K+ channel genes (Rogero and Tejedor, 1995). That the immunoreactivity observed at Type I boutons represented specific Shaker channel distribution was demonstrated by using Shaker-deficient larvae (Ferrús et al., 1990), which were devoid of anti-Shaker immunoreactivity (Fig. 1B).
Fig. 1.
Distribution of Shaker channels in wild-typeDrosophila larval neuromuscular junctions and colocalization with DLG. A, Anti-Shaker immunoreactivity at Type I boutons in a wild-type third instar larva. Muscle number designations are indicated. Arrows indicate Type I boutons. B, Absence of immunoreactive signal in a deficiency of Shaker larva, demonstrating the specificity of the staining. C–E, Synaptic colocalization of DLG and Shaker visualized in a high magnification view of Type I synaptic boutons double-labeled with anti-Shaker (C, green channel) and anti-DLG (D, red channel). E, Merged red and green channels. At this magnification, intense punctuate Shaker immunoreactivity is revealed within the area of the synaptic bouton that is stained with anti-Shaker antibodies. Scale bars: A, B, 80 μm; C–E, 4 μm.
At least three types of synapses, with different morphologies and containing different neurotransmitters, have been described inDrosophila NMJs (Johansen et al., 1989; Gorczyca et al., 1993; Monastirioti et al., 1995). Of these, only the glutamatergic Type I synapses have been shown to contain DLG protein (Lahey et al., 1994). This DLG expression at Type I synapses is regulated developmentally, being most prominent at presynaptic regions in the late embryo and at postsynaptic sites during larval stages (Guan et al., 1996). Significantly, Shaker immunoreactivity was found to be associated only with Type I synapses, and the Shaker staining pattern colocalized with that of DLG on those synapses (Fig. 1C–E). Thus, in wild-type flies, Shaker channels are concentrated at the same synaptic regions in which DLG is localized, consistent with a potential interaction of these two proteins in vivo. In contrast to anti-DLG immunoreactivity, which appears homogeneously distributed around Type I boutons, Shaker immunoreactivity exhibited more intense “hot spots” within the synaptic staining (Fig.1D).
Direct in vitro interaction between Shaker and DLG
To examine whether direct protein–protein interactions occur between DLG and fly Shaker as previously demonstrated for PSD95 and the mammalian Shaker channel subunit Kv1.4 (Kim et al., 1995), we used the semiquantitative yeast two-hybrid interaction assay (Fields and Song, 1989; Bartel et al., 1993), based on the level of induction of the reporter genes HIS3 and β-gal. PDZ1–2 or PDZ3 domains from DLG and PSD95 were tested for binding to the last 10 amino acids of the C-terminal tail of Drosophila Shaker B1 and Kv1.4.
The C-terminal Shaker tail was found to bind strongly to the first two PDZ domains of DLG (PDZ1–2; Table 1). In contrast, no binding was observed between the Shaker C-terminal tail and the PDZ3 domain of DLG (Table 1). This is identical to the binding specificity of Kv1.4 for the PDZ domains of PSD95. In fact, the C-terminal tail ofDrosophila Shaker also bound to PDZ1–2 from PSD95, and similarly, mammalian Kv1.4 bound readily to DLG as well as PSD95 (Table1). A mutant Kv1.4 carrying the C-terminal sequence–ETDE was unable to interact with either PSD95 or DLG in the two-hybrid assay (Table 1), confirming that the binding is dependent on the C-terminal PDZ binding motif. These results demonstrate that, as in mammals,Drosophila DLG and Shaker establish direct protein interactions via the binding of PDZ1–2 domains and the C terminus of the K+ channel.
Table 1.
| Shaker (ETDV) | Kv1.4 (ETDV) | Kv1.4 mut (ETDE) | ||||
|---|---|---|---|---|---|---|
| β-Gal | HIS3 | β-Gal | HIS3 | β-Gal | HIS3 | |
| dlg PDZ1–PDZ2 | +++ | +++ | +++ | +++ | − | − |
| dlg PDZ3 | − | − | − | − | − | − |
| PSD-95 PDZ1–PDZ2 | +++ | +++ | +++ | +++ | − | − |
| PSD-95 PDZ3 | − | − | − | − | − | − |
| pGAD10 | − | − | − | − | − | − |
Binding of Shaker-type K+ channels by DLG as determined by semiquantitative yeast two-hybrid assay, based on induction of yeast reporter genes HIS3 and β-gal. HIS3 activity was measured by the percentage of colonies growing on histidine-lacking medium [+++ (>60%); ++ (30–60%); + (10–30%); − (no significant growth)], and β-gal activity by determining the time taken for colonies to turn blue in X-gal filter lift assays at room temperature [+++ (<45 min); ++ (45–90 min); + (90–240 min); − (no significant β-gal activity)].
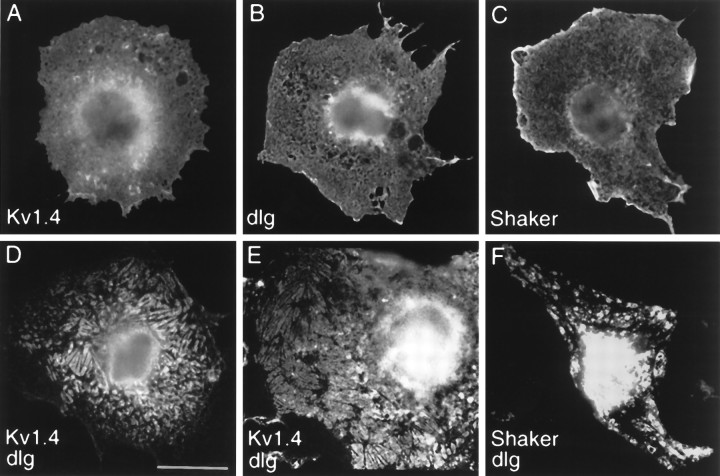
To examine the cell biological correlates of such an interaction, we cotransfected DLG with Shaker channel subunits into cultured heterologous cells (Fig. 2). When Kv1.4 or Shaker B1 cDNAs were singly transfected into COS7 cells, the K+channel immunoreactivity was distributed diffusely throughout the cell (Fig. 2A,C). DLG expressed alone also exhibited a diffuse intracellular distribution pattern (Fig. 2B). However, when Kv1.4 was cotransfected with dlg, both the mammalian Shaker protein and DLG showed a redistribution into plaque-like clusters on or near the cell surface (Fig.2D,E). The appearance of these Kv1.4 clusters was essentially identical to those resulting from Kv1.4 coexpression with PSD95 (Kim et al., 1995). Thus Drosophila DLG can function like its mammalian homolog to cluster Shaker K+ channels in heterologous cells. However, in these COS7 clustering assays, DLG was consistently less efficient than PSD-95 in clustering Kv1.4 orDrosophila Shaker K+ channels (5–10%, compared with 40–70%, respectively, as measured by the percentage of transfected cells with clear plaque-like clusters of K+channels).
Fig. 2.
Clustering of Shaker-type K+ channels by DLG in heterologous cells. A, COS7 cell singly transfected with Kv1.4 and stained with anti-Kv1.4 antibodies.B, COS7 cell singly transfected with DLG and stained with anti-DLG antibodies. C, COS7 cell singly transfected with Shaker and stained with anti-Shaker antibodies. D, E, COS7 cells cotransfected with Kv1.4 and DLG and stained with anti-Kv1.4 (D) or with anti-DLG (E) antibodies. F, COS7 cells cotransfected with Shaker and dlg and stained with anti-Shaker antibodies. When expressed alone, Kv1.4, DLG, and Shaker are distributed diffusely in the cell with some perinuclear accumulation. On coexpression of DLG and Kv1.4, or DLG and Shaker, both proteins are redistributed into plaque-like clusters (D–F) that are essentially identical to those seen with PSD-95 and Kv1.4 (Kim et al., 1995). Scale bar, 10 μm.
Much weaker clustering effect also was observed with cotransfection ofdlg and Drosophila Shaker (Fig.2F). Because clustering efficiency depends critically on the absolute and relative expression levels of PSD95 and Kv1.4 (E. Kim, unpublished observations), it is possible that poor expression ofDrosophila DLG and Shaker in monkey COS7 cells could account for the quantitative difference. Alternatively, some facilitating factor required by Drosophila DLG/Shaker may be absent in COS7 cells.
DLG is required in vivo for both Shaker K+channel clustering and synaptic targeting
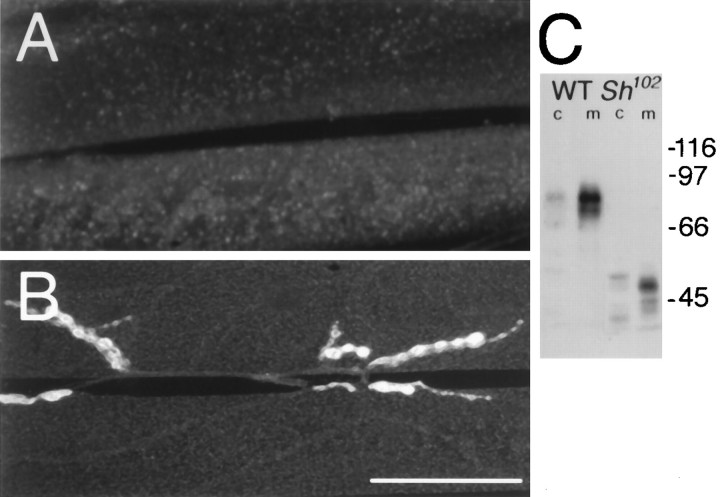
Taken together with the in vivo colocalization data (Fig. 1), the two-hybrid (Table 1) and heterologous transfection experiments (Fig. 2) suggest that a direct interaction between DLG and the C-terminal–ETDV sequence in Shaker may mediate the clustering of the K+ channel in synaptic regions. One prediction of this model is that specific deletion of the Shaker C-terminal sequence would disrupt synaptic clustering of Shaker channels at synapses in vivo. To test this hypothesis, we used a Sh mutant allele, Sh102, which lacks the C-terminal–ETDV motif (Gisselman et al., 1989). In Sh102 mutant flies, which contain a normal copy of dlg, Shaker channels failed to cluster and Type I boutons were devoid of Shaker immunoreactivity, in agreement with our model (Fig.3A). In contrast, the distribution of DLG immunoreactivity at these boutons was normal (Fig. 3B). The Shaker immunostaining in Sh102 mutants appeared diffusely distributed at the muscle membrane, barely above background levels. This apparent decrease of the Shaker immunostaining is most likely attributable to the dilution of Shaker K+channels in the whole muscle cell membrane. An alteration in the expression of the truncated Sh102 protein attributable to anomalous insertion in the cellular membrane is very unlikely, because adequate expression of the mutant Shaker protein is observed in Western blot analyses of a membrane fraction (Fig. 3C).
Fig. 3.
Lack of Shaker clustering inSh102 mutants. A, Anti-Shaker immunoreactivity in a third instar Sh102 larva, showing the absence of immunoreactivity at synaptic regions.B, Anti-DLG immunoreactivity in a differentSh102 preparation, showing normal DLG distribution at type I synapses. Scale bar, A, B, 20 μm. C, Western blot analysis of CS andSh102 cytosol (c) and membrane (m) fractions. Molecular weights (kDa) are indicated to the right of the blot. Multiple bands in the immunoblots are attributable to different Shaker isoforms produced by alternative splicing, which are detected by the antiserum (Rogero and Tejedor, 1995). Note the absence of proteolysis products and the very low levels of Sh102 protein in the cytosolic fraction, suggesting normal insertion of the truncated Sh protein in the plasma membrane.
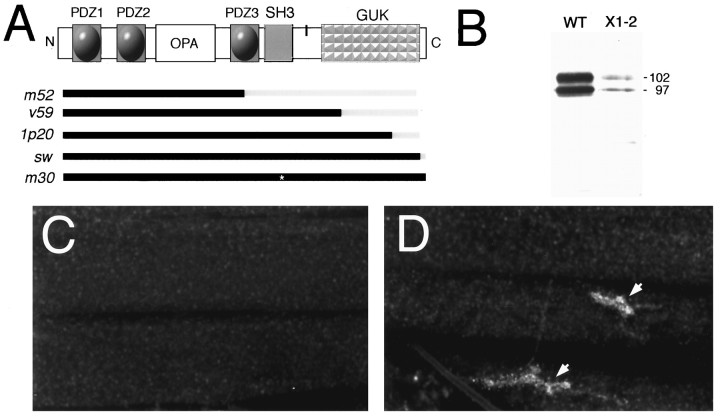
Additional genetic evidence that DLG is required for Shaker channel clustering at synapses was obtained by examining dlg mutants (Fig. 4A) (Woods and Bryant, 1991;Woods et al., 1996). dlgX1-2 is a severe hypomorphic allele, and <5% of DLG protein, as determined by Western Blot analysis, is observed during larval stages (Fig.4B). Recent molecular analysis of this allele suggests that, in addition to the reduced protein levels, a stop codon is introduced after the end of exon 8, predicting a truncated protein that lacks the GUK domain (Woods et al., 1996). However, in our studies we detected no size changes in DLGX1-2 protein (Fig. 4B). In dlgX1-2mutant larvae, Shaker immunoreactivity was absent at Type I boutons, and very low levels were observed throughout the muscle as with theSh102 mutant (Fig. 3A). This did not seem to be the result of a lack of Shaker protein expression indlgX1-2, but rather to a mislocalization or lack of clustering of the protein, because Shaker immunoreactivity was still quite intense in the larval brain (data not shown).
Fig. 4.
Genetic analysis of Shaker channel clustering and localization by DLG. A, Schematic diagram of the domain organization of the DLG protein. Bars in the bottom ofA indicate defects in several dlg mutant alleles (Woods and Bryant, 1991; Woods et al., 1996).Black, Intact protein region; gray, deleted protein region; asterisk, amino acid substitution. The vertical black bar between SH3 and GUK represents localization of the putative band 4.1 binding site.B, Western blot of body-wall muscle proteins stained with anti-DLG antibodies. Two bands of 97 and 108 kDa are observed in both wild-type and mutants, but the levels indlgX1-2 are decreased to <5% of wild-type levels. Each lane was loaded with 97 μg of body-wall muscle protein. C, Anti-Shaker immunoreactivity in dlgX1-2 body wall muscles showing lack of clustering at synaptic boutons.D, dlgv59 mutant body wall muscles labeled with anti-Shaker antibodies showing normal clustering of Shaker channels at Type I boutons. Scale bar, C, D, 40 μm.
Several domain-specific mutations in the dlg gene have been isolated, providing the opportunity to test whether Shaker channel localization is altered in these mutants and which regions of DLG may be required for this function. In dlgv59, dlg1p20, anddlgsw, different extents of the GUK domain region are deleted, but SH3 and PDZ domains are normal (Woods and Bryant, 1991; Fig. 4A). Indlgm30, a point mutation results in the substitution of a highly conserved leucine to proline in the SH3 domain, without altering PDZ and GUK domains. Although it is not clear whether this point mutation disrupts the SH3 domain,dlgm30 mutant animals develop large neoplastic tumors and die at the beginning of metamorphosis. This observation suggests that dlg function is abnormal in this mutant allele. In dlgm52 a splicing defect reveals a stop codon in intron 5, resulting in a truncated protein containing PDZ1, PDZ2, and the beginning of PDZ3 (Woods at al., 1996; Fig. 4A). Normal clustering of Shaker channels at the NMJ was observed in dlgv59 (Fig.4D), dlgsw,dlg1p20, anddlgm30, although anti-Shaker immunoreactivity was consistently lower indlgv59.
In contrast, the clustering of Shaker protein around synaptic boutons was disrupted in dlgm52 (Fig.5). In these mutants Shaker immunoreactivity was absent from Type I synapses. However, bright Shaker immunoreactive clusters were observed at nonsynaptic locations, often near the one or two muscle nuclei most proximal to the NMJ, but away from synaptic boutons (Fig. 5A,B). These results indicate that indlg mutants the formation of Shaker clusters, which depend on PDZ1–2, is still present but that the targeting of channel clusters to the appropriate synaptic regions is disrupted. This model is summarized in Figure 6.
Fig. 5.
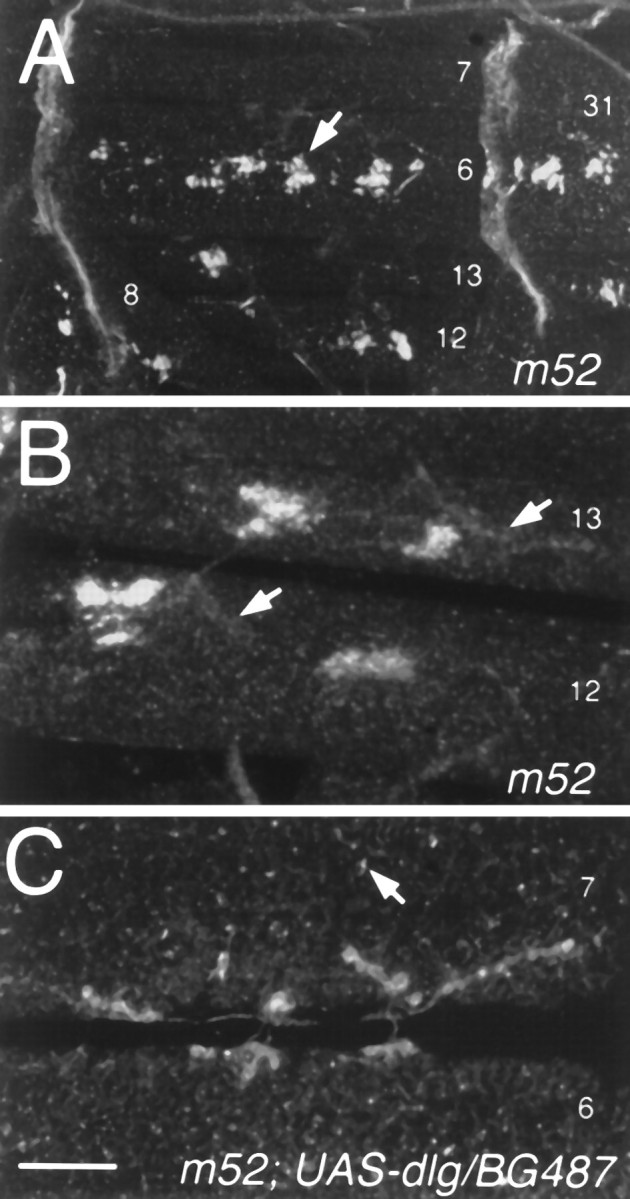
Targeting of Shaker clusters to Type I synapses is altered in dlg mutants containing only PDZ1–2 but can be rescued by postsynaptic dlg targeting. A, Anti-Shaker immunoreactivity indlgm52. In these mutants Shaker clusters (arrow) are formed at ectopic muscle regions.B, High-magnification view of ectopic clusters in muscles 12 and 13 of a dlgm52sample. Arrows indicate the localization of synaptic boutons determined by double labeling with anti-HRP antibodies (data not shown). C, Anti-Shaker immunoreactivity in adlgm52 strain carrying the P[Gal-4] element BG487, which expresses Gal-4 in a subset of muscles, and UAS-dlg. Note that in this strain clustering of Shaker channels around Type I synapses is normal and that only small ectopic clusters are observed (arrow). Scale bars: A, 90 μm; B, 25 μm;C, 20 μm.
Fig. 6.
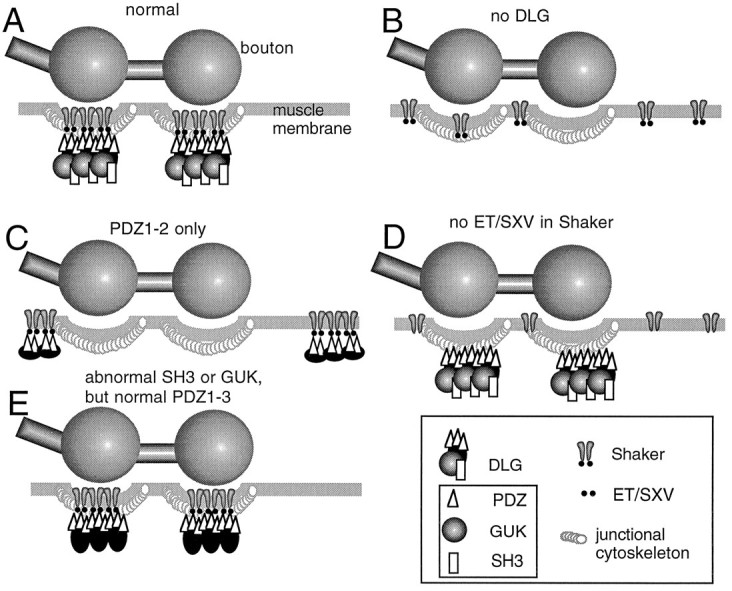
Model of Shaker clustering and Shaker synapse targeting by DLG. A, In wild-type, Shaker channels are clustered at the muscle junctional region by the interaction of their carboxyl–ETDV motif and PDZ1–2 of DLG. B, In the absence of DLG, Shaker channels fail to aggregate at the junction and remain diffusely distributed along the muscle membrane.C, When only PDZ1–2 domains are intact in DLG, Shaker channels are clustered, but at extrajunctional regions of the muscle membrane. D, Lack of ET/SXV motif in Shaker channels prevents their clustering and their localization at the junction even when DLG is normally localized. E, Abnormal SH3 or GUK domains do not prevent Shaker clustering at the junction.
To determine whether the abnormal distribution of Shaker indlgm52 mutants could be restored by the presence of normal DLG protein, we used the Gal-4 enhancer trap system to drive DLG expression in the muscle cells (Brand and Perrimon, 1993). For this experiment we used a Gal-4 P-element insertion strain, BG487, which shows strong Gal-4 expression in muscles 6 and 7 throughout the larval period, as determined in BG487/UAS–LacZ larvae. Besides a few sensory cell bodies and salivary glands, no other tissue expresses detectable levels of Gal-4 during embryonic and larval stages (Budnik et al., 1996). To drive DLG expression in muscles 6 and 7, we crosseddlgm52 mutants containing the BG487 insertion to the deficiency stain transformant containing aUAS–dlg element (Budnik et al., 1996). We found that in the progeny (dlgm52/Df;BG487/UAS–dlg) Shaker immunoreactivity was normally distributed at Type I boutons and that only small ectopic clusters could be observed (Fig.5C). This result shows that targeting DLG to muscle cells rescues the abnormal Shaker clustering phenotype ofdlgm52 mutants.
DISCUSSION
In this paper we show that in Drosophila DLG interacts directly with Shaker K+ channels and that this interaction is essential for clustering and targeting these channels to proper junctional domains in the intact animal. The clustering function had been suggested previously by in vitro studies with mammaliandlg homologs (Kim et al., 1995; Kim and Sheng, 1996). However, this is the first study to demonstrate that this clustering activity is significant for the organization of a synaptic componentin vivo. In addition, our genetic dissection of the process of Shaker channel organization at the synapse provides evidence for an additional role of DLG—the targeting of channels to synaptic regions.
The combined genetic and molecular data provide compelling evidence that the clustering of Shaker channels depends on the integrity of PDZ1–2 in DLG and the C-terminal–ETDV motif in Shaker; these are the domains that mediate the direct interaction between the two proteins. However, in dlg mutants in which only PDZ1–2 domains remain intact (dlgm52), Shaker clusters apparently still can form, but these clusters are localized at ectopic sites, away from their normal synaptic location (Fig. 6). The ectopic clustering could be rescued by targeting DLG expression to the muscle cells during the postembryonic period, demonstrating that this phenotype is dependent on the presence of intact DLG protein.
Thus, the dlgm52 phenotype suggests that a region of the DLG protein C-terminal to PDZ1–2 plays a role in targeting Shaker clusters to synapses. This synaptic targeting is unlikely to involve the SH3 and GUK domains, as evidenced by the results with dlgv59,dlg1p20,dlgsw, anddlgm30 alleles (Fig. 6). A well conserved cytoskeletal binding motif that binds to protein 4.1 has been identified in several dlg homologs (Lue et al., 1994). Band 4.1 has been associated with the function of linking membrane proteins to the underlying actin/spectrin cytoskeleton (Marchesi, 1985). The 4.1 binding motif is also present in Drosophila DLG (Lue et al., 1994) and is a potential candidate for the anchoring of Shaker clusters to synapses. A gene encoding for a band 4.1 homolog is encoded by thecoracle gene. Like DLG, COR is localized at septate junctions in epithelial tissues (Fehon et al., 1994). However, COR is not colocalized with DLG at the larval NMJ (V. Budnik, unpublished observations).
The genetic analysis of Shaker channel clustering also provides intriguing evidence for segregation of other in vivofunctions among different domains of the DLG protein. Although our results indicate that the SH3 and GUK domains are not necessary for DLG to cluster Shaker channels at synapses, the GUK domain is essential for normal development of Type I bouton postsynaptic morphology (Lahey et al., 1994; Budnik et al., 1996; Guan et al., 1996). Moreover, both SH3 and GUK domains are required for the tumor suppressor activity of DLG in the CNS and imaginal disk epithelial cells (Woods and Bryant, 1991;Woods et al., 1996). Although potential signaling roles of SH3 and GUK domains have been speculated on, the functions of these domains remain to be determined, both in epithelial cell and synaptic junctions (Woods and Bryant, 1993).
The protein rapsyn associates with nicotinic AChR and is involved in the mechanism of postsynaptic AChR clustering at the mammalian neuromuscular junction (Apel and Merlie, 1995). Because PSD95 family proteins bind and cluster the NMDA subclass of ionotropic glutamate receptors in addition to Shaker K+ channels (Kim et al., 1995, 1996; Kornau et al., 1995; Kim and Sheng, 1996; Niethammer et al., 1996), they have been suggested to play a role similar to rapsyn at glutamatergic synapses in the mammalian CNS (Kim et al., 1995, 1996;Budnik, 1996; Gomperts, 1996). In this regard, it is pertinent that DLG seems to have an important channel clustering function at the fly neuromuscular junction, which also uses glutamate as its main excitatory neurotransmitter. Interestingly, the two-hybrid and heterologous cell transfection experiments show that PDZ1–2 domains of both PSD95 and DLG can interact with either the mammalian or the fly Shaker channel. This observation shows that PDZ1–2 domains have retained their ligand-binding specificities despite some divergence in sequence (65% identity between PDZ1–2 of rat PSD95 andDrosophila DLG, as compared with 82–87% identity in this region among different members of the mammalian PSD95 family).
Our in vivo observations indicate that DLG and DLG-like proteins provide one mechanism by which the precise localization of ion channels and other proteins at synaptic regions is regulated. For example, studies of synapse structure in dlg mutants show that postsynaptic specializations at Type I boutons are underdeveloped (Lahey et al., 1994; Guan et al., 1996). This phenotype may be related to the clustering of synaptic components, such as cytoskeletal and membrane elements, required to modify synapse structure during development. Alternatively, changes in the clustering of ion channels may modify the normal synaptic activity patterns required for a correct development of NMJs (Budnik et al., 1990; Broadie and Bate, 1993; Jarecki and Keshishian, 1996). However, different domains of the DLG protein seem to be required for Shaker channel clustering and the development of postsynaptic structure. For example, postsynaptic structure is altered in both dlgv59 anddlgm52 (Lahey et al., 1994; Guan et al., 1996), whereas Shaker channel clustering appears normal indlgv59.
The diversity of channels and receptors in the central and peripheral nervous systems predicts that a large number of molecules are necessary for the proper molecular architecture of synapses. The study of DLG-like proteins, rapsyn, and gephyrin, is forming the framework by which we are beginning to understand the mechanisms of synapse assembly.
Footnotes
This work was supported by National Institutes of Health Grants RO1 NS30072 and KO4 NS01786 to V.B. and DGICYT Grant PB93-0148 and Generalitat Valenciana GV-3117/95 to F.J.T. M.S. is Assistant Investigator of the Howard Hughes Medical Institute, and V.B. is an Alfred P. Sloan Fellow.
Correspondence should be addressed to Dr. Vivian Budnik, Department of Biology, Morrill Science Center, University of Massachusetts, Amherst, MA 01003.
REFERENCES
- 1.Apel ED, Merlie JP. The assembly of the postsynaptic apparatus. Curr Opin Neurobiol. 1995;5:62–67. doi: 10.1016/0959-4388(95)80088-3. [DOI] [PubMed] [Google Scholar]
- 2.Bartel PL, Chien C-T, Sternglanz R, Fields S. Cellular interactions in development: a practical approach, pp 153–179. Oxford UP; Oxford: 1993. Using the 2-hybrid system to detect protein-protein interactions. [Google Scholar]
- 3.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development (Camb) 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 4.Broadie K, Bate M. Activity-dependent development of the neuromuscular synapse during Drosophila embryogenesis. Neuron. 1993;11:607–619. doi: 10.1016/0896-6273(93)90073-z. [DOI] [PubMed] [Google Scholar]
- 5.Budnik V (1996) Synapse maturation and structural plasticity at Drosophila neuromuscular junctions. Curr Opin Neurobiol, in press. [DOI] [PubMed]
- 6.Budnik V, Zhong Y, Wu C-F. Morphological plasticity of motor axon terminals in Drosophila mutants with altered excitability. J Neurosci. 1990;10:3754–3768. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budnik V, Koh Y-H, Guan B, Haugh C, Woods D, Gorczyca M. Regulation of synapse structure and function by the Drosophila tumor suppressor gene, dlg. Neuron. 1996;17:627–640. doi: 10.1016/s0896-6273(00)80196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho K-O, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- 9.Doyle DA, Lee A, Lewis J, Kim E, Sheng M, MacKinnon R. Crystal structures of a complexed and peptide-free membrane protein-binding domain: molecular basis of peptide recognition by PDZ. Cell. 1996;85:1067–1076. doi: 10.1016/s0092-8674(00)81307-0. [DOI] [PubMed] [Google Scholar]
- 10.Fehon RG, Dawson IA, Artavanis-Tsakonas S. A Drosophila homologue of membrane-skeleton protein 4.1 is associated with septate junctions and is encoded by the coracle gene. Development (Camb) 1994;120:545–547. doi: 10.1242/dev.120.3.545. [DOI] [PubMed] [Google Scholar]
- 11.Ferrús A, Llamazares S, de la Pompa JL, Tanouye MA, Pongs O. Genetic analysis of the Shaker gene complex of Drosophila melanogaster. Genetics. 1990;125:383–398. doi: 10.1093/genetics/125.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fields S, Song O-K. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 13.Froehner SC. Regulation of ion channel distribution at synapses. Annu Rev Neurosci. 1993;16:347–368. doi: 10.1146/annurev.ne.16.030193.002023. [DOI] [PubMed] [Google Scholar]
- 14.Garner C, Kindler S. Synaptic proteins and the assembly of synaptic junctions. Trends Cell Biol. 1996;6:429–433. doi: 10.1016/s0962-8924(96)10036-2. [DOI] [PubMed] [Google Scholar]
- 15.Gisselman G, Sewing S, Madsen BW, Mallart A, Angaut-Petit D, Müller-Holtkamp F, Ferrús A, Pongs O. The interference of truncated with normal potassium channel subunits leads to abnormal behaviour in transgenic Drosophila melanogaster. EMBO J. 1989;8:2359–2364. doi: 10.1002/j.1460-2075.1989.tb08364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomperts SN. Clustering membrane proteins: it’s all coming together with the PSD-95/SAP-90 protein family. Cell. 1996;84:659–662. doi: 10.1016/s0092-8674(00)81043-0. [DOI] [PubMed] [Google Scholar]
- 17.Gorczyca MG, Augart C, Budnik V. Insulin-like receptor and insulin-like peptide are localized at neuromuscular junctions in Drosophila. J Neurosci. 1993;13:3692–3704. doi: 10.1523/JNEUROSCI.13-09-03692.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan B, Hartmann B, Koh Y-H, Gorczyca M, Budnik V. The Drosophila tumor suppressor gene, dlg, is involved in structural plasticity at a glutamatergic synapse. Curr Biol. 1996;6:695–706. doi: 10.1016/s0960-9822(09)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall ZW, Sanes JR. Synaptic structure and development: the neuromuscular junction. Neuron. 1993;10:99–121. doi: 10.1016/s0092-8674(05)80031-5. [DOI] [PubMed] [Google Scholar]
- 20.Haugland FN, Wu C-F. A voltage-clamp analysis of gene-dosage effects of the Shaker locus on larval muscle potassium currents in Drosophila. J Neurosci. 1990;10:1357–1371. doi: 10.1523/JNEUROSCI.10-04-01357.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarecki J, Keshishian H. Role of neural activity during synaptogenesis in Drosophila. J Neurosci. 1995;15:8177–8190. doi: 10.1523/JNEUROSCI.15-12-08177.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansen J, Halpern ME, Johansen KM, Keshishian H. Stereotypic morphology of glutamatergic synapses on identified muscle cells of Drosophila larvae. J Neurosci. 1989;9:710–725. doi: 10.1523/JNEUROSCI.09-02-00710.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khuse J, Betz H, Kirsch J. The inhibitory glycine receptor: architecture, synaptic localization, and molecular pathology of a postsynaptic ion-channel complex. Curr Opin Neurobiol. 1995;5:318–323. doi: 10.1016/0959-4388(95)80044-1. [DOI] [PubMed] [Google Scholar]
- 24.Kim E, Sheng M (1996) Differential K+ channel clustering activity of PSD-95 and SAP-97, two related membrane-associated putative guanylate kinases. Neuropharmacology, in press. [DOI] [PubMed]
- 25.Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- 26.Kim E, Cho K-O, Rothschild AR, Sheng M. Heteromultimerization and NMDA receptor clustering activity of chapsyn-110, a member of the PSD-95 family of synaptic proteins. Neuron. 1996;17:103–113. doi: 10.1016/s0896-6273(00)80284-6. [DOI] [PubMed] [Google Scholar]
- 27.Kistner U, Wenzel BM, Veh RW, Cases-Langhoff C, Garner AM, Appeltauer U, Voss B, Gundelfinger ED, Garner CC. SAP90, a rat presynaptic protein related to the product of the Drosophila tumor suppressor gene dlg-A. J Biol Chem. 1993;268:4580–4583. [PubMed] [Google Scholar]
- 28.Kornau H-C, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 29.Lahey T, Gorczyca M, Jia X, Budnik V. The Drosophila tumor suppressor gene dlg is required for normal synaptic bouton structure. Neuron. 1994;13:823–835. doi: 10.1016/0896-6273(94)90249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindsley DL, Zimm GG. The genome of Drosophila melanogaster. Academic; New York: 1992. [Google Scholar]
- 31.Lue RA, Marfatia SM, Branton D, Chrishti AH. Cloning and characterization of hdlg: the human homologue of the Drosophila discs-large tumor suppressor binds to protein 4.1. Proc Natl Acad Sci USA. 1994;91:9818–9822. doi: 10.1073/pnas.91.21.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchesi VT. Stabilizing infrastructure of cell membranes. Annu Rev Cell Biol. 1985;1:531–561. doi: 10.1146/annurev.cb.01.110185.002531. [DOI] [PubMed] [Google Scholar]
- 33.Monastirioti M, Gorczyca M, Rapus J, Eckert M, White K, Budnik V. Octopamine immunoreactivity in the fruit fly Drosophila melanogaster. J Comp Neurol. 1995;356:275–287. doi: 10.1002/cne.903560210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niethammer M, Kim E, Sheng M. Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD95 family of membrane-associated guanylate kinases. J Neurosci. 1996;16:2157–2163. doi: 10.1523/JNEUROSCI.16-07-02157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perrimon N. The maternal effect of lethal(1) discs-large-1: a recessive oncogene of Drosophila melanogaster. Dev Biol. 1989;127:392–407. doi: 10.1016/0012-1606(88)90326-0. [DOI] [PubMed] [Google Scholar]
- 36.Pongs O, Kecskemethy N, Muller R, Krah-Jentgens I, Baumann A, Kiltz HH, Canal I, Llamazares S, Ferrús A. Shaker encodes a family of putative potassium channel proteins in the nervous system of Drosophila. EMBO J. 1988;7:1087–1096. doi: 10.1002/j.1460-2075.1988.tb02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogero O, Tejedor FJ. Immunochemical characterization and developmental expression of Shaker potassium channels from the nervous system of Drosophila. J Biol Chem. 1995;270:25746–25751. doi: 10.1074/jbc.270.43.25746. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz TL, Tempel BL, Papazian DM, Jan YN, Jan LY. Multiple potassium-channel components are produced by alternative splicing at the Shaker locus in Drosophila. Nature. 1988;331:137–142. doi: 10.1038/331137a0. [DOI] [PubMed] [Google Scholar]
- 39.Sheng M-L, Tsaur YN, Jan YN, Jan LY. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron. 1992;9:271–284. doi: 10.1016/0896-6273(92)90166-b. [DOI] [PubMed] [Google Scholar]
- 40.Singh S, Wu C-F. Complete separation of four potassium currents in Drosophila. Neuron. 1989;2:1325–1329. doi: 10.1016/0896-6273(89)90070-6. [DOI] [PubMed] [Google Scholar]
- 41.Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- 42.Woods DF, Bryant PJ. ZO-1, DLGA, and PSD-95/SAP-90: homologous proteins in tight, septate, and synaptic cell junctions. Mech Dev. 1993;44:85–89. doi: 10.1016/0925-4773(93)90059-7. [DOI] [PubMed] [Google Scholar]
- 43.Woods DF, Hough C, Peel D, Callaini G, Bryant P. The DLG protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J Cell Biol. 1996;134:1469–1482. doi: 10.1083/jcb.134.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu C-F, Haugland FN. Voltage-clamp analysis of membrane currents in larval muscle fibers of Drosophila: alteration of potassium currents in Shaker mutants. J Neurosci. 1985;5:2626–2640. doi: 10.1523/JNEUROSCI.05-10-02626.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]