Abstract
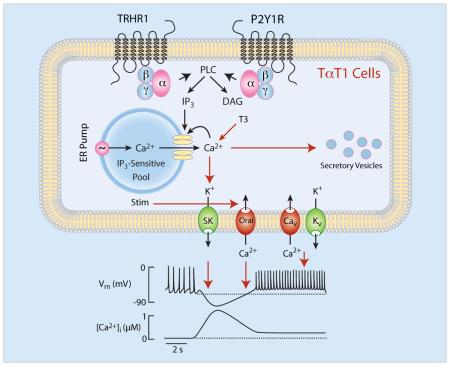
TαT1 cells are mouse thyrotroph cell line frequently used for studies on thyroid-stimulating hormone beta subunit gene expression and other cellular functions. Here we have characterized calcium-signaling pathways in TαT1 cells, an issue not previously addressed in these cells and incompletely described in native thyrotrophs. TαT1 cells are excitable and fire action potentials spontaneously and in response to application of thyrotropin-releasing hormone (TRH), the native hypothalamic agonist for thyrotrophs. Spontaneous electrical activity is coupled to small amplitude fluctuations in intracellular calcium, whereas TRH stimulates both calcium mobilization from intracellular pools and calcium influx. Non-receptor-mediated depletion of intracellular pool also leads to a prominent facilitation of calcium influx. Both receptor and non-receptor stimulated calcium influx is substantially attenuated but not completely abolished by inhibition of voltage-gated calcium channels, suggesting that depletion of intracellular calcium pool in these cells provides a signal for both voltage-independent and –dependent calcium influx, the latter by facilitating the pacemaking activity. These cells also express purinergic P2Y1 receptors and their activation by extracellular ATP mimics TRH action on calcium mobilization and influx. The thyroid hormone triiodothyronine prolongs duration of TRH-induced calcium spikes during 30-min exposure. These data indicate that TαT1 cells are capable of responding to natively feed-forward TRH signaling and intrapituitary ATP signaling with acute calcium mobilization and sustained calcium influx. Amplification of TRH-induced calcium signaling by triiodothyronine further suggests the existence of a pathway for positive feedback effects of thyroid hormones probably in a non-genomic manner.
Keywords: ATP, Calcium influx, Calcium mobilization, P2Y receptors, Thyrotrophs, Thyrotropin-releasing hormone, Triiodothryonine
1. Introduction
The anterior pituitary gland consists of at least five secretory cell types, corticotrophs, gonadotrophs, lactotrophs, somatotrophs and thyrotrophs, as well as of non-endocrine folliculostellate cells and vascular cells [1]. In vivo, these cells form a functional unit, called lobular structure, which is surrounded by extracellular matrix [2]. Within the lobule, five secretory cells are not randomly distributed but are organized with specific topographical affinities. For example, thyrotrophs are in close proximity with somatotrophs and especially with lactotrophs [3]. Folliculostellate cells are located in the center of lobule and form an excitable network that signals through gap junctions [4]. Extracellular matrix components, including laminin and collagens, contribute to the control of secretory cell types [5]. It also appears that at least some components of extracellular matrix are produced by secretory cell types, as indicated by ability of gonadotropin-releasing hormone to stimulate expression and secretion of dentin matrix protein-1 in gonadotrophs [6].
Numerous cell lines of all five secretory cell types of the anterior pituitary have been established. The most commonly used cell models are: mouse AtT-20 cells for corticotrophs, rat GC cells for somatotrophs, rat GH3/GH4C1 cells for lacto-somatotrophs, mouse LβT2 cells for gonadotrophs, and rat MMQ cells for lactotrophs [7]. These cells have provided valuable information on genealogy of the various cell lineages, the role of specific transcriptional factors determining each cell phenotype, cloning and characterization of receptors for hypothalamic neurohormones, feedback control of hormone synthesis and secretion, and signaling – transcription, and signaling – secretion coupling [7]. There is also a significant progress in understanding the calcium signaling properties and function of AtT-20 cells [8, 9], GC cells [10], GH3/GH4-C1 cells [11, 12], LβT2 [13, 14], and MMQ cells [15, 16].
Mellon and co-workers developed TαT1 cells, which represent differentiated thyrotrophs of the pars distalis [17, 18] and became a useful model for studies on thyroid-stimulating hormone beta subunit gene (Tshb) expression and other cellular functions [19-23]. At the present time, however, there is no information about calcium signaling pathways in these cells and the role of calcium in their functions. Also, very little is known about calcium signaling in native thyrotrophs. Ashworth and Hinkle showed that thyrotropin-releasing hormone (TRH) caused increase in intracellular calcium concentration ([Ca2+]i) in individual rat thyrotrophs and that the pattern of response was heterogeneous, as well as that potassium-induced depolarization of cells leads to increase in [Ca2+]i, indicating the presence of voltage-gated calcium (Cav) channels [24]. We also observed variable patterns of TRH-induced calcium signaling in identified thyrotrophs [3] as well as their spontaneous firing of action potentials (APs) [25].
Here we studied activation of calcium mobilization and influx pathways in single isolated TαT1 cells in response to application of TRH, a native hypothalamic agonist for thyrotrophs [26], and extracellular ATP, an intrapituitary agonist for two classes of receptors: G protein-coupled P2Y receptors and ATP-gated P2X channels [27]. We also examined contribution of voltage-gated and voltage-independent calcium influx pathway to calcium signaling, and the role of triiodothyronine (T3) in cellular calcium homeostasis. Moreover, we studied the coupling of TRH receptors and thyroid-stimulating hormone beta subunit gene (Tshb) expression in these cells in vitro.
2. Material and Methods
2.1. Cell culture
Coverslips and plates were coated with Matrigel (BD Biosciences, Bedford, MA) diluted 30-fold in PBS, to facilitate adhesion of TαT1 cells, provided by Dr. Pamela L. Mellon (University of California, San Diego, CA). TαT1 cells were plated during 24 or 48 h in DMEM containing 10% fetal bovine serum (FBS) treated (T3 experiments) or not with charcoal, 4.5 mg/ml glucose, 110 μg/ml pyruvate, 584 μg/ml glutamine, 100 U/ml penicillin, and 0.1 mg/ml streptomycin (Life Technologies, Grand Island, NY) and maintained at 37 oC in an environment of 5% CO2. After that, cells were used for qRT-PCR analysis and intracellular calcium or electrophysiological measurements according to protocols described below.
2.2. qRT-PCR analysis
At 60% of confluence, the cells were exposed to 100 nM TRH for 0-6 hours. Afterwards, the cells were lysed and the total RNA was extracted using Trizol® reagent. Five hundred ng of total RNA were used for the Reverse Transcriptase (RT) assay by M-MLV Reverse Transcriptase (50,000U; Promega®, EUA). The first-strand cDNA (product of the RT) was submitted to real-time quantitative PCR using GoTaq® qPCR Master Mix (Promega, WI, USA) and specific oligonucleotides for Tshb (forward: GGCAAACTGTTTCTTCCCAA; reverse: GTTGGTTTTGACAGCCTCGT; 198 bp) and Gapdh, used as an internal control, (forward: GGGCTGCCCAGAACATCAT; reverse: CCGTTCAGCTCTGGGATGAC; 76 bp). The conditions for PCR were: 95 ºC – 2 min, followed by 40 cycles of 95 ºC – 15 s, 60 ºC – 1 min, and 72 ºC – 20 s. The reactions were performed in a Corbett Research system (Corbett Life Sciences, Australia). Ct values were recorded for each target gene, normalized to the results obtained with the internal control gene according to the ddCT method previously described [28]. The efficiency and slope values for Tshb and Gapdh amplification were close to the optimal values required for the ddCT analysis and the results were expressed as fold increase relative to Gapdh expression [29].
2.3. Intracellular calcium measurements
TαT1 cells plated on matrigel-coated coverslips were bathed in KR-like medium containing 2.5 μM Fura-2 AM or Fluo-3 AM for 1 h at room temperature. After that, the coverslips were washed in KR-like media and they were mounted on the stage of an inverted Observer-D1 microscope (Carl Zeiss, Oberkochen, Germany) attached to an ORCA-ER camera (Hamamatsu Photonics, Hamamatsu City, Japan) and a Lambda DG-4 wavelength switcher (Sutter, Novato, CA). Hardware control and image analysis were performed using Metafluor software (Molecular Devices, Downingtown, PA). Experiments were done under a 40× oil-immersion objective during exposure to alternating 340- and 380 nm excitation beams for Fura-2 or 488 nm for Fluo-3 loaded cells, and the intensity of light emission at 520 nm was followed simultaneously in about 20 single cells. Changes in [Ca2+]i are presented by the ratio of fluorescence intensities F340/F380 in case of Fura-2. For Fluo-3 measurements, measured fluorescence intensities are divided by the initial fluorescence at given region and further subtracted by the same ratio at the background (region with no cells). Since the illumination of cells with beams of 340 and 380 nm wavelengths caused [Ca2+]i increase, measurements with Fura-2 had to be performed with the reduced sampling rate (about 1 point per second), while the measurements with Fluo-3 were done with 2 points per second. Traces shown are representative or mean values from at least 20 cells.
2.4. Electrophysiological measurements
Whole-cell currents and membrane voltage were recorded in single isolated TαT1 cells using nystatin (300 μg/mL) perforated patch clamp technique. Cells were seeded 4 h before the experiments in 12 mm round coverslips (10.000 per coverslip). Cells were transferred to a recording chamber and continuously perfused at 2 mL/min at room temperature with extracellular buffer containing (in mM): 140 NaCl, 5 KCl, 1. 3 MgCl2, 2.5 CaCl2, 10 HEPES, 10 Glucose, pH 7.4 adjusted with NaOH, 295 mOsm. All experiments were recorded using a Multiclamp 700A or Axopatch 200B amplifiers (Molecular Devices, Sunnyvale, CA), voltage and currents were low pass filtered at 10 kHz and digitally sampled at 20 kHz using a Digidata 1440A A/D converter and data were acquired using pClamp 10.3 software (Molecular Devices). The whole cell pipettes were pulled to a tip resistance of 3-5 MOhms and filled with an intracellular buffer containing (in mM): 120 potassium gluconate, 10 KCl, 8 mM NaCl, 10 HEPES, 0.5 mM EGTA (270 mOsmol/kg-1). All buffers and drugs were locally perfused with a peristaltic pump (BioRad, Hercules, CA) coupled to a perfusion system (ALA Scientific Instruments, Farmingdale, NY). Membrane potentials were corrected post-hoc for liquid junction potential of 14.9 mV. In voltage-clamp experiments, whole cell capacitance and series resistance were compensated by 70%. To induce the activation of voltage-dependent current, we used a 20 mV steps from −80 −100 mV (100 ms) from a holding potential of −60 mV. Leak currents were subtracted using a P/4 protocol. In current-clamp experiments the cells were held at zero and rheobase current was measured with a series of current 1 s, 2 pA current injections steps from 0 to 8 pA and defined as the smallest current injection needed to elicit an AP. Bridge balance and access resistance were monitored during the recording and experiments with >20% change were discarded.
3. Results
3.1. Electrophysiological properties of TαT1 cells
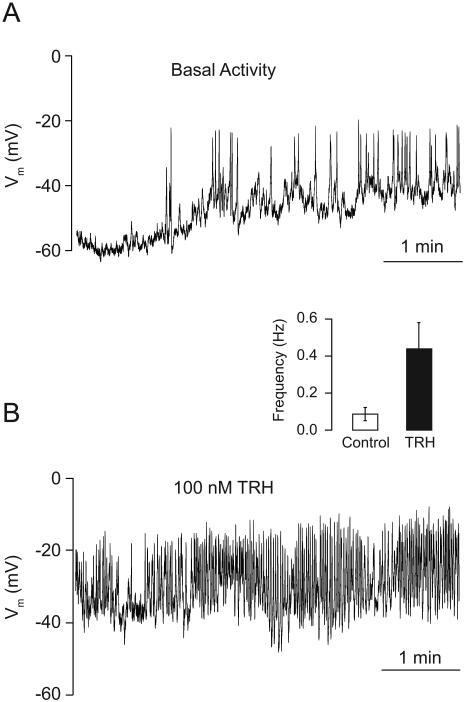
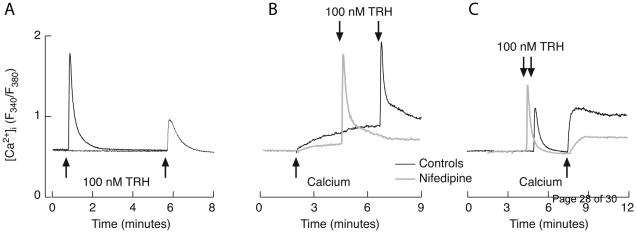
To address whether the TαT1 cells are excitable and how do they respond to TRH stimulation, we recorded the electrical activity in nystatin-perforated patch mode. After establishing a whole-cell patch, TαT1 cells showed a spontaneous electrical activity with a resting membrane potential of −55 ± 4 mV (Fig. 1A), characterized by small depolarizing events (<10 mV) with a mean frequency of 0.3 Hz, and big depolarizing non-overshooting APs with amplitudes of 40 ± 5 mV, 1.5 ± 0.3 s half width and 0.08 ± 0.03 Hz frequency. The acute stimulation with TRH causes a transient hyperpolarization due to activation of a calcium-controlled potassium channels (data not shown). The sustained stimulation with 100 nM TRH induced a depolarization of 10 mV and increased the firing frequency of the AP to 0.4 ± 0.1 Hz, with amplitudes of 35 ± 6 mV and decreasing the half width to 1.0 ± 0.2 s (Fig. 1B). The resting value of the membrane capacitance was 17 ± 2 pF before and 18.6 ± 2.3 pF after TRH stimulation. Altogether, these results indicate that TαT1 cells are spontaneously active and TRH stimulation depolarizes the resting membrane potential, leading to increase in the spiking frequency.
Fig. 1.
Characterization of spontaneous and TRH-stimulated electrical activity in TαT1 cells. (A) The electrical activity measured in current clamp mode in nystatin perforated patch showed a resting membrane potential of −55 ± 4 mV and spontaneous activity, characterized by small (< 10 mV; mean frequency 0.3 Hz) and big depolarizing events (> 10 mV; frequency 0.08 ± 0.03 Hz). (B) The TRH stimulation (100 nM) induced a depolarization of 10 mV and increased the firing frequency of the action potentials to 0.4 ± 0.1 Hz, with amplitudes of 35 ± 6 mV. (Inset) Frequency of spiking in unstimulated and TRH-stimulated cells.
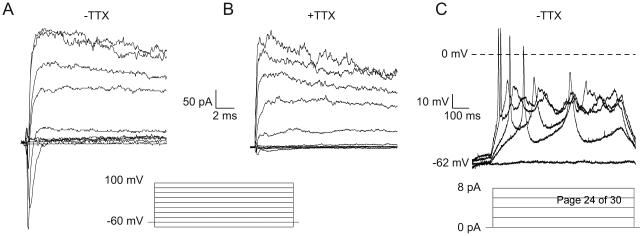
To determine the ionic conductance underlying the electrical activity, we used a voltage step protocol from −80 to +100 mV for 100 ms (Fig. 2A) in order to activate voltage dependent currents. With this protocol in the perforated-patch configuration, we observed a development of a fast inactivating Na+ current starting at −20 mV, with a peak of −250 pA at 0 mV (Fig. 2A), the application of 1 μM tetrodotoxin (TTX) reduced the inward current by 96 ± 2% (Fig. 2B) indicating the presence of TTX-sensitive voltage-dependent sodium channels. On the other hand, at depolarized potential (> −20 mV), we observed the development of an outward slow-inactivating K+ current with a peak of 300 pA (Fig. 2A and B) that was not inhibited by TTX. In summary, these results suggest that TTX-sensitive voltage-gated sodium channels and voltage-gated potassium channels contribute to TαT1 cell excitability.
Fig. 2.
Voltage-gated ion currents in TαT1 cells. (A) Representative traces in TαT1 cells elicited by 100 ms voltage steps from −80 mV to 100 mV, from a holding potential of −60 mV, showing an inward peak amplitude of −250 pA at 0 mV and an outward current peak amplitude of 300 pA at 100 mV. (B) The application of 1 μM TTX inhibited the inward current. (C) Representative traces of current injection steps showing the rheobase at 2 pA.
To determine the rheobase current threshold for APs, we performed current steps protocols from 0 to 8 pA (Fig. 2C). This experiment shows that TαT1 cells start to fire APs at 2 pA of current injection, these spikes overshoot 0 mV, and at higher current injection, the amplitude of these events decrease. Altogether, this result shows that TαT1 needs small charge transfer to trigger an AP; this behavior is in accordance with the observed high input resistance (180 ± 12 MOhms).
3.2. Calcium mobilization and influx
When bathed in Ca2+-containing medium, TαT1 cells exhibit small amplitude spontaneous [Ca2+]i transients (Fig. 3A). These transients were abolished in cells bathed in calcium-deficient medium (Fig. 4D-F), and in the presence of nifedipine, a Cav channel blocker (data not shown). These data are consistent with electrophysiological experiments shown in Fig. 1A, indicating that spontaneous electrical activity leads to voltage-gated calcium influx.
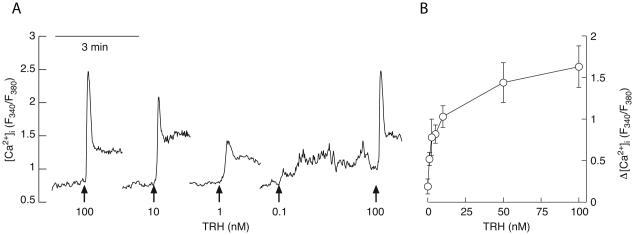
Fig. 3.
Concentration-dependent effects of TRH on [Ca2+]i response in TαT1 cell line bathed in Ca2+-containing medium. (A) Representative traces for each TRH concentration. The arrows indicate the start of TRH application. (B) Concentration-response curve depicting the dependence of the peak amplitude response subtracted by the basal values. The circles are averages calculated from three independent experiments with at least 20 cells in each one. The bars denote standard errors. The dye used was Fura-2 and changes in [Ca2+]i are represented by the ratio of the fluorescence emitted at 340 and 380 nm.
Fig. 4.
The store depletion-activated Ca2+ influx in TαT1 cells. Store depletion in Ca2+ deficient medium with the help of 100 nM TRH (A, D-F), 1 μM thapsigargin (TG), an inhibitor of SERCA type ATPase (B), or even by the simple Ca2+ leak from intracellular stores (C) is accompanied by significant Ca2+ influx once Ca2+ (2 mM) is returned to the medium. Panels A, B, and C show averaged values from at least 20 cells obtained from Fura-2 measurements, while panels D, E, and F show examples of single cell measurements obtained from Fluo-3 fluorescence. Notice fluctuations in [Ca2+]i during plateau phase in single cell recording.
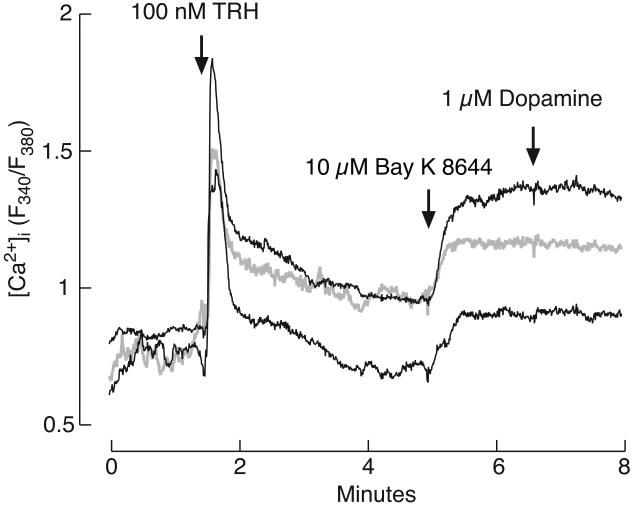
When bathed in calcium-containing medium, application of TRH caused a rapid increase in [Ca2+]i, followed by a more gradual decline to a plateau. The spike amplitude was dependent on TRH concentration. Figure 3A shows representative traces and panel B shows mean ± SEM values for spike amplitude response. The rapid [Ca2+]i response was preserved in cells bathed in calcium-deficient medium (Fig. 4A, D, E, and F), suggesting that the calcium mobilization pathway is operative in TαT1 cells. In contrast, the plateau phase was abolished in Ca2+-deficient medium, indicating that TRH also facilitates calcium influx during the sustained agonist application. The influx can be further enhanced by Bay K 8644 (Fig. 5), indicating that L-type Cav channels are expressed and operative in these cells. In contrast, dopamine was not capable of suppressing it (Fig. 5), arguing against the expression of functional dopamine DA-2 receptors in this cell type.
Fig. 5.
Effect of Bay K 8644, an L-type Cav channel agonist, and the lack of effect of dopamine on [Ca2+]i in cells bathed in calcium-containing medium and treated with TRH. Three representative traces from the same experiment are shown; single cells measurements obtained from Fura-2 fluorescence.
3.3. The nature of TRH-activated calcium influx
To examine in more details TRH-induced calcium influx, cells were initially bathed in Ca2+-deficient medium and stimulated with TRH, followed by introduction of calcium in bath solution in the presence of TRH. Figure 4 shows that, upon TRH-induced Ca2+ mobilization in extracellular Ca2+ deficient medium, Ca2+ influx can be re-initiated by returning Ca2+ to the extracellular medium. This rise was observed in both Fura-2 and Fluo-3 measurements. Figure 4A represents the mean value from over 20 cells using Fura-2 measurements, whereas Fig. 4D, E, and F show single cell traces using Fluo-3. Notice fluctuations in [Ca2+]i in single cell recording during the plateau response.
Calcium influx could also be stimulated by non-receptor-mediated Ca2+ mobilization. Figure 4B illustrates depletion of the intracellular pool with thapsigargin, an inhibitor of SERCA type ATPase, in Ca2+-deficient medium, followed by the secondary rise in [Ca2+]i induced by return of bath calcium. Keeping the cells in calcium deficient medium for a prolonged time was also sufficient to provoke Ca2+ influx (Fig. 4C). Furthermore, TRH-induced [Ca2+]i peak in cells that have spent several minutes in Ca2+-deficient medium was much lower than in cells stimulated soon after removing Ca2+ from the medium (Fig. 6A). These observations indicate that TαT1 cells have extremely leaky intracellular Ca2+ pool and that its depletion in both receptor and non-receptor manner activates calcium influx.
Fig. 6.
Involvement of Cav channels in Ca2+ influx induced by the intracellular store depletion. (A) Ca2+ leak from the intracellular pool is demonstrated by the reduction of TRH-induced [Ca2+]i amplitude after ~6 min spent in Ca2+-deficient medium. (B) Returning Ca2+ to the extracellular medium after a shorter time (~ 2 min) induced smaller Ca2+ influx (black trace) that was inhibited by 1 μM nifedipine (gray trace). Notice the recovery of TRH-induced Ca2+ signal upon returning of Ca2+ to the medium. (C) Ca2+ influx induced by returning Ca2+ to the extracellular medium of cells with depleted pools by prolonged exposure to Ca2+-deficient medium and TRH (black trace) was partially inhibited by 1 μM nifedipine (gray trace). Experiments were done using Fura-2 as a Ca2+ indicator and traces shown are averages of at least 20 cells per experiment.
In cells bathed in Ca2+-deficient medium for a short period of time, return of bath calcium causes a gradual increase in [Ca2+]i (Fig 6B, black trace). The same effect was observed in the presence of nifedipine, an L-type Cav channel blocker, but with smaller magnitude and at slower rate (Fig. 6B, gray trace). TRH-induced depletion of intracellular Ca2+ pool is also associated with a rise in [Ca2+]i after return of bath calcium (Fig. 6C, black trace), the effect that was also attenuated by application of nifedipine (Fig. 6C, gray trace). These results indicate that spontaneous and TRH-stimulated release of Ca2+ from intracellular pools triggers calcium influx through L-type Cav channels and other Ca2+ influx pathways.
3.4. The lack of effect of TRH on Tshb expression
To examine effects of TRH on Tshb expression, TαT1 cells in static cultures were exposed to 100 nM TRH or to medium alone for variable times. The RT-PCR analysis revealed a substantial basal Tshb expression and the lack of significant effect of TRH treatment on the gene expression during the first 6 h of stimulation (Table 1).
Table 1.
The lack of effect of TRH on Tshb expression in TαT1 cells in static cultures. Cells were exposed to 100 nM TRH for the time indicated. Values shown are mean ± SEM of Tshb/Gapdh expression.
| Time (h) | Basal | N | TRH-stimulated | N |
|---|---|---|---|---|
| 0 | 1.05 ± 0.27 | 6 | 0.95 ± 0.30 | 7 |
| 1 | 1.11 ± 0.06 | 3 | 1.70 ± 0.10 | 3 |
| 2 | 1.42 ± 0.25 | 6 | 1.72 ± 0.31 | 5 |
| 4 | 1.18 ± 0.19 | 4 | 1.61 ± 0.12 | 6 |
| 6 | 1.39 ± 0.13 | 5 | 1.86 ± 0.06 | 5 |
3.5. Expression of calcium-mobilizing P2Y1 receptors
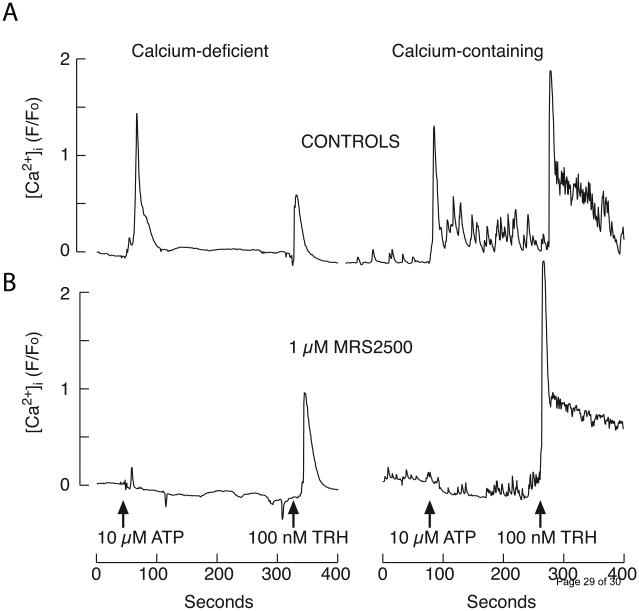
The presence of calcium-mobilizing purinergic P2Y receptors was evidenced by the calcium signaling after administration of 10 μM ATP in TαT1 cells bathed in calcium-deficient (Fig 7A, left) or − containing (Fig 7A, right) medium. In parallel to TRH action, ATP generated a monophasic response in Ca2+-deficient medium and subsequent application of TRH induced a small amplitude calcium signals. In cells bathed in Ca2+-containing medium, ATP induced biphasic response and application of TRH in the presence of ATP induced high amplitude biphasic response, indicating the refilling of intracellular Ca2+ content during the first plateau response.
Fig. 7.
TαT1 cells express the Ca2+-mobilizing P2Y1 receptor. (A) ATP- and TRH-induced [Ca2+]i responses in cells bathed in calcium-deficient (left) and calcium-containing (right) media. (B) ATP-induced rise in [Ca2+]i was blocked by 1 μM MRS2500 in both calcium deficient (left) and calcium containing medium (right), without affecting response to TRH application. Arrows indicate the time of application of agonists. Shown are representative traces of at least 20 cells. Experiments were done using Fluo-3 as a Ca2+ indicator.
In further work, we focused on identification of purinergic receptors expressed in these cells using pharmacological tools. In the presence of 1 μM MRS2500 [2-Iodo-N6-methyl-(N)-methanocarba- 2′-deoxyadenosine-3′,5′-bisphosphate], a potent and selective antagonist of P2Y1 receptor type, stimulatory effect of 10 μM ATP on [Ca2+]i was abolished, while leaving TRH induced signaling unaffected in both Ca2+ deficient (Fig 7B, left panel) and Ca2+ containing (Fig 7B, right panel). This experiment clearly indicates the expression of P2Y1 and no other calcium-mobilizing P2Y receptor subtypes, as well as the absence of functional P2X receptor channels capable of generating global calcium signals detectable by methods used in this study.
3.6. Effects of T3 on TRH-induced calcium signaling
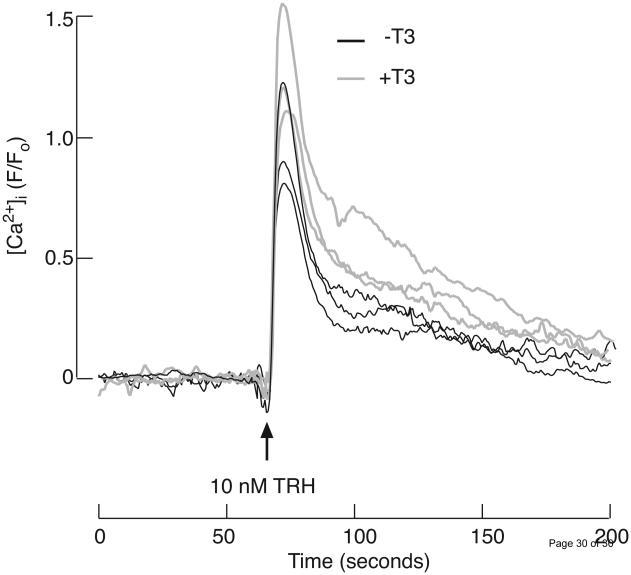
For this experiment, TαT1 cells were cultured in DMEM containing 10% charcoal-treated fetal bovine serum to exclude influence of T3 present in it. The pretreatment of TαT1 cells with 10 nM T3 for 30 min prior to TRH application modified TRH-induced [Ca2+]i signals, making the peaks broader and the decline to plateau levels slower (Fig. 8). The basal and plateau levels were not affected by T3. Because T3 effects occur within 30 min of application, these results indicate its non-genomic effects on calcium signaling in TαT1 cells.
Fig. 8.
Effects of triiodothyronine (T3) on TRH induced [Ca2+]i response in TαT1 cells. Prior to measurements, the cells were cultured for 48 h in medium containing fetal bovine serum treated with charcoal. Shown are average values of three control experiments (black traces) and three experiments where the cells where pre-treated for 30 min with 10 nM T3 (gray traces). Average values are derived from at least 20 cells. The arrow indicates the time of application of 10 nM TRH. The dye used is Fluo-3 and [Ca2+]i profiles are presented as ratios between fluorescence intensities and basal fluorescence at the corresponding region. Further background subtraction was also done.
4. Discussion
Here we established that calcium-signaling pathways of the TαT1 cell line are complex and resemble that of differentiated secretory pituitary cells. TαT1 cells are excitable and fire APs spontaneously, accompanied with voltage-gated calcium influx. Their calcium-mobilizing pathway is also operative, causing a rapid calcium release in a non-oscillatory manner and sustained facilitation of electrical activity and voltage-gated calcium influx. It appears that depletion of intracellular calcium pool in these cells not only provides a signal for voltage-independent calcium influx, but also facilitates the pacemaking activity accompanied with calcium influx. Such synchronized activity of two pathways results in a biphasic calcium signaling: the predominantly extracellular calcium-independent early spike phase and the extracellular calcium-dependent sustained plateau phase.
In general, endocrine pituitary cells are excitable and generate APs of different patterns. Some cells generate tall and narrow APs, with amplitudes of more than 60 mV, half-widths of less than 50 ms, and spiking frequencies that are typically ~0.7 Hz. Other cells generate bursting type of APs, consisting of periodic depolarized potentials with superimposed small-amplitude spikes, the membrane potential rarely goes above −10 mV during a plateau burst, and the spikes are quite small. Gonadotrophs usually fire the first type of APs [30] and somatotrophs and lactotrophs fire the second type [30-32]. However, cells from same lineage may exhibit both types of electrical activity [10, 11, 30, 33-35]. The type of APs we observed in TαT1 cells is more comparable to plateau bursting, with non-overshooting APs of 40 ± 5 mV amplitudes, 1.5 s half width, and 0.1 Hz frequency.
Calcium-mobilizing receptors are expressed in all endocrine pituitary cells. In gonadotrophs, Ca2+ mobilization is triggered by GnRH, endothelins, PACAP, substance P, and vasopressin/oxytocin. Lactotrophs express Ca2+-mobilizing receptors activated by acetylcholine, angiotensin II, ATP, ETs, oxytocin, 5-HT, substance P, TRH and galanin. In corticotrophs, the Ca2+-mobilizing pathway is activated by vasopressin, norepinephrine and potentially 5-HT. Somatotrophs express Ca2+-mobilizing ghrelin and ETA receptors [1]. Here we show that, like native thyrotrophs and lactotrophs, TαT1 cells express calcium-mobilizing TRH receptor and TRH activates these receptors with an EC50 value in subnanomolar concentrations. We also show that these cells express calcium mobilizing P2Y1 receptors, which are activated by extracellular ATP. Earlier studies with dispersed pituitary cells revealed the presence of this type of receptors in lactotrophs and other non-identified cell type [36]. Immunoreactive P2Y1 receptors were identified in rat thyrotrophs [37] and our experiments shows that mouse TαT1 cells also express functional P2Y1 receptors. In contrast to TRH-responsive lactotrophs, which express P2X4 receptor channels as well [38], the present data suggest that P2X receptor channels capable of generating global calcium signaling are not expressed in TαT1 cells.
In pituitary cells, the pattern of calcium mobilization is not determined by receptors but by cell type [1]. In lactotrophs, somatotrophs, thyrotrophs and cells from the GH cell lines, the initial rapid rise in [Ca2+]i is typically followed by a slow decline to a plateau in [Ca2+]i, with spike phase, predominantly reflecting the contribution of calcium mobilizing pathway, and the plateau phase, reflecting sustained activity of calcium influx pathway. This non-oscillatory pattern of Ca2+ response is termed biphasic. In mammalian gonadotrophs, the pulse is typically followed by large baseline [Ca2+]i oscillations [39, 40]. The present data show that TαT1 cells also respond to TRH and ATP application with biphasic response and that the ratio between the peak amplitude of spike and plateau response is determined by agonist concentration.
The coupling between calcium mobilization and influx pathways during activation of calcium mobilizing receptors is well established in both non-excitable and excitable cells. In non-excitable cells, depletion of the intracellular pool via receptor activation and non-receptor manner triggers activation of store-operated calcium entry mediated by Stim1 and Orai1 [41]. In excitable cells, including pituitary cells, voltage-gated calcium entry is known to contribute to sustained calcium entry activated by calcium-mobilizing receptors. In pituitary cells, it has been suggested that M [42] or erg-like [43, 44] potassium channels account for sustained depolarization and associated facilitation of voltage-gated calcium entry.
However, here we show that non-receptor-mediated store depletion in TαT1 cells also facilitates voltage-gated calcium entry, suggesting that the store-operated calcium entry mechanism is responsible for cell depolarization. Consistent with this, we observed thapsigargin-induced inward depolarizing current in immortalized hypothalamic GT1 cells [45] and others reported that Stim and Orai proteins play important role in neuronal calcium signaling and excitability (reviewed in [46]). We suggest that in TαT1 cells store depletion facilitates Ca2+ influx through Orai channels, leading to depolarization of cells and activation of Cav channels. In that scenario, the sustained calcium influx activated by TRH and ATP reflects activation of both Orai and Cav channels, with Orai also playing a role of pacemaking due to a depolarizing nature of ICRAC. Additional work is needed to clarify whether this feature is a unique characteristic of TαT1 cells, reflecting their embryonic origin or their immortalization, or is also operative in native thyrotrophs.
Further important information obtained in this study is a relatively rapid effect of T3 on TRH-induced calcium signaling. Exposure of TαT1 cells to T3 for up to 30 min has a small but consistent amplifying effect on biphasic response, caused by delaying the decay in [Ca2+]i after the spike in calcium response was reached. The time course of T3 effect is consistent with the non-genomic effect of this hormone on TRH-induced calcium signaling. In accordance with this hypothesis, previous studies using anterior pituitary dispersed cells have shown that perifusion of T3 for only 30 min potentiated the TRH-induced TSH release [47]. T3 also affects cardiomyocyte calcium handling [48]. In further agreement with our data, it has been shown that T3 increases mitochondrial metabolism non-transcriptionally, leading to stimulation of 1,4,5-trisphosphate-mediated calcium waves [49].
Although TαT1 cells express functional high affinity TRH receptors, their activation does not trigger Tshb expression up to 6 h of TRH administration. Such uncoupling, however, is not unique for these cells; the lack of the effect of TRH on Tshb expression was also observed in dispersed rat pituitary cells [3]. This observation reinforces the hypothesis that influence of other cell types and/or extracellular matrix proteins is/are needed for TRH-induced Tshb expression, which is commonly observed in vivo [50, 51].
In contrast to expression of two calcium-mobilizing receptor subtypes, it appears that TαT1 cells do not express functional dopamine DA2 receptors and/or sufficient number of receptors to alter spontaneous and receptor-stimulated voltage-gated calcium influx. These receptors are expressed in lactotrophs, which are also Pit-1-derived cell lineage [1], and provide critical control of spontaneous voltage-gated calcium influx and basal cAMP production and prolactin secretion [52]. Because basal cAMP production in pituitary cells is substantial [53] and this messenger interacts strongly with calcium-secretion coupling in pituitary cells [54, 55], it is reasonable to speculate that basal cyclic nucleotide production in thyrotrophs influences spontaneous and receptor controlled TSH secretion.
In conclusion, the expression of functional TRH and P2Y1 receptors in TαT1 cells indicates that embryonic thyrotrophs are equipped to respond to feed-forward action of hypothalamic TRH and intrapituitary effects of released ATP with a rapid calcium mobilization and sustained calcium influx. Moreover, TRH-induced calcium mobilization in TαT1 cells is amplified by T3, an action consistent with the positive feedback effect of this hormone on calcium-secretion coupling, presumably through a non-transcriptional mechanism, in contrast to the transcriptional and post-transcriptional negative effects of T3 on Tshb expression. Further experiments are needed to clarify the status of cyclic nucleotide production in these cells and its influence on calcium signaling and calcium-secretion coupling.
Highlights.
TαT1 thyrotrophs are excitable and generate spontaneous action potentials.
These cells express functional calcium-mobilizing TRH and P2Y1 receptors.
Their activation triggers rapid Ca2+ mobilization coupled to sustained Ca2 influx.
Both store-operated and voltage-gated calcium entry contribute to sustained calcium influx.
Cellular calcium homeostasis is affected by triiodothyronine.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Child Health and Human Development (ZIA DH000195-20) and by a grant from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP: Proc. 2013/05629-4) to MTN. MTN is recipient of Conselho Nacional de Pesquisa e Desenvolvimento (CNPq) fellowships and PBS is recipient of a FAPESP fellowship (2012/06643-8).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflicts of interest.
References
- [1].Stojilkovic SS, Tabak J, Bertram R. Ion channels and signaling in the pituitary gland. Endocr Rev. 2010;31:845–915. doi: 10.1210/er.2010-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Soji T, Mabuchi Y, Kurono C, Herbert DC. Folliculo-stellate cells and intercellular communication within the rat anterior pituitary gland. Microsc Res Tech. 1997;39:138–149. doi: 10.1002/(SICI)1097-0029(19971015)39:2<138::AID-JEMT5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- [3].Bargi-Souza P, Kucka M, Bjelobaba I, Tomic M, Janjic MM, Nunes MT, Stojilkovic SS. Loss of basal and TRH-stimulated Tshb expression in dispersed pituitary cells. Endocrinology. 2015;156:242–254. doi: 10.1210/en.2014-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fauquier T, Guerineau NC, McKinney RA, Bauer K, Mollard P. Folliculostellate cell network: a route for long-distance communication in the anterior pituitary. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8891–8896. doi: 10.1073/pnas.151339598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kuchenbauer F, Hopfner U, Stalla J, Arzt E, Stalla GK, Paez-Pereda M. Extracellular matrix components regulate ACTH production and proliferation in corticotroph tumor cells. Molecular and cellular endocrinology. 2001;175:141–148. doi: 10.1016/s0303-7207(01)00390-2. [DOI] [PubMed] [Google Scholar]
- [6].Kucka M, Bjelobaba I, Clokie SJ, Klein DC, Stojilkovic SS. Female-specific induction of rat pituitary dentin matrix protein-1 by GnRH. Mol Endocrinol. 2013;27:1840–1855. doi: 10.1210/me.2013-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ooi GT, Tawadros N, Escalona RM. Pituitary cell lines and their endocrine applications. Molecular and cellular endocrinology. 2004;228:1–21. doi: 10.1016/j.mce.2004.07.018. [DOI] [PubMed] [Google Scholar]
- [8].Fiekers JF, Konopka LM. Spontaneous transients of [Ca2+]i depend on external calcium and the activation of L-type voltage-gated calcium channels in a clonal pituitary cell line (AtT-20) of cultured mouse corticotropes. Cell calcium. 1996;19:327–336. doi: 10.1016/s0143-4160(96)90073-1. [DOI] [PubMed] [Google Scholar]
- [9].Fiekers JF, Gelbspan D, Heppner TJ. Calcium homeostasis in a clonal pituitary cell line of mouse corticotropes. Canadian journal of physiology and pharmacology. 2001;79:502–511. [PubMed] [Google Scholar]
- [10].Kwiecien R, Robert C, Cannon R, Vigues S, Arnoux A, Kordon C. C. Hammond, Endogenous pacemaker activity of rat tumour somatotrophs. J Physiol. 1998;508:883–905. doi: 10.1111/j.1469-7793.1998.883bp.x. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schlegel W, Winiger BP, Mollard P, Vacher P, Wuarin F, Zahnd GR, Wollheim CB, Dufy B. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987;329:719–721. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- [12].Willmott NJ, Asselin J, Galione A. Calcium store depletion potentiates a phosphodiesterase inhibitor- and dibutyryl cGMP-evoked calcium influx in rat pituitary GH3 cells. FEBS Lett. 1996;386:39–42. doi: 10.1016/0014-5793(96)00413-9. [DOI] [PubMed] [Google Scholar]
- [13].Naidich M, Shterntal B, Furman R, Pawson AJ, Jabbour HN, Morgan K, Millar RP, Jia J, Tomic M, Stojilkovic S, Stern N, Naor Z. Elucidation of mechanisms of the reciprocal cross talk between gonadotropin-releasing hormone and prostaglandin receptors. Endocrinology. 2010;151:2700–2712. doi: 10.1210/en.2009-1335. [DOI] [PubMed] [Google Scholar]
- [14].Zemkova H, Kucka M, Bjelobaba I, Tomic M, Stojilkovic SS. Multiple cholinergic signaling pathways in pituitary gonadotrophs. Endocrinology. 2013;154:421–433. doi: 10.1210/en.2012-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lecchi M, Redaelli E, Rosati B, Gurrola G, Florio T, Crociani O, Curia G, Cassulini RR, Masi A, Arcangeli A, Olivotto M, Schettini G, Possani LD, Wanke E. Isolation of a long-lasting eag-related gene-type K+ current in MMQ lactotrophs and its accommodating role during slow firing and prolactin release. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:3414–3425. doi: 10.1523/JNEUROSCI.22-09-03414.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Login IS, Kuan SI, Judd AM, MacLeod RM. Regulation of the intracellular calcium concentration in MMQ pituitary cells by dopamine and protein kinase C. Cell calcium. 1990;11:525–530. doi: 10.1016/0143-4160(90)90028-s. [DOI] [PubMed] [Google Scholar]
- [17].Alarid ET, Windle JJ, Whyte DB, Mellon PL. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development. 1996;122:3319–3329. doi: 10.1242/dev.122.10.3319. [DOI] [PubMed] [Google Scholar]
- [18].Yusta B, Alarid ET, Gordon DF, Ridgway EC, Mellon PL. The thyrotropin beta-subunit gene is repressed by thyroid hormone in a novel thyrotrope cell line, mouse T alphaT1 cells. Endocrinology. 1998;139:4476–4482. doi: 10.1210/endo.139.11.6283. [DOI] [PubMed] [Google Scholar]
- [19].Chiamolera MI, Sidhaye AR, Matsumoto S, He Q, Hashimoto K, Ortiga-Carvalho TM, Wondisford FE. Fundamentally distinct roles of thyroid hormone receptor isoforms in a thyrotroph cell line are due to differential DNA binding. Mol Endocrinol. 2012;26:926–939. doi: 10.1210/me.2011-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kim KK, Song SB, Kang KI, Rhee M, Kim KE. Activation of the thyroid-stimulating hormone beta-subunit gene by LIM homeodomain transcription factor Lhx2. Endocrinology. 2007;148:3468–3476. doi: 10.1210/en.2006-1088. [DOI] [PubMed] [Google Scholar]
- [21].Zatelli MC, Gentilin E, Daffara F, Tagliati F, Reimondo G, Carandina G, Ambrosio MR, Terzolo M, Degli Uberti EC. Therapeutic concentrations of mitotane (o,p'-DDD) inhibit thyrotroph cell viability and TSH expression and secretion in a mouse cell line model. Endocrinology. 2010;151:2453–2461. doi: 10.1210/en.2009-1404. [DOI] [PubMed] [Google Scholar]
- [22].Sharma V, Hays WR, Wood WM, Pugazhenthi U, St Germain DL, Bianco AC, Krezel W, Chambon P, Haugen BR. Effects of rexinoids on thyrotrope function and the hypothalamic-pituitary-thyroid axis. Endocrinology. 2006;147:1438–1451. doi: 10.1210/en.2005-0706. [DOI] [PubMed] [Google Scholar]
- [23].Aninye IO, Matsumoto S, Sidhaye AR, Wondisford FE. Circadian regulation of Tshb gene expression by Rev-Erbalpha (NR1D1) and nuclear corepressor 1 (NCOR1) The Journal of biological chemistry. 2014;289:17070–17077. doi: 10.1074/jbc.M114.569723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ashworth R, Hinkle PM. Thyrotropin-releasing hormone-induced intracellular calcium responses in individual rat lactotrophs and thyrotrophs. Endocrinology. 1996;137:5205–5212. doi: 10.1210/endo.137.12.8940336. [DOI] [PubMed] [Google Scholar]
- [25].Kretschmannova K, Kucka M, Gonzalez-Iglesias AE, Stojilkovic SS. The expression and role of hyperpolarization-activated and cyclic nucleotide-gated channels in endocrine anterior pituitary cells. Mol Endocrinol. 2012;26:153–164. doi: 10.1210/me.2011-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zoeller RT, Tan SW, Tyl RW. General background on the hypothalamic-pituitary-thyroid (HPT) axis. Crit Rev Toxicol. 2007;37:11–53. doi: 10.1080/10408440601123446. [DOI] [PubMed] [Google Scholar]
- [27].Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- [28].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [29].Dussault AA, Pouliot M. Rapid and simple comparison of messenger RNA levels using real-time PCR. Biol Proced Online. 2006;8:1–10. doi: 10.1251/bpo114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Van Goor F, Li YX, Stojilkovic SS. Paradoxical role of large-conductance calcium-activated K+ (BK) channels in controlling action potential-driven Ca2+ entry in anterior pituitary cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:5902–5915. doi: 10.1523/JNEUROSCI.21-16-05902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tsaneva-Atanasova K, Sherman A, van Goor F, Stojilkovic SS. Mechanism of spontaneous and receptor-controlled electrical activity in pituitary somatotrophs: experiments and theory. J Neurophysiol. 2007;98:131–144. doi: 10.1152/jn.00872.2006. [DOI] [PubMed] [Google Scholar]
- [32].Chen C, Zhang J, Vincent JD, Israel JM. Sodium and calcium currents in action potentials of rat somatotrophs: their possible functions in growth hormone secretion. Life Sci. 1990;46:983–989. doi: 10.1016/0024-3205(90)90021-i. [DOI] [PubMed] [Google Scholar]
- [33].Kuryshev YA, Childs GV, Ritchie AK. Corticotropin-releasing hormone stimulates Ca2+ entry through L- and P-type Ca2+ channels in rat corticotropes. Endocrinology. 1996;137:2269–2277. doi: 10.1210/endo.137.6.8641175. [DOI] [PubMed] [Google Scholar]
- [34].Kuryshev YA, Haak L, Childs GV, Ritchie AK. Corticotropin releasing hormone inhibits an inwardly rectifying potassium current in rat corticotropes. J Physiol. 1997;502(Pt 2):265–279. doi: 10.1111/j.1469-7793.1997.265bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Le Foll F, Castel H, Soriani O, Vaudry H, Cazin L. Gramicidin-perforated patch revealed depolarizing effect of GABA in cultured frog melanotrophs. J Physiol. 1998;507:55–69. doi: 10.1111/j.1469-7793.1998.055bu.x. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].He ML, Gonzalez-Iglesias AE, Stojilkovic SS. Role of nucleotide P2 receptors in calcium signaling and prolactin release in pituitary lactotrophs. The Journal of biological chemistry. 2003;278:46270–46277. doi: 10.1074/jbc.M309005200. [DOI] [PubMed] [Google Scholar]
- [37].Yu Q, Guo W, Song X, Liu X, Xiang Z, He C, Burnstock G. Expression of P2Y receptors in the rat anterior pituitary. Purinergic Signal. 2011;7:207–219. doi: 10.1007/s11302-011-9236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zemkova H, Kucka M, Li S, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. Characterization of purinergic P2X4 receptor channels expressed in anterior pituitary cells. Am J Physiol Endocrinol Metab. 2010;298:E644–651. doi: 10.1152/ajpendo.00558.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kukuljan M, Stojilkovic SS, Rojas E, Catt KJ. Apamin-sensitive potassium channels mediate agonist-induced oscillations of membrane potential in pituitary gonadotrophs. FEBS Lett. 1992;301:19–22. doi: 10.1016/0014-5793(92)80201-q. [DOI] [PubMed] [Google Scholar]
- [40].Tse A, Hille B. GnRH-induced Ca2+ oscillations and rhythmic hyperpolarizations of pituitary gonadotropes. Science. 1992;255:462–464. doi: 10.1126/science.1734523. [DOI] [PubMed] [Google Scholar]
- [41].Hogan PG, Rao A. Store-operated calcium entry: Mechanisms and modulation. Biochemical and biophysical research communications. 2015;460:40–49. doi: 10.1016/j.bbrc.2015.02.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sankaranarayanan S, Simasko SM. Characterization of an M-like current modulated by thyrotropin-releasing hormone in normal rat lactotrophs. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:1668–1678. doi: 10.1523/JNEUROSCI.16-05-01668.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schafer R, Wulfsen I, Behrens S, Weinsberg F, Bauer CK, Schwarz JR. The erg-like potassium current in rat lactotrophs. J Physiol. 1999;518:401–416. doi: 10.1111/j.1469-7793.1999.0401p.x. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bauer CK, Schafer R, Schiemann D, Reid G, Hanganu I, Schwarz JR. A functional role of the erg-like inward-rectifying K+ current in prolactin secretion from rat lactotrophs. Molecular and cellular endocrinology. 1999;148:37–45. doi: 10.1016/s0303-7207(98)00241-x. [DOI] [PubMed] [Google Scholar]
- [45].Van Goor F, Krsmanovic LZ, Catt KJ, Stojilkovic SS. Coordinate regulation of gonadotropin-releasing hormone neuronal firing patterns by cytosolic calcium and store depletion. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4101–4106. doi: 10.1073/pnas.96.7.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Moccia F, Zuccolo E, Soda T, Tanzi F, Guerra G, Mapelli L, Lodola F, D'Angelo E. Stim and Orai proteins in neuronal Ca(2+) signaling and excitability. Frontiers in cellular neuroscience. 2015;9:153. doi: 10.3389/fncel.2015.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Roussel JP, Grazzini E, Zumbihl R, Rodriguez E, Astier H. Triiodo-L-thyronine enhances TRH-induced TSH release from perifused rat pituitaries and intracellular Ca2+ levels from dispersed pituitary cells. European journal of pharmacology. 1995;289:205–215. doi: 10.1016/0922-4106(95)90096-9. [DOI] [PubMed] [Google Scholar]
- [48].Forini F, Paolicchi A, Pizzorusso T, Ratto GM, Saviozzi M, Vanini V, Iervasi G. 3,5,3'- Triiodothyronine deprivation affects phenotype and intracellular [Ca2+]i of human cardiomyocytes in culture. Cardiovascular research. 2001;51:322–330. doi: 10.1016/s0008-6363(01)00287-5. [DOI] [PubMed] [Google Scholar]
- [49].Saelim N, John LM, Wu J, Park JS, Bai Y, Camacho P, Lechleiter JD. Nontranscriptional modulation of intracellular Ca2+ signaling by ligand stimulated thyroid hormone receptor. The Journal of cell biology. 2004;167:915–924. doi: 10.1083/jcb.200409011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Persani L. Hypothalamic thyrotropin-releasing hormone and thyrotropin biological activity. Thyroid. 1998;8:941–946. doi: 10.1089/thy.1998.8.941. [DOI] [PubMed] [Google Scholar]
- [51].Taylor T, Weintraub BD. Thyrotropin (TSH)-releasing hormone regulation of TSH subunit biosynthesis and glycosylation in normal and hypothyroid rat pituitaries. Endocrinology. 1985;116:1968–1976. doi: 10.1210/endo-116-5-1968. [DOI] [PubMed] [Google Scholar]
- [52].Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiological reviews. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- [53].Kucka M, Bjelobaba I, Tomic M, Stojilkovic SS. The Role of Cyclic Nucleotides in Pituitary Lactotroph Functions. Front Endocrinol (Lausanne) 2013;4:122. doi: 10.3389/fendo.2013.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Calejo AI, Jorgacevski J, Kucka M, Kreft M, Goncalves PP, Stojilkovic SS, Zorec R. cAMP-mediated stabilization of fusion pores in cultured rat pituitary lactotrophs. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:8068–8078. doi: 10.1523/JNEUROSCI.5351-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Calejo AI, Jorgacevski J, Rituper B, Gucek A, Pereira PM, Santos MA, Potokar M, Vardjan N, Kreft M, Goncalves PP, Zorec R. Hyperpolarization-activated cyclic nucleotide-gated channels and cAMP-dependent modulation of exocytosis in cultured rat lactotrophs. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:15638–15647. doi: 10.1523/JNEUROSCI.5290-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]