Introduction
Stair climbing is a daily activity that is important for maintaining mobility in the home and community, a high quality of life, and independence. Due to the increased physical demand of stair climbing compared to level ground walking [Andriacchi et al., 1980, Costigan et al., 2002, Jevsevar et al., 1993, Kirkwood et al., 1999, McFadyen and Winter, 1988], clinicians and researchers use stair climbing performance to track and describe function in patients with knee pathology such as osteoarthritis (OA) or total knee arthroplasty (TKA). The stair climbing test (SCT) is a clinical measure frequently used to quantify stair climbing function and performance [Adegoke et al., 2012, Mizner et al., 2005, Schmitt et al., 2008, Stevens-Lapsley et al., 2011, Yoshida et al., 2008, Zeni et al., 2010]. The SCT tracks the functional status of populations of individuals with knee pathology by measuring the time it takes for the individual to ascend and descend a flight of stairs as fast as possible, with lower values indicative of better performance [Almeida et al., 2010, Kennedy et al., 2005]. Understanding the typical differences in biomechanics with respect to stair climbing speed in a healthy population may help aid clinicians’ interpretation of the SCT and development of rehabilitation protocols to enhance stair climbing performance and improve quality of life.
Stair climbing biomechanics have been described in healthy populations in order to understand the support necessary to manage the applied loads [Andriacchi, Andersson, 1980, Costigan, Deluzio, 2002, Kirkwood, Culham, 1999, Kowalk et al., 1996, Livingston et al., 1991, McFadyen and Winter, 1988, Nadeau et al., 2003, Protopapadaki et al., 2007, Riener, 2002, Yu et al., 1997], but only at self-selected speeds. During stair ascent, the greatest support contribution comes from the knee extensors, while the knee extensors and ankle plantar flexors provide relatively similar support during the weight acceptance phase of stair descent [McFadyen and Winter, 1988]. Compared to gait, larger sagittal plane range of motion is required at the knee for both stair ascent and descent, at the hip for stair ascent, and ankle for stair descent. External peak flexion moments are reported to be 3 times larger for the knee during stair ascent than gait [Andriacchi, Andersson, 1980], and 1.5 times larger for the hip during stair descent [Jevsevar, Riley, 1993]. However, stair climbing data collection methods in the literature have not accounted for speed of ascent/descent.
Speed has been investigated during level ground walking, where angles and moments at the hip, knee and ankle have a strong positive association with speed [Andriacchi et al., 1985, Bejek et al., 2006, Kirtley et al., 1985, Lelas et al., 2003, Yang and Winter, 1985]. When investigating abnormal gait biomechanics in pathological populations, it has been strongly recommended to separate the effects due to pathology from the influence of slower gait speed [Chaudhari and Andriacchi, 2008]. Along these same lines, since stair climbing is commonly used to assess the function of a patient it is important to identify whether variables should be speed-matched in order to accurately identify clinical and statistically significant differences.
The purpose of the present study was to identify how peak lower extremity angles, moments and muscle activations change with stair climbing speed in a healthy population. We hypothesized that the lower extremity joint angles, moments and muscle activation magnitudes would increase with increasing stair climbing speed during both stair ascent and descent, as they do during level ground walking [Andriacchi, Strickland, 1985, Kirtley, Whittle, 1985, Lelas, Merriman, 2003, Yang and Winter, 1985].
Materials and Methods
Investigated subjects
A convenience sample of thirty healthy subjects (15 males, 15 females, age=27.5±10.7 years, height=1.8±0.08m, weight=73.9±12.6kg) with no history of lower extremity or abdominal surgery participated in this study after providing IRB-approved informed consent.
Data Collection Methods
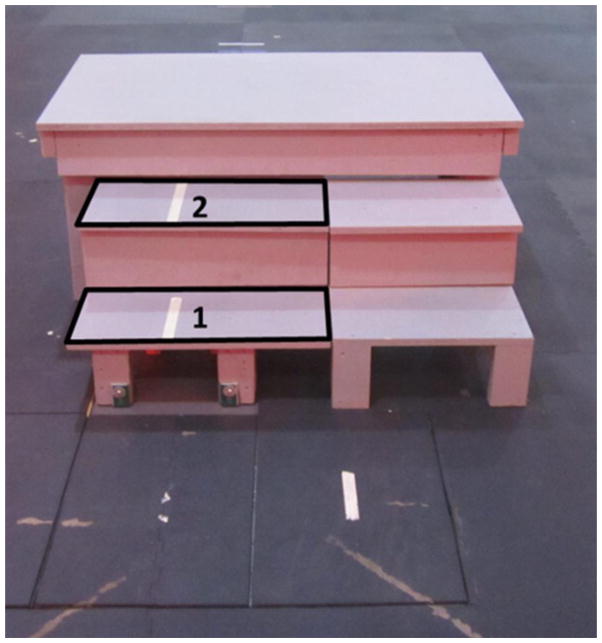
Each subject performed stair ascent and descent trials on a custom made three-step staircase (tread depth: 25.5 cm, step height: 20 cm) (Figure 1) at three different self-selected speeds. At least three trials starting with both right and left legs for each direction and speed were collected. The subjects initially stepped forward with their preferred limb and were only instructed to step with a specific limb if necessary to collect all trials. The subjects started with their normal self-selected (SS) speed, then were instructed to navigate the stairs slower than SS, and finally, faster than SS speed but without running (i.e. without a flight phase). The first two steps of the staircase were attached to force plates (Bertec 4060) embedded in the floor, and kinetic data was recorded at 1500 Hz. A modified point-cluster marker set was used on the upper and lower extremities [Jamison et al., 2012]. Three-dimensional marker data were captured with 10 Vicon MX-F40 cameras (Vicon; Oxford, UK) at 150 Hz. Unilateral lower extremity muscle activation from a randomly assigned limb was quantified using a wireless surface electromyography (EMG) system (Telemyo DTS, Noraxon USA, Inc; Scottsdale, AZ) collected at 1500 Hz. Electrode locations, placed according to the SENIAM model [Seniam], were shaved, lightly abraded and cleaned with alcohol pads. Pre-gelled, rectangular Ag/AgCL surface dual electrodes with a 42 mm inter-electrode distance (Vermed, Inc; Bellows Falls, VT) were placed on the muscle belly and oriented parallel to the fibers of the gluteus maximus (GMAX), gluteus medius (GMED), rectus femoris (RF), vastus lateralis (VL), vastus medialis (VM), semimembranosus (SM), biceps femoris (long head (BF-L) and short head (BF-S)), medial gastrocnemius (MG), lateral gastrocnemius (LG) and soleus (SOL). Each subject also performed a sit to stand task which was used to normalize the EMG signals recorded during the stair climbing task. For the sit to stand task, subjects were instructed to keep their arms crossed across their chest and to sit down and stand up four times.
Figure 1.
Stair Setup. The first and second steps, in the identified areas, are instrumented. The peak angles and moments during stance were measured on step 2 during ascent and step 1 during descent.
Data Analysis
Ground reaction force data were translated to the point of application according to the equations in the Bertec Force Plate Manual [Bertec, 2009]. Marker and force data were filtered with fourth order low-pass Butterworth filters at a cutoff frequency of 6 Hz, to minimize skin artifact and artifacts due to resonance of the staircase structure and to avoid introducing filtering artifacts from mismatched cutoff frequencies [Bisseling and Hof, 2006, Kristianslund et al., 2012]. Force data were visually examined to confirm that no flight phase occurred. Inverse dynamics for the lower extremity were calculated using custom Matlab and Vicon BodyBuilder scripts. All moments are expressed as externally applied to the joint of interest.
EMG data were hardware band pass filtered with 10 and 500 Hz cutoff frequencies to remove motion artifact [Konrad, 2005]. Centering was not needed after inspection of the signal activity while resting revealed that the RMS signal amplitude in a 500ms window was less than 0.01 V, and therefore did not introduce any bias. The muscle activation signals obtained during stair climbing were smoothed using a Root Mean Square (RMS) filter with a 100ms window, and the maximum activation during stance phase was identified for each muscle. The stair climbing muscle activation data were normalized to the maximum 500ms running average muscle activation after RMS smoothing for each muscle during the sit to stand task. Quality control of EMG was accomplished by visual inspection of each of the muscles raw EMG signals. EMG with questionable magnitude indicating possible artificial signal due to electrode motion, or missing data due to signal loss was removed from analysis [Jamison et al., 2013].
Statistical Analysis
Linear mixed models for repeated measures were used to study the association of lower extremity peak joint angles, peak external moments in the sagittal and frontal planes (Listed in Table 1), and peak muscle activations versus the stair climbing speed (slow, SS and fast). Peak angles, moments and muscle activation magnitude during stance phase were calculated on step 2 during ascent and step 1 during descent (Figure 1). Peak angles during swing phase were calculated when the leg swung over step 2 during ascent and step 1 during descent.
Table 1.
Abbreviations key.
| Key | |
|---|---|
| pKFA (°) | Peak Knee Flexion Angle |
| pKFA_swing (°) | Peak Knee Flexion Angle (swing) |
| pKFM (%BW*H) | External Peak Knee Flexion Moment |
| PHFA (°) | Peak Hip Flexion Angle |
| pHFA_swing (°) | Peak Hip Flexion Angle (swing) |
| pHFM (%BW*H) | External Peak Hip Flexion Moment |
| pHEM (%BW*H) | External Peak Hip Extension Moment |
| pADFA (°) | Peak Ankle Dorsiflexion Angle |
| pAPFA_swing (°) | Peak Ankle Plantarflexion Angle (swing) |
| pADFM (%BW*H) | External Peak Ankle Dorsiflexion Moment |
| pKAdA (°) | Peak Knee Adduction Angle |
| pKAdA_swing (°) | Peak Knee Adduction Angle (swing) |
| pKAbA (°) | Peak Knee Abduction Angle |
| pKAdM (%BW*H) | External Peak Knee Adduction Moment |
| pKAbM (%BW*H) | External Peak Knee Abduction Moment |
| pHAdA (°) | Peak Hip Adducion Angle |
| pHAbA (°) | Peak Hip Abduction Angle |
| pHAdM (%BW*H) | External Peak Hip Adduction Moment |
| pHAbM (%BW*H) | External Peak Hip Abduction Moment |
| pAAdA (°) | Peak Ankle Adduction Angle |
| pAAbA (°) | Peak Ankle Abdution Angle |
| pAAdM (%BW*H) | External Peak Ankle Adduction Moment |
| pAAbM (%BW*H) | External Peak Ankle Abduction Moment |
Unless ‘swing’ is indicated, all variables are calculated during stance phase
The mixed models for kinematics and kinetics included limb (right/left) and gender (male/female) as covariates. The kinematic and kinetic data were separated by stair climbing direction (ascent/descent) since previous literature and preliminary analysis show that peak joint angles and moments are significantly different depending on direction [Protopapadaki, Drechsler, 2007]. Subject was included as a random effect for all mixed models to account for variability between subjects. Normality tests were conducted for all of the variables of interest, and non-normal variables were log transformed for statistical analysis.
The mixed models for peak muscle activation included the interaction between direction and speed and gender as a covariate. All muscle activation data was log transformed to get a normally distributed data set.
Sensitivity analyses were conducted using a mixed model where the stair climbing speed was considered as a continuous variable, speed on step (SOS), which was calculated as the inverse of the stance time on the step. Consistent results were obtained from the sensitivity analyses. All p-values reported here have not been adjusted for multiple comparisons. An alpha of 0.01, rather than 0.05 was used to determine significance due to the number of comparisons in this data analysis.
Results
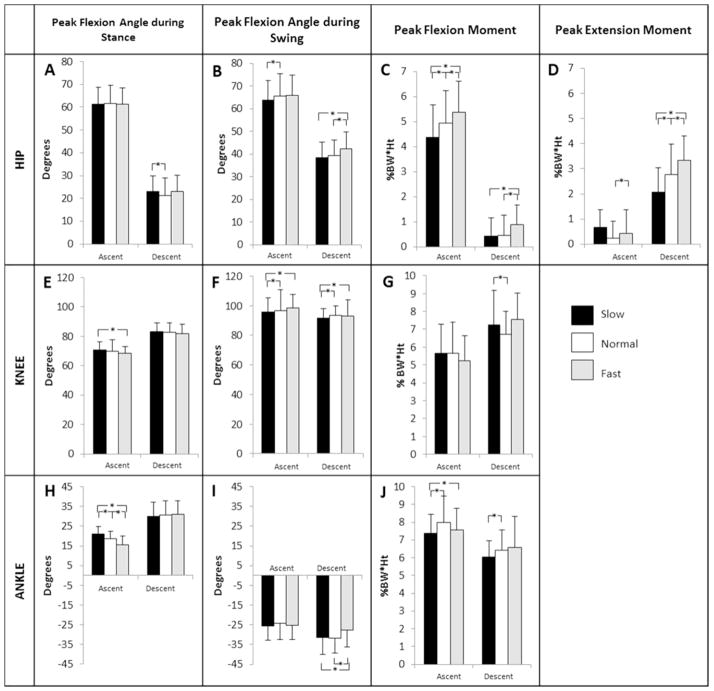
All sagittal plane angles and moments at the hip, knee, and ankle varied with speed to some extent. The pHFM during ascent (Fig 2C) and pHEM during descent (Fig 2D) increased with increasing speed (pHFM: ascent: p=0.0041, fast vs. SS; p<0.0001, fast vs. slow; descent: p<0.0001, fast vs. SS; p=0.0004, fast vs. slow; pHEM: descent: p=0.0001). The pKFA_swing was greater during the SS and fast speeds compared to the slow speed for both ascent (p=0.0003, p<0.0001, respectively) and descent (p=0.0003, p<0.0001, respectively) (Fig 2F). Only pKFM at the SS speed during descent was significantly different than the slow speed (Fig 2G). All other pKFM variables did not demonstrate differences with speed for ascent (p=0.033, fast vs. SS; p=0.052, fast vs. slow; p=0.843, SS vs. slow) and only SS to slow pKFM was significant during descent (p=0.20, fast vs. SS; p=0.31, fast vs. slow; p=0.0038, SS vs. slow) across subjects (Figure 3). The pADFA decreased with increasing speed during stair ascent (p<0.0001) (Fig 2H). The pAPFA_swing was significantly less during the fast speed compared to the SS and slow speeds during stair descent (p<0.0001) (Fig 2I). The pADFM was greatest at the SS speed during stair ascent (Fig 2J). There were no significant differences between sides (right/left limb) or gender. The peak mean and standard deviation for each of the sagittal plane kinematic and kinetic variables are displayed in Table 2. Table 3 indicates the p-values of the sagittal plane kinematic and kinetic variables that had statistically significant differences between the different speeds. Figure 2 graphically shows the means with standard deviations and indicates significance.
Figure 2.
Sagittal plane kinematic and kinetic variables of interest for the hip, knee and ankle. Statistically significant differences between speeds are indicated with an ‘*’ (p <0.01).
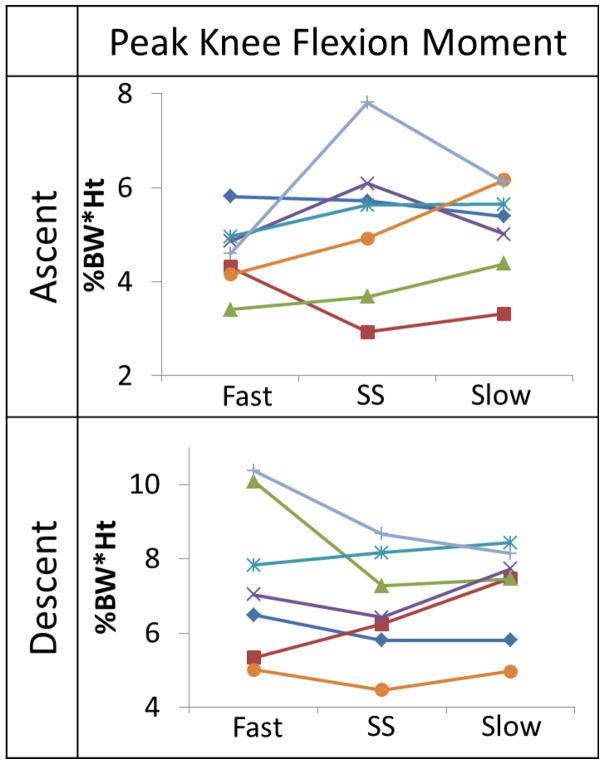
Figure 3.
Subject sample showing the inconsistent pKFM patterns as stair climbing speed changed between fast, self-selected (SS) and slow.
Table 2.
Mean ± Standard Deviation of sagittal plane kinematics and kinetics during ascent and descent.
| ASCENT | DESCENT | |||||
|---|---|---|---|---|---|---|
| FAST | SELF-SELECTED | SLOW | FAST | SELF-SELECTED | SLOW | |
| pKFA (°) | 68.64±5.68 | 69.81±7.84 | 70.72±4.42 | 81.97±6.10 | 82.56±6.58 | 83.11±6.35 |
| pKFA_swing (°) | 98.31±9.81 | 96.68±14.18 | 95.78±9.42 | 93.04±6.37 | 93.69±6.10 | 91.72±10.76 |
| pKFM (%BW*H) | 5.35±1.62 | 5.66±1.75 | 5.66±1.37 | 7.53±1.92 | 6.73±1.28 | 7.26±1.50 |
| pHFA (°) | 61.4±7.49 | 61.75±8.02 | 61.37±7.06 | 22.9±6.79 | 21.15±7.74 | 23.08±7.39 |
| pHFA_swing (°) | 65.8±9.11 | 65.47±9.90 | 63.94±8.68 | 42.27±7.41 | 39.23±6.87 | 38.35±7.11 |
| pHFM (%BW*H) | 5.38±1.24 | 4.93±1.32 | 4.37±1.31 | 0.88±0.79 | 0.45±0.81 | 0.42±0.75 |
| pHEM (%BW*H) | 0.42±0.96 | 0.25±0.66 | 0.66±0.70 | 3.33±0.97 | 2.76±1.22 | 2.08±0.95 |
| pADFA (°) | 15.39±4.65 | 18.48±3.78 | 20.87±3.82 | 31.06±6.72 | 30.64±7.31 | 29.92±7.13 |
| pAPFA_swing (°) | 25.37±7.03 | 24.32±8.21 | 25.68±6.98 | 27.64±8.65 | 31.73±7.45 | 31.32±8.70 |
| pADFM (%BW*H) | 7.55±1.24 | 8.0±1.49 | 7.37±1.07 | 6.59±1.74 | 6.44±1.13 | 6.05±0.90 |
Variable abbreviations defined in Table 1.
Table 3.
Sagittal Plane Kinematic and Kinetic p-values
| Ascent | Descent | |||||
|---|---|---|---|---|---|---|
| Fast vs. SS | Fast vs. Slow | SS vs. Slow | Fast vs. SS | Fast vs. Slow | SS vs. Slow | |
| pKFA (°) | 0.0900 | 0.0014* | 0.0256 | 0.1565 | 0.0879 | 0.5323 |
| pKFA_swing (°) | 0.3343 | 0.0003* | <.0001* | 0.7433 | 0.0003* | <.0001* |
| pKFM (%BW*H) | 0.0325 | 0.0516 | 0.8400 | 0.2032 | 0.3126 | 0.0038* |
| pHFA (°) | 0.1162 | 0.7481 | 0.0855 | 0.0271 | 0.6943 | 0.0033* |
| pHFA_swing (°) | 0.5357 | 0.0455 | <.0001* | 0.0002* | 0.0002* | 0.0846 |
| pHFM (%BW*H) | 0.0041* | <.0001* | <.0001* | <.0001* | 0.0004* | 0.7680 |
| pHEM (%BW*H) | 0.0348 | 0.6589 | 0.0434 | 0.0001* | <.0001* | <.0001* |
| pADFA (°) | <.0001* | <.0001* | <.0001* | 0.3790 | 0.0967 | 0.1903 |
| pAPFA_swing (°) | 0.1471 | 0.5472 | 0.0149 | <.0001* | <.0001* | 0.9686 |
| pADFM (%BW*H) | 0.1084 | 0.0040* | <.0001* | 0.1137 | 0.0219 | 0.0002* |
Self-selected abbreviated by SS. Variable abbreviations defined in Table 1. Significant p-values (p<0.01) are denoted with an ‘*’ and a greyed cell.
Few of the frontal plane kinematic and kinetic variables varied with speed at the hip, knee and ankle. When significant differences were found, the magnitudes of the changes were small, suggestive of minimal clinical significance. For example, while speed was a significant factor in mean pKAdA during descent and pHAdA during ascent, the mean values at the three speeds were separated by less than a degree. There were also no significant differences between sides (right/left limb) or gender. The means and standard deviations of all the frontal plane kinematic and kinetic variables are shown in Table 4. The p-values of the kinematic and kinetic variables with respect to speed are specified in Table 5. Lower extremity muscle activations varied with speed in ways that were consistent with joint moment variations. Of the quadriceps muscle activations measured with surface EMG, only the RF had the distinct pattern of increasing activation with increasing speed for ascent. For the RF during descent, the activation magnitude during the SS and slow speeds were not different from each other (p=0.75); however, the activation during the fast speed was significantly greater than at the SS and slow speeds (p<0.0001). VM and VL activations did not vary with speed during descent. During ascent, the activations during the SS and fast speeds were not different from each other (p=0.40 (VM), p=0.16 (VL)); however, activations at the slow speed were significantly less than those measured during the SS and fast speeds (p=0.0001, p=0.0063 (VM) and p<0.0001, p=0.0039 (VL), respectively).
Table 4.
Mean ± Standard Deviations of frontal plane kinematics and kinetics for ascent and descent.
| ASCENT | DESCENT | |||||
|---|---|---|---|---|---|---|
| FAST | SELF-SELECTED | SLOW | FAST | SELF-SELECTED | SLOW | |
| pKAdA (°) | 11.44±5.70 | 11.07±6.17 | 11.02±5.96 | 7.91±4.45 | 7.24±4.79 | 6.94±5.08 |
| pKAdA_swing (°) | 12.76±6.30 | 13.03±6.51 | 12.2±6.69 | 8.46±5.15 | 9.43±5.80 | 9.225.52 |
| pKAbA (°) | 1.19±3.33 | 1.48±2.75 | 1.77±3.03 | N/A | 0.13±3.00 | 0.60±2.96 |
| pKAdM (%BW*H) | 3.52±1.29 | 3.32±1.32 | 3.28±0.97 | 3.48±1.27 | 3.49±0.98 | 3.75±1.08 |
| pKAbM (%BW*H) | 0.49±0.43 | 0.46±0.41 | 0.44±0.44 | 0.25±0.31 | 0.27±0.53 | 0.17±0.29 |
| pHAdA (°) | 6.52±3.57 | 7.41±3.68 | 8.26±3.92 | 5.61±3.21 | 5.26±3.15 | 5.75±3.56 |
| pHAbA (°) | 5.07±2.87 | 5.22±3.05 | 5.16±2.78 | 6.08±3.20 | 6.35±2.83 | 6.42±3.32 |
| pHAdM (%BW*H) | 4.43±1.23 | 4.26±1.21 | 4.25±1.10 | 4.92±1.33 | 4.93±1.41 | 4.92±1.26 |
| pHAbM (%BW*H) | 0.91±0.55 | 0.70±0.50 | 0.46±0.42 | 0.58±0.60 | 0.60±0.87 | 0.23±0.42 |
| pAAdA (°) | 5.97±3.04 | 4.57±13.23 | 6.09±3.00 | 6.96±3.63 | 7.00±4.20 | 6.82±3.66 |
| pAAbA (°) | 2.01±1.69 | 2.52±12.91 | 1.64±1.59 | 2.81±2.14 | 2.95±2.50 | 2.79±2.65 |
| pAAdM (%BW*H) | 0.83±0.47 | 0.75±0.37 | 0.67±0.33 | 0.81±1.25 | 0.72±0.40 | 0.73±0.43 |
| pAAbM (%BW*H) | 0.20±0.22 | 0.17±0.21 | 0.18±0.19 | 0.20±0.20 | 0.21±0.20 | 0.19±0.18 |
Variable abbreviations defined in Table 1.
Table 5.
Frontal Plane kinematic and kinetic p-values
| Ascent | Descent | |||||
|---|---|---|---|---|---|---|
| Fast vs. SS | Fast vs. Slow | SS vs. Slow | Fast vs. SS | Fast vs. Slow | SS vs. Slow | |
| pKAdA (°) | 0.8736 | 0.1349 | 0.1083 | 0.0009* | 0.0007* | 0.7583 |
| pKAdA_swing (°) | 0.8345 | 0.2534 | 0.0624 | 0.1007 | 0.0058* | 0.0566 |
| pKAbA (°) | 0.1104 | 0.8622 | 0.0469 | 0.2190 | 0.5018 | 0.0587 |
| pKAdM (%BW*H) | 0.9220 | 0.0179 | 0.0005* | 0.1960 | 0.9133 | 0.3979 |
| pKAbM (%BW*H) | 0.6448 | 0.0599 | 0.0227 | 0.8692 | 0.0540 | 0.1554 |
| pHAdA (°) | 0.0010* | 0.0001* | 0.2423 | 0.4145 | 0.0301 | 0.1224 |
| pHAbA (°) | 0.2334 | 0.0287 | 0.2908 | 0.9154 | 0.1977 | 0.1022 |
| pHAdM (%BW*H) | 0.1732 | 0.7165 | 0.2570 | 0.1257 | 0.1950 | 0.9451 |
| pHAbM (%BW*H) | 0.0304 | <.0001* | <.0001* | 0.0407 | <.0001* | <.0001* |
| pAAdA (°) | 0.4736 | 0.6410 | 0.0845 | 0.1392 | 0.5976 | 0.0164 |
| pAAbA (°) | 0.6689 | 0.8350 | 0.7150 | 0.4139 | 0.9888 | 0.3392 |
| pAAdM (%BW*H) | 0.0256 | 0.0107 | 0.9171 | 0.3275 | 0.1578 | 0.4555 |
| pAAbM (%BW*H) | 0.1889 | 0.0005* | 0.0016* | 0.9362 | 0.0652 | 0.0089* |
Self-selected abbreviated as SS. Variable abbreviations defined in Table 1. Significant p-values (p<0.01) are denoted with an ‘*’ and in a greyed cell.
The surface EMG of all hamstring muscles; SM, BF-L, and BF-S, showed that peak activation magnitudes increased with increasing speed during both ascent and descent. The activation for GMAX also increased significantly with increasing speed for descent, but during ascent, only a trend between speed and GMAX activation was observed. The muscle activation for GMED did not vary with speed during ascent (p = 0.43, fast vs. SS; p=0.47, fast vs. slow; p=0.96, SS vs. slow). During descent, speed was a significant factor in GMED activation overall; however, only the activation at the slow speed was significantly less than the activations at the normal and fast speeds (p=0.0066, p=0.0015, respectively). The activation magnitude at the normal speed was not significantly less than the fast speed (p=0.22).
The activation magnitude of the plantar flexors; MG, LG, and SOL, increased with increasing speed during ascent, but the activation magnitude was not significantly greater between the SS and fast speeds (p=0.37, p=0.28, p=0.18). During descent, the LG and SOL significantly increased with increasing speed, while the MG was not statistically different between the SS and fast speeds. The means and standard deviation of the peak muscle activation magnitudes at each speed are presented in Table 6. The p-values of all the peak muscle activations with respect to speed are specified in Table 7.
Table 6.
Mean ± Standard Deviation of peak muscle activation magnitudes for ascent and descent.
| ASCENT | DESCENT | |||||
|---|---|---|---|---|---|---|
| SLOW | SELF-SELECTED | FAST | SLOW | SELF-SELECTED | FAST | |
| Rectus Femoris | 0.65±0.44 | 0.80±0.53 | 0.96±0.60 | 0.48±0.28 | 0.46±0.24 | 0.59±0.31 |
| Vastus Medialis | 2.41±1.97 | 2.58±1.95 | 2.59±1.93 | 1.48±1.09 | 1.48±1.13 | 1.74±1.54 |
| Vastus Lateralis | 3.86±2.68 | 4.77±3.39 | 4.60±3.31 | 2.10±1.27 | 1.98±1.07 | 2.36±1.55 |
| Semimembranosus | 1.13±0.59 | 1.45±0.80 | 1.91±1.20 | 0.61±0.53 | 0.60±0.56 | 0.54±0.36 |
| Biceps Femoris – S | 0.36±0.24 | 0.41±0.24 | 0.49±0.31 | 0.18±0.14 | 0.21±0.15 | 0.26±0.20 |
| Biceps Femoris – L | 0.35±0.28 | 0.39±0.25 | 0.48±0.31 | 0.15±0.12 | 0.18±0.12 | 0.22±0.14 |
| Gluteus Maximus | 0.30±0.20 | 0.34±0.21 | 0.36±0.24 | 0.09±0.07 | 0.11±0.06 | 0.13±0.09 |
| Gluteus Medius | 0.50±0.43 | 0.50±0.48 | 0.50±0.42 | 0.22±0.20 | 0.24±0.18 | 0.22±0.14 |
| Medial Gastrocnemius | 1.65±0.85 | 1.81±0.91 | 2.10±1.12 | 1.00±0.65 | 1.14±0.57 | 1.25±0.76 |
| Lateral Gastrocnemius | 0.99±0.48 | 1.16±0.51 | 1.28±0.58 | 0.49±0.38 | 0.46±0.26 | 0.67±0.39 |
| Soleus | 1.64±0.73 | 1.87±1.04 | 1.99±1.10 | 0.83±0.50 | 0.89±0.43 | 1.18±0.57 |
Table 7.
Peak muscle activation p-values.
| Ascent | Descent | |||||
|---|---|---|---|---|---|---|
| Fast vs. SS | Fast vs. Slow | SS vs. Slow | Fast vs. SS | Fast vs. Slow | SS vs. Slow | |
| Rectus Femoris | 0.0012* | <.0001* | 0.0003* | <.0001* | <.0001* | 0.7518 |
| Vastus Medialis | 0.4020 | 0.0063* | 0.0001* | 0.1871 | 0.7071 | 0.0564 |
| Vastus Lateralis | 0.1623 | 0.0039* | <.0001* | 0.2718 | 0.5017 | 0.6648 |
| Semimembranosus | 0.0022* | <.0001* | <.0001* | 0001* | <.0001* | <.0001* |
| Biceps Femoris – S | 0.0060* | <.0001* | 0.0001* | 0.0011* | <.0001* | <.0001* |
| Biceps Femoris – L | 0.0006* | <.0001* | 0.0035* | <.0001* | <.0001* | 0.0008* |
| Gluteus Maximus | 0.2482 | 0.0064* | 0.0152 | 0.0001* | <.0001* | 0.0013* |
| Gluteus Medius | 0.4344 | 0.4712 | 0.9579 | 0.2168 | 0.0015* | 0.0066* |
| Medial Gastrocnemius | 0.3710 | 0.0055* | 0.0014* | 0.1537 | 0.0085* | 0.0181 |
| Lateral Gastrocnemius | 0.2756 | 0.0002* | <.0001* | <.0001* | <.0001* | 0.0003* |
| Soleus | 0.1763 | <.0001* | 0.0001* | <.0001* | <.0001* | <.0001* |
Self-selected abbreviated ‘SS’. Significant p-values (p<0.01) are denoted with an ‘*’ and in a greyed cell.
Discussion
Significant differences were observed in lower extremity sagittal plane peak joint angles and moments, when stair climbing speed was changed between SS, slow, and fast in a healthy population. These changes have important implications for clinical interpretation of SCT times as well as research study design and interpretation of results. Stair climbing places an increased demand on the quadriceps compared to level ground walking, exhibited primarily by the peak external knee flexion moment [Andriacchi, Andersson, 1980, Costigan, Deluzio, 2002, Protopapadaki, Drechsler, 2007] and the magnitude of the quadriceps muscle activations [Andriacchi, Andersson, 1980, McFadyen and Winter, 1988]. In this study, several variables significantly increased when changing from the slow to the SS speed during stair ascent (pADFM (Fig 2J), pHFM (Fig 2C)) and during stair descent (pKFM (Fig 2G), pADFM (Fig 2J), pHEM (Fig 2D)), indicating that stair climbing at slower than SS speed may be less demanding on the lower extremity joints. The peak muscle activations associated with these joint moments also increased going from the slow to SS speed (VM, VL, SM, BF-L, BF-S, GMAX during stair ascent, and VM, VL, and RF during stair descent) indicating that the necessary torques and peak muscle activations are reduced when navigating stairs more slowly. Overall, slower stair climbing speeds may be used by subjects as an adaptation mechanism to reduce demand on the knee extensors, ankle plantar flexors, hip extensors during ascent, and hip flexors during descent.
Contrary to expectations, when increasing stair climbing speed from SS to fast, subjects more consistently increased the contribution of the hip (Fig 2C, 2D) rather than the knee (Fig 2G). The pKFM did not change as the participants changed speed from SS to fast during ascent or descent, which is different from what is seen during level ground walking [Andriacchi, Strickland, 1985]. This result suggests that, unlike in walking, healthy individuals do not appear to require the increased knee extensor contribution to attain faster-than-normal speeds during stair climbing. The pKFM in this healthy population had inconsistent patterns when changing speeds (Figure 3). Some subjects increased pKFM with increasing speed, others decreased pKFM with increasing speed, while others had the highest or lowest pKFM at SS rather than a monotonic relationship across all three speeds. This suggests that pKFM may be a subject specific measure. Within this population, pKFM did not show an association with speed; however, several participants did display a pattern of increasing pKFM with speed, indicating that some people may use the knee musculature to increase speed, while others use predominantly the hip or ankle, so further study is needed to determine if these subgrouping exist. Also, due to the inconsistency seen in the pKFM pattern as stair climbing speed increased within this population, it is possible that there is more than one strategy to modulate speed.
Conversely, the pHFM did significantly increase with increasing speed for both stair ascent and descent (Fig 2C), and pHEM increased with increasing speed during descent (Fig 2D). These results are both consistent with increased contributions of the hip musculature (SM, BL-L, and GMAX) when increasing stair climbing speed above SS. Of the quadriceps muscles measured, only the RF muscle activation showed a statistically significant increase as stair climbing speed increased to the fast speed. Given that the RF is both a knee extensor and a hip flexor, the change in RF peak activation while the VL and VM did not change further suggests that navigating stairs faster appears to require greater contribution from the hip flexors, consistent with the result that pHEM increases as speed increases.
In the frontal plane, only the kinematics changed as speed increased to fast. The only kinetic variables that significantly changed were pKAdM (slow to SS during ascent) and pHAdM and pAAbM (slow to SS during ascent and descent) These results suggest that in a healthy population, frontal plane motion and loading reach a plateau at SS speed, and increasing speed further, without the presence of a flight phase, does not increase frontal plane loading to any significant degree. This result is different from what has been reported in level ground walking in healthy subjects, where a linear relationship between moments and speed has been observed across the full range of walking speeds [Andriacchi, Strickland, 1985].
Although this study was performed with a healthy population, it provides insight into the biomechanics of stair climbing at different speeds. These results indicate that slower stair climbing may contribute to the abnormal kinematic and kinetic patterns previously reported in knee pathology populations. Patients with knee pathology often present with slower SCT times and abnormal kinematic and kinetic patterns. Patients with severe knee OA utilize a forward trunk lean which decreases the peak external knee flexion moment and the demand placed on the quadriceps during stair climbing [Asay et al., 2009]. Although slower SCT times are correlated with diminished quadriceps function [Hurley, 1998, Hurley and Newham, 1993], these results indicate that once the minimum amount of quadriceps strength is reached to support the body, stair climbing speed and the SCT time may be further improved by focusing on the hip musculature. However, further investigation is warranted in order to determine if the same speed relationships at the hip and knee hold true in populations with knee pathology. Knee pathology populations with weaker muscles compared to healthy controls may simply scale muscle activations with the same relative activation patterns and kinematic and kinetic patterns when climbing stairs faster [Reeves et al., 2008, 2009], and the SCT could depend more on muscle endurance rather than solely muscle strength. Alternatively, it is possible that individuals with specific pathologies experience lower extremity muscle weakness or inhibition may use different muscle activation patterns. These patterns could lead to abnormal kinetic patterns compared to what we have observed in this healthy population. Further research is needed to test these hypotheses.
When designing future studies of stair climbing, speed matching may be critical for most biomechanical variables based on the results of this study because all sagittal plane kinetic variables and muscle activation magnitudes were significantly associated with speed, either going from slow to SS, or SS to fast, or both. These associations could potentially confound any cross-sectional comparisons between healthy and pathological populations or assessments of interventions to improve stair climbing biomechanics.
There are several limitations associated with this study that should be considered. First, the age distribution of our population was not uniform, leading to our inability to examine changes in stair ascent/descent with age or to provide an ideal comparison to pathologies associated with age such as osteoarthritis. The standard limitations that are associated with skin based markers should also be mentioned. However, this study used the Point-Cluster technique on the lower extremities to reduce errors associated with soft-tissue deformation [Andriacchi, 1998], and even the fast stair climbing was slow enough that the additional soft-tissue deformations associated with ballistic activities like running and jumping were avoided. Another important limitation was the use of a three-step staircase instead of a full flight of stairs. End effects from approaching the top or bottom of the staircase may have affected the subject’s movement, and subjects were not able to achieve a steady-state speed as they might in the middle of a full flight of stairs. The lack of ability to achieve a steady-state speed was most pronounced in the fast speeds, which are most relevant in healthy populations and likely less so in populations with lower extremity pathology.
Conclusion
This study observed several significant effects of stair ascent and descent speed that could influence the clinical interpretation of a patient’s performance on the SCT. All lower extremity joints showed a decrease in moment when speed was slower than SS, indicating slower SCT times may be an adaptation by patients with lower extremity pathology to decrease the demand on the affected joint. Contrary to expectations, the knee and ankle flexion moments did not increase when changing from the SS to fast speed, while hip flexion and extension moments did increase with increasing speed. Further studies are needed to determine if the same patterns hold true for knee pathology populations. From a research design standpoint, many biomechanical variables were associated with stair climbing speed, especially in the sagittal plane; therefore, future stair climbing research studies should consider matching stair climbing speed in order to accurately assess differences between subjects or changes over time.
Acknowledgments
Funding from award number R01AR056700 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases supported this study.
Biographies

Jacqueline Lewis received her Ph.D. from The Ohio State University in Biomedical Engineering in 2015. Her dissertation research investigated the factors that affect stair climbing ability in patients with severe knee osteoarthritis and following total knee arthroplasty. Jackie is currently a senior biomechanist at the forensic biomechanics firm, ARCCA, Inc.

Greg Freisinger received his Ph.D. in mechanical engineering from The Ohio State University in 2015. His dissertation research examined intra-operative knee laxity in patients with severe osteoarthritis and following total knee arthroplasty. Greg is currently a Davies Fellow at the United States Military - West Point and Army Research Laboratory, where he teaches in the Civil and Mechanical Engineering department and investigates the effect of gait training with powered exosuits.

Dr. Xueliang (Jeff) Pan is a biostatistician at the Center for Biostatistics from the Ohio State University Wexner Medical Center. He specialized in the design of experiment and clinical trial driven by clearly defined hypotheses, and data analysis using proper statistical models. His primary interests include: to generate statistical hypotheses that address scientifically important questions; to optimize the experiment/clinical trial design with advanced statistical testing strategies; and to transform complex data into formats that can be easily and correctly interpreted. As a collaborator and co investigators, he received multiple grants from NIH and other agencies.

Laura Schmitt received her Master’s in Physical Therapy and PhD in Biomechanics and Movement Science from the University of Delaware. She has been on faculty at Ohio State University since 2010, where she teaches in the musculoskeletal curriculum. Dr. Schmitt’s research focuses on understanding neuromuscular control of the knee, as related to optimizing rehabilitation outcomes following musculoskeletal knee injury.

Robert Siston received his Ph.D. in mechanical engineering from Stanford University in 2005, and has been a member of the faculty at Ohio State since 2006. He applies principles of mechanical engineering to establish a scientific basis for the treatment of human movement disorders. His recent work has focused on knee osteoarthritis, from understanding how muscle weakness contributes to abnormal motion to optimizing surgical technique during total knee replacement to improve patient post-operative outcomes.

Ajit Chaudhari received his Ph.D. in mechanical engineering from Stanford University in 2003, and has been a member of the faculty at OSU since 2006. His research focuses on the basic question: Can we better understand how athletic injuries happen by studying the human body as a mechanical system? In particular, Dr. Chaudhari is interested in the role of core stability in the prevention and treatment of injuries across the entire body, the mechanisms behind overuse running injuries, balance and gait in patients with neurological and orthopedic pathologies, and the biomechanics of baseball pitching.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adegoke BO, Babatunde FO, Oyeyemi AL. Pain, balance, self-reported function and physical function in individuals with knee osteoarthritis. Physiother Theory Pract. 2012;28:32–40. doi: 10.3109/09593985.2011.570858. [DOI] [PubMed] [Google Scholar]
- Almeida GJ, Schroeder CA, Gil AB, Fitzgerald GK, Piva SR. Interrater reliability and validity of the stair ascend/descend test in subjects with total knee arthroplasty. Arch Phys Med Rehabil. 2010;91:932–8. doi: 10.1016/j.apmr.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriacchi TP. A point cluster method for In Vivo motion anlaysis: Applied to a study of knee kinematics. Journal of Biomechanical Engineering. 1998;120:743–99. doi: 10.1115/1.2834888. [DOI] [PubMed] [Google Scholar]
- Andriacchi TP, Andersson GB, Fermier RW, Stern D, Galante JO. A Study of Lower-Limb Mechanics during Stair-Climbing. Journal of Bone and Joint Surgery. 1980;62-A:749–57. [PubMed] [Google Scholar]
- Andriacchi TP, Strickland AB, Berme N, Engin AE, Correia Da Silva KM. Gait Analysis as a Tool to Assess Joint Kinetics In: , editor. Biomechanics of Normal and Pathological Human Articulating Joints. Dordrecht: Martinus Nijhoff; 1985. pp. 83–102. [Google Scholar]
- Asay JL, Mündermann A, Andriacchi TP. Adaptive patterns of movement during stair climbing in patients with knee osteoarthritis. Journal of Orthopaedic Research. 2009;27:325–9. doi: 10.1002/jor.20751. [DOI] [PubMed] [Google Scholar]
- Bejek Z, Paroczai R, Illyes A, Kiss RM. The influence of walking speed on gait parameters in healthy people and in patients with osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2006;14:612–22. doi: 10.1007/s00167-005-0005-6. [DOI] [PubMed] [Google Scholar]
- Bertec. Bertec Force Plates Manual. MIE Medical Research Ltd; 2009. [Google Scholar]
- Bisseling RW, Hof AL. Handling of impact forces in inverse dynamics. Journal of Biomechanics. 2006;39:2438–44. doi: 10.1016/j.jbiomech.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Chaudhari AMW, Andriacchi TP. Biomechanics and Gait. In: Fischgrund JS, editor. Orthopaedic Knowledge Update. 9. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008. pp. 379–87. [Google Scholar]
- Costigan PA, Deluzio KJ, Wyss UP. Knee and hip kinetics during normal stair climbing. Gait and Posture. 2002:31–7. doi: 10.1016/s0966-6362(01)00201-6. [DOI] [PubMed] [Google Scholar]
- Hurley MV. Quadriceps weakness in osteoarthritis. Curr Opin Rheumatol. 1998;10:246–50. doi: 10.1097/00002281-199805000-00015. [DOI] [PubMed] [Google Scholar]
- Hurley MV, Newham DJ. The influence of arthrogenous muscle inhibition on quadriceps rehabilitation of patients with early, unilateral osteoarthritic knees. Br J Rheumatol. 1993;32:127–31. doi: 10.1093/rheumatology/32.2.127. [DOI] [PubMed] [Google Scholar]
- Jamison ST, McNally MP, Schmitt LC, Chaudhari AM. The effects of core muscle activation on dynamic trunk position and knee abduction moments: implications for ACL injury. J Biomech. 2013;46:2236–41. doi: 10.1016/j.jbiomech.2013.06.021. [DOI] [PubMed] [Google Scholar]
- Jamison ST, Pan X, Chaudhari AM. Knee moments during run-to-cut maneuvers are associated with lateral trunk positioning. J Biomech. 2012;45:1881–5. doi: 10.1016/j.jbiomech.2012.05.031. [DOI] [PubMed] [Google Scholar]
- Jevsevar DS, Riley PO, Hodge WA, Krebs DE. Knee kinematics and kinetics during locomotor activities of daily living in subjects with knee arthroplasty and in healthy control subjects. Phys Ther. 1993;73:229–39. doi: 10.1093/ptj/73.4.229. discussion 40–2. [DOI] [PubMed] [Google Scholar]
- Kennedy DM, Stratford PW, Wessel J, Gollish JD, Penney D. Assessing stability and change of four performance measures: a longitudinal study evaluating outcome following total hip and knee arthroplasty. BMC Musculoskelet Disord. 2005;6:3. doi: 10.1186/1471-2474-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood RN, Culham EG, Costigan P. Hip moments during level walking, stair climbing, and exercise in individuals aged 55 years or older. Phys Ther. 1999;79:360–70. [PubMed] [Google Scholar]
- Kirtley C, Whittle MW, Jefferson RJ. Influence of walking speed on gait parameters. J Biomed Eng. 1985;7:282–8. doi: 10.1016/0141-5425(85)90055-x. [DOI] [PubMed] [Google Scholar]
- Konrad P. The ABC of EMG. A Practical Introduction to Kinesiological Electromyography from Noraxon. 2005 [Google Scholar]
- Kowalk DL, Duncan JA, Vaughan CL. Abduction–adduction moments at the knee during stair ascent and descent. J Biomech. 1996;29:383–8. doi: 10.1016/0021-9290(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Kristianslund E, Krosshaug T, van den Bogert AJ. Effect of low pass filtering on joint moments from inverse dynamics: Implications for injury prevention. Journal of Biomechanics. 2012;45:666–71. doi: 10.1016/j.jbiomech.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Lelas JL, Merriman GJ, Riley PO, Kerrigan DC. Predicting peak kinematic and kinetic parameters from gait speed. Gait Posture. 2003;17:106–12. doi: 10.1016/s0966-6362(02)00060-7. [DOI] [PubMed] [Google Scholar]
- Livingston LA, Stevenson JM, Olney SJ. Stairclimbing kinematics on stairs of differing dimensions. Arch Phys Med Rehabil. 1991;72:398–402. [PubMed] [Google Scholar]
- McFadyen BJ, Winter DA. An integrated biomechanical analysis of normal stair ascent and descent. J Biomech. 1988;21:733–44. doi: 10.1016/0021-9290(88)90282-5. [DOI] [PubMed] [Google Scholar]
- Mizner RL, Petterson SC, Stevens JE, Axe MJ, Snyder–Mackler L. Preoperative quadriceps strength predicts functional ability one year after total knee arthroplasty. J Rheumatol. 2005;32:1533–9. [PubMed] [Google Scholar]
- Nadeau S, McFadyen BJ, Malouin F. Frontal and sagittal plane analyses of the stair climbing task in healthy adults aged over 40 years: what are the challenges compared to level walking? Clinical Biomechanics. 2003;18:950–9. doi: 10.1016/s0268-0033(03)00179-7. [DOI] [PubMed] [Google Scholar]
- Protopapadaki A, Drechsler WI, Cramp MC, Coutts FJ, Scott OM. Hip, knee, ankle kinematics and kinetics during stair ascent and descent in healthy young individuals. Clinical Biomechanics. 2007;22:203–10. doi: 10.1016/j.clinbiomech.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Spanjaard M, Mohagheghi AA, Baltzopoulos V, Maganaris CN. The demands of stair descent relative to maximum capacities in elderly and young adults. J Electromyogr Kinesiol. 2008;18:218–27. doi: 10.1016/j.jelekin.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Spanjaard M, Mohagheghi AA, Baltzopoulos V, Maganaris CN. Older adults employ alternative strategies to operate within their maximum capabilities when ascending stairs. J Electromyogr Kinesiol. 2009;19:e57–68. doi: 10.1016/j.jelekin.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Riener R. Stair ascent and descent at different inclinations. Gait & Posture. 2002:32–44. doi: 10.1016/s0966-6362(01)00162-x. [DOI] [PubMed] [Google Scholar]
- Schmitt LC, Fitzgerald GK, Reisman AS, Rudolph KS. Instability, laxity, and physical function in patients with medial knee osteoarthritis. Phys Ther. 2008;88:1506–16. doi: 10.2522/ptj.20060223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seniam. SENIAM.
- Stevens-Lapsley JE, Schenkman ML, Dayton MR. Comparison of self-reported knee injury and osteoarthritis outcome score to performance measures in patients after total knee arthroplasty. PM R. 2011;3:541–9. doi: 10.1016/j.pmrj.2011.03.002. quiz 9. [DOI] [PubMed] [Google Scholar]
- Yang JF, Winter DA. Surface EMG profiles during different walking cadences in humans. Electroencephalogr Clin Neurophysiol. 1985;60:485–91. doi: 10.1016/0013-4694(85)91108-3. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Mizner RL, Ramsey DK, Snyder-Mackler L. Examining outcomes from total knee arthroplasty and the relationship between quadriceps strength and knee function over time. Clin Biomech (Bristol, Avon) 2008;23:320–8. doi: 10.1016/j.clinbiomech.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Stuart MJ, Kienbacher T, Growney ES, An KN. Valgus-varus motion of the knee in normal level walking and stair climbing. Clin Biomech (Bristol, Avon) 1997;12:286–93. doi: 10.1016/s0268-0033(97)00005-3. [DOI] [PubMed] [Google Scholar]
- Zeni JA, Jr, Axe MJ, Snyder-Mackler L. Clinical predictors of elective total joint replacement in persons with end-stage knee osteoarthritis. BMC Musculoskelet Disord. 2010;11:86. doi: 10.1186/1471-2474-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]