Abstract
The ability to maintain balance deteriorates with increasing age. The aim was to investigate the role of age in generation of anticipatory (APA) and compensatory (CPA) postural adjustments during pushing an object. Older (68.8 ± 1.0 years) and young adults (30.1 ± 1.4 years) participated in the experiment involving pushing an object (a pendulum attached to the ceiling) using both hands. Electrical activity of six leg and trunk muscles and displacements of the center of pressure (COP) were recorded and analyzed during the APA and CPA phases. The onset time, integrals of muscle activity, and COP displacements were determined. In addition, the indexes of co-activation and reciprocal activation of muscles for the shank, thigh, and trunk segments were calculated. Older adults, compared to young adults, showed less efficient postural control seen as delayed anticipatory muscle onset times and delayed COP displacements. Moreover, older adults used co-activation of muscles during the CPA phase while younger subjects utilized reciprocal activation of muscles. The observed diminished efficiency of postural control during both anticipatory and compensatory postural adjustments observed in older adults might predispose them to falls while performing tasks involving pushing. The outcome provides a background for future studies focused on the optimization of the daily activities of older adults.
Keywords: aging, anticipatory, compensatory, muscle activity, pushing
1. Introduction
Age is an important factor affecting the ability to maintain standing posture while performing voluntary arm movements (Bleuse et al., 2006, Carvalho et al., 2010, Kubicki et al., 2012) or dealing with external impacts to the body (Bugnariu and Sveistrup, 2006, Kanekar and Aruin, 2014a, b). Both arm movements and external impacts induce body perturbations and require implementation of the anticipatory and compensatory postural strategies to maintain and restore balance. Anticipatory postural adjustments (APA) are a feed-forward control mechanism, which reflects changes in the activity of postural muscles prior to the expected postural perturbations (Aruin and Latash, 1995, 1996, Belen'kii et al., 1967, Massion, 1992). The compensatory postural adjustments (CPA) are a feedback-based control mechanism, which reflects changes in muscle activity during the balance restoration phase following the perturbations (Alexandrov et al., 2005, Macpherson et al., 1989, Maki and McIlroy, 2006, Park et al., 2004).
Pushing, which involves using upper extremities exerting force away from the body, is the activity people commonly use in daily life for example, while moving strollers or grocery carts; it is also used by older adults pushing a wheeled walker with a seat while ambulating. Furthermore, aerobic activity involving for example, pushing a lawn mower, has beneficial effects on health outcomes for older adults (Elsawy and Higgins, 2010). However, how older adults perform daily activity involving pushing was not reported. Moreover, pushing tasks involve force exertion performed simultaneously with maintenance of balance, which could be challenging for the older adults. In addition, efficient pushing requires well-organized postural control utilizing both anticipatory and compensatory postural strategies (Lee and Aruin, 2013). It was reported that older adults performing pushing exhibited different compared to young adults distal-to-proximal postural response (Inglin and Woollacott, 1988) and higher amplitude of COP excursions (Blaszczyk et al., 1997). It was also suggested that physical activities involving pushing could be a risk factor for falls and fractures in the elderly (Palvanen et al., 2000).
The role of age in the generation of anticipatory postural adjustments was studied during performance of a pull-and-push arm movements (Blaszczyk, Lowe, 1997, Inglin and Woollacott, 1988, Stelmach et al., 1990), arm arising movements (Bleuse, Cassim, 2006, Carvalho, Vasconcelos, 2010, Kubicki, Bonnetblanc, 2012), or externally induced perturbations (Kanekar and Aruin, 2014b). It was reported that when older and young adults were exposed to external perturbations, ventral and dorsal postural muscles of older adults were activated during the APA phase about 50 to 80 ms later than the muscles of young adults (Kanekar and Aruin, 2014b). Moreover, the COP displacements were seen 100 ms later in older adults during both self- and externally-triggered perturbations (Bugnariu and Sveistrup, 2006, Kanekar and Aruin, 2014b).
Studies of compensatory postural adjustments using external perturbations applied to the upper body revealed that magnitudes of muscle activity and COP displacements in older adults were significantly larger than in young participants (Claudino et al., 2013, Kanekar and Aruin, 2014a). However, postural control of older adults performing tasks involving pushing an object is not well understood. Therefore, the objective of this study was to investigate effects of age on anticipatory and compensatory postural adjustments when performing a pushing task. Moreover, prior literature suggests that the central nervous system (CNS) controls muscles as task-specific structural units and not at a single muscle level (Bernshtein, 1967, Slijper and Latash, 2000, Slijper and Latash, 2004). Additionally, since the CNS uses either reciprocal activation or co-activation of ventral and dorsal muscles for postural control, sum and difference between ventral and dorsal muscle activities characterizing co-activation (C value) and reciprocal activation (R value) of postural muscles respectively (Slijper and Latash, 2004, Staude and Wolf, 1999) could be used to describe postural control. Furthermore, postural sway in older adults increase with the increase of the task demands (Prioli et al., 2006) and larger COP displacements were observed during both anticipatory and compensatory phases of postural control in older adults exposed to external perturbations (Claudino, dos Santos, 2013, Kanekar and Aruin, 2014b). Hence, our first hypothesis was that the activation of leg and trunk muscles and COP displacements will be delayed in older adults compared to young subjects. Secondly, we hypothesized that older adults will control muscle activity using co-activation pattern with larger COP displacement while young adults will utilize pattern of reciprocal activation of muscles with smaller COP displacement during the anticipatory phase (APAs) and the compensatory (CPAs) phase of postural control when performing the pushing task.
2. Methods
2.1. Participants
Eight older adults (4 males and 4 females) and eight young adults (5 males and 3 females) without any neurological or musculoskeletal disorders participated in the study. All participants had normal or corrected to normal vision and were able to understand and follow instructions. Older adults were independent community ambulators, were not on any sedative medications, and had not undergone any surgery in the six months prior to study participation. The mean (SE) age of the older group was 68.8 ± 1.0 years, the mean height was 1.73 ± 0.03 m, and the mean body mass was 64.9 ± 6.8 kg. The mean (SE) age of the young group was 30.1 ± 1.4 years, the mean height was 1.73 ± 0.02 m and the mean body mass was 73.9 ± 4.6 kg. Both, body height and mass were not significantly different between the two groups. All subjects signed a written informed consent approved by the Institutional Review Board of the University of Illinois at Chicago.
2.2. Experimental procedure
The subjects were required to stand on a force platform (Model OR-5, AMTI, USA) in front of an aluminum pendulum (affixed to the ceiling) and push the horizontal flat wooden handle (62 × 9 × 2 cm) attached to it. An extra load (30% body mass) was fastened to the opposite side of the handle. The subjects were instructed to stand upright with feet shoulder width apart. Their upper arms were by the sides of their trunk at 90 degrees of elbow flexion and wrist extension, and palms slightly contacting the wooden handle. The height of the pendulum was adjusted to match the subject's hand position (Fig. 1). The subjects were instructed to push the pendulum handle straight forward with both hands using only trunk motion without wrist flexion and elbow extension as well as without taking a step or lifting the heels from the surface of the force platform. The subjects performed each trial in a self-paced manner after receiving the experimenter's command “push.” After the pendulum was pushed away, it was caught by the experimenter. Then, the subjects returned to the starting position and waited for the experimenter's command to perform the next trial (5 trials in total). All the subjects were provided with several practice trials to familiarize themselves with the task. The study was conducted during one session that lasted approximately 20 minutes.
Fig. 1.
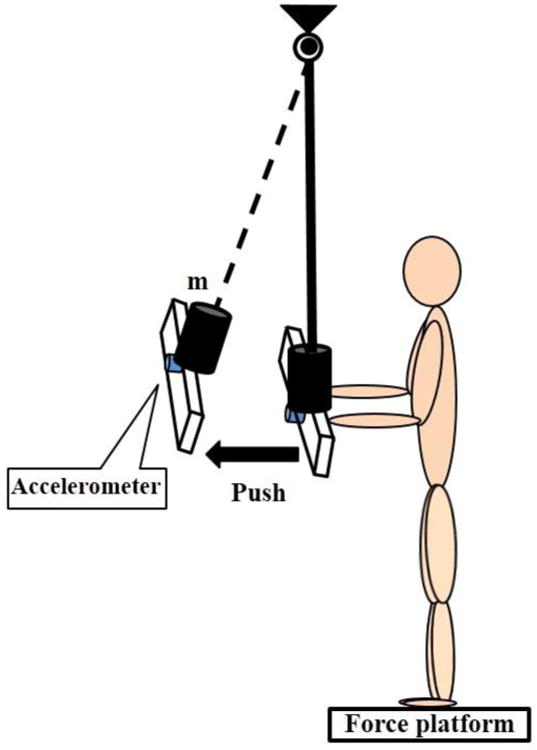
The schematic representation of the experimental setup. m- is the additional weight attached to the pendulum.
2.3 Data collection
The electrical activity of muscles (EMG) was recorded using disposable surface electrodes (Red Dot, 3M, USA). After cleaning the skin with alcohol, electrodes were attached to the bellies of the following muscles: tibialis anterior, TA (at one-third on the line between the tip of the fibula and the tip of the medial malleolus), medial gastrocnemius, MG (on the most prominent bulge of the muscle), rectus femoris, RF (at 50% on the line from the anterior superior iliac spine to the superior part of the patella), biceps femoris, BF (half way between the ischial tuberosity and the lateral epicondyle of the tibia), rectus abdominis, RA (3 cm lateral to the umbilicus), and erector spinae, ES (3 cm lateral to L1). The selected muscles have been used in previous studies of anticipatory and compensatory postural adjustments involving externally induced body perturbations (Aruin and Latash, 1996, Mohapatra et al., 2012, Santos et al., 2010). The placement of electrodes was based on recommendations reported in the literature (Basmajian, 1980). The EMG activity was recorded from the muscles on the right side only because both the posture and induced body perturbation were symmetrical. EMG signals were band-pass filtered (10-500 Hz) and amplified (gain 2000) using the EMG system (Myopac, RUN Technologies, USA).
Ground reaction forces and moments of forces were recorded from the force platform. An accelerometer (Model 333B42, PCB Piezotronics Inc., USA; weight 7.5 mg, sensitivity 51.0 mv/(m/s2) and measurement range ± 98 m/s2 pk) was attached to the pendulum and its signal was used to determine the timing when the pendulum was pushed away. The forces, moments of forces, EMGs, and accelerometer signals were synchronized and digitized with a 16-bit resolution at 1,000 Hz by means of an analog-to-digital converter and customized LabVIEW 8.6.1 software (National Instruments, Austin TX, USA). The data were stored on a computer for further processing.
2.4. Data processing
All data were processed offline using MATLAB software (MathWorks, Natick, MA, USA). The accelerometer signal was used to determine the timing the pendulum started moving away (T0) by applying the Teager-Kaiser onset time detection method (Li et al., 2007).
In order to minimize the effects of electrical activity of the heart, EMG data of the trunk muscles (RA and ES) was subjected to an additional procedure. This contamination was identified by means of independent component analysis and removed from the signals (Lee et al., 2010). Then, all EMG data were high-pass filtered at 20 Hz, full-wave rectified, and low-pass filtered at 2 Hz (2nd order Butterworth). The timing of changes in the muscle activity (EMG onset time) was estimated with an approximate generalized likelihood ratio algorithm (concepts of statistically optimal change detection in random process) by using a combination of a fixed-size and sliding test window shifted along a data sequence (Staude and Wolf, 1999).
The EMG data were integrated during the two phases: 1) from -100 ms to +50 ms (anticipatory postural adjustment, APA) and 2) from +50 ms to +200 ms (compensatory postural adjustment, CPA) in relation to T0. Moreover, ∫EMGs of the baseline activity during the 150 ms time window (0-150 ms) were obtained at the beginning of the trial. Two approaches were used to describe changes in muscle activity during the APA and CPA phases. One approach was focused on normalization of activity of each muscle using integral of baseline activity; as a result, activation of muscles was described by values larger than zero (∫EMG>0) and inhibition by values smaller than zero (∫EMG<0). Thus, the ∫APA and ∫CPA were normalized by ∫baseline for each muscle as:
According to the framework of the equilibrium-point hypothesis (Feldman, 1986), C indexes describe co-activation and R indexes describe reciprocal activation of agonist–antagonist muscle pairs (Slijper and Latash, 2004). Thus, the second approach was based on using the sums (C) and differences (R) between normalized ∫EMG values and calculated separately for the shank, thigh, and trunk in the APA and CPA phases as:
The ground reaction forces and the moments of forces were filtered with a 20Hz low-pass, 2nd order, zero-lag Butterworth filter. Time-varying COP traces in the anterior-posterior direction were calculated using the approximations described in the literature (Winter et al., 1996). An approximate generalized likelihood ratio algorithm (Staude and Wolf, 1999) was used to determine the timing of COP shifting away from the baseline (COPonset). Then, the COP values obtained at T0 (COPAPA) were used to describe the COP displacement during the APA phase and the COP peak values (COPCPA) were acquired to reflect the COP displacement during the CPA phase. All variables were calculated for each trial and averaged over five trials.
2.6. Statistics
Paired t tests were performed to compare young (Y) and older (O) groups on EMG onset time, COPonset, COPAPA, and COPCPA. In addition, paired t tests were used to evaluate differences between C and R values for both groups during the APA and CPA phases, separately. Statistical difference was set at p <0.05. Means and standard errors are presented in the results section and figures.
3. Results
Onset of muscle activity was seen in all leg and trunk muscles prior to T0 in both of the older and young groups. The ES in the older group, however, was activated after T0. All muscles in the older group became active later than in the young group. Figure 2 illustrates onset of muscle activities in the older and young groups at the group level. Age significantly affected onset time in the MG, BF, and ES. Thus, the MG onset was -59.33 ± 70.94 ms in the older group and –292.43 ± 32.10 ms in the young group (t = -3.225; p = 0.015). The BF onset was -62.75 ± 47.81 ms in the older group and –281.02 ± 16.30 ms in the young group (t = -3.601; p = 0.009). For ES muscle, it was 49.13 ± 36.46 ms and -86.14 ± 30.51 ms in the older and young group, respectively (t = -2.548; p = 0.038). In the older group, the onset of TA was -259.25 ± 45.26 ms, RF was -244.71 ± 36.17 ms, and RA was -27.50 ± 48.24ms, which were closer to the timing of the pendulum moving away than the young group (TA: -278.63 ± 41.58 ms, RF: -268.86 ± 28.65 ms and RA: -84.71 ± 24.92). The difference was not statistically significant.
Fig. 2.
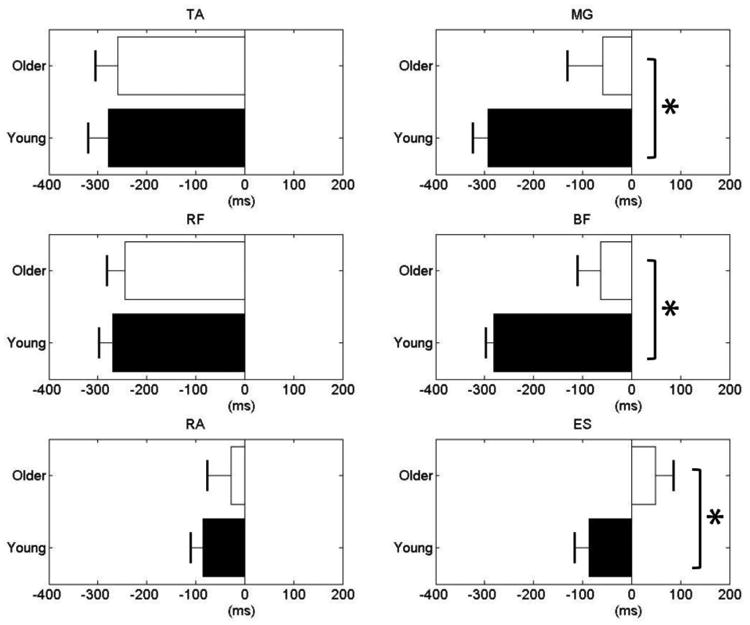
Onset times in the ventral (left panels) and dorsal (right panels) muscles. Data are presented from -400 ms to 200 ms in relation to the moment of the start of pushing (T0).
Comparisons between the C and R values in the older (white bars) and young groups (black bars) revealed different patterns (Fig. 3). Positive values indicate that there was an increase in the electrical activity of a muscle during the APA and CPA phases while negative values reflect an inhibition of the electrical activity. In the young group, C and R values calculated for the shank segment were significantly different (t = -4.859, p = 0.002) and the mean difference between C and R value (C – R) was -1.02, indicating that young participants used reciprocal activation of shank muscles in the APA phase. In the older group, smaller C value was only observed in the shank segment (C – R = -0.26); both thigh and trunk segments showed higher C value than R value (C – R = 1.23 and 0.26 for thigh and trunk, separately). Figure 3 illustrates that significantly larger C values than R values were seen in the shank (t = 2.790, p = 0.03), thigh (t = 3.067, p = 0.02), and trunk (t = 2.679, p = 0.03) muscles in the older group. Furthermore, the mean differences between the C and R values were 3.68 for the shank, 4.31 for the thigh, and 3.78 for the trunk segments. In the young group, the mean difference between the C and R values for the shank was -0.95 and significantly smaller C than R value were seen in the shank segment (t = -4.758, p < 0.01). The mean differences were -0.18 for the thigh (C > R) and 0.16 for the trunk (C < R), however, the difference was not statistically significant.
Fig. 3.
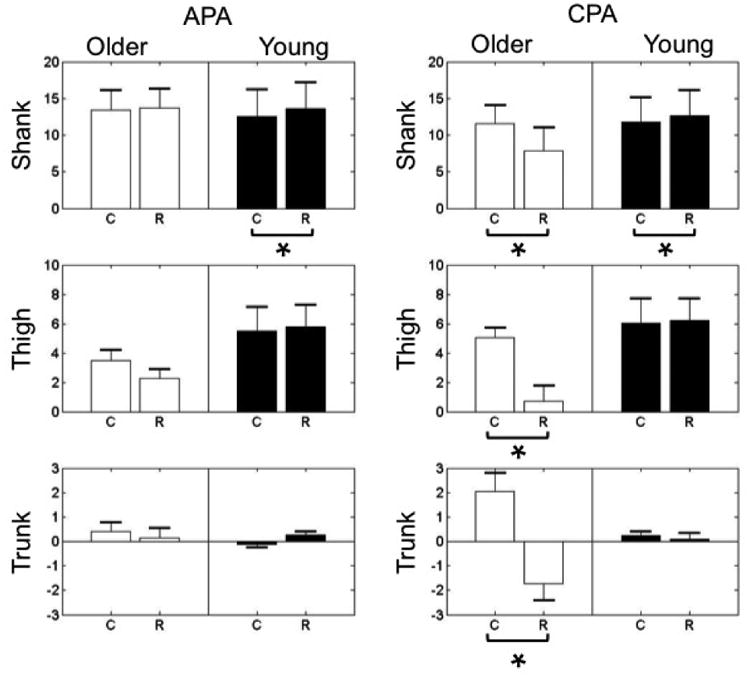
C and R indexes calculated for the shank, thigh and trunk segments during the APA (left panels) and CPA (right panels) phases of postural control.
The timing of the onset of the COP displacement was -321.93 ± 21.78 ms in relation to T0 in the younger group and -253.08 ± 21.51 ms in the older group; the difference between the groups was statistically significant (t = -3.476; p = 0.01). The COP displacement was always shifted backward during the APA phase and continuously shifted backward during the CPA phase. At the final stage of the pushing task, the COP displacement moved forward and returned to the original position. This pattern was observed in both of the older and young groups. Furthermore, the magnitudes of the COP displacement in the older group were larger than the young group during both phases but not significantly different between the two groups (Fig 4).
Fig. 4.
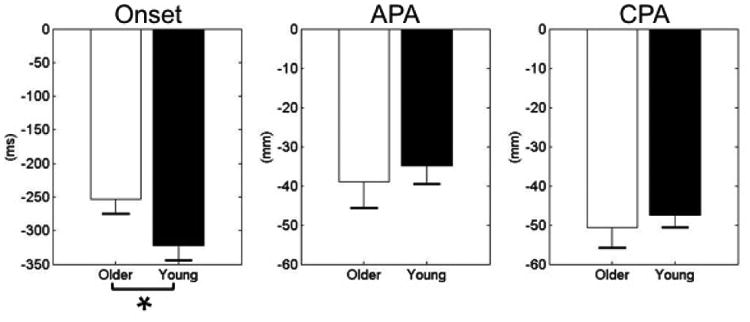
Onset times show the moment when COP shifted away from the baseline prior to T0. Changes in the COP displacement at T0 and the peak value represent the APA and CPA phases, respectively.
4. Discussion
The study was conducted to investigate the effect of age on the anticipatory and compensatory postural adjustments during the performance of a pushing task. The main result was that anticipatory activation of postural muscles was delayed in the older adults compared to the young adults. Moreover, in the APA phase, young subjects showed more reciprocal activation of shank muscles than co-contraction (R indexes were larger than C indexes). In the CPA phase, older adults showed more co-contraction of shank, thigh, and trunk segments muscles (C indexes were larger than R indexes); on the contrary, young subjects showed more reciprocal activation of shank muscles (R indexes were larger than C indexes). Meanwhile, older participants, as compared to young subjects, showed delayed COP onset time in the APA and CPA phases. However, the maximal accelerations of the pushing in the current study were similar between older (14.05 m/s2) and young (14.64 m/s2) groups. This outcome, taken together with the literature reporting that older adults could maintain arm velocities comparable to young adults during performance of tasks involving body perturbations (Woollacott and Manchester, 1993), suggests that the magnitudes of self-initiated perturbations were similar in both of the groups. Hence, the observed differences in the muscle activity and the COP displacements between the groups are attributed to the effects of age.
4.1. Muscle activation patterns
While subjects in both the groups activated muscles prior to the start of the pushing movement, as compared to young, older adults demonstrated delayed patterns of muscle activation. This study outcome supports our first hypothesis. The observed delayed activation of postural muscles in older adults is in line with previous literature describing age-related delays of muscle latency during performance of the arm raising tasks (Inglin and Woollacott, 1988, Rogers et al., 1992) and while being exposed to external perturbations (Kanekar and Aruin, 2014b). Large differences (about 200 ms) in the muscle activity onset time between older and young groups were observed in the dorsal muscles only. However, the differences in latencies of the ventral muscles between older and young groups were small (from 19 to 57 ms). This large variance indicated that analysis based on comparing timing of muscle activation might not be sensitive enough to distinguish differences between activation and inhibition of muscle activity as well as between patterns of co-activation or reciprocal activation of muscles. Hence, the results of C-R comparisons using the sum (C value) and difference (R value) between the activation of ventral and dorsal muscles of the shank, thigh, and trunk segments confirmed the importance of considering activity of the agonist and antagonist muscles at the joint level (Slijper and Latash, 2004, Staude and Wolf, 1999).
In the APA phase, older participants showed muscle activation patterns that are not associated with either specific co-activation or reciprocal activation of muscles serving the shank, thigh, and trunk segments. On the contrary, young participants demonstrated reciprocal patterns of muscle activation in the shank segment. These results were partially in line with our second hypothesis that young adults use pattern of reciprocal activation of muscles during the APA phase. These results are also supported by the findings obtained from young adults dealing with perturbations in the anterior-posterior direction (Kanekar and Aruin, 2014b, Santos and Aruin, 2008). However, the observed comparable C and R values in older adults suggests that they most likely produced activation of the dorsal muscles that were not drastically different from the baseline activity. As such, it is quite possible that older adults use predominately ventral muscles during anticipatory postural adjustments associated with the pushing task. As such, one could suggest that one of the consequences of age is that older adults, compared to young adults, utilize inefficient postural adjustments when exposed to similar perturbations.
Increases in muscle activation and significant co-activation of muscles in the shank, thigh, and trunk segments were seen in the CPA phase. Increased compensatory co-activation of the leg and trunk muscles was reported in older adults exposed to lateral perturbations (Claudino, dos Santos, 2013). Meanwhile, young participants utilized reciprocal activation of muscles in the shank segments (Fig 2). When task demands are relatively lenient, the CNS uses reciprocal activations of ventral and dorsal muscles for postural control and co-activation of muscles for handling high demanding tasks (Santos and Aruin, 2008). Absence of muscle inhibition and subsequent increase in muscle co-activation indicates that older adults consider the pushing task as a challenging perturbation, and as such, they utilize additional muscle activities to maintain balance. This information taken together with the observed delayed initiation of anticipatory activation of muscles in older adults (Fig 1) suggests that the elderly may have problems when performing activities involving pushing an object (e.g. pushing a wheeled walker with a grocery bag attached to it) or while performing other high demanding daily activities. Future studies involve recording kinematics would provide body positions to investigate a potential role of differences between postural performances of older and young subjects during pushing.
4.2. COP displacements
Delayed anticipatory muscle activities in the older adults were also associated with the delay in the COP displacements. The onsets of the COP displacement in relation to the onset of the pushing movement in older and young adults were -253.08 ms and -321.93 ms respectively. Onsets of the COP displacement described in the literature associated with the unilateral arm rising were -148 ms in older and -256 ms in young adults (Bleuse, Cassim, 2006). The difference in the onsets of the COP displacement between older and young adults in the current study was around 70 ms and it was about 100 ms in the previous publications. Such a difference between the outcomes of the two studies could be due to using different experimental tasks (pushing vs. arm rising). Regardless of the experimental protocol used, older adults as compared to young adults demonstrated delayed onsets of COP displacement.
In the present study, the older group showed no significantly different COP displacement from the young group in the APA and CPA phases, which was contrary to the second hypothesis and the outcome of the previous study involving external perturbations (Kanekar and Aruin, 2014b). This discrepancy could be due to the dissimilarity of the experimental tasks: pushing used in the current study is a voluntary self-induced perturbation while externally-induced perturbations were used in the previous study. Relatively similar COP displacements between the older and young adults in the APA and CPA phases could potentially be associated with increased risk of losing balance during pushing in older adults. This suggestion is based on two factors. First, prior to the pushing movement, older participants demonstrated comparable with young participants COP displacements. Second, the increased COP displacement during the balance restoration phase after the pushing movement, combined with slow reaction time seen in older adults (Stelmach, Populin, 1990), might predispose them to even larger instability making it more difficult to regain balance. It was also demonstrated that when young subjects push heavier objects, they show increased magnitudes of COP displacement and vertical torque (Lee and Aruin, 2015). As such, one could suggest that when older adults are required to push heavier objects, their inability to control the COP magnitude and displacement might predispose them to losing their balance and potentially falling.
5. Conclusions
The outcome of the study demonstrates that young and older adults use different patterns of postural adjustments while performing the task of pushing a heavy object. Thus, older adults compared to young subjects use larger and delayed activation of muscles during the anticipatory phase of postural control and larger co-activation of muscles. Moreover, during the compensatory phase of postural control, older adults utilize larger co-activation of muscles while reciprocal activation of muscles was seen in the young group. The observed diminished efficiency of anticipatory postural adjustments and larger compensatory postural adjustments in older adults might predispose them to falls while performing daily activities involving pushing.
Acknowledgments
We thank the study participants for their exceptional cooperation. We also thank Mohan Ganesan, Charlie Ma and Sailee Jagdhane for help in data collection. This work was supported in part by the NIDRR, grant no. H133P110004 and NIH, grant no. HD064838.
Biography
Yun-Ju Lee received her PhD in Biomechanics from VU University Amsterdam, Netherlands after receiving her Bachelor's degree in Physical Therapy and Master's degree in Rehabilitation Science from Chang Gung University, Taiwan. She is currently doing her postdoctoral fellowship in the Department of Physical therapy at University of Illinois at Chicago. Her Primary area of research is related to biomechanics, ergonomics, motor control and rehabilitation. She is actively involved in investigating mechanism of perturbations and functional activities for improving movement performances and developing rehabilitation exercise in older adults and individuals with neurological disorders.

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexandrov AV, Frolov AA, Horak FB, Carlson-Kuhta P, Park S. Feedback equilibrium control during human standing. Biological cybernetics. 2005;93:309–22. doi: 10.1007/s00422-005-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. Directional specificity of postural muscles in feed-forward postural reactions during fast voluntary arm movements. Experimental brain research. 1995;103:323–32. doi: 10.1007/BF00231718. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. Anticipatory postural adjustments during self-initiated perturbations of different magnitude triggered by a standard motor action. Electroencephalography and clinical neurophysiology. 1996;101:497–503. doi: 10.1016/s0013-4694(96)95219-4. [DOI] [PubMed] [Google Scholar]
- Basmajian JV. Electromyography--dynamic gross anatomy: a review. Am J Anat. 1980;159:245–60. doi: 10.1002/aja.1001590302. [DOI] [PubMed] [Google Scholar]
- Belen'kii VE, Gurfinkel VS, Pal'tsev EI. Control elements of voluntary movements. Biofizika. 1967;12:135–41. [PubMed] [Google Scholar]
- Bernshtein NA. The co-ordination and regulation of movements. 1st English. Oxford, New York: Pergamon Press; 1967. [Google Scholar]
- Blaszczyk JW, Lowe DL, Hansen PD. Age-related differences in performance of stereotype arm movements: movement and posture interaction. Acta neurobiologiae experimentalis. 1997;57:49–57. doi: 10.55782/ane-1997-1210. [DOI] [PubMed] [Google Scholar]
- Bleuse S, Cassim F, Blatt JL, Labyt E, Derambure P, Guieu JD, et al. Effect of age on anticipatory postural adjustments in unilateral arm movement. Gait Posture. 2006;24:203–10. doi: 10.1016/j.gaitpost.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Bugnariu N, Sveistrup H. Age-related changes in postural responses to externally- and self-triggered continuous perturbations. Archives of gerontology and geriatrics. 2006;42:73–89. doi: 10.1016/j.archger.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Carvalho R, Vasconcelos O, Goncalves P, Conceicao F, Vilas-Boas JP. The effects of physical activity in the anticipatory postural adjustments in elderly people. Motor control. 2010;14:371–9. doi: 10.1123/mcj.14.3.371. [DOI] [PubMed] [Google Scholar]
- Claudino R, dos Santos EC, Santos MJ. Compensatory but not anticipatory adjustments are altered in older adults during lateral postural perturbations. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2013;124:1628–37. doi: 10.1016/j.clinph.2013.02.111. [DOI] [PubMed] [Google Scholar]
- Elsawy B, Higgins KE. Physical activity guidelines for older adults. American family physician. 2010;81:55–9. [PubMed] [Google Scholar]
- Feldman AG. Once more on the equilibrium-point hypothesis (lambda model) for motor control. Journal of motor behavior. 1986;18:17–54. doi: 10.1080/00222895.1986.10735369. [DOI] [PubMed] [Google Scholar]
- Inglin B, Woollacott M. Age-related changes in anticipatory postural adjustments associated with arm movements. Journal of gerontology. 1988;43:M105–13. doi: 10.1093/geronj/43.4.m105. [DOI] [PubMed] [Google Scholar]
- Kanekar N, Aruin AS. Aging and balance control in response to external perturbations: role of anticipatory and compensatory postural mechanisms. Age. 2014a;36:9621. doi: 10.1007/s11357-014-9621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekar N, Aruin AS. The effect of aging on anticipatory postural control. Experimental brain research. 2014b;232:1127–36. doi: 10.1007/s00221-014-3822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki A, Bonnetblanc F, Petrement G, Ballay Y, Mourey F. Delayed postural control during self-generated perturbations in the frail older adults. Clinical interventions in aging. 2012;7:65–75. doi: 10.2147/CIA.S28352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Aruin A. Three components of postural control associated with pushing in symmetrical and asymmetrical stance. Experimental brain research. 2013;228:341–51. doi: 10.1007/s00221-013-3567-4. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Aruin AS. Effects of asymmetrical stance and movement on body rotation in pushing. Journal of biomechanics. 2015;48:283–9. doi: 10.1016/j.jbiomech.2014.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Hoozemans MJ, van Dieen JH. Oblique abdominal muscle activity in response to external perturbations when pushing a cart. Journal of biomechanics. 2010;43:1364–72. doi: 10.1016/j.jbiomech.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Li X, Zhou P, Aruin AS. Teager-Kaiser energy operation of surface EMG improves muscle activity onset detection. Annals of biomedical engineering. 2007;35:1532–8. doi: 10.1007/s10439-007-9320-z. [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Horak FB, Dunbar DC, Dow RS. Stance dependence of automatic postural adjustments in humans. Experimental brain research. 1989;78:557–66. doi: 10.1007/BF00230243. [DOI] [PubMed] [Google Scholar]
- Maki BE, McIlroy WE. Control of rapid limb movements for balance recovery: age-related changes and implications for fall prevention. Age and ageing. 2006;35(Suppl 2):ii12–ii8. doi: 10.1093/ageing/afl078. [DOI] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Progress in neurobiology. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Mohapatra S, Krishnan V, Aruin AS. Postural control in response to an external perturbation: effect of altered proprioceptive information. Experimental brain research. 2012;217:197–208. doi: 10.1007/s00221-011-2986-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palvanen M, Kannus P, Parkkari J, Pitkajarvi T, Pasanen M, Vuori I, et al. The injury mechanisms of osteoporotic upper extremity fractures among older adults: a controlled study of 287 consecutive patients and their 108 controls. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2000;11:822–31. doi: 10.1007/s001980070040. [DOI] [PubMed] [Google Scholar]
- Park S, Horak FB, Kuo AD. Postural feedback responses scale with biomechanical constraints in human standing. Experimental brain research. 2004;154:417–27. doi: 10.1007/s00221-003-1674-3. [DOI] [PubMed] [Google Scholar]
- Prioli AC, Cardozo AS, de Freitas Junior PB, Barela JA. Task demand effects on postural control in older adults. Human movement science. 2006;25:435–46. doi: 10.1016/j.humov.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Rogers MW, Kukulka CG, Soderberg GL. Age-related changes in postural responses preceding rapid self-paced and reaction time arm movements. Journal of gerontology. 1992;47:M159–65. doi: 10.1093/geronj/47.5.m159. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Aruin AS. Role of lateral muscles and body orientation in feedforward postural control. Experimental brain research. 2008;184:547–59. doi: 10.1007/s00221-007-1123-9. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Kanekar N, Aruin AS. The role of anticipatory postural adjustments in compensatory control of posture: 1. Electromyographic analysis. Journal of electromyography and kinesiology : official journal of the International Society of Electrophysiological Kinesiology. 2010;20:388–97. doi: 10.1016/j.jelekin.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Slijper H, Latash M. The effects of instability and additional hand support on anticipatory postural adjustments in leg, trunk, and arm muscles during standing. Experimental brain research. 2000;135:81–93. doi: 10.1007/s002210000492. [DOI] [PubMed] [Google Scholar]
- Slijper H, Latash ML. The effects of muscle vibration on anticipatory postural adjustments. Brain Res. 2004;1015:57–72. doi: 10.1016/j.brainres.2004.04.054. [DOI] [PubMed] [Google Scholar]
- Staude G, Wolf W. Objective motor response onset detection in surface myoelectric signals. Med Eng Phys. 1999;21:449–67. doi: 10.1016/s1350-4533(99)00067-3. [DOI] [PubMed] [Google Scholar]
- Stelmach GE, Populin L, Muller F. Postural muscle onset and voluntary movement in the elderly. Neuroscience letters. 1990;117:188–93. doi: 10.1016/0304-3940(90)90142-v. [DOI] [PubMed] [Google Scholar]
- Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. Journal of neurophysiology. 1996;75:2334–43. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]
- Woollacott MH, Manchester DL. Anticipatory postural adjustments in older adults: are changes in response characteristics due to changes in strategy? Journal of gerontology. 1993;48:M64–70. doi: 10.1093/geronj/48.2.m64. [DOI] [PubMed] [Google Scholar]


