Abstract
Background
The interaction between baseline kidney function and the performance of biomarkers of acute kidney injury (AKI) on the development of AKI is unclear.
Study Design
Post-hoc analysis of prospective cohort study.
Setting & Participants
The 1,219 TRIBE-AKI Consortium adult cardiac surgery cohort participants.
Predictor
Unadjusted post-operative urinary biomarkers of AKI measured within 6 hours of surgery.
Outcome
AKI was defined as greater than or equal to AKI Network stage 1 (any AKI) as well as a doubling of serum creatinine from the pre-operative value or the need for emergent dialysis (severe AKI).
Measurements
Stratified analyses by a pre-operative eGFR ≤ 60 ml/min/1.73 m2 vs. > 60 ml/min/1.73 m2.
Results
180 (42%) patients with a pre-operative eGFR ≤ 60 ml/min/1.73m2 developed clinical AKI compared to 246 (31%) in those with an eGFR >60 ml/min//1.73m2 (p<0.001). For log2-transformed biomarker concentrations there was a significant interaction between any AKI and baseline eGFR for interleukin 18 (IL-18; p=0.007) and borderline significance for liver-type fatty acid binding protein (p=0.06). For all biomarkers, the adjusted relative risk (RR) point estimates for the risk of any AKI were higher in those with elevated baseline eGFRs compared to those with an eGFR ≤ 60 ml/min/1.73m2. However the difference in magnitude of these risks were quite low (adjusted RRs were 1.04 [95% CI, 0.99–1.09] and 1.11 [95% CI, 1.07–1.15] for those with a pre-operative eGFR ≤ 60 ml/min/1.73 m2 and those with higher eGFRs, respectively). Although no biomarker displayed an interaction for baseline eGFR and severe AKI, log2-transformed IL-18 and kidney injury molecule 1 (KIM-1) had significant adjusted RRs for severe AKI in those with and without baseline eGFR ≤ 60 ml/min/1.73 m2.
Limitations
Limited numbers of patients with severe AKI and emergent dialysis.
Conclusions
The association between early post-operative AKI urinary biomarkers and AKI is modified by preoperative eGFR. The degree of this modification and its impact on the biomarker-AKI association is small across biomarkers. Our findings suggest that distinct biomarker cut-offs for those with and without a pre-operative eGFR ≤ 60 ml/min/1.73 m2 is not necessary.
Keywords: urine biomarkers, interleukin 18 (IL-18), liver-type fatty acid binding protein (L-FABP), Acute Renal Failure (ARF), acute kidney injury (AKI), perioperative AKI, effect modification, estimated glomerular filtration rate (eGFR), prognosis, cardiac surgery, surgical complication
Acute kidney injury (AKI) is the most common reason for inpatient nephrology consultation. Significant research efforts are focused on translating biomarkers of AKI so they become available for clinical use. 1,2 While several urine biomarkers have demonstrated promise for their ability to detect AKI earlier than traditional markers or to predict disease progression, clinical factors that impact biomarker performance, such as decreased pre-operative glomerular filtration rate (GFR), remain unclear. 3–6 Chronic kidney disease (CKD) as defined by decreased GFR is a common risk factor for the development of AKI, occurring in one third to half of all those with AKI, 6–8 and is heavily weighted in a variety of AKI risk assessment tools.9–11 Biomarkers like urinary neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1) act as markers of renal tubular injury and, as such, have recently been shown to be increased in the presence of CKD but absence of AKI. 12–15 However, when evaluated as a prognostic tool in the setting of AKI, these same markers have demonstrated varied performance in those with and without pre-existing decreased baseline GFR.16–18 It stands to reason that biomarker concentrations and kinetics will be different in those with and without decreased baseline GFR as decreasing functional nephron mass and renal reserve will affect the kidney’s ability to filter or produce these proteins. Additionally, if biomarker concentrations are higher in those with lower baseline GFRs, smaller renal insults resulting in phenotypically milder AKI (as evidenced by change in serum creatinine) may lead to a disproportionate increase in biomarker concentration despite a weaker association with AKI. The interplay between AKI, GFR and urinary biomarkers of renal tubular injury remain unclear.
To clarify the interaction between urinary biomarkers, GFR and AKI, we performed a secondary analysis of the prospectively collected Translational Research Investigating Biomarker Endpoints in AKI (TRIBE-AKI) adult cardiac surgery cohort to evaluate the effect modification by pre-operative GFR on the association of six urinary biomarkers of AKI (NGAL, KIM-1, IL-18, liver-type fatty acid binding protein [L-FABP], cystatin C and albumin), assessed in the hours following surgery, and AKI during the hospital stay.
Methods
Patient Cohorts and Samples
The detailed methods of the TRIBE AKI cardiac surgery cohort (clinicaltrials.gov identifier NCT00774137) have been previously described. 6,19 Adults deemed high risk for AKI following cardiac surgery (CABG and/ or valve repair or replacement) were prospectively enrolled at six academic medical centers in North America in July 2007–December 2009. All participants provided written informed consent and the study was approved by each institution’s research ethics board. The first postoperative samples were collected within 6 hours of admission to the ICU. For the first 24 hours postoperatively, urine samples were collected every 6 hours. The remaining daily blood and urine samples were obtained at the time of routine morning blood collection done for clinical care. Specimen collection was stopped on postoperative day 3 in patients who did not demonstrate any AKI in the 3 days following surgery. Details on sample collection and processing have been previously described. 6,19
Outcomes: Study Variables
The definition of AKI was development, at a minimum, of a 50% increase or absolute increase of 0.3 mg/dL in serum creatinine from pre-operative baseline during the hospital stay (AKI Network [AKIN] stage 1). Severe AKI was defined as developing at least a 100% increase in serum creatinine from baseline or the need for renal replacement therapy. 20,21
All pre-operative creatinine and biomarker values were measured within two months prior to surgery. Pre- and post-operative serum creatinine concentrations were measured in the same clinical laboratory for each patient at all sites. Serum creatinine values were recorded for every patient throughout the hospital stay. For adults we estimated preoperative GFR (eGFR) using the CKD-EPI (CKD Epidemiology Collaboration) creatinine equation. 22 As previously described we collected preoperative characteristics, operative details, and post-operative complications using definitions of the Society of Thoracic Surgeons.6,19
Biomarker Assays
All biomarkers were measured in blinded fashion with assay information and inter and intra-assay coefficients of variation as previously described (Parikh et al6 [IL-18 and NGAL], Parikh et al23 [KIM-1 and L-FABP], Koyner et al24 [CysC], and Molonar et al25 [albumin]).
Statistical Analysis
Continuous variables were compared with a two-sample t-test or Wilcoxon rank sum test and dichotomous variables with the chi-square test or fisher’s exact test. The adult population was divided into unique quintiles using the first post-operative value of each individual biomarker. Unadjusted trends across biomarker quintiles were assessed by Cochran-Armitage test for dichotomous outcomes. To evaluate the association between biomarkers and AKI, modified Poisson regression models with a log link function were used. 26 We adjusted for age, gender, race, history of hypertension, history of diabetes, history of congestive heart failure, surgery status (elective vs. non-elective), type of surgery (bypass alone, valve alone or bypass and valve), myocardial infarction and cardiopulmonary bypass pump time. Biomarkers were included in models as log2-transformed continuous variables or as quintiles. To explore the possibility of effect modification by pre-operative eGFR, we included an interaction term in the model between eGFR >60 vs. ≤60 ml/min/1.73m2 and the biomarker or CKD-EPI eGFR (continuously) and the biomarker. The same clinical adjustment variables were used across all analyses. All analyses were performed in SAS version 9.2 (SAS Institute Inc, Cary, NC) and R 2.12.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
Figure 1 provides information about those TRIBE-AKI subjects included in this analysis. Of the 1,219 adults in the TRIBE-AKI cohort, 424 (34.8%) had a pre-operative eGFR ≤ 60 ml/min/1.73 m2.22 Defined as AKIN stage 1, AKI occurred more commonly in those with a pre-operative eGFR ≤60 ml/min/1.73m2: 180 of 424 (42.5%) compared to 246 of 795 (30.9%) in those without decreased baseline eGFR (p < 0.001). Severe AKI, defined as AKIN stage 2 or higher, was not different in patients with and without decreased baseline GFR, (p =0.3). Table 1 demonstrates the patient characteristics of those with and without a pre-operative eGFR ≤60 ml/min/1.73m2. Those with a pre-operative eGFR ≤ 60 ml/min/1.73m2 were more likely to be older, female and have a history of hypertension and congestive heart failure (p<0.05 for all). Those with lower baseline eGFRs were more likely to undergo a valve surgery or a combined valve and CABG procedure and had longer cross clamp and cardiopulmonary bypass times (p<0.05 for all). (Table 1)
Figure 1.
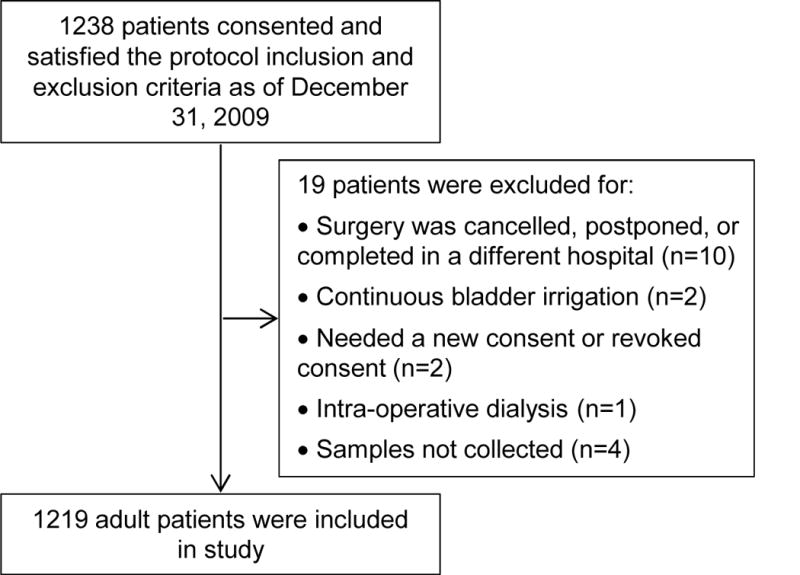
TRIBE-AKI subjects included in the analyses
Table 1.
Clinical Characteristics of those patients with and without a Pre-operative eGFR≤60 ml/min/1.73m2
| Characteristic | eGFR ≤60 ml/min/1.73m2 (n= 424) | eGFR >60 ml/min/1.73m2 (n=795) | P-value† |
|---|---|---|---|
| Age at time of surgery, y | 74.86 (8.49) | 69.66(10.43) | <0.001 |
| Male sex | 252 (59%) | 574 (72%) | <0.001 |
| White race | 394 (93%) | 747 (94%) | 0.5 |
| Diabetes | 174(41%) | 337 (42%) | 0.6 |
| Hypertension | 350 (83%) | 611 (77%) | 0.02 |
| Congestive Heart Failure | 133(31%) | 181 (23%) | 0.001 |
| Elective Surgery | 104(25%) | 151 (19%) | 0.02 |
| Surgery | |||
| CABG | 161 (38%) | 424 (53%) | <0.001 |
| CABG and Valve | 117 (28%) | 162(20%) | <0.001 |
| Valve | 146(34%) | 209 (26%) | <0.001 |
| Myocardial Infarction | 114(28%) | 199(26%) | 0.5 |
| Cardiac catheterization in past 48 h | 27 (6%) | 46 (6%) | 0.7 |
| Preop Serum Creatinine (mg/dL) | 1.41 (0.36) | 0.92(0.17) | <0.001 |
| Preop eGFR (ml/min/1.73m2) | 46.26(10.04) | 78.44(12.77) | <0.001 |
| Perfusion Time* | 118.29(55.89) | 112.02(61.86) | 0.02 |
| Cross Clamp time (min) | 81.84(42.08) | 75.77 (45.55) | 0.001 |
| Cardioplegia | 384(91%) | 693 (87%) | 0.08 |
| IABP | 25 (6%) | 32 (4%) | 0.1 |
| Severe AKI | 25 (6%) | 35 (4%) | 0.3 |
| Any AKI | 180(42%) | 246(31%) | <0.001 |
Note: Values tor categorical variables are given as number (percentage); tor continuous variables, as mean ± standard deviation. Conversion factor for serum creatinine in mg/dL to μmol/L, ×88.4.
Perfustion Time is reported for the patients who had cardiopulmonary bypass
Pvalues are for the comparison of patients with and without decreased pre-op GFR AKI, acute kidney injury; CABG = Coronary Artery Bypass Grafting; eGFR= estimated Glomerular Filtration Rate IABP = Intra-Aortic Balloon Pump; preop, preoperative
Biomarker Kinetics and Preoperative eGFR
Figure 2 displays the biomarker concentrations over the pre and early post-operative period for those patients with and without a pre-operative eGFR ≤ 60 ml/min/1.73m2 stratified by their post-operative AKI status. Table S1 (provided as online supplementary material) compares the pre-operative biomarker concentrations in those with and without a pre-operative eGFR ≤ 60 ml/min/1.73m2. Amongst those with lower pre-operative eGFR, values of urine albumin and L-FABP were higher in those who went on to develop AKI (median albumin levels, 32.9 [IQR, 10–92.8] vs. 14.0 [5.8–60] mg/L [p <0.01]; median L-FABP concentrations, 3.98 [IQR, 1.6–12.9] vs. 2.35 [1.1–4.7] ng/mL [P <0.01]). In those with a higher pre-operative eGFR, only the pre-operative urinary albumin levels were different amongst those who did and did not develop AKI (AKI: 13.9 [IQR, 6.7–42] g/dL; without AKI: 9.9 [IQR, 5.0–22.0] mg/L; p <0.01). Figure 2 also shows that individual biomarkers were significantly different at several post-operative timepoints with values being higher in those with baseline eGFR >60 mL/min/1.73 m2. Table S2 provides the AUCs for biomarkers measured in the first 6 post-operative hours to predict all AKI and severe AKI stratified by pre-operative eGFR.
Figure 2. Peri-operative biomarker distributions stratified along baseline eGFR and AKI status.
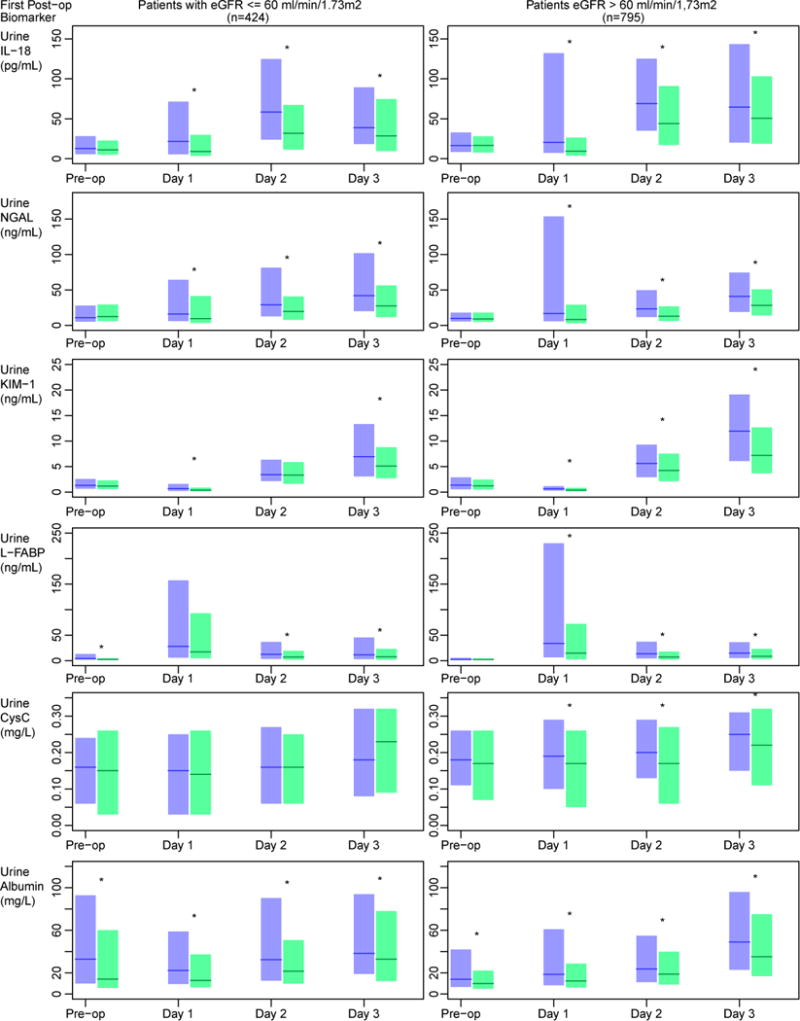
The bar plots represent the median (horizontal line) and 25–75% IQR(boxes) for those subjects with (blue) and without (green) postoperative AKI. In those with a preoperative eGFR ≤ 60 ml/min/1.73m2, pre-operative L-FABP and albumin were higher in those who went on to develop AKI, while only urinary albumin levels were elevated pre-operatively in those with an eGFR > 60 ml/min/1.73m2. At the majority of post-operative timepoints biomarkers were elevated in those with AKI regardless of baseline eGFR. (* p <0.05). CysC, cystatin C.
Association of Continuous Early Postoperative Biomarker Values With AKI and Interactions With Preoperative eGFR
Table 2 demonstrates the association of the log2-transformed continuous early postoperative biomarker values for the development of any AKI while accounting for the interaction with baseline eGFR. In those with a preoperative eGFR ≤ 60 ml/min/1.73m2, after adjusting for the clinical model, only KIM-1 and albumin were independently associated with the development of any AKI. In those with higher baseline eGFR, all biomarkers except urine Cystatin C were independently associated with any AKI in the adjusted analyses. However, no log2-transformed biomarker had an adjusted relative risk (RR) greater than 1.16. The only biomarker that performed significantly differently across the dichotomized cohort was IL-18 (Table 2).Table S3 provides the analyses for the urine creatinine-adjusted log2-transformed continuous biomarker values. Again, IL-18 remained the only biomarker with a significant difference between those with and without decreased baseline eGFR, and overall the adjusted RR was not greater than 1.25. Table 2 also shows the association of the log2-transformed continuous biomarker values for the development of severe AKI while accounting for the interaction with baseline eGFR.
Table 2.
Association of Log-transformed Continuous Early Post-operative Biomarkers with Any and Severe AKI and interaction with Pre-operative eGFR
| Urinary Biomarker | eGFR ≤ 60 ml/min/1.73m2 (n= 424) | eGFR > 60 ml/min/1.73m2 (n=795) | eGFR X Biomarker p-value | ||
|---|---|---|---|---|---|
| unadjusted RR (95% CI) | adjusted RR (95% CI) | unadjusted RR (95% CI) | adjusted RR (95% CI) | ||
| Association With Any AKI | |||||
| IL-18 | 1.08 [1.04, 1.12] | 1.04 [0.99, 1.09] | 1.14 [1.10, 1.17] | 1.11 [1.07, 1.15] | 0.007 |
| NGAL | 1.05 [1.01, 1.09] | 1.03 [0.99, 1.07] | 1.09 [1.06, 1.12] | 1.05 [1.02, 1.09] | 0.2 |
| KIM-1 | 1.14 [1.08, 1.21] | 1.09 [1.03, 1.16] | 1.16 [1.09, 1.23] | 1.16 [1.09, 1.23] | 0.3 |
| L-FABP | 1.04 [1.00, 1.08] | 1.02 [0.98, 1.06] | 1.09 [1.06, 1.12] | 1.06 [1.03, 1.10] | 0.06 |
| Cystatin C | 0.95 [0.87, 1.04] | 0.95 [0.87, 1.04] | 1.08 [0.97, 1.20] | 1.03 [0.95, 1.12] | 0.1 |
| Albumin | 1.09 [1.03, 1.15] | 1.06 [1.00, 1.13] | 1.12 [1.06, 1.18] | 1.09 [1.03, 1.16] | 0.5 |
| Association With Severe AKI | |||||
| IL-18 | 1.31 [1.16, 1.47] | 1.26 [1.11, 1.43] | 1.35 [1.23, 1.49] | 1.25 [1.11, 1.40] | 0.6 |
| NGAL | 1.22 [1.09, 1.37] | 1.18 [1.03, 1.35] | 1.20 [1.09, 1.33] | 1.09 [0.98, 1.22] | 0.8 |
| KIM-1 | 1.42 [1.13, 1.80] | 1.40 [1.05, 1.87] | 1.53 [1.29, 1.82] | 1.58 [1.28, 1.95] | 0.3 |
| L-FABP | 1.10 [0.94, 1.29] | 1.04 [0.90, 1.20] | 1.18 [1.04, 1.34] | 1.07 [0.96, 1.21] | 0.6 |
| Cystatin C | 1.40 [0.86, 2.26] | 1.36 [0.81, 2.28] | 1.01 [0.64, 1.58] | 0.88 [0.67, 1.15] | 0.2 |
| Albumin | 1.22 [0.98, 1.52] | 1.26 [0.85, 1.86] | 1.41 [1.18, 1.68] | 1.28 [1.04, 1.58] | 0.6 |
Note: Biomarkers were log2-transformed; RRs are expressed per doubling of biomarker concentration. Adjusted for age, gender, race, history of hypertension, history of diabetes, history of congestive heart failure, surgery status (elective vs. non-elective), type of surgery (bypass alone, valve alone or bypass and valve), myocardial infarction and cardiopulmonary bypass pump time.
AKI, acute kidney injury; CI, confidence interval; eGFR, estimated glomerular filtration rate; IL-18, interleukin 18; KIM-1, kidney injury molecule 1; L-FABP, liver-type fatty acid binding protein; NGAL, neutrophil gelatinase-associated lipocalin; RR. relative risk
To further investigate the interaction between biomarkers and eGFR, we plotted the log2-transformed biomarkers against the logit of any AKI for those with and without pre-operative eGFR >60 ml/min/1.73m2. These plots (Figure 3) demonstrate that there is an interaction present for IL-18 (p=0.007) and borderline statistical significance for an interaction for cystatin C (p=0.1) and L-FABP (p=0.06). Importantly, in these plots the interactions occur at biomarker values that are significantly higher than the post-operative values seen in the population. We investigated the interaction between continuous log2-transformed biomarkers and continuous eGFR and the development of post-operative AKI (tables a–d of Item S1). Item S1 shows coefficients and p-values for biomarkers and eGFR when examining any AKI and Severe AKI in adjusted and unadjusted analyses.
Figure 3. Log-transformed (base 2) Continuous biomarkers and the Logit of Any AKI.
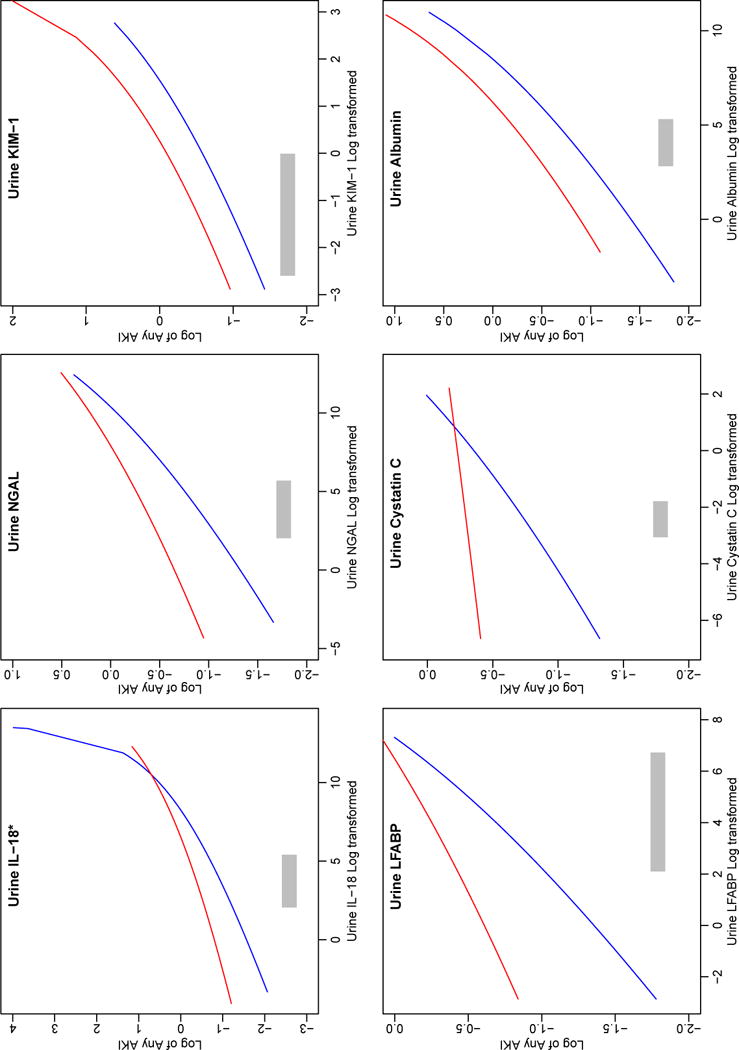
The plots demonstrate the presence or absence of an interaction between continuous biomarkers, AKI and baseline eGFR (dichotomized at 60 ml/min/1.73m2). The red line represents patients with eGFR ≤60 and blue line represents patients with eGFR > 60. The plots demonstrate the Interquartile range of values for the individual biomarkers at this early postoperative timepoint (grey bars). The plots that cross or nearly intersect are those that demonstrate a significant (or trend to significant) interaction (IL-18: p=0.007; L-FABP: p=0.06; cystatin C: p=0.1). Importantly for those biomarkers which displayed an interaction (or trend) that this interaction takes place at values on the extreme high end of the spectrum well above what we saw in our cohort.
Association of Early Postoperative Biomarker Quintiles With AKI and Interactions With Preoperative eGFR
We further analyzed the incidence and association of AKI in the cohort dichotomized by baseline eGFR across quintiles of the first post-operative biomarker values (Figure S1). The incidence of AKI was significantly different across biomarker quintiles for a majority of biomarkers (Cochran-Armitage p<0.05), regardless of baseline eGFR with the exception of urine cystatin C in those with eGFR ≤ 60 ml/min/1.73m2 (p=0.7). Tables a and b of Item S2 display the unadjusted and adjusted RRs for individual biomarker quintiles (for biomarkers unadjusted and adjusted for urine creatinine). The interaction tests were significant for some biomarkers but the adjusted RRs were not meaningfully different.
The Spearman Correlation coefficients of the first post-operative biomarkers values with each other are found in Table S4 (all correlations p<0.001).
Association of Preoperative Biomarker With AKI and Interactions With Preoperative eGFR
Table 3 demonstrates the adjusted and unadjusted RRs for pre-operative log2-transformed pre-operative biomarkers and the development of AKI. Urinary albumin and cystatin C were the only biomarkers associated with increased risk of any AKI in those with and without a preoperative eGFR ≤ 60 ml/min/1.73m2 (with albumin being significant for both); However, the adjusted magnitude of this risk for albumin was small (eGFR≤ 60 ml/min/1.73m2: RR, 1.08 [95% confidence interval, 1.03–1.13]; eGFR >60 ml/min/1.73m2: RR, 1.09 [95% confidence interval, 1.04–1.14]). Despite no significant ability to detect an increased risk for AKI, L-FABP was the only biomarker different across the two baseline eGFR strata (p=0.03). The data were similar when looking at the pre-operative biomarkers association with severe AKI (Table 3). Table S5 demonstrates the performance of pre-operative biomarkers to predict AKI across biomarker quintiles stratified by baseline eGFR.
Table 3.
Pre-operative log-transformed continuous biomarker for the prediction of Any and Severe AKI
| Urinary biomarker | eGFR ≤ 60 ml/min/1.73m2 | eGFR > 60 ml/min/1.73m2 | eGFR x Biomarker p-value | ||
|---|---|---|---|---|---|
| unadjusted RR (95% CI) | adjusted RR (95% CI) | unadjusted RR (95% CI) | adjusted RR (95% CI) | ||
| Prediction of Any AKI | |||||
| IL-18 | 1.04 [0.98, 1.11] | 1.04 [0.98, 1.11] | 1.02 [0.96, 1.08] | 1.02 [0.96, 1.08] | 0.7 |
| NGAL | 0.98 [0.92, 1.04] | 1.00 [0.94, 1.06] | 1.03 [0.96, 1.10] | 1.04 [0.97, 1.11] | 0.4 |
| KIM-1 | 0.98 [0.89, 1.08] | 0.99 [0.90, 1.10] | 1.00 [0.90, 1.10] | 1.02 [0.92, 1.13] | 0.8 |
| L-FABP | 1.03 [0.96, 1.11] | 1.03 [0.96, 1.10] | 1.05 [0.99, 1.11] | 1.05 [0.99, 1.11] | 0.03 |
| Cystatin C | 1.10 [1.05, 1.15] | 1.09 [1.04, 1.15] | 1.02 [0.96, 1.07] | 1.01 [0.96, 1.07] | 0.8 |
| Albumin | 1.08 [1.03, 1.13] | 1.08 [1.03, 1.13] | 1.09 [1.03, 1.14] | 1.09 [1.04, 1.14] | 0.9 |
| Prediction of Severe AKI | |||||
| IL-18 | 1.26 [1.04, 1.52] | 1.27 [1.06, 1.53] | 1.11 [0.90, 1.37] | 1.17 [1.00, 1.37] | 0.8 |
| NGAL | 1.06 [0.91, 1.25] | 1.07 [0.91, 1.27] | 1.01 [0.85, 1.20] | 1.08 [0.88, 1.32] | 0.8 |
| KIM-1 | 1.19 [0.90, 1.58] | 1.22 [0.92, 1.62] | 1.09 [0.91, 1.31] | 1.12 [0.95, 1.33] | 0.8 |
| L-FABP | 1.17 [1.01, 1.35] | 1.18 [0.99, 1.39] | 0.99 [0.80, 1.23] | 0.98 [0.81, 1.19] | 0.3 |
| Cystatin C | 1.27 [0.95, 1.71] | 1.43 [1.01, 2.03] | 1.05 [0.87, 1.28] | 1.11 [0.88, 1.39] | 0.5 |
| Albumin | 1.16 [0.99, 1.35] | 1.20 [1.04, 1.39] | 1.10 [0.96, 1.25] | 1.10 [0.96, 1.26] | 0.5 |
Note: Biomarkers were log2-transformed; RRs are expressed per doubling of biomarker concentration. Adjusted for age, gender, race, history of hypertension, history of diabetes, history of congestive heart failure, surgery status (elective vs. non-elective), type of surgery (bypass alone, valve alone or bypass and valve), myocardial infarction and cardiopulmonary bypass pump time.
AKI, acute kidney injury; CI, confidence interval; eGFR, estimated glomerular filtration rate; IL-18, interleukin 18; KIM-1, kidney injury molecule 1; L-FABP, liver-type fatty acid binding protein; NGAL, neutrophil gelatinase-associated lipocalin; RR. relative risk
Finally, we investigated the association of the change in biomarker concentration from pre-operative to early post-operative values and the development of AKI in those with and without a preoperative eGFR ≤ 60 ml/min/1.73m2. The results were nearly identical to those presented for pre-operative and post-operative values alone (Table S6).
Discussion
In our analysis of a prospective multi-center observational cohort of adults undergoing cardiac surgery, we discovered that there is statistically significant interaction between urinary biomarkers and its association with AKI in the presence of pre-operative eGFR. We have demonstrated that these statistical interactions were present, but varied across biomarkers depending on whether biomarkers were analyzed continuously (IL-18) or across quintiles (NGAL, L-FABP and albuminuria). Additionally, the presence and magnitude of these interactions varied if baseline eGFR was assessed as a dichotomous (≤ vs >60 ml/min/1.73m2) or continuous variable. For most biomarkers, their median levels and association with mild AKI was slightly larger in patients with higher baseline eGFR. However, the magnitude of effect of these interactions is small, and the differences are not clinically meaningful at the average biomarker concentrations to require separate reporting of these sub-groups.
These results provide some clarity on an issue that has been discussed throughout the urinary biomarker literature for the past several years. 16–18 McIlroy and colleagues measured urinary NGAL in a prospective observational study of 426 adult cardiac surgery patients and demonstrated that, when AKI was defined as a 50% increase in serum creatinine from baseline (over 7 days), NGAL was not able to detect AKI in those with a eGFR less than 60 ml/min/1.73m2. This was in contrast to its performance in those with an eGFR > 60 ml/min/1.73m2, where it was able to predict AKI at several timepoints in the first 24 hours following the completion of cardiopulmonary bypass. 17 Additionally, urinary NGAL measured 24 hours after surgery performed the best in the subset of their patients who had an eGFR of 90–120 ml/min/1.73m2 (AUC of 0.88 for the detection of AKI [stage 1 AKI]). In contrast, our data demonstrated a clear pattern of significant association when pre and post-operative NGAL values were assessed as continuous variables adjusting for the model (Tables 2 and 3).
Conversely, in a post-hoc analysis of the Early Intervention in Acute Renal Failure (EARLYARF) study18, Endre and colleagues measured several urinary biomarkers in their mixed surgical–medical ICU cohorts, and assessed their ability to predict AKI (defined by the AKIN criteria20) stratified by GFR status. They demonstrated that several biomarkers, including NGAL, IL-18, KIM-1 and cystatin C, all displayed individual patterns in their timing and ability to detect AKI in those with variable baseline eGFRs. In this study, while several biomarkers (including NGAL, IL-18 and KIM-1) were associated with the development of AKI in those with an eGFR less than 60 ml/min/1.73m2, these same biomarkers were not able to consistently predict AKI in those with eGFRs of 60-<90, 90-<120 and ≥120 ml/min/1.73 m2.18 Our data differ slightly from those of the EARLYARF study as well as the aforementioned McIlroy et al study. 17 These differences may stem from our patient populations. McIlroy et al was single-center cohort of cardiac surgery patients, where patient care and unique practice patterns may affect biomarker results and outcomes, and the EARLYARF study was a mixed ICU cohort with only 17.8% of patients undergoing cardiac surgery and, as such, the AKI and its associated biomarker fingerprint from this cohort will likely be very different than those in our multi-center, high-risk adult cardiac surgery cohort. 17,18
Our study has several strengths. Our data, samples and biomarker measurements were all from a large prospective multi-center investigation. We also utilized standardized, internationally accepted definitions of AKI20 and eGFR equations22. However, as previously reported, we are limited in that there was a low number of severe AKI events. By their nature, post-hoc interaction analyses are often underpowered and this undoubtedly affected our analyses. Additionally, our findings are specific to the setting of cardiac surgery-associated AKI and, as such, our findings may be setting specific and not generalizable to other AKI phenotypes. Additionally, we are limited in that we relied on serum creatinine as our gold standard to define AKI. Serum creatinine has been repeatedly demonstrated to be an imperfect biomarker of renal tubular injury and does not adequately reflect the underlying renal reserve/ functioning nephron mass27,28 and may in fact negatively affect biomarker performance.29 In spite of these limitations, our data is strengthened by reporting on our complete panel of urinary biomarkers and providing data for these biomarkers at multiple timepoints, for multiple definitions of AKI as well as adjusted for urinary creatinine. Finally we provide thorough and complete analyses investigating the biomarkers and eGFR as categorical and continuous variables and attempted to account for several clinical variables known to affect AKI and patient outcomes.
In summary, in this secondary analysis of the TRIBE-AKI adult cardiac surgery cohort, biomarkers of AKI performed differently in those with and without a preoperative eGFR ≤60 ml/min/1.73m2. Although there were statistically significant interactions between AKI biomarkers and the baseline eGFR, the effects were weak, inconsistent across biomarkers, varied across definitions of both AKI and baseline kidney function. Even when these interactions are significant, their clinical implication is modest and, as such, at this time applying distinct biomarker cut-offs for those with and without decreased pre-operative eGFR is not necessary in the peri-operative setting.
Supplementary Material
Acknowledgments
Members of the TRIBE-AKI Consortium are Dr. Jai Raman, Dr. Valluvan Jeevanandam, and Dr. Shahab Akhter (University of Chicago); Dr. Prasad Devarajan, Michael Bennett, Qing Ma, and Rachel Griffiths (University of Cincinnati Children’s Hospital); Dr. Charles Edelstein (University of Colorado); Dr. Cary Passik and Ms. Judy Nagy (Danbury Hospital); Dr. Madhav Swaminathan (Duke University); Dr. Michael Chu, Dr. Martin Goldbach, Dr. Lin Ruo Guo, Dr. Neil McKenzie, Dr. Mary Lee Myers, Dr. Richard Novick, Dr. Mac Quantz, Virginia Schumann, and LauraWebster (University of Western Ontario); Dr. Michael Zappitelli and Dr. Ana Palijan (Montreal Children’s Hospital); and Dr. Michael Dewar, Dr. Umer Darr, Dr. Sabet Hashim, Dr. John Elefteriades, Dr. Arnar Geirsson, Dr. Susan Garwood, Ms. Rowena Kemp, and Dr. Isabel Butrymowicz (Yale-New Haven Hospital).
Support: This study was supported by the National Institutes of Health (NIH) (R01HL085757 to C.R.P.) to fund the TRIBE-AKI Consortium to study novel biomarkers of AKI in cardiac surgery. J.L.K. and S.G.C. have been supported by NIH Career Development Awards (K23DK081616 and K23DK080132, respectively). C.R.P. is supported by the NIH (K24DK090203). S.G.C., A.X.G., and C.R.P. are also members of the NIH-sponsored Assess, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury Consortium (U01DK082185). The urine biomarker assays were donated by Abbott Diagnostics (IL-18 and NGAL) and Sekisui Diagnostics LLC (cystatin C, KIM-1 and L-FABP). The granting agencies, Abbott Diagnostics, and Sekisui Diagnostics, Inc did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding sources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Dr Parikh, with Dr Charles Edelstein, is a named co-inventor on the IL-18 patent. Dr Devarajan is co-inventor on the NGAL patents.
Contributions: Research idea and study design: JLK, SGC, HT-P, CRP; data acquisition JLK, SGC, UDP, MS, AXG, CRP; data analysis and interpretation: JLK, SGC, HT-P, UDP MS, AXG, CRP; statistical analysis: HT-P, supervision: CRP. Each author contributed important intellectual content during the manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy and integrity of any portion of the work are appropriately investigated and resolved. JLK and CRP take responsibility that this study has been reported honestly, accurately and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned and registered have been explained.
References
- 1.Koyner JL, Cerda J, Goldstein SL, et al. The Daily Burden of Acute Kidney Injury: A World Kidney Day Survey of U.S. Nephrologists. Journal of the American Society of Nephrology. 2013;24:3A. doi: 10.1053/j.ajkd.2014.03.018. Oral Abstract Presented at ASN Renal Week - Thursdayvember 7, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Koyner JL, Parikh CR. Clinical Utility of Biomarkers of AKI in Cardiac Surgery and Critical Illness. Clin J Am Soc Nephrol. 2013;8(6):1034–1042. doi: 10.2215/CJN.05150512. [DOI] [PubMed] [Google Scholar]
- 3.Bihorac A, Chawla LS, Shaw AD, et al. Validation of Cell-Cycle Arrest Biomarkers for Acute Kidney Injury Using Clinical Adjudication. Am J Respir Crit Care Med. 2014;189(8):932–9. doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 4.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17(1):R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koyner JL, Garg AX, Coca SG, et al. Biomarkers Predict Progression of Acute Kidney Injury after Cardiac Surgery. J Am Soc Nephrol. 2012;23(5):905–14. doi: 10.1681/ASN.2011090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parikh CR, Coca SG, Thiessen-Philbrook H, et al. Postoperative Biomarkers Predict Acute Kidney Injury and Poor Outcomes after Adult Cardiac Surgery. J Am Soc Nephrol. 2011;22(9):1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. Jama. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 8.Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med. 2009;37(9):2552–2558. doi: 10.1097/CCM.0b013e3181a5906f. [DOI] [PubMed] [Google Scholar]
- 9.Mehta RH, Grab JD, O’Brien SM, et al. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114(21):2208–2216. doi: 10.1161/CIRCULATIONAHA.106.635573. quiz 2208. [DOI] [PubMed] [Google Scholar]
- 10.Thakar C, Liangos O, Yared J-P, Nelson D, Hariachar S, Paganini E. Predicting acute renal failure after cardiac surgery: Validation and redefinition of a risk stratification alogorithm. Hemodial Int. 2003;7:143–147. doi: 10.1046/j.1492-7535.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 11.Wijeysundera DN, Karkouti K, Dupuis JY, et al. Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. Jama. 2007;297(16):1801–1809. doi: 10.1001/jama.297.16.1801. [DOI] [PubMed] [Google Scholar]
- 12.Malyszko J, Malyszko JS, Bachorzewska-Gajewska H, Poniatowski B, Dobrzycki S, Mysliwiec M. Neutrophil gelatinase-associated lipocalin is a new and sensitive marker of kidney function in chronic kidney disease patients and renal allograft recipients. Transplant Proc. 2009;41(1):158–161. doi: 10.1016/j.transproceed.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 13.Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(2):337–344. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dieplinger B, Mueller T, Kollerits B, et al. Pro-A-type natriuretic peptide and pro-adrenomedullin predict progression of chronic kidney disease: the MMKD Study. Kidney Int. 2009;75(4):408–414. doi: 10.1038/ki.2008.560. [DOI] [PubMed] [Google Scholar]
- 15.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 16.Koyner JL, Vaidya VS, Bennett MR, et al. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol. 2010;5(12):2154–2165. doi: 10.2215/CJN.00740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McIlroy DR, Wagener G, Lee HT. Neutrophil Gelatinase-Associated Lipocalin and acute kidney injury after cardiac surgery: the effect of baseline renal function on diagnostic performance. Clin J Am Soc Nephrol. 2010;5(2):211–219. doi: 10.2215/CJN.04240609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endre ZH, Pickering JW, Walker RJ, et al. Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int. 2011;79(10):1119–1130. doi: 10.1038/ki.2010.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parikh CR, Devarajan P, Zappitelli M, et al. Postoperative Biomarkers Predict Acute Kidney Injury and Poor Outcomes after Pediatric Cardiac Surgery. J Am Soc Nephrol. 2011;22(9):1737–1747. doi: 10.1681/ASN.2010111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parikh CR, Thiessen-Philbrook H, Garg AX, et al. Performance of Kidney Injury Molecule-1 and Liver Fatty Acid-Binding Protein and Combined Biomarkers of AKI after Cardiac Surgery. Clin J Am Soc Nephrol. 2013;8(7):1079–88. doi: 10.2215/CJN.10971012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koyner JL, Garg AX, Shlipak MG, et al. Urinary cystatin C and acute kidney injury after cardiac surgery. Am J Kidney Dis. 2013;61(5):730–738. doi: 10.1053/j.ajkd.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molnar AO, Parikh CR, Sint K, et al. Association of postoperative proteinuria with AKI after cardiac surgery among patients at high risk. Clin J Am Soc Nephrol. 2012;7(11):1749–1760. doi: 10.2215/CJN.13421211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou G. A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 27.Bosch JP, Saccaggi A, Lauer A, Ronco C, Belledonne M, Glabman S. Renal functional reserve in humans. Effect of protein intake on glomerular filtration rate. Am J Med. 1983;75(6):943–950. doi: 10.1016/0002-9343(83)90873-2. [DOI] [PubMed] [Google Scholar]
- 28.Ronco C, Rosner MH. Acute kidney injury and residual renal function. Crit Care. 2012;16(4):144. doi: 10.1186/cc11426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waikar SS, Betensky RA, Emerson SC, Bonventre JV. Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol. 2012;23(1):13–21. doi: 10.1681/ASN.2010111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


