Abstract
The combination of prescription opioid dependence and chronic pain is increasingly prevalent and hazardous to public health. Variability in pain may explain poor prescription opioid addiction treatment outcomes in persons with chronic pain. This study examined pain trajectories and pain volatility in patients with chronic pain receiving treatment for prescription opioid addiction. We conducted secondary analyses of adults with chronic pain (N = 149) who received buprenorphine-naloxone (BUP-NLX) and counseling for 12 weeks in an outpatient, multi-site clinical trial. Good treatment outcome was defined as urine-verified abstinence from opioids at treatment endpoint (Week 12) and during at least two of the previous three weeks. Pain severity significantly declined over time during treatment (b = − 0.36, p < .001). Patients with greater pain volatility were less likely to have a good treatment outcome (OR = 0.55, p < .05), controlling for baseline pain severity and rate of change in pain over time. A one standard deviation increase in pain volatility was associated with a 44% reduction in the probability of endpoint abstinence. The significant reduction in subjective pain during treatment provides observational support for the analgesic effects of BUP-NLX in patients with chronic pain and opioid dependence. Patients with greater volatility in subjective pain during treatment have increased risk for returning to opioid use by the conclusion of an intensive treatment with BUP-NLX and counseling. Future research should examine underlying mechanisms of pain volatility and identify related therapeutic targets to optimize interventions for prescription opioid addiction and co-occurring chronic pain.
Keywords: chronic pain, prescription opioids, buprenorphine, treatment
Prescription opioid abuse and addiction have reached epidemic levels, with rates of prescribing, non-medical use and abuse, fatal overdoses and emergency room visits, and treatment admissions increasing rapidly in the past 10–15 years (Substance Abuse and Mental Health Services Administration (SAMHSA), 2013; Atluri, Sudarshan, & Manchikanti, 2014). Prescription opioids are currently the second-most commonly abused drug in the nation (SAMHSA, 2013). Increased rates of prescription opioid addiction in adults with chronic non-cancer pain have contributed significantly to these trends (Ballantyne & LaForge, 2007), and the combination of chronic pain and prescription opioid addiction is particularly challenging clinically (Garland, Froeliger, Zeidan, Partin, & Howard, 2013; Sullivan & Howe, 2013). Currently, no treatments for prescription opioid addiction in chronic pain patients have strong empirical support (Windmill et al., 2013). As such, the development of better treatment regimens for prescription opioid addiction in patients with chronic pain is a high priority across the diverse areas of primary care, pain management, addiction treatment, and public health (Ling, Mooney, & Hillhouse, 2011).
Buprenorphine-naloxone (BUP-NLX), a partial mu-opioid agonist/antagonist with established efficacy and FDA-approval for opioid dependence treatment (Campbell & Lovell, 2012; Ling et al., 2005), is a promising pharmacological intervention for co-occurring chronic pain and prescription opioid addiction. Compared to full opioid agonists, BUP-NLX offers an improved safety profile and diminished abuse liability due to its reduced risk of respiratory depression and attenuated rewarding effects (Dahan, 2006; Jones, 2004; Kamei et al., 1995; Walsh & Eissenberg, 2003). While BUP-NLX is not FDA-approved for treatment of pain, its off-label use for chronic pain management in opioid-dependent patients has been described (Chen, Chen, & Mao, 2014; Rosenblum et al., 2012), and transdermal buprenorphine is an FDA-approved treatment for chronic pain (Cote & Montgomery, 2014). Recent experimental and observational research suggests BUP-NLX may offer sufficient analgesia in this population (Daitch et al., 2012; Pade, Cardon, Hoffman, & Geppert, 2012; Roux et al., 2013), with one randomized trial finding similar reductions in pain from BUP-NLX and low-dose methadone (Neumann et al., 2013). These findings are promising but further studies are necessary to characterize the course of analgesic response to BUP-NLX in patients with prescription opioid addiction and its impact on opioid treatment outcomes.
Despite the overall efficacy of BUP-NLX for opioid dependence, relapse to opioid use during treatment is common and difficult to predict (Alford et al., 2011; Garcia-Portilla, Bobes-Bascaran, Bascaran, Saiz, & Bobes, 2014; Hser et al., 2014), but the specific factors that contribute to poor outcomes are not well-understood. One such factor could be severity of pain, as patients with severe pain often have worse outcomes from addiction treatments (Caldeiro et al., 2008; Larson et al., 2007). However, results from previous studies of pain and BUP-NLX outcomes have been mixed. In one study severe pain at baseline predicted better opioid use outcomes at the end of a brief BUP-NLX detoxification, but severe pain at the end of detoxification predicted worse opioid use outcomes two weeks later (Potter et al., 2010). Other studies did not find worse BUP-NLX treatment outcomes in patients with chronic pain or more severe pain at baseline (Chakrabarti, Woody, Griffin, Subramaniam, & Weiss, 2010; Fox et al., 2012; Weiss et al., 2011). Given the few available studies it is unclear whether persons with chronic pain usually have worse outcomes from BUP-NLX treatment.
In contrast to these previous studies that examined pain as a static or “fixed” variable, it is possible that more dynamic aspects of pain predict treatment outcomes within the chronic pain population. Risks for drug relapse are often dynamic (Witkiewitz & Marlatt, 2005), such that temporal variations in risks can be more predictive of treatment outcomes than distal factors (e.g., baseline pain severity). Such temporal variability may be especially relevant for chronic pain, which is characterized by unpredictable fluctuations in pain intensity (Webster, 2008). In particular, the magnitude of “volatility” in pain, defined as the degree of unsystematic fluctuation in pain severity over time, may be an important clinical feature of pain that relates to opioid treatment outcomes. This hypothesis is supported by prior studies of smoking, where volatility in withdrawal and urges predicted smoking lapses during treatment (Cofta-Woerpel et al., 2011; Piasecki, Jorenby, Smith, Fiore, & Baker, 2003), above and beyond severity of baseline withdrawal. To our knowledge no study has directly compared baseline pain severity, change in pain over time, and pain volatility in the prediction of prescription opioid dependence treatment outcomes.
In a previous multi-site clinical trial for prescription opioid addiction, patients with chronic pain did not have worse treatment outcomes overall (Weiss et al., 2011), but changes in pain over time and within-person pain volatility have not been investigated in this sample. The three aims of the current study were to (a) examine changes in pain severity during BUP-NLX treatment for prescription opioid addiction, (b) characterize pain trajectories and pain volatility, and (c) examine whether baseline pain, pain trajectories, or pain volatility may impact prescription opioid addiction treatment. We hypothesized that patients with greater pain over time and greater pain volatility during treatment would have worse treatment outcomes.
Methods
Study Design
This study involved secondary analyses of Phase 2 of the Prescription Opioid Addiction Treatment Study (POATS), a multi-site, randomized, adaptive clinical trial for treatment of prescription opioid addiction (Weiss et al., 2011; Weiss et al., 2010). The original POATS sample involved 653 patients in Phase 1, a 4-week BUP-NLX detoxification with randomization to standard or enhanced counseling. Most of the Phase 1 sample (n = 610, 93%) did not achieve sustained abstinence, and 360 of these patients enrolled in Phase 2. About 41% (n = 149) of the Phase 2 sample had chronic pain and were included in the current study. The primary POATS study revealed no significant effects of treatment condition or chronic pain status on primary opioid use outcomes (Weiss et al., 2011).
Treatment in Phase 2 consisted of open-label BUP-NLX stabilization for 12 weeks and re-randomization to enhanced counseling vs. standard medical management. Study physicians dispensed BUP/NLX at scheduled medical visits, which occurred twice in the first week of Phase 2 and once-weekly thereafter. Sublingual BUP/NLX tablets were dispensed for once-daily dosing ranging from 8/2 mg to 32/8 mg BUP/NLX per day. The BUP/NLX protocol allowed physicians to adjust the dose by as much as 8mg/day at each visit according to patients’ opioid use, withdrawal, craving, and adverse side effects.
Sample
Key inclusion criteria for POATS included the following: at least 18 years old, DSM-IV diagnosis of current prescription opioid dependence, physiological dependence on opioids, agreement to birth control (women), and absence of unstable medical/psychiatric conditions. Key exclusion criteria included use of heroin on ≥ 4 days in the past month, physiological dependence on alcohol/sedatives/stimulants, and lifetime injection of heroin. Complete inclusion and exclusion criteria are in previous reports (Weiss et al., 2011; Weiss et al., 2010). The current study includes Phase 2 participants who had chronic pain (n = 149), defined as having current pain that is “greater than usual aches and pain” for at least three months. Participants receiving prescribed opioid treatment for pain were cleared by their prescribing physician prior to inclusion in the study, and agreed to withdraw from opioid analgesics at least 12 hours prior to BUP/NLX induction. Non-opioid concomitant medications for pain (e.g., ibuprofen, acetaminophen) were permitted and 60% of the sample reported use of these medications. With the exception of greater baseline pain, the chronic pain subsample and the non-pain sample did not differ significantly on any demographic or pretreatment clinical variables (see Table 1 for pretreatment characteristics). The majority of the sample was male (55%) and most were Caucasian (88%), and on average participants used prescription opioids nearly every day (M = 27.9 days) in the past month. Most of the sample (95%) had been using prescription opioids for at least a year and most (82%) reported relief of physical pain as their primary reason for initial use of opioids. The most common physical site of pain was back (43%), followed by lower extremities (21%), sacrum/hip (14%), shoulder/arms (12%), and head/neck (10%). Significant pain in multiple areas of the body was reported by 77% of the sample.
Table 1.
Demographic and clinical characteristics of adults with chronic pain receiving treatment for prescription opioid dependence (N = 149).
| Variable | % (n) or M (SD) |
|---|---|
| Sex: % (n) male | 55% (82) |
| Race: % (n) white | 88% (131) |
| Years of education: M (SD) | 12.8 (2.4) |
| Marital status: % (n) currently married | 30% (45) |
| Baseline pain severity: M (SD) | 4.5 (3.0) |
| Days of prescription opioid use in past 30: M (SD) | 27.9 (3.8) |
| Heroin history: % (n) ever used | 24% (36) |
| Prescription opioid route history: % (n) ever used non-orally | 86% (128) |
| Prescription opioid treatment history: % (n) ever received treatment | 34% (50) |
Measures
Opioid Use
At the phase 2 randomization visit and at each subsequent weekly visit, participants provided urine samples for drugs of abuse, including prescription analgesics, illicit opioids, and methadone. Participants also reported recent drug use via a calendar-assisted retrospective interview (Sobell & Sobell, 1992). In this study we used the same primary outcome variable as the original trial (Weiss et al., 2011). “Successful outcome” was defined as abstinence from all opioids during the final week and at least 2 of the previous 3 weeks, which was assessed via self-report and confirmed by urine drug screen (UDS). Weekly opioid UDS, coded positive (1) vs. negative (0), was also utilized as a time-varying covariate in analyses of pain scores. The number of opioid-free urine samples in Weeks 1–8 was also a covariate in treatment outcome analyses.
Subjective pain severity
Participants in this sample completed the Brief Pain Inventory (BPI)-Short Form (Keller et al., 2004) at randomization and monthly visits and an abbreviated BPI at all other weekly visits. Although both versions assessed worst, average, least, and current pain on a 0–10 scale, the target time frame for worst, average, and least pain differed between the full (24 hours) and abbreviated versions (7 days) of the BPI. Therefore, to assess weekly pain in this study we only utilized the current pain severity item which was consistent across both versions (“right now”). Average baseline pain was in the moderate range (M = 4.5, SD = 3.0).
Baseline demographic and clinical characteristics
Demographics were captured with a brief demographics questionnaire and the Addiction Severity Index-Lite (Cacciola, Alterman, McLellan, Lin, & Lynch, 2007). Baseline measures of opioid use and treatment history were captured with the Pain and Opiate Analgesic Use History (Weiss et al., 2010). Baseline demographic and clinical characteristics used in this study included sex, race, marital status, history of heroin use, history of non-oral prescription opioid use, history of opioid dependence treatment, and Phase 1 and 2 treatment condition.
Buprenorphine/naloxone dose
A drug dispensing and compliance log completed at medical visits collected information on BUP/NLX dosing. This data was used to extract peak BUP/NLX dose during the treatment phase (M = 20 mg/day, SD = 6.75). The most common peak dose was 16mg/day reported by 26% of the sample, followed by 24mg/day (20%) and 32mg/day (14%). Peak BUP-NLX dose was tested as a covariate of treatment outcome.
Opiate withdrawal
The Clinical Opiate Withdrawal Scale (COWS) was completed at baseline and every four weeks during treatment to measure opiate withdrawal severity (Wesson & Ling, 2003). In this study the average COWS score during treatment was used to examine the association between opiate withdrawal and pain volatility.
Non-opioid drug use
Use of other (non-opioid) drugs was examined via weekly UDS and tested as a covariate of weekly pain. Each UDS tested for amphetamines, benzodiazepines, cocaine, methamphetamine, and cannabis. The most common other illicit drugs used were cannabis (21% of UDS positive) and benzodiazepines (18% of UDS positive); all drugs were used at very low rates (1–7%) during treatment.
Statistical analyses
Individual pain trajectories
Repeated measures of pain scores were nested within individuals therefore hierarchical linear models (HLMs) estimated individual trajectories of pain, with fixed effects for person-level and time-varying covariates. Individual heterogeneity in intercepts and covariate slopes were estimated with random effects. All available data was included via maximum-likelihood (ML) estimation, a preferred method when longitudinal data is considered missing-at-random (Schafer & Graham, 2002). Analyses revealed no significant differences on any study variables between participants with any missing data vs. those with full data, supporting this assumption. The HLM of pain estimated weekly pain ratings as a function of individual-specific intercepts (initial level) and slopes for linear time, quadratic time, and opioid UDS, along with the fixed effects of time, time-varying opioid UDS, demographics, and clinical covariates. Following from prior research (McCarthy, Piasecki, Fiore, & Baker, 2006) empirical bayes estimates of significant random effects (e.g., intercept, time slope) were extracted and standardized for use in further analyses. Individual plots of estimated pain trajectories were also produced to illustrate the variability in pain trajectory intercepts and slopes and to display the effects of any significant covariates.
Pain volatility
Pain volatility scores captured the dispersion of each individual’s pain scores around their own pain trajectory. To separate volatility from potential confounders, such as baseline pain and opioid use, the volatility score only utilized remaining variability in pain after accounting for intercepts, slopes, and significant covariate effects. Similar to prior research (Cofta-Woerpel et al., 2011) we used mean absolute deviation for the volatility score, by obtaining pain score residuals from the final HLM, converting residuals to absolute values, and calculating the mean absolute residual for each participant. This created a single volatility score for each participant for the 12-week treatment phase, with larger volatility reflecting greater week-to-week deviations above or below their individualized pain trajectory. To illustrate the range of pain volatility, the actual pain score residual values were plotted across the pain volatility scores. We also plotted pain score residual values over the overall pain trajectory after splitting the sample at the median volatility score, which illustrated the deviation in pain scores around the pain trajectory over time.
Analysis of opioid use outcomes
Potential covariates of Phase 2 treatment outcome included Phase 1 treatment group, Phase 2 treatment group, demographics, and baseline clinical characteristics. The number of opioid-free urine samples provided in Weeks 1–8 (i.e., prior to the treatment outcome period) and peak BUP/NLX dose were also tested as covariates. A preliminary logistic regression examined these covariate effects on treatment outcome and statistically significant covariates (at p < .05) were retained for further models. To test the improvement in model fit provided by dynamic vs. static pain variables, an initial logistic regression examined Phase 2 baseline pain as a predictor. A subsequent logistic regression examined pain intercepts, slopes, and volatility obtained from the model of weekly pain scores during treatment as predictors of treatment outcome. Model comparisons were conducted via likelihood-ratio (LR) tests, with outcome classification examined with sensitivity and specificity metrics. All analyses were conducted in Stata 13.0 (StataCorp., 2013).
Results
Weekly pain scores during BUP-NLX stabilization
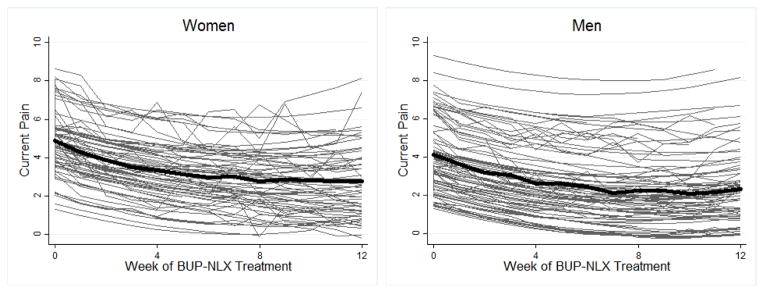
A total of 1,635 weekly pain scores were obtained for the sample, with an average of 10.97 (SD = 2.98) pain scores provided by each participant during treatment. Individual pain trajectories estimated by the final model are displayed in Figure 1. The sample-wide trajectory of pain was characterized by initially moderate levels of pain (4.51) that declined significantly over the 12-week treatment (linear time b = − 0.36, p < .001), with significant upward curvature over time as indicated by a positive quadratic time effect (b = 0.02, p < .001). Individuals varied significantly around the average pain trajectory in both initial level and linear change over time (see Figure 1), as evidenced by significant variance in the random intercept, σ2 = 3.16, 95% CI [2.39, 4.19], and random linear time slope, σ2 = 0.02, 95% CI [0.01, 0.03]. A statistically significant fixed effect for opioid UDS (b = 0.50, p < .01) indicated positive opioid UDS were associated with greater pain, as displayed by time-specific offsets in pain in Figure 1. Individuals varied significantly in the magnitude of this UDS association, as indicated by significant variance in the random UDS slope, σ2 = 0.92, 95% CI [0.46, 1.84]. Sex was the only significant person-level predictor of pain, with men reporting lower pain than women (b = − 0.76, p < .05). Phase 1 and 2 treatment group, race, prior heroin use, non-oral POA use, prior POA treatment, and non-opioid drug use did not predict pain severity.
Figure 1.
Model-estimated trajectories of subjective pain in adults with chronic pain receiving treatment for prescription opioid addiction. The average trajectory (bold line) is displayed separately for women and men. Thin lines display individualized trajectories which illustrate the significant variability in baseline pain and rate of change in pain over time.
Pain volatility
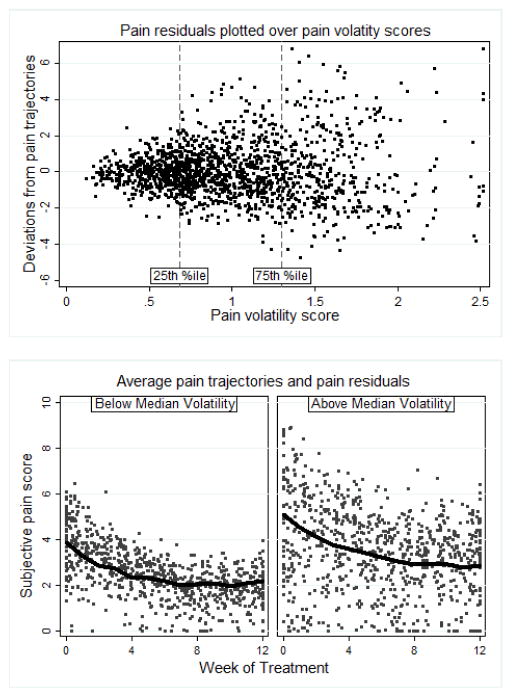
Measures of pain volatility scores, obtained from the HLM of weekly pain scores, captured the average magnitude of each individual’s deviation in pain away from their own pain trajectory during the 12-week treatment phase. The average pain volatility score was 1.00 (SD = 0.45, Range = 0.20 2.51), indicating that in a given week the average participant deviated one point above or below by their own trajectory as predicted by systematic time effects, sex, and weekly opioid use. To illustrate the relationship between volatility scores and the observed weekly deviations in pain, we plotted the weekly pain residuals against individual pain volatility scores (see Figure 2), noting that participants below the 25th percentile had pain scores that always adhered relatively closely (within 2.5 points) to their own trajectory, while those above the 75th percentile had pain scores with larger deviations (up to 6.5 points). To examine the relationship between pain volatility and pain trajectories, the sample was split at median volatility (0.91) and weekly pain residuals were plotted around each group’s average pain trajectory (see Figure 2). Note that the above-median group appeared to have greater initial pain and greater reduction in pain over time. Pain volatility scores were also positively correlated with pain intercepts (r = 0.23, p < .01) and negatively correlated with linear time slopes (r = −0.28, p < .001). Pain volatility scores were also positively correlated with participants’ mean COWS score (r = .26, p < .001).
Figure 2.
Pain score residuals from model-estimated individual pain trajectories plotted across individual volatility scores (top panel) illustrate greater deviations associated with greater pain volatility. Pain score residuals plotted around the average pain trajectories for median-split volatility groups (bottom panel) illustrate scatter around the average trajectories over time.
Prediction of treatment outcome
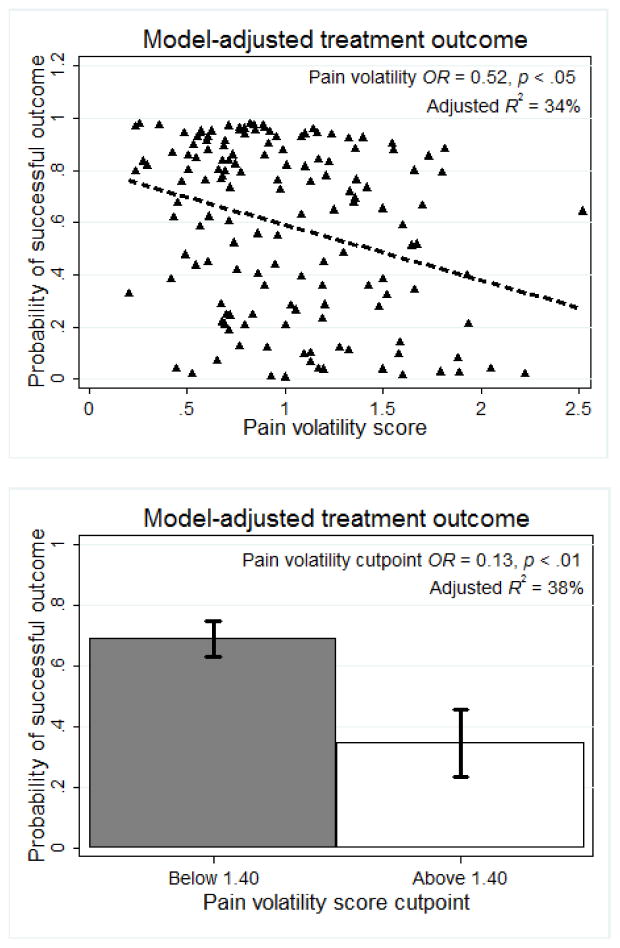
In the covariate logistic regression treatment outcome was not predicted by Phase 1 or 2 treatment group, race, sex, marital status, non-oral prescription opioid use, or prior opioid treatment. No prior heroin use, OR = 3.65, p < .05, 95% CI [1.16, 11.49], and greater number of opioid-free UDS in Weeks 1–8, OR = 1.69, p < .001, 95% CI [1.33, 2.13] predicted greater probability of successful outcome, while greater peak BUP/NLX dose predicted lower probability of successful outcome, OR = 0.92, p < .05, 95% CI [0.85, 0.99]. Controlling for these significant covariates, baseline pain severity did not predict treatment outcome, OR = 0.95, p = .52, 95% CI [0.81, 1.11]. In contrast, greater pain volatility predicted lower probability of successful outcome, OR = 0.51, p < .05, 95% CI [0.30, 0.89], controlling for heroin use history, Week 1–8 opioid UDS, peak BUP/NLX dose, pain intercept, linear pain slope, and opioid UDS slope (see Table 2). Adding pain volatility as a predictor of treatment outcome (in addition to significant clinical covariates and baseline pain) significantly improved model fit (LR χ2(1) = 5.34, p < .05), and the overall model explained a moderate amount of variance in treatment outcomes (pseudo R2 = 34.3%). Figure 3 displays the adjusted probability of good treatment outcome as a function of pain volatility scores.
Table 2.
Logistic regressions of successful treatment outcomea following 12 weeks of buprenorphine-naloxone stabilization and counseling.
| Variables | OR | OR | OR |
|---|---|---|---|
| Race (white vs. other) | 3.28 | 3.68 | 3.86 |
| Heroin use (never used vs. ever) | 3.55* | 4.09* | 4.71* |
| Count of opioid-free urines (Week 1–8) | 1.69*** | 1.76*** | 1.76*** |
| Peak buprenorphine/naloxone dose | 0.92* | 0.92* | 0.91* |
| Baseline pain | - | 1.05 | |
| Pain intercept | 1.35 | - | |
| Pain linear slope | 0.84 | - | |
| Urine drug screen slope | 0.93 | - | |
| Pain volatility | 0.51* | 0.52* | |
|
| |||
| Model fit statistics | |||
|
| |||
| Likelihood-ratio X2 (df) | 46.2 (4) | 52.6 (8) | 53.4 (6) |
| Pseudo-R2 (in %) | 29.1 | 33.1 | 34.2 |
p < .05,
p < .001.
Defined as abstinence from opioids during week 12 and at least two of weeks 9–11, assessed via self-report and urine drug screen
Figure 3.
Controlling for heroin use, ethnicity, early-treatment opioid use, and peak buprenorphine-naloxone dose, higher pain volatility scores were significantly associated with reduced probability of endpoint abstinence, when examined as a continuous volatility score (top panel) and as a cutpoint (bottom panel).
Further analyses examined whether a specific cutpoint in pain volatility would accurately predict treatment outcome. A volatility score of 1.40 met Bonferroni-corrected alpha of .005 (χ2(1) = 10.40, p < .001), as successful outcomes were significantly more likely for participants below this cutpoint of pain volatility (71%) compared to participants above it (36%). In a logistic regression controlling for clinical covariates and other pain variables, individuals above this cutpoint were significantly less likely to have successful outcome (OR = 0.13, p < .01, 95% CI [0.04, 0.47]), with the mean adjusted probabilities of successful outcome displayed in Figure 3.
Discussion
In this study we sought to further understand contributions to poor prescription opioid treatment outcomes in adults with chronic pain, finding that patients with greater volatility in pain were less likely to achieve sustained opioid abstinence at the end of treatment. Our pain volatility index captured each patient’s typical weekly deviation in pain from their own pain trajectory, and the association between volatility and treatment outcome was independent of baseline pain severity, change in pain, prior heroin use, opioid use in early treatment, and peak BUP-NLX dose. In prior studies chronic pain status or baseline pain severity did not predict BUP-NLX treatment outcomes (Chakrabarti et al., 2010; Fox et al., 2012; Weiss et al., 2011). These findings would suggest that the mere presence of chronic pain does not impact BUP-NLX treatment outcomes. However, our findings suggest that among persons with chronic pain, greater within-person volatility in pain reflects greater risk for poor opioid use outcomes from BUP-NLX and counseling. Our findings also suggest that within this population, pain volatility may be a better indicator of risk than other aspects of pain, such as baseline pain severity and increased pain over time. Chronic pain is often characterized by unpredictable fluctuations in pain (Webster, 2008), and our study may be the first that links pain volatility to prescription opioid treatment outcomes.
Because this study did not directly manipulate pain we cannot conclude that pain volatility itself causes relapse to opioid use, and it is unclear from our findings why some individuals had pain that was more volatile than others. However, it is possible that identifying the mechanisms of pain volatility could reveal novel therapeutic targets with the potential to improve opioid treatment outcomes in this population. Such mechanisms are currently unknown as pain volatility is not a well-developed construct, but pain sensitivity is a potentially relevant factor with some known correlates. Greater acute pain sensitivity has been linked to greater default mode network-insula connectivity (Loggia et al., 2013), greater self-reported stress (Cathcart, Bhullar, Immink, Della Vedova, & Hayball, 2012; Crettaz et al., 2013), more severe depression (Kwon & Chang, 2013), and unpleasant physical activity (Cook, Stegner, & Ellingson, 2010). Pain sensitivity and opioid craving are also correlated in abstinent heroin users (Ren, Shi, Epstein, Wang, & Lu, 2009). In related findings our study found that participants’ pain volatility was positively correlated with their severity of opioid withdrawal during treatment. Future studies could investigate whether temporal pain volatility is linked to neural connectivity, emotional distress, or opioid craving, and whether interventions targeting these mechanisms could reduce pain volatility and improve treatment outcomes in patients with chronic pain. Of note, the association between pain volatility and endpoint abstinence was independent of opioid use in early treatment, suggesting that pain volatility was a distinct risk for poor endpoint outcome that was not simply due to greater opioid use early in treatment in patients with more volatile pain.
In addition to pain volatility, our study examined changes in pain during 12 weeks of BUP-NLX treatment and counseling, finding an overall decrease in pain over time. Although causation cannot be concluded from this open-label BUP-NLX design, this observational finding replicates previous findings of analgesic benefits of BUP-NLX for persons with chronic pain and opioid dependence (Daitch et al., 2012; Neumann et al., 2013; Pade et al., 2012; Rosenblum et al., 2012; Roux et al., 2013). The overall pattern of subjective pain was a gradual reduction during treatment, but we also found significant variability in pain trajectories, meaning some patients did not experience as much reduction in pain. Future studies should identify factors that influence the analgesic response to BUP-NLX in prescription opioid-dependent patients with chronic pain. An enhanced counseling condition did not impact pain over standard counseling, but POATS was foremost an opioid treatment study and both counseling models primarily targeted opioid use. Efficacious psychosocial interventions for pain exist (Bushnell, Ceko, & Low, 2013; Ehde, Dillworth, & Turner, 2014), and it is possible that an adjunct therapy primarily targeting chronic pain could potentially enhance the analgesic effects of BUP-NLX in patients whose pain is unresponsive to medication alone.
In this sample urine-verified opioid use was associated with greater concurrent pain. Although the concurrent assessments in this study prevent any determinations of temporal directionality between opioid use and pain, this finding may reflect episodic opioid use in response to increased pain (“self-medication”), with pain persisting due to the partial agonist/antagonist properties of BUP-NLX. Patients also may have experienced increased subjective pain in response to opioid use and subsequent withdrawal. Research designs with more frequent, fine-grained assessments would be necessary to examine precisely how opioid use, withdrawal, and pain unfold over time during an episode of opioid dependence treatment.
Covariate analyses revealed that non-white ethnicity, any heroin use, and stronger peak BUP-NLX dose were associated with worse treatment outcomes. Race did not predict treatment outcome in the full POATS sample (Dreifuss et al., 2013), but within this chronic pain subsample only 38% of non-white patients achieved successful outcome compared to 68% of white patients. Although based on a small percentage of the sample (12%) in the current study, non-white patients have had poorer outcomes in previous studies of BUP-NLX (Alford et al., 2011), suggesting tailored interventions for non-white patients may be necessary in some settings. The poorer treatment outcomes for heroin users replicated findings from the full POATS sample (Weiss et al., 2011). In persons with prescription opioid dependence a history of heroin use typically conveys a more severe course of opioid dependence and these patients often respond more poorly to a wide range of treatments (Dreifuss et al., 2013; Jones, 2013; Nielsen, Hillhouse, Thomas, Hasson, & Ling, 2013). In the original trial BUP-NLX doses could be adjusted during treatment according to opioid use, craving, and withdrawal. Our results showed patients who were titrated to greater peak BUP-NLX doses were also least likely to achieve endpoint abstinence, perhaps owing to more severe opioid dependence.
Our findings must be interpreted in light of several limitations. This study involved secondary analyses that examined pain-related predictors of treatment outcome in a subsample of the original trial. Because the original study was not designed specifically to examine these aims, these findings are preliminary and in need of prospective confirmation. In particular, BUP-NLX was open-label and adjusted variably, so the causal impact and dose-response effect of BUP-NLX on pain and pain volatility cannot be determined from this design. Furthermore, because the pain metrics utilized here are sample-dependent and not immediately applicable to the broader population, more generalized research is warranted before these findings can be incorporated into specific clinical recommendations. Although inclusion criteria in the original trial were carefully designed to closely represent the prescription opioid-dependent population, this study was conducted in research-oriented treatment clinics with the necessary resources to support a controlled clinical trial, which may not represent the typical clinical care setting. Furthermore, these findings may not generalize to chronic pain patients that do not meet criteria for prescription opioid dependence or desire opioid dependence treatment. Although we detected a positive association between time-varying opioid use and pain severity, we could not disentangle the causal relationship between these factors due to their concurrent measurement. Finally, because these secondary analyses were limited to the BUP-NLX stabilization phase, it is unknown whether the risk for opioid use associated with pain volatility persists beyond the completion of active treatment.
In persons with chronic pain, this study revealed significant decreases in subjective pain during BUP-NLX treatment and counseling for prescription opioid addiction. Furthermore, patients with lower volatility in subjective pain were more likely to achieve endpoint abstinence. These findings indicate that intra-individual volatility in subjective pain may be a better prognostic marker for future opioid use than initial pain severity or rate of change in pain over time. Future research is needed to identify underlying neurobiological or psychosocial mechanisms of pain volatility that can be targeted with interventions. Furthermore, clinicians providing treatment for co-occurring prescription opioid addiction and chronic pain may consider monitoring pain volatility to monitor risk for poor treatment outcomes and adjust treatment regimens accordingly.
Contributor Information
Matthew J. Worley, Department of Family Medicine, University of California, Los Angeles
Keith G. Heinzerling, Department of Family Medicine, University of California, Los Angeles
Steven Shoptaw, Department of Family Medicine, University of California, Los Angeles
Walter Ling, Integrated Substance Abuse Program, University of California Los Angeles
References
- Alford DP, LaBelle CT, Kretsch N, Bergeron A, Winter M, Botticelli M, Samet JH. Collaborative care of opioid-addicted patients in primary care using buprenorphine: five-year experience. Archives of Internal Medicine. 2011;171:425–431. doi: 10.1001/archinternmed.2010.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atluri S, Sudarshan G, Manchikanti L. Assessment of the trends in medical use and misuse of opioid analgesics from 2004 to 2011. Pain Physician. 2014;17:E119–128. [PubMed] [Google Scholar]
- Ballantyne JC, LaForge KS. Opioid dependence and addiction during opioid treatment of chronic pain. Pain. 2007;129:235–255. doi: 10.1016/j.pain.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nature reviews: Neuroscience. 2013;14:502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciola JS, Alterman AI, McLellan AT, Lin YT, Lynch KG. Initial evidence for the reliability and validity of a “Lite” version of the Addiction Severity Index. Drug and Alcohol Dependence. 2007;87:297–302. doi: 10.1016/j.drugalcdep.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Calcaterra S, Glanz J, Binswanger IA. National trends in pharmaceutical opioid related overdose deaths compared to other substance related overdose deaths: 1999–2009. Drug and Alcohol Dependence. 2013;131:263–270. doi: 10.1016/j.drugalcdep.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeiro RM, Malte CA, Calsyn DA, Baer JS, Nichol P, Kivlahan DR, Saxon AJ. The association of persistent pain with out-patient addiction treatment outcomes and service utilization. Addiction. 2008;103:1996–2005. doi: 10.1111/j.1360-0443.2008.02358.x. [DOI] [PubMed] [Google Scholar]
- Campbell ND, Lovell AM. The history of the development of buprenorphine as an addiction therapeutic. Annals of the New York Academy of Sciences. 2012;1248:124–139. doi: 10.1111/j.1749-6632.2011.06352.x. [DOI] [PubMed] [Google Scholar]
- Cathcart S, Bhullar N, Immink M, Della Vedova C, Hayball J. Pain sensitivity mediates the relationship between stress and headache intensity in chronic tension-type headache. Pain Research & Management. 2012;17:377–380. doi: 10.1155/2012/132830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti A, Woody GE, Griffin ML, Subramaniam G, Weiss RD. Predictors of buprenorphine-naloxone dosing in a 12-week treatment trial for opioid-dependent youth: secondary analyses from a NIDA Clinical Trials Network study. Drug and Alcohol Dependence. 2010;107:253–256. doi: 10.1016/j.drugalcdep.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KY, Chen L, Mao J. Buprenorphine-Naloxone Therapy in Pain Management. Anesthesiology. 2014;120:1262–1274. doi: 10.1097/ALN.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cofta-Woerpel L, McClure JB, Li Y, Urbauer D, Cinciripini PM, Wetter DW. Early cessation success or failure among women attempting to quit smoking: trajectories and volatility of urge and negative mood during the first postcessation week. Journal of Abnormal Psychology. 2011;120:596–606. doi: 10.1037/a0023755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug and Alcohol Dependence. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Cook DB, Stegner AJ, Ellingson LD. Exercise alters pain sensitivity in Gulf War veterans with chronic musculoskeletal pain. The Journal of Pain. 2010;11:764–772. doi: 10.1016/j.jpain.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Cote J, Montgomery L. Sublingual buprenorphine as an analgesic in chronic pain: a systematic review. Pain Medicine. 2014;15:1171–1178. doi: 10.1111/pme.12386. [DOI] [PubMed] [Google Scholar]
- Crettaz B, Marziniak M, Willeke P, Young P, Hellhammer D, Stumpf A, Burgmer M. Stress-induced allodynia--evidence of increased pain sensitivity in healthy humans and patients with chronic pain after experimentally induced psychosocial stress. PloS One. 2013;8:e69460. doi: 10.1371/journal.pone.0069460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A. Opioid-induced respiratory effects: new data on buprenorphine. Palliative Medicine. 2006;20S1:S3–8. [PubMed] [Google Scholar]
- Daitch J, Frey ME, Silver D, Mitnick C, Daitch D, Pergolizzi J., Jr Conversion of chronic pain patients from full-opioid agonists to sublingual buprenorphine. Pain Physician. 2012;15:ES59–66. [PubMed] [Google Scholar]
- Dreifuss JA, Griffin ML, Frost K, Fitzmaurice GM, Potter JS, Fiellin DA, Weiss RD. Patient characteristics associated with buprenorphine/naloxone treatment outcome for prescription opioid dependence: Results from a multisite study. Drug and Alcohol Dependence. 2013;131:112–118. doi: 10.1016/j.drugalcdep.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehde DM, Dillworth TM, Turner JA. Cognitive-behavioral therapy for individuals with chronic pain: Efficacy, innovations, and directions for research. The American Psychologist. 2014;69:153–166. doi: 10.1037/a0035747. [DOI] [PubMed] [Google Scholar]
- Fox AD, Sohler NL, Starrels JL, Ning Y, Giovanniello A, Cunningham CO. Pain is not associated with worse office-based buprenorphine treatment outcomes. Substance Abuse. 2012;33:361–365. doi: 10.1080/08897077.2011.638734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Portilla MP, Bobes-Bascaran MT, Bascaran MT, Saiz PA, Bobes J. Long term outcomes of pharmacological treatments for opioid dependence: does methadone still lead the pack? British Journal of Clinical Pharmacology. 2014;77:272–284. doi: 10.1111/bcp.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Zeidan F, Partin K, Howard MO. The downward spiral of chronic pain, prescription opioid misuse, and addiction: cognitive, affective, and neuropsychopharmacologic pathways. Neuroscience and Biobehavioral Reviews. 2013;37:2597–2607. doi: 10.1016/j.neubiorev.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, Ling W. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109:79–87. doi: 10.1111/add.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002–2004 and 2008–2010. Drug and Alcohol Dependence. 2013;132:95–100. doi: 10.1016/j.drugalcdep.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Jones HE. Practical considerations for the clinical use of buprenorphine. Science & Practice Perspectives. 2004;2:4–20. doi: 10.1151/spp04224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei J, Saitoh A, Suzuki T, Misawa M, Nagase H, Kasuya Y. Buprenorphine exerts its antinociceptive activity via mu 1-opioid receptors. Life Sciences. 1995;56:PL285–290. doi: 10.1016/0024-3205(95)00078-x. [DOI] [PubMed] [Google Scholar]
- Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. The Clinical Journal of Pain. 2004;20:309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Kwon JK, Chang IH. Pain, catastrophizing, and depression in chronic prostatitis/chronic pelvic pain syndrome. International Neurourology Journal. 2013;17:48–58. doi: 10.5213/inj.2013.17.2.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MJ, Paasche-Orlow M, Cheng DM, Lloyd-Travaglini C, Saitz R, Samet JH. Persistent pain is associated with substance use after detoxification: a prospective cohort analysis. Addiction. 2007;102:752–760. doi: 10.1111/j.1360-0443.2007.01759.x. [DOI] [PubMed] [Google Scholar]
- Ling W, Amass L, Shoptaw S, Annon JJ, Hillhouse M, Babcock D, Ziedonis D. A multi-center randomized trial of buprenorphine-naloxone versus clonidine for opioid detoxification: findings from the National Institute on Drug Abuse Clinical Trials Network. Addiction. 2005;100:1090–1100. doi: 10.1111/j.1360-0443.2005.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Mooney L, Hillhouse M. Prescription opioid abuse, pain and addiction: clinical issues and implications. Drug and Alcohol Review. 2011;30:300–305. doi: 10.1111/j.1465-3362.2010.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, Napadow V. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. 2013;154:24–33. doi: 10.1016/j.pain.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: an electronic diary study. Journal of Abnormal Psychology. 2006;115:454–466. doi: 10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- Neumann AM, Blondell RD, Jaanimagi U, Giambrone AK, Homish GG, Lozano JR, Azadfard M. A preliminary study comparing methadone and buprenorphine in patients with chronic pain and coexistent opioid addiction. Journal of Addictive Diseases. 2013;32:68–78. doi: 10.1080/10550887.2012.759872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Hillhouse M, Thomas C, Hasson A, Ling W. A comparison of buprenorphine taper outcomes between prescription opioid and heroin users. Journal of Addiction Medicine. 2013;7:33–38. doi: 10.1097/ADM.0b013e318277e92e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pade PA, Cardon KE, Hoffman RM, Geppert CM. Prescription opioid abuse, chronic pain, and primary care: a Co-occurring Disorders Clinic in the chronic disease model. Journal of Substance Abuse Treatment. 2012;43:446–450. doi: 10.1016/j.jsat.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: II. Improved tests of withdrawal-relapse relations. Journal of Abnormal Psychology. 2003;112:14–27. [PubMed] [Google Scholar]
- Potter JS, Chakrabarti A, Domier CP, Hillhouse MP, Weiss RD, Ling W. Pain and continued opioid use in individuals receiving buprenorphine-naloxone for opioid detoxification: secondary analyses from the Clinical Trials Network. Journal of Substance Abuse Treatment. 2010;38:S80–86. doi: 10.1016/j.jsat.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren ZY, Shi J, Epstein DH, Wang J, Lu L. Abnormal pain response in pain-sensitive opiate addicts after prolonged abstinence predicts increased drug craving. Psychopharmacology. 2009;204:423–429. doi: 10.1007/s00213-009-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum A, Cruciani RA, Strain EC, Cleland CM, Joseph H, Magura S, Portenoy RK. Sublingual buprenorphine/naloxone for chronic pain in at-risk patients: development and pilot test of a clinical protocol. Journal of Opioid Management. 2012;8:369–382. doi: 10.5055/jom.2012.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux P, Sullivan MA, Cohen J, Fugon L, Jones JD, Vosburg SK, Comer SD. Buprenorphine/naloxone as a promising therapeutic option for opioid abusing patients with chronic pain: reduction of pain, opioid withdrawal symptoms, and abuse liability of oral oxycodone. Pain. 2013;154:1442–1448. doi: 10.1016/j.pain.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- StataCorp. Stata: Release 13. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: 2013. HSDUH Series H-46, HHS Publication No. (SMA) 13-4795. HSDUH Series H-46, HHS Publication No. (SMA) 13-4795. [Google Scholar]
- Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain. 2013;154(Suppl 1):S94–100. doi: 10.1016/j.pain.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Eissenberg T. The clinical pharmacology of buprenorphine: extrapolating from the laboratory to the clinic. Drug and Alcohol Dependence. 2003;70:S13–27. doi: 10.1016/s0376-8716(03)00056-5. [DOI] [PubMed] [Google Scholar]
- Webster LR. Breakthrough pain in the management of chronic persistent pain syndromes. The American Journal of Managed Care. 2008;14:S116–122. [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, Ling W. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Archives of General Psychiatry. 2011;68:1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Provost SE, Huang Z, Jacobs P, Hasson A, Ling W. A multi-site, two-phase, Prescription Opioid Addiction Treatment Study (POATS): rationale, design, and methodology. Contemporary Clinical Trials. 2010;31:189–199. doi: 10.1016/j.cct.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) Journal of Psychoactive Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- Windmill J, Fisher E, Eccleston C, Derry S, Stannard C, Knaggs R, Moore RA. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. The Cochrane Database of Systematic Reviews. 2013;9:CD010323. doi: 10.1002/14651858.CD010323.pub2. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt GA. Emphasis on interpersonal factors in a dynamic model of relapse. The American Psychologist. 2005;60:341–342. doi: 10.1037/0003-066X.60.4.341. [DOI] [PubMed] [Google Scholar]