Abstract
Introduction
There is limited work that has examined the effect of quitting smoking on anxious arousal, an underlying dimension of anxiety symptoms and psychopathology.
Method
Smokers (n = 185, 54.1% female) enrolled in a smoking cessation treatment trial were monitored post-cessation in terms of abstinence status (biochemically verified; at Weeks 1, 2, and Month 1 post-quit) and severity of panic-relevant symptoms (self-reported; at Month 1 and 3 post-quit). Structural equation models were conducted, adjusting for participant sex, age, treatment condition, and pre-cessation nicotine dependence, presence of depressive/anxiety disorders, anxious arousal, and anxiety sensitivity.
Results
After adjusting for covariates, participants who remained abstinent for one month (n = 80; 43.2%) relative to those who did not (n = 105; 56.8%) demonstrated significant reductions in anxious arousal at Month 1 (β=−26, p = .04) and Month 3 post-quit (β = −36, p = .006); abstinence status had a nonsignificant effect on anxious arousal severity at Month 3 after controlling for Month 1 anxious arousal (β = −.18, p = .09).
Discussion
Findings align with theoretical models of smoking-anxiety interplay and suggest that smoking cessation can result in reductions in anxious arousal.
Keywords: Smoking cessation, Tobacco, Prevention, Hyperarousal
1. Introduction
Smokers with comorbid psychiatric disorders show less success in being able to quit smoking, which contribute to the stagnation of smoking cessation rates well-documented over recent years (Goodwin, Zvolensky, Keyes, & Hasin, 2012; Ziedonis et al., 2008). Anxiety symptoms and disorders, in particular, serve to maintain and even promote tobacco use and dependence (Morissette, Tull, Gulliver, Kamholz, & Zimering, 2007) and impair quit success (Piper, Cook, Schlam, Jorenby, & Baker, 2011). Indeed, there are bidirectional associations between smoking and anxiety symptoms/disorders (Amering et al., 1999; Cosci, Knuts, Abrams, Griez, & Schruers, 2010; Zvolensky & Bernstein, 2005; Zvolensky, Feldner, Leen-Feldner, & McLeish, 2005). For example, the initiation of cigarette smoking typically precedes the initial onset of anxiety psychopathology (e.g., Bernstein, Zvolensky, Schmidt, & Sachs-Ericcson, 2007) and smoking increases the later risk for anxiety psychopathology (Breslau, Novak, & Kessler, 2004; Isensee, Wittchen, Stein, Hofler, & Lieb, 2003; Johnson et al., 2000; Jamal, Does, Penninx, & Cuijpers, 2011). Additionally, successful smoking cessation is associated with reductions in anxiety symptoms and decreased likelihood of anxiety disorders among those with preexisting disorders (Cavazos-Rehg et al., 2014; McDermott, Marteau, Hollands, Hankins, & Aveyard, 2013; Shahab, Andrew, & West, 2014; Taylor et al., 2014).
Given the phenotypic heterogeneity in the expression of symptoms across anxiety disorders (Watson, 2005), transdiagnostic models of emotional disorders (anxiety and depressive conditions) have suggested underlying dimensional constructs may serve to explain between-individual variability in symptom presentation (i.e., tripartite model; Clark & Watson, 1991; Watson & Clark et al., 1995; Watson & Weber et al., 1995). Anxious arousal, reflecting the extent to which one experiences somatic arousal and tension (e.g., shortness of breath, dizziness, lightheadedness, trembling, shacking), is a core, cross-cutting feature of many anxiety disorders (Watson & Clark et al., 1995). Anxious arousal also is related to smoking (see review; Ameringer & Leventhal, 2010). For example, anxious arousal is associated with higher levels of nicotine dependence (Zvolensky, Stewart, Vujanovic, Gavric, & Steeves, 2009) and a history of a greater number of unsuccessful smoking cessation attempts (Zvolensky, Johnson, Leyro, Hogan, & Tursi, 2009). Further, poorer perceptions of physical health are associated with higher levels of anxious arousal among daily smokers (McLeish, Zvolensky, Bonn-Miller, & Bernstein, 2006). Evidence also suggests that smoking heaviness and anxious arousal may be bi-directionally linked by affect-regulatory smoking motives (Johnson, Stewart, Zvolensky, & Steeves, 2009). Moreover, anxious arousal is associated with experiencing greater increases in abstinence-induced depression and fatigue (Leventhal, Ameringer, Osborn, Zvolensky, & Langdon, 2013), which was not seen in other tripartite aspects of anxiety and depression (e.g., anhedonic depression). Yet, there is no empirical data on the nature of anxious arousal after a smoking cessation attempt. This gap in knowledge is clinically important to address in order to better understand anxiety phenomena broadly during the process of quitting, which may have cross-cutting implications across specific forms of anxiety psychopathology. That is, based on complex heterogeneity across and within different anxiety disorders, examining anxious arousal, a more parsimonious trans-diagnostic construct, would provide more precise and informative information regarding the patterning of (anxiety) symptoms after a quit attempt.
The majority of empirical evidence suggests that smoking cessation yields on reductions of anxiety symptoms (Becoña, Vázquez, &Míguez, 2002; Dawkins, Powell, Pickering, Powell, &West, 2009; McDermott et al., 2013; Solomon et al., 2006), although there is large heterogeneity across studies (see meta-analysis; Taylor et al., 2014). It is possible that variability in study findings is, in part, a function of motivation to quit smoking (Taylor et al., 2014), but also may be due to broad types of measurement of anxiety symptoms utilized in existing studies that do not permit an examination of ‘pure’ anxious arousal (i.e, they cannot distinguish between anxiety symptoms that do and do not overlap with depression; Taylor et al., 2014). For this reason, it is important to examine the nature of anxious arousal as a function of smoking cessation. Reductions in anxious arousal after smoking cessation would be expected based on the understanding that nicotine has anxiogenic properties over time and can actually promote greater levels of anxiety over time (Kassel, Stroud, & Paronis, 2003; Leventhal & Zvolensky, 2015; Zvolensky & Bernstein, 2005), despite the (perceived or actual) affect regulation and modulation properties of smoking (nicotine) in the immediate context of use—likely due to narrowing of attentional focus to most immediate stimuli in environment and away from subjective distressing thoughts, feelings or sensations (Kassel & Unrod, 2000). Thus, although abstinence from smoking may induce symptoms in the short-term (Vessichhio, Termine, & George, 2002; Zvolensky, Lejuez, Kahler, & Brown, 2004), perhaps due to heightened cognitive-affective reactivity to interoceptive perturbation (Zvolensky & Bernstein, 2005), quitting smoking should theoretically lessen the severity of anxious arousal during periods of sustained abstinence. To address this question, the present study tested the hypothesis that abstainers, relative to non-abstainers, would report less severe anxious arousal at one- and three-months post cessation. These effects were expected to be significant after adjusting for participant gender, age, study treatment condition, and severity of pre-quit levels of nicotine dependence and anxious arousal. The effects were also expected to be significant after adjusting for the presence of depressive/anxiety disorders pre-cessation. Additionally, unlike past work (Taylor et al., 2014), to strengthen the test of these effects, pre-quit levels of anxiety sensitivity (i.e., tendency to misinterpret the meaning of anxious arousal or fear of anxious arousal) was adjusted for in analyses.
2. Material and methods
2.1. Participants
Participants (n = 185; 54.1% female; Mage = 39.41, SD = 13.76) were adult daily smokers recruited as part of a smoking cessation and panic disorder prevention trial (clinicaltrials.gov #NCT01753141). Eligibility criteria for the parent study included: smoking ≥8 cigarettes per day for at least the past year, and motivation to quit rated at least 5 or higher on a 10-point scale. Exclusionary criteria included current use of smoking cessation products or treatment, regular use of other tobacco products, unstable psychotropic medication (had to be stable ≥3 months), history of panic disorder (defined by the DSM-IV-TR), past-month suicidality, a history of psychotic-spectrum disorders, current pregnancy or nursing and inability to provide informed consent. Participants were included in the current study if smoking cessation data were available for at least two of three post-quit follow-up appointments (i.e., Week 1, Week 2, and/or Month 1).
The majority of participants identified race as white (85.4%) and completed at least some college (80.6%). The average daily smoking rate was of this sample was 16.8 (SD = 8.39) cigarettes per day and on average participants reported daily smoking for 21.0 years (SD = 13.86). Moderate levels of nicotine dependence were reported among the sample per the Fagerström for Nicotine Dependence (M=5.1, SD = 2.27) and baseline expired carbon monoxide (CO) averaged 20.6 ppm (SD = 11.99). Current (past-month) Axis I primary diagnoses were as follows: depressive/mood disorder (7.0%), alcohol use disorder (3.8%), substance use disorder (2.7%), social anxiety disorder (9.2%), specific phobia (3.8%), obsessive-compulsive disorder (1.6%), posttraumatic stress disorder (2.2%), and generalized anxiety disorder (5.4%).
2.2. Procedure
Participants were recruited through community-based advertisements at two treatment sites (University of Vermont, Burlington VT and Florida State University, Tallahassee, FL). Potentially-eligible participants were scheduled for a baseline assessment during which participants were assessed using a structured clinical diagnostic assessment, provided a CO analysis to verify smoking status and completed a computerized battery of self-report questionnaires. Eligible participants were randomly assigned to one of two smoking cessation treatment programs and scheduled for treatment initiation approximately 1–2 weeks after the baseline assessment. Smoking cessation treatment consisted of either (1) a standard smoking cessation program (Fiore et al., 2008), or (2) anxiety-focused smoking cessation treatment (Zvolensky, Yartz, Gregor, Gonzalez, & Bernstein, 2008), both included use of nicotine replacement therapy via the transdermal nicotine patch, which was initiated at treatment session four (quit day). Treatment consisted of four 60-min weekly individual sessions conducted by a trained doctoral-level graduate student. Quit day occurred during the last treatment session (Session 4). All treatment was supervised by study authors (MJZ and NBS) and checked for treatment fidelity by independent reviewers. Follow-up assessments occurred at Week 1, Week 2, Month 1, Month 3 post-quit attempt. All participants provided informed consent prior to participation and the study protocol was approved by the Institutional Review Board at both institutions, where the study was conducted.
2.3. Measures
2.3.1. Descriptive measures
The Smoking History Questionnaire (SHQ; Brown, Lejuez, Kahler, & Strong, 2002) is a self-report questionnaire used to assess smoking history (e.g., onset of regular daily smoking), pattern (e.g., number of cigarettes consumed per day), and quit history. In the present study, the SHQ was employed to describe the sample on smoking history and patterns of use (e.g., smoking rate, years as a regular smoker).
The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/NP; First, Spitzer, Gibbon, & Williams, 2007) was used to assess current (past-year) and lifetime Axis I psychological disorders. This non-patient version of the SCID is commonly used in research with community participants (First et al., 2007). Interviews were administered by doctoral level graduate students or highly trained post-baccalaureate clinical research assistants with diagnostic assessment experience; interviewers were supervised by independent doctoral-level psychologists. All random selection of 12.5% of SCID assessments were checked to ensure diagnostic accuracy (no discrepancies were noted; 100% agreement). The SCID-I/NP was used in the current study to describe psychopathology among the sample. Further, the presence of a primary depressive/mood or anxiety disorder was included as a covariate.
2.3.2. Anxious arousal
The Inventory of Depression and Anxiety Symptoms (IDAS; Watson et al., 2007) is a 64-item self-report measure of symptoms of major depression and related anxiety disorders. This measure yields one broad scale and several symptom scales. Respondents are asked to rate the degree to which they have experienced symptoms in the past two weeks, scored on a 5-point Likert-type scale (1 =“not at all” to 5 = “extremely”). One scale from the IDAS includes items that reflect sympathetic arousal, which is often seen in panic attacks and panic disorder (thus is labeled the IDAS-Panic scale). This scale includes 8 items that were adapted from the Mood and Anxiety Symptom Questionnaire—Anxious Arousal Scale (MASQ; Watson & Clark et al., 1995), which includes items like “I was trembling or shaking”, “My heart was racing or pounding”, or “I was short of breath”. The IDAS-Panic subscale can be viewed as a short-form version of the MASQ-Anxious Arousal scale (Watson et al., 2007), thus was used in the current study as an index of the severity of anxious arousal at baseline, Month 1 and Month 3. Psychiatric populations have been found to average scores of 15.1 (SD = 6.10) on the IDAS-Panic scale (possible range = 8–40). This scale has strong psychometric properties, including test-retest reliability, internal consistency, and convergent validity with self-report measure of anxiety and diagnostic assessments of anxiety disorders (Watson et al., 2007).
2.3.3. Covariates
The Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991) is a six-item scale designed to assess gradations in tobacco dependence. Scores range from 0 to 10, with higher scores reflecting higher levels of physiological dependence on nicotine. The FTND measure has shown adequate levels of internal consistency, positive relations with key smoking variables (e.g. saliva cotinine), and high test-retest reliability (Heatherton et al., 1991).
The Anxiety Sensitivity Index-3 (ASI-3; Taylor et al., 2007) is an 18-item psychometrically-sound self-report measure in which respondents indicate the extent to which they are concerned about possible negative consequences of anxiety-related symptoms (e.g., “It scares me when my heart beats rapidly”). Responses are rated on a 5-point Likert scale ranging from 0 (very little) to 4 (very much) and summed to generate a total score. The ASI-3 has strong and improved psychometric properties relative to previous measures of the construct (Taylor et al., 2007).
2.3.4. Biochemical verification of smoking
Carbon monoxide (CO) analysis of breath samples was used as a biochemical verification of smoking status. Expired air CO levels were assessed using a CMD/CO Carbon Monoxide Monitor (Model 3110; Spirometrics, Inc.). CO breath samples at each time point were used as an indicator of abstinence (expired CO ≤4 ppm, as abstinent; per Perkins, Karelitz, & Jao, 2013).
2.4. Definition of quit status
Quit status (i.e., smoking cessation following the intervention; categorized as 0 = non-quitters, 1 = successful quitters) was based on Week 1, Week 2, and Month 1 post-intervention biochemical verification via CO levels ≤4 ppm. To balance accurate classification with missing data in verification of quit status, it was determined that individuals would be included in the analyses if they had data available for at least two time points, and for whom a consistent pattern (i.e., non-quitter or successful quitter) was present. For example, if an individual had data available at Week 1 and at Month 1, but the data conflicted regarding quit status categorization, this individual was excluded from data analysis. However, if an individual had data available at Week 1 and at Month 1, and both time points were consistent regarding quit status, then this individual would be classified accordingly.
Compared to individuals with CO data at the Quit Week appointment (n = 254), 86.2% (n = 219) had CO data at Week 1, 81.1% (n = 206) had CO data at Week 2, and 71.3% (n = 181) had CO data at Month 1. Of the 254 individuals with CO data at Quit Week, 215 participants had CO data available for at least two of three time points. There were no differences in gender, treatment condition, presence of depressive/anxiety disorders, or baseline FTND scores between individuals with and without CO data available at two or more post-intervention appointments. Baseline ASI-3 scores were significantly different (F[1,252] = 5.55, p = .02) such that individuals who had CO data available at two or more post-intervention appointments had significantly lower baseline ASI-3 scores (M = 13.8, SD = 11.56) than individuals who did not have data present at two or more post-intervention time points (M=18.7, SD = 13.61). In addition, baseline levels of anxious arousal were significantly different [F(1, 252) = 5.95, p = .02], such that individuals who had CO data available had lower IDAS-Panic scores (M = 10.5, SD = 3.28) than did individuals who did not have CO data available (M = 12.1, SD = 5.42). Finally, age was also significantly different (F[1,252] = 12.96, p<.001) such that individuals who had CO data available at two or more post-intervention appointments were significantly older (Mage = 39.5 years, SD = 13.75) than individuals who did not have data present at two or more post-intervention time points (Mage = 31.0, SD = 13.11). Of the 215 participants with CO data available for at least two time points, 80 (37.2%) were classified as smoking, 105 (48.8%) were classified as abstinent, and 30 (14.0%) were unable to be classified because no consistent pattern for quit status was detected. There were no differences in gender, age, treatment condition, or baseline nicotine dependence, panic symptoms, or anxiety sensitivity between individuals included in the analyses and those excluded because of inconsistent quit status.
2.5. Data analytic plan
To examine the effects of quit status on anxious arousal, structural equation modeling (SEM) was conducted in Mplus version 7.1 (Muthén & Muthén, 2012). IDAS-Panic items at baseline, Month 1, and Month 3 were modeled as categorical indicators of anxious arousal factors using the robust weighted least squares estimator (WLSMV in Mplus). Overall model fit was assessed using the χ2 statistic and several fit indices, including the comparative fit index (CFI) and the root mean square error of approximation (RMSEA), with accompanying 90% confidence intervals (CIs). A nonsignificant χ2 test statistic indicates good model fit; however, this statistic can be too restrictive, especially when numerous items are used per factor (Hu & Bentler, 1999; Moshagen, 2012; Mulaik, 2007). CFI values greater than .95 and RMSEA values less than .06 indicate good fit. CFI values greater than .90 and RMSEA values less than .08 indicate adequate fit. Finally, lower bound 90% CIs less than .05 indicate that good model fit cannot be ruled out whereas upper bound 90% CIs greater than .10 indicate that poor model fit cannot be ruled out (Brown, 2006; Browne & Cudeck, 1992).
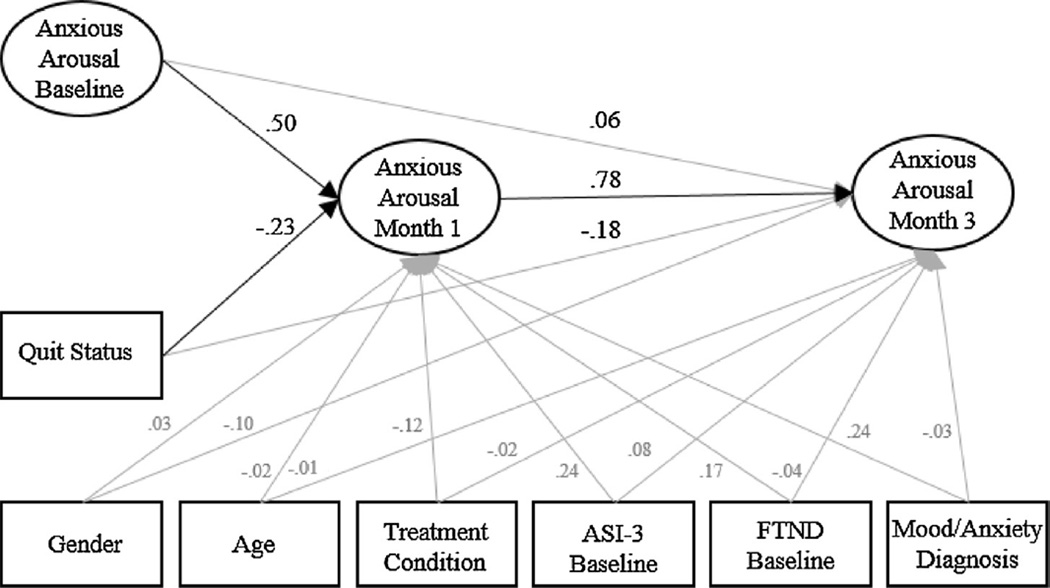
A baseline measurement model of Baseline, Month 1, and Month 3 anxious arousal factors with correlations between all factors was first fit to the data. Following this, an SEM model with directionality of effects, quit status, and covariates (e.g., gender, age, treatment condition, and baseline ASI-3 and FTND scores, and presence of depressive/anxiety disorders) predicting anxious arousal factors at Month 1 and Month 3, was fit to the data (Fig. 1).
Fig. 1.
Structural equation model of the standardized effects.
Note: ASI-3 = Anxiety Sensitivity Index-3. FTND = Fagerström Test of Nicotine Dependence. Grey lines indicate non-significant pathways. Items and residual errors are omitted for clarity. The effects of covariates are in gray for clarity.
3. Results
3.1. Descriptive statistics and correlations
Based on quit status, 185 individuals could be classified in the current study and were either classified as non-quitters (n = 80) or as successful quitters (n = 105). Differences between non-quitters and successful quitters on baseline demographic variables, rates of depressive mood and anxiety disorders, smoking status, anxiety sensitivity, and nicotine dependence are provided in Table 1. Correlations (Pearsons for continuous-by-continuous variables, point-biserial for categorical-by-continuous, and phi coefficients for categorical-by-categorical) and means are provided by quit status for all variables in Table 2.
Table 1.
Comparison of demographics, mood and anxiety disorder status, baseline anxiety sensitivity, nicotine dependence, and anxious arousal between cigarette non-quitters and quitters.
| Non-quitters (n = 80) (%) | Quitters (n = 105) (%) | χ2 | |||
|---|---|---|---|---|---|
| Gender (% male) | 50.0 | 42.9 | .93 | ||
| Diagnoses | |||||
| Depression | 32.5 | 26.7 | .75 | ||
| SAD | 7.5 | 10.5 | .48 | ||
| Specific phobia | 7.5 | 1.0 | 5.35* | ||
| OCD | 1.3 | 1.9 | .12 | ||
| PTSD | 2.5 | 1.9 | .08 | ||
| GAD | 8.8 | 2.9 | 3.08 | ||
| Mean | SD | Mean | SD | F | |
| Age | 38.6 | 14.06 | 40.0 | 13.57 | .47 |
| FTND | 5.3 | 2.21 | 5.0 | 2.33 | .56 |
| ASI-3 | 14.9 | 11.35 | 13.3 | 11.11 | .92 |
| IDAS panic | 10.7 | 2.70 | 10.6 | 3.80 | .09 |
Note: SAD = social anxiety disorder. OCD = obsessive-compulsive disorder. PTSD = posttraumatic stress disorder. GAD = generalized anxiety disorder. FTND = Fagerström Test for Nicotine Dependence. ASI-3 = Anxiety Sensitivity Index-3. SD = standard deviation.
p < .05.
Table 2.
Descriptive statistics and correlations for anxious arousal and control variables by smoking status.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | M (SD) or % quit | |
|---|---|---|---|---|---|---|---|---|---|
| BL anxious arousal | – | .61* | .66* | .52* | .15 | .01 | −.03 | −.11 | 10.6 (3.80) |
| M1 anxious arousal | .47* | – | .78* | .58* | .21 | .14 | .02 | .03 | 9.9 (2.74 |
| M3 anxious arousal | .39* | .68* | – | .42* | .14 | .14 | −.06 | −.01 | 9.7 (2.77) |
| BLASI-3 | .54* | .47* | .59* | – | .18 | .10 | −.03 | .08 | 13.3 (11.11) |
| BLFTND | .18 | .01 | −.03 | .09 | – | .26* | .07 | .15 | 5.0 (2.33) |
| Age | −.02 | −.03 | −.15 | −.14* | .52* | – | .11 | .07 | 40.0 (13.57) |
| Gender (male) | .24* | .12 | −.03 | .12* | .004 | .07 | – | .08 | 43% Male |
| Condition (active) | −.21* | −.16 | −.09 | −.21* | .01 | .12 | −.04 | – | 60% Active |
| M (SD) or % non-quit | 10.7 (2.70) | 11.1 (4.26) | 12.1 (9.71) | 14.9 (13.30) | 5.3 (5.01) | 38.6 (14.06) | 50% Male | 49% Active |
Note: top diagonal contains correlations for participants still smoking (n = 80) and bottom diagonal contains correlations for participants who quit smoking (n = 105). M = mean. SD = standard deviation. BL=baseline. M1 = Month 1. M3 = Month 3. IDAS= Inventory of Depression and Anxiety Symptoms. ASI-3 = Anxiety Sensitivity Index-3. FTND = Fagerström Test for Nicotine Dependence. Active = in active treatment condition, relative to standard control treamtent.
p < .05.
3.2. Structural equation model examining the effects of quit status on anxious arousal
A confirmatory factor analysis (CFA) of Baseline, Month 1, and Month 3 anxious arousal factors provided adequate fit to the data (χ2 =485.42, p<.05, CFI = .92, RMSEA =.07, 90% CI [.06, .08]). A model including directionality of anxious arousal factors (i.e., Baseline anxious arousal predicting Month 1 and Month 3 anxious arousal and Month 1 anxious arousal predicting Month 3 anxious arousal), quit status and the covariates, including, gender, age, baseline anxiety sensitivity and nicotine dependence, mood/anxiety disorder diagnosis, and treatment condition provided adequate fit to the data (χ2 = 582.77, p<.05, CFI = .93, RMSEA =.05, 90% CI [.04, .06]). Controlling for the baseline anxious arousal factor and covariates, quit status significantly predicted anxious arousal at Month 1 (β = −.23, p = .04), indicating that individuals who successfully achieved abstinence showed a .23 SD reduction in anxious arousal at Month 1 as compared to individuals who did not achieved abstinence. Controlling for the Baseline and Month 1 anxious arousal factors as well as covariates, quit status was non-significantly associated with anxious arousal at Month 3 (β = −.18, p = .09).
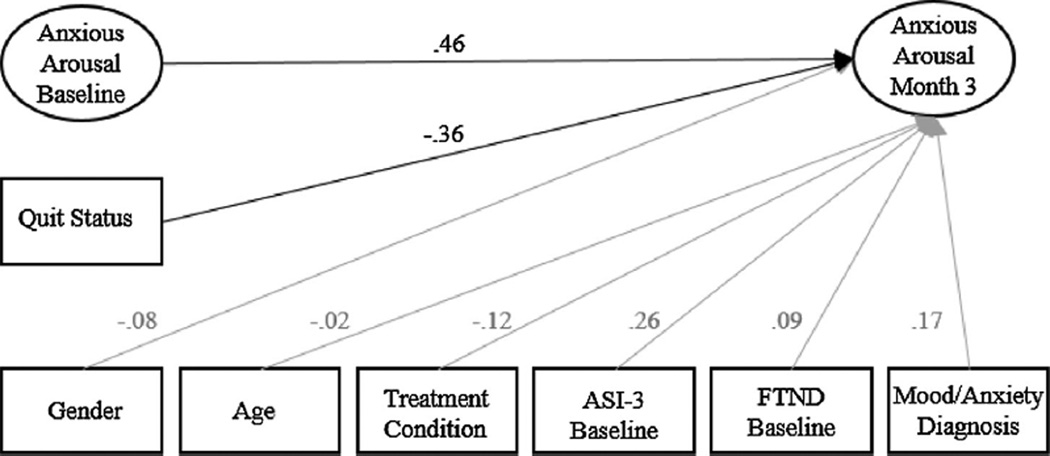
To determine if the effects of quit status was as directly predictor of Month 3 anxious arousal, the effect of quit status on anxious arousal at Month 3 was examined controlling for anxious arousal at baseline only (see Fig. 2). This model provided adequate fit to the data (χ2 = 332.06, p<.05, CFI = .92, RMSEA =.06, 90% CI [.04,.07]).As expected, after controlling for baseline anxious arousal and covariates, quit status significantly predictive anxious arousal at Month 3 (β = −.36, p = .006), indicating that individual who successfully quit smoking showing a .36 SD reduction in anxious arousal at Month 3 as compared to individual who did not quit smoking.
Fig. 2.
Structural equation model of the standardized effects of Quit Status and covariates on Month 3 Panic.
Note: ASI-3 = Anxiety Sensitivity Index-3. FTND = Fagerström Test of Nicotine Dependence. Items and residual errors are omitted for clarity. The effects of covariates are in gray for clarity.
4. Discussion
The current study examined the impact of biochemically verified continued smoking abstinence (for one month), relative to non-abstinence, in terms of its effect on anxious arousal at one- and three-months post-intervention, among a sample of treatment-seeking smokers. Of those included in analyses, approximately 43% of participants achieved biochemically-verified sustained abstinence for one-month post-quit attempt, whereas approximately 57% did not. As expected, after accounting for baseline level of anxious arousal, abstainers, relative to non-abstainers, evidenced statistically significant reductions in anxious arousal at Month 1 and Month 3 post-cessation; the effects were similar magnitude in size. After accounting for Month 1 reductions anxious arousal, the effect of abstinence status on Month 3 anxious arousal was non-significant, suggesting that the effect of abstinence status on Month 3 anxious arousal occurs through Month 1 reductions in anxious arousal. This set of findings compliments and uniquely extends existing work documenting the role of smoking cessation in terms of reduction of anxiety symptoms (Taylor et al., 2014). Indeed, relative to past work, the current findings are bolstered by the biochemical verification of smoking abstinence (across multiple time-points) and use of a conservative cut-point for defining abstinence (Perkins et al., 2013). These effects were apparent after accounting for the (non-significant) effects of gender, age, baseline levels of anxiety sensitivity and nicotine dependence, presence of depressive/anxiety disorders, and treatment condition.
It is also worth noting that the group of smokers who did not achieve abstinence did not demonstrate statistically significant decreases or increases in anxious arousal during follow-up assessments. Moreover, the current sample did not present with current/past panic psychopathology (panic attacks, panic disorder, agoraphobia) due to the nature of the parent study. Thus, even among smokers without panic-spectrum psychopathology who may have had other emotional disorders where anxious arousal may be relevant, sustained smoking is related to reductions in anxious arousal. These data therefore provide compelling evidence that (successful) smoking cessation contributes to reductions in anxious arousal over time. Future work may benefit by examining the sub-set of smokers who struggled to maintain smoking abstinence (those who demonstrated a relapsing-remitting pattern of smoking). It has been proposed that those smokers who continue to persist in attempting to quit (the ‘struggling quitter’) may be more prone to experience increases in smoking-relevant symptoms (e.g., protracted withdrawal, craving) and subsequent increases in depressive symptoms during early quit phases (Berlin, Chen, & Covey, 2010) although this not consistently documented (Capron, Allan, Norr, Zvolensky, & Schmidt, 2014). To the best of our knowledge, no work has addressed this type of prediction in relation to anxious arousal (or anxiety symptoms).
There are a number of study limitations. First, quit status abstinence was assessed by three expired CO measurements collected during the four weeks following the quit attempt. Although biochemical verification of smoking status is superior to self-reported abstinence, expired CO measurement is sensitive to recency of smoking. Thus, it is possible that a smoker may have engaged in between-assessment smoking, but achieved smoking abstinence in the 24-h prior to follow-up study appointment, and would have been classified as abstinent. Bolstering this measurement with salivary cotinine data would further confirm sustained abstinence. Second, the process of quitting smoking is dynamic (Kirchner, Shiffman, & Wileyto, 2012). One-month of smoking abstinence was demarcated based on its alignment with designated study follow-up visits; however, this target point is nonetheless somewhat arbitrary. Further investigations may consider modeling individual trajectories in sustained periods of abstinence regardless of when it occurs post-quit day. Third, although important to understand the longer-term mental health outcomes of smokers who quit, attrition at follow-up time points (beyond 3-month post-quit day) prohibited tests of the effects of one-month abstinence on longer-term anxious arousal. Fourth, the current sample did not include individuals with panic disorder or psychotic-spectrum psychopathology. Therefore, it is important to consider the extent to which these findings generalize to smokers who potentially have these psychological disorders. Additionally, while the presence/absence of depressive/anxiety disorders was controlled for in the current analyses, we were under-powered to test a model stratified on this variable. Given smokers with emotional disorders would be naturally predisposed to experiencing higher levels of anxious arousal, relative to smokers without these disorders, the latter test would be important to understand if and how these smokers differentially experience changes in anxious arousal after quitting. Lastly, sample characteristics limited the generalizability of the current findings to more racially/ethnically and socioeconomically-diverse smokers, and those who attempt to quit without aid of treatment (self-guided cessation attempt) or with use of first-line pharmacotherapy (e.g., bupropion). For example, the current sample consisted of smokers who primarily identified race was white (85.4%) and completed at least some college (80.6%); factors that may be associated with better smoking cessation outcomes (Piper et al., 2010). Future work could explore these questions to better contextualize the larger generalizability of the current observations.
Overall, the current findings are in accord with theoretical and conceptual models of smoking-anxiety (Ameringer & Leventhal, 2010; Morissette et al., 2007) and suggest that even short-term smoking abstinence (one month) can result in reductions in anxious arousal, a core construct underlying anxiety disorders (Clark & Watson, 1991). Smokers may therefore benefit from psychoeducation regarding the mental health (in addition to physical health) benefits of smoking cessation and how smoking and anxious hyperarousal interplay with one another in the context of quitting. Future research is needed to explore moderators that identify smokers who after achieving abstinence, continue to experience anxiety-relevant somatic arousal, and by extension, greater relative risk for subsequent smoking lapse/relapse.
Acknowledgements
This work was funded by a National Institute of Mental Health grant awarded to Drs. Michael J. Zvolensky and Norman B. Schmidt (R01-MH076629-01A1). Ms. Farris is supported by a pre-doctoral National Research Service Award from the National Institute of Drug Abuse (F31-DA035564).
Footnotes
No authors have any conflicts of interests or financial disclosures to report.
References
- Ameringer KJ, Leventhal AM. Applying the tripartite model of anxiety and depression to cigarette smoking: an integrative review. Nicotine & Tobacco Research. 2010;12(12):1183–1194. doi: 10.1093/ntr/ntq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amering M, Bankier B, Berger P, Griengl H, Windhaber J, Katschnig H. Panic disorder and cigarette smoking behavior. Comprehensive Psychiatry. 1999;40(1):35–38. doi: 10.1016/s0010-440x(99)90074-3. [DOI] [PubMed] [Google Scholar]
- Bernstein A, Zvolensky MJ, Schmidt NB, Sachs-Ericcson N. Developmental course(s) of lifetime cigarette use and panic attack comorbidity: an equifinal phenomenon? Behavior Modification. 2007;31(1):117–135. doi: 10.1177/0145445506295056. [DOI] [PubMed] [Google Scholar]
- Becoña E, Vázquez FL, Míguez MDC. Smoking cessation and anxiety in a clinical sample. Personality and Individual Differences. 2002;32(3):489–494. http://dx.doi.org/10.1016/S0191-8869(01)50-2. [Google Scholar]
- Berlin I, Chen H, Covey LS. Depressive mood, suicide ideation and anxiety in smokers who do and smokers who do not manage to stop smoking after a target quit day. Addiction. 2010;105(12):2209–2216. doi: 10.1111/j.1360-0443.2010.03109.x. http://dx.doi.org/10.1111/j.1360-0443.2010.03109.x. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. Sociological Methods and Research. 1992;21:230–258. [Google Scholar]
- Breslau N, Novak SP, Kessler RC. Psychiatric disorders and stages of smoking. Biological Psychiatry. 2004;55(1):69–76. doi: 10.1016/s0006-3223(03)00317-2. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. Journal of Abnormal Psychology. 2002;111(1):180. [PubMed] [Google Scholar]
- Brown TA. Confirmatory factor analysis for applied research. New York, NY: The Guilford Press; 2006. [Google Scholar]
- Capron DW, Allan NP, Norr AM, Zvolensky MJ, Schmidt NB. The effect of successful and unsuccessful smoking cessation on short-term anxiety, depression, and suicidality. Addictive. Behaviors. 2014;39(4):782–788. doi: 10.1016/j.addbeh.2013.12.014. http://dx.doi.org/10.1016/j.addbeh.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosci F, Knuts IJE, Abrams K, Griez EJL, Schruers KRJ. Cigarette smoking and panic: a critical review of the literature. Journal of Clinical Psychiatry. 2010;71(5):606–615. doi: 10.4088/JCP.08r04523blu. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100(3):316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Cavazos-Rehg PA, Breslau N, Hatsukami D, Krauss MJ, Spitznagel EL, Grucza RA, et al. Smoking cessation is associated with lower rates of mood/anxiety and alcohol use disorders. Psychological Medicine. 2014;44(12):2523–2535. doi: 10.1017/S0033291713003206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, Pickering A, Powell J, West R. Patterns of change in withdrawal symptoms, desire to smoke, reward motivation and response inhibition across 3 months of smoking abstinence. Addiction. 2009;104(5):850–858. doi: 10.1111/j.1360-0443.2009.02522.x. http://dx.doi.org/10.1111/j.1360-0443.2009.02522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, Curry SJ. Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. Treating tobacco use and dependence: 2008 update. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patent edition (SCIDI/NP) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2007. [Google Scholar]
- Goodwin RD, Zvolensky MJ, Keyes KM, Hasin DS. Mental disorders and cigarette use among adults in the United States. American Journal on Addictions. 2012;21(5):416–423. doi: 10.1111/j.1521-0391.2012.00263.x. http://dx.doi.org/10.1111/j.1521-0391.2012.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6(1):1–55. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström K. The fagerström test for nicotine dependence: a revision of the fagerström tolerance questionnaire. British Journal of Addiction. 1991 doi: 10.1111/j.1360-0443.1991.tb01879.x. http://dx.doi.org/10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Isensee B, Wittchen HU, Stein MB, Hofler M, Lieb R. Smoking increases the risk of panic: findings from a prospective community study. Archives of General Psychiatry. 2003;60(7):692–700. doi: 10.1001/archpsyc.60.7.692. http://dx.doi.org/10.1001/archpsyc.60.7.692. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Pine DS, Klein DF, Kasen S, Brook JS. Association between cigarette smoking and anxiety disorders during adolescence and early adulthood. Journal of the American Medical Association. 2000;284(18):2348–2351. doi: 10.1001/jama.284.18.2348. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Stewart SH, Zvolensky MJ, Steeves D. Evaluating the mediating role of coping-based smoking motives among treatment-seeking adult smokers. Nicotine & Tobacco Research. 2009;11(11):1296–1303. doi: 10.1093/ntr/ntp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal M, Does AJWVD, Penninx BWJH, Cuijpers P. Age at smoking onset and the onset of depression and anxiety disorders. Nicotine & Tobacco Research. 2011;13(9):809–819. doi: 10.1093/ntr/ntr077. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129(2):270–304. doi: 10.1037/0033-2909.129.2.270. http://dx.doi.org/10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Unrod M. Smoking, anxiety, and attention: support for the role of nicotine in attentionally mediated anxiolysis. Journal of Abnormal Psychology. 2000;109(1):161–166. doi: 10.1037//0021-843x.109.1.161. [DOI] [PubMed] [Google Scholar]
- Kirchner TR, Shiffman S, Wileyto EP. Relapse dynamics during smoking cessation: recurrent abstinence violation effects and lapse-relapse progression. Journal of Abnormal Psychology. 2012;121(1):187–197. doi: 10.1037/a0024451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Ameringer KJ, Osborn E, Zvolensky MJ, Langdon KJ. Anxiety and depressive symptoms and affective patterns of tobacco withdrawal. Drug and Alcohol Dependence. 2013;133(2):324–329. doi: 10.1016/j.drugalcdep.2013.06.015. http://dx.doi.org/10.1016/j.drugalcdep.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Zvolensky MJ. Anxiety, depression, and cigarette smoking: a transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychological Bulletin. 2015;141(1):176–212. doi: 10.1037/bul0000003. http://dx.doi.org/10.1037/bul0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeish AC, Zvolensky MJ, Bonn-Miller MO, Bernstein A. Perceived health moderates the association between smoking rate and panic vulnerability variables among daily smokers. Depression and Anxiety. 2006;23(5):257–265. doi: 10.1002/da.20170. [DOI] [PubMed] [Google Scholar]
- Muthén BO, Muthén LK. Mplus (versin 7.1) Los Angeles, CA: 2012. [Google Scholar]
- Moshagen M. The model size effect in SEM: inflated goodness-of-fit statistics are due to the size of the covariance matrix. Structural Equation Modeling: A Multidisciplinary Journal. 2012;19:86–98. [Google Scholar]
- McDermott MS, Marteau TM, Hollands GJ, Hankins M, Aveyard P. Change in anxiety following successful and unsuccessful attempts at smoking cessation: Cohort study. The British Journal of Psychiatry. 2013;202(1):62–67. doi: 10.1192/bjp.bp.112.114389. [DOI] [PubMed] [Google Scholar]
- Morissette SB, Tull MT, Gulliver SB, Kamholz BW, Zimering RT. Anxiety, anxiety disorders, tobacco use, and nicotine: a critical review of interrelationships. Psychological Bulletin. 2007;133(2):245–272. doi: 10.1037/0033-2909.133.2.245. http://dx.doi.org/10.1037/0033-2909.133.2.245. [DOI] [PubMed] [Google Scholar]
- Mulaik S. There is a place for approximate fit in structural equation modelling. Personality and Individual Differences. 2007;42:883–891. [Google Scholar]
- Perkins KA, Karelitz JL, Jao NC. Optimal carbon monoxide criteria to confirm 24-hr smoking abstinence. Nicotine & Tobacco Research. 2013;15(5):978–982. doi: 10.1093/ntr/nts205. http://dx.doi.org/10.1093/ntr/nts205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Baker TB. Anxiety diagnoses in smokers seeking cessation treatment: relations with tobacco dependence, withdrawal, outcome and response to treatment. Addiction. 2011;106(2):418–427. doi: 10.1111/j.1360-0443.2010.03173.x. http://dx.doi.org/10.1111/j.1360-0443.2010.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, et al. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine & Tobacco Research. 2010;12(6):647–657. doi: 10.1093/ntr/ntq067. http://dx.doi.org/10.1093/ntr/ntq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab L, Andrew S, West R. Changes in prevalence of depression and anxiety following smoking cessation: results from an international cohort study (ATTEMPT) Psychological Medicine. 2014;44(1):127–141. doi: 10.1017/S0033291713000391. [DOI] [PubMed] [Google Scholar]
- Solomon JL, Higgins ST, Heil SH, Badger GJ, Mongeon JA, Bernstein IM. Psychological symptoms following smoking cessation in pregnant smokers. Journal of Behavioral Medicine. 2006;29(2):151–160. doi: 10.1007/s10865-005-9041-4. http://dx.doi.org/10.1007/s10865-005-9041-4. [DOI] [PubMed] [Google Scholar]
- Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P. Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ: British Medical Journal. 2014;348 doi: 10.1136/bmj.g1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, et al. Robust dimensions of anxiety sensitivity: development and initial validation of the Anxiety Sensitivity Index-3. Psychological Assessment. 2007;19(2):176–188. doi: 10.1037/1040-3590.19.2.176. http://dx.doi.org/10.1037/1040-3590.19.2.176 (Supplemental) [DOI] [PubMed] [Google Scholar]
- Vessichhio JC, Termine A, George TP. Smoking cessation and panic attacks: a report of 2 cases. Journal of Clinical Psychiatry. 2002;63(7):594–595. doi: 10.4088/jcp.v63n0710b. [DOI] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: a quantitative hierarchical model for DSM-V. Journal of Abnormal Psychology. 2005;114(4):522–536. doi: 10.1037/0021-843X.114.4.522. http://dx.doi.org/10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology. 1995;104(1):15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, et al. Development and validation of the inventory of depression and anxiety symptoms (IDAS) Psychological Assessment. 2007;19(3):253–268. doi: 10.1037/1040-3590.19.3.253. http://dx.doi.org/10.1037/1040-3590.19.3.253. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology. 1995;104(1):3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, et al. Tobacco use and cessation in psychiatric disorders: national institute of mental health report. Nicotine & Tobacco Research. 2008;10(12):1691–1715. doi: 10.1080/14622200802443569. http://dx.doi.org/10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Bernstein A. Cigarette smoking and panic psychopathology. Current Directions in Psychological Science. 2005;14(6):301–305. doi: 10.1177/0963721410388642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Feldner MT, Leen-Feldner EW, McLeish AC. Smoking and panic attacks, panic disorder, and agoraphobia: a review of the empirical literature. Clinical Psychology Review. 2005;25(6):761–789. doi: 10.1016/j.cpr.2005.05.001. http://dx.doi.org/10.1016/j.cpr.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Johnson KA, Leyro TM, Hogan J, Tursi L. Quit-attempt history: relation to current levels of emotional vulnerability among adult cigarette users. Journal of Studies on Alcohol Drugs. 2009;70(4):551–554. doi: 10.15288/jsad.2009.70.551. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Lejuez C, Kahler CW, Brown RA. Nonclinical panic attack history and smoking cessation: an initial examination. Addictive Behaviors. 2004;29(4):825–830. doi: 10.1016/j.addbeh.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Stewart SH, Vujanovic AA, Gavric D, Steeves D. Anxiety sensitivity and anxiety and depressive symptoms in the prediction of early smoking lapse and relapse during smoking cessation treatment. Nicotine &Tobacco Research. 2009;11(3):323–331. doi: 10.1093/ntr/ntn037. http://dx.doi.org/10.1093/ntr/ntn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Yartz AR, Gregor K, Gonzalez A, Bernstein A. Interoceptive exposure-based cessation intervention for smokers high in anxiety sensitivity: a case series. Journal of Cognitive Psychotherapy. 2008;22(4):346–365. http://dx.doi.org/10.1891/0889-8391.22.4.346. [Google Scholar]