Abstract
Background
Patients with end-stage renal disease (ESRD) receiving dialysis have poor health-related quality of life (HRQoL). Physical symptoms are highly prevalent among dialysis-dependent patients and play important roles in HRQoL. A range of symptom assessment tools have been used in dialysis-dependent patients, but there has been no previous systematic assessment of the existing symptom measures’ content, validity, and reliability.
Study Design
systematic review of the literature
Settings & Population
ESRD patients on maintenance dialysis
Selection Criteria for Studies
instruments with ≥3 physical symptoms previously used in dialysis-dependent patients and evidence of validity or reliability testing
Intervention
patient-reported physical symptom assessment instrument
Outcomes
instrument symptom-related content, validity, and reliability
Results
From 3,148 screened abstracts, 89 full-text articles were eligible for review. After article exclusion and further article identification via reference reviews, 58 articles on 23 symptom assessment instruments with documented reliability or validity testing were identified. Of the assessment instruments, 43.5% were generic and 56.5% were ESRD-specific. Symptoms most frequently assessed were fatigue, shortness of breath, insomnia, nausea and vomiting, and appetite. The instruments varied widely in respondent time burden, recall period, and symptom attributes. Few instruments considered recall periods less than 2 weeks and few assessed a range of symptom attributes. Psychometric testing was completed for congruent validity (70%), known group validity (25%), responsiveness (30%), internal consistency (78%), and test-retest reliability (65%). Content validity was assessed in dialysis populations in 57% of the 23 instruments.
Limitations
Consideration of physical symptoms only and exclusion of single symptom-focused instruments
Conclusions
The number of available instruments focused exclusively on physical symptoms in dialysis patients is limited. Few symptom-containing instruments have short recall periods, assess diverse symptom attributes, and have undergone comprehensive psychometric testing. Improved symptom-focused assessment tools are needed to improve symptom evaluation and symptom responsiveness to intervention among dialysis-dependent patients.
INDEX WORDS: maintenance dialysis, end-stage renal disease (ESRD), health-related quality of life (HRQoL), physical symptoms, patient-reported symptom tool, patient-reported outcome instrument, patient-centered care, comorbidity burden, fatigue, shortness of breath, insomnia, poor appetite, nausea, systematic review
Patients with end-stage renal disease (ESRD) on dialysis have poor health-related quality of life (HRQOL) compared to members of the general population.1–4 A high burden of co-morbid illness, impaired physical function, and other factors contribute to this suboptimal HRQOL, and existing data suggest that physical symptoms also play important roles.5, 6
Dialysis-dependent patients have numerous physical symptoms with more than half of patients reporting fatigue, pain, cramps, sleep disturbance, and sexual dysfunction.7–9 Despite the relevance of symptoms to HRQOL, healthcare providers are not adept at recognizing them. One study found that providers frequently do not identify key symptoms, and when symptoms are recognized, providers underestimate their severity.8 Additionally, evidence-based dialysis treatment interventions and symptom-targeted pharmaceutical therapies are lacking. Erythropoiesis-stimulating agent use is associated with improved HRQOL and reduced fatigue,10, 11 but few other dialysis prescription changes have been shown to modulate HRQOL or symptoms. To inform the development of new symptom interventions, an accurate understanding of symptom prevalence, patient prioritization of symptoms, and the pathophysiology underlying common symptoms is needed.
To assess symptoms, clinicians and investigators rely on a range of patient-reported symptom tools including instruments that measure HRQOL,12–18 dialysis-specific symptom indices,5, 19 and symptom questionnaires originally developed for non-dialysis patients.20–23 As a result, the type and quality of data collected is widely varied, thus limiting precise conclusions about patient prioritization of symptoms and symptom responsiveness to mitigation strategies. Understanding symptoms related to dialysis procedures may inform symptom pathophysiology comprehension and may help identify therapeutic treatment modifications.
We undertook this systematic review to identify measures used to assess patient-reported physical symptoms in the dialysis-dependent population and to describe instrument development, symptom-related content, and psychometric properties of the identified measures. We limited our review to physical symptoms to capture symptoms most likely to fluctuate on a treatment-to-treatment basis. To establish a baseline quality threshold for considered instruments, we limited the review to measures with published validity and/ or reliability assessments.
Methods
Study Overview
We conducted a systematic literature review according to guidelines provided by the US Department of Health and Human Services Agency for Healthcare Research and Quality,24, 25 and we used the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines to guide data collection and reporting of evidence.26
Selection Criteria for Articles and Instruments
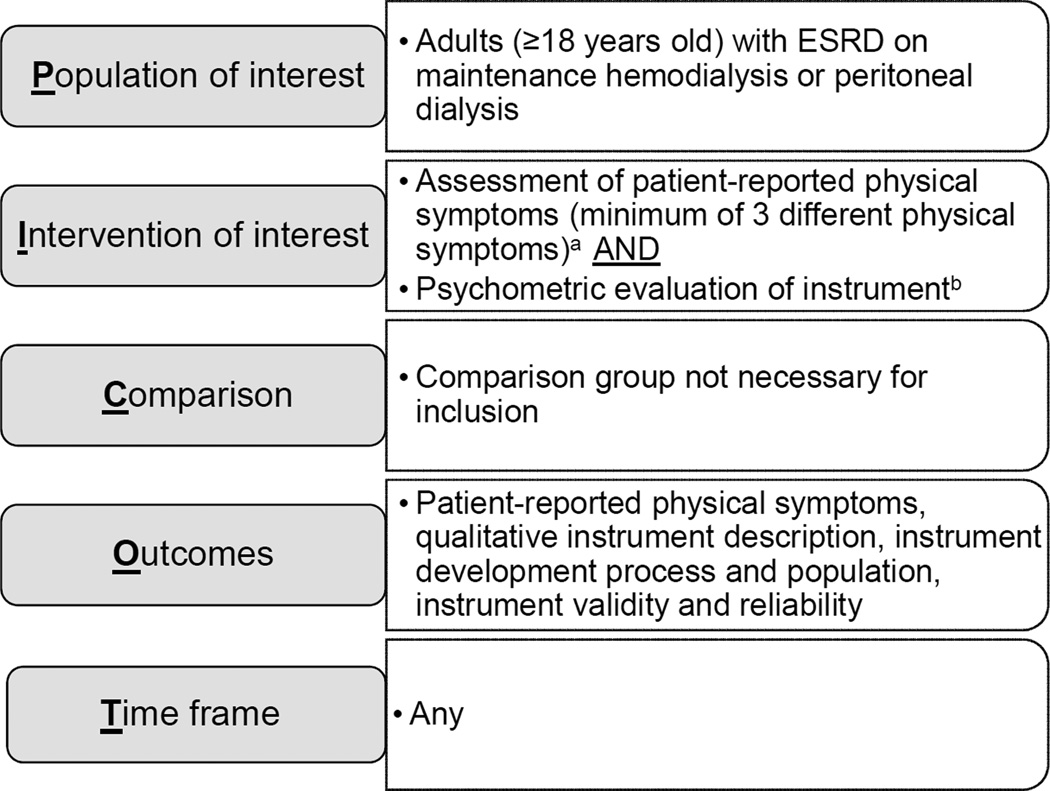
Eligibility criteria were developed using modified PICOT (population of interest, intervention of interest, comparison, outcomes, time frame) criteria (Figure 1).27 Full inclusion and exclusion criteria are reported in Table 1. We began by identifying relevant articles for review, but the unit of analysis was the patient-reported outcome instrument ascertained from the identified articles.
Figure 1.
PICOT criteria and search strategy.27
a Instruments focused on a single symptom such as pruritus, thirst, fatigue, sleep, or sexual dysfunction and instruments with mood symptoms only were excluded. Physical function and capacity were not considered symptoms. Instruments focused exclusively on physical function were excluded.
b Instrument psychometric assessment included content validity, construct validity, responsiveness, internal consistency reliability, and test-retest reliability. Instruments with no retrievable information on validity or reliability were excluded.
Table 1.
Article and instrument selection criteria.
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Article-level |
|
|
|
Instrument- level |
|
|
Physical function and capacity were not considered symptoms.
Article and Instrument Identification
Articles for review were identified from MEDLINE (via PubMed) and Embase (via Elsevier), which were searched from 1946 (MEDLINE) and 1966 (Embase) to December 31, 2014 with the assistance of an experienced reference librarian (L.H.). Key words and controlled vocabulary were used for each database, and searches were constructed using a combination of medical subheadings, keywords, and text words. As physical symptom assessments are often embedded in HRQOL assessments, we conducted searches for HRQOL or symptoms. Complete search strings are available in Table S1 (provided as online supplementary material). Reference lists of selected studies were further searched for additional instruments and articles. Individual instruments were identified within each of the articles. Focused searches to identify psychometric analyses of the identified instruments were then performed.
Data Abstraction and Psychometric Assessment
A pre-determined methodology was followed to determine articles for inclusion. Two non-clinicians with training in literature reviews and patient-centered outcomes (J.D.P., C.J.P.) independently reviewed all article titles and abstracts in accordance with the selection criteria. If either reviewer deemed an article potentially eligible based on the title or abstract, a full-text review was completed. Articles marked for possible inclusion by either reviewer underwent independent, full-text review by two investigators (J.D.P., J.E.F.) to determine final inclusion or exclusion. In the full text review, investigators used a standardized spreadsheet to extract each article and determine eligibility. Reviewer disagreement was resolved by consensus.
Trained reviewers (J.D.P., K.D.W.) extracted relevant data from each included article into a standardized abstraction form. The structured abstraction tool included the following: instrument descriptive data (symptoms assessed, symptom attributes, recall period, response format, burden), instrument development data (year and country of development, intended use, target population, population involved in questionnaire development, and development process), and instrument psychometric assessment (content validity, construct validity, responsiveness to change, internal consistency reliability, and test-retest reliability). A third team member (J.E.F.) compared all extractions with original articles for completeness and accuracy.
Assessment of Instrument Reliability and Validity
An overview of the considered instrument psychometric properties, definitions, and common assessment methods is provided in Table 2. For this review, content validity was deemed present if the target population (dialysis patients) was involved in instrument item development, and a clear description of concepts being measured was provided.28 Construct validity was considered as congruent validity or known-group validity as these were the two most commonly reported forms of construct validity in the evaluated instruments. Responsiveness to change was deemed present if score change statistics were assessed in an ESRD population.29 With the exception of content validity and responsiveness, testing in an ESRD population was not required.30 We elected to report on the presence or absence of selected psychometric testing rather than rendering a quality assessment of the reported psychometric measures. Lack of consensus regarding quality thresholds for many of the psychometric measures exists, and there are few accepted standards for rating subjective aspects of psychometric evaluations such as content and construct validity.31, 32 To facilitate the interested reader’s assessment of psychometric testing quality, we provided a summary of available psychometric results for each instrument in Table S2.
Table 2.
Psychometric measures considered in patient-reported outcomes instrument evaluations.
| Measure | Definition | Common methods of assessment |
Interpretationa |
|---|---|---|---|
| Validity (degree to which an instrument measures the concept it is intended to measure)28,30,31,59 | |||
| Content validity | Extent to which the instrument includes the most relevant and important aspects of a concept54 |
Stakeholder focus groups, interviews, surveys |
Qualitative evidence from development and pre-testing60 |
| Construct validityb | Evidence that relationships among items, domains, and concepts conform to a priori hypotheses28 |
Correlation statistics |
≥0.70 supports strong correlation |
| Congruent | Extent to which measure correlates with measure assessing the same construct |
||
| Known group | Extent to which measure is sensitive to differences and similarities in groups with known attributes |
||
| Responsiveness | Extent to which instrument can detect changes in the construct being measured over time30 |
Score change statistics |
Statistically significant difference in scores pre- and post-clinically relevant events29 |
| Reliability (degree to which an instrument is free from measurement error)30, 31,59 | |||
| Internal consistency reliability |
Degree of the interrelatedness among the items in a multi-item measure30,31 |
Cronbach’s α | ≥0.70: adequate internal consistency60 |
| Test-retest reliability |
Measure of the ability to provide consistent scores over time in a stable population30 |
Intraclass correlation coefficient; Kappa statistic |
≥0.70: supports test-retest reliability29 |
Interpretation score thresholds are not well established and may differ across populations and sources.
Construct validity was considered as congruent and known group validity as these were the construct validity sub-types most commonly assessed in identified instruments.
Data Analysis
Physical symptoms assessed by the included instruments were tabulated, descriptive statistics were reported, and instrument symptom assessment criteria were tabulated. To examine the frequency of validity and reliability testing, descriptive statistics for the percentage of instruments that underwent such assessment were compiled. We considered instruments overall and categorized as ESRD-specific and non-ESRD-specific. We considered psychometric assessments specific to the symptom domain and specific to non-symptom domains or to the overall instrument.
Results
Literature Search
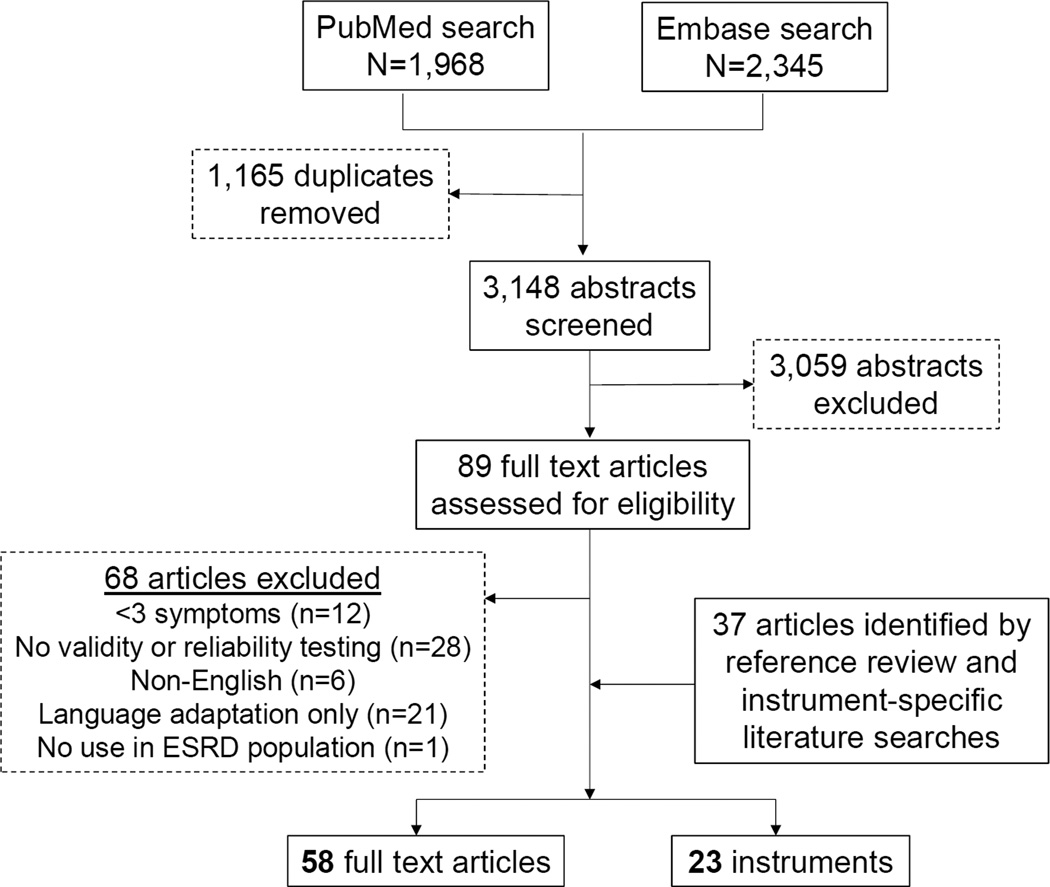
Figure 2 is a flow diagram of article and instrument selection. The initial search identified 1,968 articles/abstracts from MEDLINE and 2,345 articles/abstracts from Embase. After duplicates were removed, 3,059 articles/abstracts were excluded based on selection criteria, leaving 89 for full-text review. Sixty-eight full text articles were excluded and 37 additional articles were identified from reference review and instrument-specific literature searches. A total of 58 full text articles and 23 instruments were included in the final analysis (Table S2).
Figure 2.
Flow chart of article and instrument selection.
Abbreviations: ESRD, end stage renal disease.
While 23 instruments met selection criteria, there were several notable exclusions. We excluded the Merkus questionnaire, a symptom-based HRQOL instrument developed specifically for dialysis-dependent patients, because instrument validation was reported only in a Dutch language publication.6, 33 Furthermore, symptom-specific instruments such as those focused exclusively on sleep, pain, and fatigue were excluded. Patients often experience multiple symptoms simultaneously, and symptoms can have important interactions, such as pruritus and insomnia.34 Single symptom-focused instruments preclude the study of such interactions. To ensure inclusion of a range of symptoms and to limit the scope of this review, we excluded measures focused on <3 symptoms. This choice led to the exclusion of such commonly used instruments as the Pittsburgh Sleep Quality Index, McGill Pain Inventory, Fatigue Severity Scale, International Index of Erectile Function Index, Restless Leg Syndrome Questionnaire, and Dialysis Thirst Index.35–43
Instrument Symptom Assessment
Table 3 provides an overview of the included instruments. We identified 13 instruments assessing ≥3 physical symptoms developed specifically for use among dialysis-dependent patients and 10 instruments used in dialysis-dependent patients but developed in non-dialysis populations. Four of the 13 dialysis-specific instruments focused exclusively on symptoms, and the others included symptom assessment as a relevant HRQOL domain. Four of the 10 non-dialysis-specific instruments focused exclusively on symptoms, and 6 instruments included symptoms as one of multiple instrument domains.
Table 3.
Description of 23 included physical symptom instruments
| Instrument a | Brief description |
|---|---|
| Developed for Dialysis Populations (n=13) | |
| 100 Category Checklist; Japan (2009) | Developed to assess physical and psychosocial problems as well as functional and environmental factors affecting QoL in hemodialysis patients |
| CHOICE Health Experience Questionnaire (CHEQ); US (2000) |
Developed to complement the generic 36-Item Short Form Health Survey (SF-36), be sensitive to the effectiveness of alternative dialysis modalities and dosing regimens, and be useful for longitudinal collection in routine practice |
| Curtin, et al.63; US (2002) | Developed to catalogue symptoms experienced by dialysis patients with the goal of improving functional status |
| Dialysis Symptom Index (DSI); US (2003) | Developed to assess the physical and emotional symptom burdens of hemodialysis patients |
| Fluid Management Survey; US (2014) | Developed to assess hemodialysis patient-stated preferences regarding fluid management |
| Hemodialysis Quality of Life Questionnaire (HQL); Canada (1990) |
Developed to assess hemodialysis patient QoL and physical and emotional symptoms |
| Kidney Disease Quality of Life Instrument (KDQoL); US (1994) |
Developed to assess disease-specific health-related QoL encompassing both generic and disease-specific elements |
| Kidney Disease Questionnaire (KDQ) Canada (1990) |
Developed to assess disease-specific QoL for use in clinical trials of maintenance hemodialysis patients |
| Modified Edmonton Symptom Assessment System (ESAS); Canada (2006) |
Modification of existing instrument specific to dialysis population developed to assess the physical and emotional symptom burdens of dialysis patients |
| National Kidney Dialysis and Kidney Transplantation Study (NKDKTS); US (1980s) |
Developed as part of government-commissioned study to investigate QoL, quality of care, rehabilitation, and health status of US patients undergoing dialysis |
| Parfrey Symptom Assessment; Canada (1987) | Developed to measure QoL among ESRD patients |
| Physical Symptom Distress Scale; Taiwan (1997) |
Developed to estimate the degree of symptom distress experienced by ESRD patients |
| Short-Version Checklist; Japan (2013) | Developed as a shortened version of the 100 Category Checklist to assess physical problems as well as functional and environmental factors affecting QoL in hemodialysis patients |
| Developed for Non-dialysis Populations (n=10) | |
| Bowel Disease Questionnaire; US (1989) | Developed to elicit gastrointestinal symptoms relevant to functional disorders. |
| European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC-QLQ-C30); Belgium (1987) |
Developed to assess health-related QoL of cancer patients in clinical trials |
| McGill Quality of Life Questionnaire (MQOL); Canada (1995) |
Developed to assess general domains of QoL in patients at all stages of life- threatening illnesses from diagnosis to cure or death |
| Memorial Symptom Assessment Scale (MSAS); US (1990) |
Developed to measure the prevalence and characteristics of physical and emotional symptoms experienced by diverse types of cancer patients |
| Nottingham Health Profile (NHP); UK (1980) | Developed to assess an individual’s perception of his or her own health status |
| Palliative Care Outcome Symptom Scale (POS- S Renal); UK (1998) |
Developed to improve outcome measurement by evaluating different outcomes in palliative care for patients with advanced disease; disease-specific modules were developed |
| Quality of Life at the End of Life (QUAL-E); US (2001) |
Developed to assess QoL at the end of life in a range of diseases and degrees of illness across care settings |
| Quality of Well Being Self-Administered Scale (QWB-SA); US (1970s) |
Developed to estimate QoL-adjusted years (cost utility analysis metric) |
| Rotterdam Symptom Checklist (RSCL); Netherlands (1980s) |
Developed to measure the symptoms reported by cancer patients participating in clinical research |
| Symptom Distress Scale (SDS); US (1970s) | Developed to measure the construct of symptom distress from the specific symptoms being experienced as reported by the patient |
Instrument; country of development (estimated year of development).
ESRD, end-stage renal disease; QoL, quality of life; US, United States
Table 4 displays the physical symptoms included in the identified instruments. The symptoms most commonly assessed were: fatigue or low energy (17 instruments (81%)); cough or shortness of breath (15 (71%)); insomnia or trouble falling asleep, (14 (67%)), poor appetite (14 (67%)), and nausea or vomiting, (14 (67%)). The symptoms of feeling sick, restless, experiencing muscle loss, easy bruising, and perceived hypotension appeared in only one instrument each (5%). Seven (33%) instruments had blank fields to allow for patient reporting of additional symptoms not listed elsewhere. Three instruments contained blank fields for patient-identified symptoms only and had no standardized symptom questions.
Table 4.
Physical symptoms included in patient-reported outcome instruments used for patients on dialysis (N=21).a,b
| Symptom | No. (%) of Instruments |
|---|---|
| General | |
| fatigue/ low energy | 17 (81%) |
| dizziness/ faintness/ lightheadedness | 12 (57%) |
| skin: dry/ itchy/ color change | 11 (52%) |
| numbness/ tingling | 8 (38%) |
| weakness | 7 (33%) |
| restless legs | 4 (19%) |
| sweats/ fever/ chills/ shivering | 3 (14%) |
| weight loss or gain | 3 (14%) |
| stiffness | 2 (10%) |
| hair loss | 2 (10%) |
| easy bruising | 1 (5%) |
| feeling sick | 1 (5%) |
| muscle loss | 1 (5%) |
| restless | 1 (5%) |
| Cardiovascular/Respiratory | |
| cough/ shortness of breath/ dyspnea | 15 (71%) |
| chest pain/ angina | 7 (33%) |
| swelling (legs/ arms/ nonspecific) | 7 (33%) |
| asthma or wheeze | 2 (10%) |
| heart palpitations | 2 (10%) |
| perceived hypotension | 1 (5%) |
| Sleep | |
| insomnia/ trouble falling asleep | 14 (67%) |
| awaken during sleep | 5 (24%) |
| drowsiness | 5 (24%) |
| Gastrointestinal | |
| loss of appetite/ poor appetite | 14 (67%) |
| nausea/ vomiting | 14 (67%) |
| diarrhea/ constipation | 10 (48%) |
| abdominal pain/ ulcer | 5 (24%) |
| dry mouth | 5 (24%) |
| stomach cramps/ gas pain | 3 (14%) |
| fullness/ bloating | 3 (14%) |
| thirst | 3 (14%) |
| change in taste/ metallic taste | 2 (10%) |
| difficulty swallowing/ mouth sore | 2 (10%) |
| Pain | |
| Pain/aches | 10 (48%) |
| Headache | 9 (43%) |
| Muscle cramps/ cramps | 8 (38%) |
| Backache | 5 (24%) |
| Bone/joint pain | 5 (24%) |
| Muscle soreness/muscle pain | 4 (19%) |
| Spasm | 2 (10%) |
| Other | |
| Confusion or memory difficulty | 4 (19%) |
| Difficulty is sexual arousal | 4 (19.%) |
| Dialysis access problem or pain | 3 (14%) |
| Self-reported symptom* | 8 (38%) |
Note: Excludes mood-related symptoms such as depression, anxiety, irritability, and boredom. Excludes 100 Category Checklist61 and Short-Version Checklist62 as these instruments each contained 2 symptom domains referred to as “body function component” and “body structure component.” Each component contained multiple categories. Many of the categories were non-specific, and we were unable to re-categorize them as discrete symptoms comparable to the other instruments. These 2 instruments were developed in the Japanese language, likely contributing to such discrepancies in categorization. The “body function component” of the Short-Version Checklist contained the following 17 categories: sleep functions, seeing functions, sensations associated with hearing and vestibular function, sensation of pain, blood pressure functions, hematological system functions, general physical endurance, aerobic capacity, fatigability, defecation functions, urinary excretory functions, urination functions, mobility of joint functions, sensations related to muscles and movement functions, protective functions of the skin, sensation related to the skin, and functions of hair. The “body structure component” contained the following 5 categories: structure of eyeball, structure of urinary system, kidneys, structure of upper extremity, and structure of nails.62 The 100 Category Checklist followed a similar pattern, and specific symptoms could not be tabulated.61
Patient fill-in.
Table 5 summarizes symptom categories, instrument burdens (time for completion), and symptom attributes assessed across instruments. More detailed instrument symptom question descriptions are available in Table S3. The least time-intensive instruments were the Symptom Distress Scale and Physical Symptom Distress Scale, each requiring 5 minutes for completion.14, 44 The most burdensome instruments were HRQOL-focused measures such as the CHOICE (Choices for Healthy Outcomes in Caring for ESRD) Health Experience Questionnaire (CHEQ), Kidney Disease Questionnaire (KDQ), and Kidney Disease Quality of Life (KDQoL), all requiring nearly 30 minutes for completion.13, 45–47 Symptom recall periods ranged from 1 year (the Bowel disease questionnaire) to “present” (modified Edmonton Symptom Assessment System [ESAS]). Five instruments considered symptoms with respect to the dialysis procedure (e.g. inter-, intra-, or post-dialysis). The majority of dialysis patient-specific instruments considered symptoms over 2–4 weeks. The modified ESAS instrument was the only dialysis patient-specific instrument that assessed symptoms at “present.”
Table 5.
Physical symptom evaluation in patient-reported outcome instruments used in patients on dialysis.
| Instrument | Completion time |
No. of symptoms |
Symptom Categoriesc | Dialysis treatment specific |
Recall | Symptom attributes** |
Symptom frequency ** |
Symptom QoL impact** |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gen. |
CV/ Pulm |
Sleep | GI | Pain | Other |
Self- report |
||||||||
| Developed for Dialysis Populations | ||||||||||||||
| 100 Category Checkliste, f |
NR | 35 | Y | Y | Y | Y | Y | Y | N | N | u/k | N | N | N |
| CHOICE Health Experience Questionnaire |
25–30 min13 | 17 | Y | Y | Y | Y | Y | Y | N | Y (intra-dialysis) |
4 wk; 3 mo |
bother (14) severity (2) problem (3) |
Y(8) | N |
| Curtin, et al.63 | NR | 47* | Y | Y | Y | Y | Y | Y | N | N | 4 wk | N | Y(47) | N |
| Dialysis Symptom Index |
NR | 30* | Y | Y | Y | Y | Y | Y | N | N | 1 wk | bother (30) | N | N |
| Fluid management Survey |
10–28 min53 | 10 | Y | Y | N | Y | Y | N | N | Y (intra-dialysis) |
2 wk | bother (8) | Y(2) | N |
| Hemodialysis QoL Questionnairee14,64 |
NR | 51 | Y | Y | Y | Y | Y | N | N | Y (intra- and inter-dialysis) |
NR | NR | NR | NR |
| Kidney Disease QoL Instrument |
5–25 min47 | 30 | Y | Y | Y | Y | Y | Y | N | N | 4 wk | bother (13) severity (2) problem (2) |
Y(13) | N |
| Kidney Disease Questionnairee 15,65,66 |
10–38 min45,46 | self-report only |
N | N | N | N | N | N | Y | N | 2 wk | NR | NR | NR |
| Modified Edmonton Symptom Assessment System |
NR | 10* | Y | Y | Y | Y | Y | N | Y | N | present | severity (10) | N | N |
| National Kidney Dialysis and Kidney Transplantation Studye 16, 67 |
NR | 13 | Y | Y | Y | Y | Y | Y | N | Y (post-dialysis) |
4 wk last dialysis |
N | Y(13) | N |
| Parfrey Symptom Assessment |
15–20 min17 | 12 + | Y | Y | Y | Y | Y | N | Y | Y (intra- and post-dialysis) |
previous few wk |
severity (12) | Y(12) | Y(12) |
| Physical Symptom Distress Scale |
5 min68 | 16* | Y | Y | Y | Y | Y | N | N | N | 1 wk | bother (16) | N | N |
| Short-Version Checkliste, f |
NR | 22 | Y | Y | Y | Y | Y | N | N | N | NR | N | N | N |
| Developed for Nondialysis Populations | ||||||||||||||
| Bowel disease questionnaire |
NR | 17d | Y | Y | Y | Y | Y | N | N | N | 1 y | bother (17) | Y(17) | N |
| European Organization for Research and Treatment of Cancer Quality of Life Questionnaire |
12 ± 7.5 min20 | 18 | Y | Y | Y | Y | Y | N | N | N | 1 wk | burden (18) | N | N |
| McGill Quality of Life Questionnaire |
15–20 min18 | self-report only |
N | N | N | N | N | N | Y | N | 2 d | problem | N | N |
| Memorial Symptom | NR | 32* | Y | Y | Y | Y | Y | Y | Y | N | 1 wk | bother (32) | Y(24) | N |
| Assessment Scale | severity (32) | |||||||||||||
| Nottingham Health Profile |
11 min45 | 33 | Y | N | Y | N | Y | N | N | N | present | N | N | N |
| Palliative Care Outcome Symptom Scale |
<10 min69 | 17* | Y | Y | Y | Y | Y | N | Y | N | 1 wk | bother (17) | N | N |
| Quality of Life at the End of Life |
NR | self-report only |
N | N | N | N | N | N | Y | N | 1 wk | bother severity |
Y | Y |
| Quality of Well Being Self-Administered Scale |
14 min70 | 58 | Y | Y | Y | Y | Y | Y | Y | N | 3 d | N | N | N |
| Rotterdam Symptom Checklist |
8 min71 | 30* | Y | Y | Y | Y | Y | Y | N | N | 2 wk | bother (30) | N | N |
| Symptom distress scale |
5 min44 | 10* | Y | Y | Y | Y | Y | N | N | N | lately | severity (4) | Y(8) | N |
Numbers in parentheses denote numbers of symptoms for which each attribute (bother, severity, problem, burden) or parameter was evaluated when known. Bother and interference reported as bother.
CHOICE: Choices for Healthy Outcomes in Caring for ESRD; CV/Pulm, cardiovascular/pulmonary; Gen, general; GI, gastrointestinal; NR, not reported; QoL, quality of life; u/k, unknown
General (fatigue, low energy, dizziness, faintness, lightheadedness, skin changes, numbness, tingling, weakness, restless legs, sweats, chills, weight loss or gain, stiffness, hair loss, easy bruising, feeling sick, muscle loss restless); CV/Pulm (cough, shortness of breath, dyspnea, chest pain, angina, swelling, asthma, wheeze, heart palpitations, perceived hypotension); Sleep (insomnia, trouble falling asleep, awaken during sleep, drowsiness, lack of sleep); GI (loss of appetite, poor appetite, nausea, vomiting, diarrhea, constipation, abdominal pain, ulcer, dry mouth, stomach cramps, gas pain, fullness, bloating, thirst, change in taste, metallic taste, difficulty in swallowing, mouth sore); Pain (pain, aches, headache, muscle cramps, cramps, backache, bone pain, joint pain, muscle soreness, muscle pain, spasm); Other (confusion, memory difficulty, difficulty in sexual arousal, lack of sexual interest, dialysis access problem or pain).
Instrument contains a non-gastrointestinal symptom checklist which is the basis for this report.
Copy of instrument could not be obtained for investigator review (n=5). In these instances, symptom evaluation criteria were garnered from associated references noted in the table.
The 100 Category Checklist61 and Short-Version Checklist62 contained 2 symptom domains referred to as “body function component” and “body structure component.” Each component contained multiple categories. Many of the categories were broad and potentially inclusive of multiple symptoms. In this table, we report the total number of symptom-related categories.
instrument evaluates symptoms only;
Overall, the instruments selectively addressed symptom attributes (severity, bother, frequency, timing, HRQOL impact). The Parfrey Symptom Assessment tool assessed the most symptom attributes including severity, frequency, necessity of drug treatment, sleep and daily activity interference, and quality of life impact. The non-dialysis specific Memorial Symptom Assessment Scale (MSAS) considered symptom bother, severity, and frequency and served as the basis for the Dialysis Symptom Index (DSI). However, when modified for a dialysis-dependent population, the DSI was simplified to include assessment of symptom bother only. Other instruments selectively addressed symptom attributes, often varying symptom attribute evaluation by discrete symptom. For example, the KDQOL, the most widely used HRQOL instrument among dialysis-dependent patients, assessed bother for all symptoms, but considered severity, life interference, and frequency for only select symptoms (pain, sexual dysfunction, sleep, and fatigue).
Instrument Validity and Reliability Assessment
Table 6 displays the validity and reliability testing of the symptom domains of the included instruments. Complete psychometric assessment results and an overall summary of psychometric testing are available in Tables S2 and S4. Overall, 13 (57%) instruments met criteria for content validity assessment. One of the 10 non-ESRD-specific instruments met such criteria, while 12 of the 13 ESRD-specific instruments displayed content validity evidence. For generic and ESRD-specific instruments, congruent construct validity was the most commonly tested form of construct validity. Fifteen (65%) instruments underwent congruent construct validity assessment and 8 (35%) underwent known groups construct validity assessment for symptom-related domains. Only 7 (30%) instruments were tested for responsiveness among patients with ESRD. Symptom-related domain internal consistency was assessed in 16 (70%) instruments. Overall, 15 (65%) instruments underwent test-retest reliability testing with only 11 (48%) undergoing such testing for the symptom-specific domain.
Table 6.
Presence of validity and reliability testing of the symptom domain in instruments assessing physical symptoms.
| Measures of Validity | Measures of Reliability | |||||
|---|---|---|---|---|---|---|
| Instrument | Content | Congruent Construct |
Known Group Construct |
Responsiveness | Internal Consistency |
Test-Retest |
| Developed for Dialysis Populations | ||||||
| 100 Category Checklist | Y | Y | Y | N | Y | N |
| CHOICE Health Experience Questionnaire | Y | N | Y | N | Y | N |
| Curtin, et al.63 | Y | Y | N | N | Y | N |
| Dialysis Symptom Index | Y | N | N | N | N | Y |
| Fluid management Survey | Y | N | N | N | Y | N |
| Hemodialysis Quality of Life Questionnaire | Y | N | N | Y | N | Y |
| Kidney Disease Quality of Life Instrument | Y | Y | Y | Y | Y | |
| Kidney Disease Questionnaire | Y | Y | N | Y | Y | Y |
| Modified Edmonton Symptom Assessment System |
Y | Y | N | Y | Y | Y |
| National Kidney Dialysis and Kidney Transplantation Study |
Y | N | Y | Y | Y | Y |
| Parfrey Symptom Assessment | Y | N | Y | Y | N | N |
| Physical Symptom Distress Scale | N | Y | N | N | Y | Y |
| Short-Version Checklist | Y | Y | Y | N | Y | N |
| Developed for Nondialysis Populations | ||||||
| Bowel disease questionnaire | N | N | N | N | N | N |
| European Organization for Research and Treatment of Cancer Quality of Life Questionnaire |
N | Y | N | N | Y | N |
| McGill Quality of Life Questionnaire | N | Y | N | N | Y | Y |
| Memorial Symptom Assessment Scale | N | Y | Y | N | Y | N |
| Nottingham Health Profile | N | Y | N | Y | N | Y |
| Palliative Care Outcome Symptom Scale | N | Y | N | N | N | Y |
| Quality of Life at the End of Life | Y | Y | N | N | Y | Y |
| Quality of Well Being Self-Administered Scale | N | N | N | N | N | N |
| Rotterdam Symptom Checklist | N | Y | N | N | Y | N |
| Symptom distress scale | N | Y | Y | N | Y | Y |
Note: All psychometric measures except for content validity were deemed present if assessed quantitatively. Content validity was deemed present if a clear description of the measurement aim was provided and if dialysis patients were involved in instrument item development. Responsiveness was deemed present only if analyzed in an end-stage renal disease population.
A detailed list of validity and reliability testing for each instrument can be found in Table S3. The KDQOL showed evidence of content validity, known group validity, congruent validity, internal consistency reliability, and responsiveness for the symptom-specific domains. The DSI, the most commonly used dialysis-specific, symptom-focused instrument, demonstrated content validity and test-retest reliability but had no reported testing of construct validity, internal consistency reliability, or responsiveness. The modified ESAS demonstrated content validity, congruent validity, test-retest reliability, and responsiveness. We found no evidence of internal consistency reliability of the modified ESAS in dialysis patients, but did find such testing of the non-modified instrument in cancer patients.48, 49
Discussion
We identified 23 instruments with reported validity and/ or reliability testing that were used to assess a wide range of patient-reported physical symptoms in dialysis-dependent populations. Few measures considered short symptom recall periods (<1 week), and few assessed a range of symptom attributes. Additionally, the number of instruments focused exclusively on symptoms was limited, and psychometric testing of the available symptom-focused instruments was variable. A valid, symptom-focused instrument with short recall and assessment of multiple symptom attributes is needed to improve symptom assessment among maintenance dialysis patients.
Symptoms are a critical contributor to overall HRQOL, satisfaction with care, and medical decision-making among dialysis-dependent patients.5, 6, 50, 51 In a survey of patients on or nearing dialysis, caregivers, and healthcare providers, 3 of the top research priorities were symptom-related. Patient-identified research priorities included improved treatments for itching, poor energy, sleep disorders, restless leg syndrome, and cramping.52 Additionally, patient acceptance of different dialysis modalities, and dialysis treatment length or frequency, may be influenced by the treatment’s perceived symptom impact. Ramkumar, et al. administered a utility measure questionnaire assessing patient preferences for 3 in-center, intensive HD schedules and found that anticipated improvement in energy and sleep increased patient acceptance of all 3 proposed schedules.51 Despite the high importance of symptoms to dialysis-dependent patients, few symptom-targeted therapies exist, and we have limited understanding of the effect of treatment modifications on symptoms.
Improved symptom recognition and assessment may enhance provider-patient communication about therapy plans. In contrast to the findings of Ramkumar et al. that symptoms influence treatment acceptance, a study of patient preferences in fluid management found that patient-reported cramping, dyspnea, and swelling did not increase patient willingness to extend treatment times or try alternative HD schedules.53 While longer treatment times might relieve intradialytic cramping and hypotension, and more frequent HD might mitigate dyspnea and swelling, symptom presence did not influence the acceptance of such treatment changes. A potential explanation for this seeming incongruity is poor patient understanding of potential treatment and symptom associations. Improved evidence regarding symptom-treatment associations may allow providers to better educate patients about potential benefits of different modalities and treatment aspects.
To identify symptoms and to assess the efficacy of interventions, valid, reliable, and responsive symptom assessment tools are needed. In current practice, symptoms are typically assessed as part of broader HRQOL evaluations. The KDQOL, the most commonly administered HRQOL survey, contains a symptom domain, but the associated items cover a limited range of symptom attributes and have long recall periods (4 weeks). Additionally, the instrument requires up to 30 minutes for completion. These features make the KDQOL and other HRQOL instruments less suitable for frequent symptom assessment compared to more concise, symptom-focused measures with shorter recall periods and greater symptom attributes.
Of the dialysis-specific instruments four instruments focused exclusively on symptoms: Curtin et al., DSI, modified ESAS, and Physical Symptom Distress Scale. Curtin et al. and Physical Symptom Distress Scale have no reported use outside of their development. The modified ESAS has been used more extensively and underwent robust psychometric testing. As modified ESAS measures “present” symptoms, it is a potentially sensitive tool for assessing treatment-to-treatment symptom fluctuation. However, the modified ESAS examines symptom severity only and does not assess symptom frequency or impact on HRQOL. Similar to the modified ESAS, the DSI also had extensive patient and expert input during development, but psychometric testing outside of test-retest reliability was not reported.
While not focused exclusively on symptoms, the Parfrey Symptom Assessment is a HRQOL tool that considers a wide range of symptom attributes. The Parfrey tool addresses symptom severity and frequency and symptom impact on HRQOL, daily living, and sleep. With the inclusion of other aspects of HRQOL such as overall life satisfaction and general affect, the Parfrey tool is longer than symptom-limited tools and requires 15–20 minutes for completion.17 This tool has many advantages over the other identified instruments, but its recall period of weeks limits its utility for assessment of short-term symptom fluctuation. Improved physical symptom assessment tools with short recall periods (<1 week), multi-attribute symptom assessment, and consideration of symptoms relevant to the timing of the dialysis procedure may enhance our understanding of symptom pathophysiology and response to intervention.
The ideal symptom assessment tool for dialysis patients must capture symptoms important to dialysis patients. Content validity measures the extent to which instruments capture concepts relevant to the targeted population. Two key elements of content validity are 1) incorporation of target population input into item generation and prioritization, and 2) evaluation of respondent comprehension of survey items.54, 55 Engagement of the target population in instrument development is fairly routine: 12 of the 13 symptom-related instruments developed for dialysis patients reported dialysis patient involvement in item generation. Evaluation of patient comprehension of measure content is an equally important, but often neglected aspect of content validity. Cognitive interviewing, the process of probing respondent thought processes to elucidate question understanding, is often performed to assess item understanding as it can identify problems with comprehension, recall, and decision processes. Furthermore, it can also detect structural defects in a questionnaire.56, 57 We identified no instrument in which in-depth cognitive interviewing was described in measure development. The fluid management survey did undergo “comprehension testing” in which dialysis patients were interviewed following survey completion and asked to point out ambiguous questions or other points of confusion. However, these interviews were not standardized and were dependent on patient-identified survey ambiguities.53 As new symptom instruments are developed for dialysis patients, greater attention to cognitive interviewing and other comprehension testing is warranted.
Finally, the selection of the optimal symptom evaluation measure depends on the purpose of the symptom assessment. For example, when testing new drugs, devices, or other therapeutic interventions directed at specific symptoms such as pruritus, restless legs, or sleep disorders, in-depth, symptom-specific instruments are appropriate. These measures need to be symptom-targeted, inclusive of a range of symptom attributes, and be responsive to change over time and to intervention. Measures assessing the broader concept of symptom burden and its contribution to HRQOL require different characteristics. Such symptom assessments should include a wide range of potential symptoms and may benefit from greater focus on life interference and burden rather than symptom timing and duration. Furthermore, whether the purpose of the symptom instrument is for detailed research purposes or broader clinical assessments, it is our recommendation that measures include blank, respondent-generated items to ensure that the full range of the patient symptom experience is captured.
While we approached this systematic review with methodological rigor, our review does have limitations. We included instruments with ≥3 physical symptoms, excluding those focused on single symptoms and those assessing only mood-related symptoms. This led to the exclusion of symptom measures focused exclusively on sleep, restless legs, sexual dysfunction, and depression, all important symptoms and co-morbid conditions among dialysis-dependent patients. Review of these symptom-specific instruments is warranted. Additionally, we excluded instruments that had no evidence of validity or reliability testing. This choice resulted in a higher percentage of included instruments with validity and reliability testing compared to prior reports.58 Finally, we did not provide an assessment of the quality of instrument psychometric testing as standards, particularly for the subjective aspects of psychometric testing, are controversial, and gold standards do not exist. Instrument reliability and validity must ultimately be determined by individual providers and investigators with consideration given to the patient population, intervention, and desired outcome. We provided psychometric testing results to help inform these decisions in Table S2.
In summary, our review highlights the diversity of methods used for physical symptom assessment among dialysis-dependent patients and identifies the lack of a valid, symptom-focused instrument with short recall and assessment of multiple symptom attributes. Improved symptom assessment tools are needed to improve symptom evaluation and symptom responsiveness to intervention among dialysis-dependent patients.
Supplementary Material
Acknowledgements
Support: This work was partially supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through grant award 1UL1TR001111. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: JEF, BBR, TSC; data acquisition: LH, JDP, CJP; data analysis/interpretation: JEF, JDP, CJP, KDW, BBR, TSC; statistical analysis: JEF, BBR; supervision or mentorship: BBR, TSC. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. JEF takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Supplementary Material
Table S1: Search strategies.
Table S2: Instrument validity and reliability testing.
Table S3: Instrument intended population, recall periods, and symptom attributes assessed.
Table S4: Summary of validity and reliability testing in instruments assessing physical symptoms.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
References
- 1.Valderrábano F, Jofre R, López-Gómez JM. Quality of life in end-stage renal disease patients. Am J Kidney Dis. 2001;38:443–464. doi: 10.1053/ajkd.2001.26824. [DOI] [PubMed] [Google Scholar]
- 2.Evans RW, Manninen DL, Garrison LP, Hart LG, Blagg CR, Gutman RA, Hull AR, Lowrie EG. The quality of life of patients with end-stage renal disease. N Engl J Med. 1985;312:553–559. doi: 10.1056/NEJM198502283120905. [DOI] [PubMed] [Google Scholar]
- 3.Bremer BA, McCauley CR, Wrona RM, Johnson JP. Quality of life in end-stage renal disease: a reexamination. Am J Kidney Dis. 1989;13:200–209. doi: 10.1016/s0272-6386(89)80053-8. [DOI] [PubMed] [Google Scholar]
- 4.Unruh ML, Weisbord SD, Kimmel PL. Health-related quality of life in nephrology research and clinical practice. Semin Dial. 2005;18:82–90. doi: 10.1111/j.1525-139X.2005.18206.x. [DOI] [PubMed] [Google Scholar]
- 5.Davison SN, Jhangri GS, Johnson JA. Cross-sectional validity of a modified Edmonton symptom assessment system in dialysis patients: a simple assessment of symptom burden. Kidney Int. 2006;69:1621–1625. doi: 10.1038/sj.ki.5000184. [DOI] [PubMed] [Google Scholar]
- 6.Merkus MP, Jager KJ, Dekker FW, de Haan RJ, Boeschoten EW, Krediet RT. Physical symptoms and quality of life in patients on chronic dialysis: results of The Netherlands Cooperative Study on Adequacy of Dialysis (NECOSAD) Nephrol Dial Transplant. 1999;14:1163–1170. doi: 10.1093/ndt/14.5.1163. [DOI] [PubMed] [Google Scholar]
- 7.Weisbord SD, Fried LF, Arnold RM, Fine MJ, Levenson DJ, Peterson RA, Switzer GE. Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. J Am Soc Nephrol. 2005;16:2487–2494. doi: 10.1681/ASN.2005020157. [DOI] [PubMed] [Google Scholar]
- 8.Weisbord SD, Fried LF, Mor MK, Resnick AL, Unruh ML, Palevsky PM, Levenson DJ, Cooksey SH, Fine MJ, Kimmel PL, Arnold RM. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2007;2:960–967. doi: 10.2215/CJN.00990207. [DOI] [PubMed] [Google Scholar]
- 9.Rosas SE, Joffe M, Franklin E, Strom BL, Kotzker W, Brensinger C, Grossman E, Glasser D, Feldman HI. Prevalence and determinants of erectile dysfunction in hemodialysis patients. Kidney Int. 2001;59:2259–2266. doi: 10.1046/j.1523-1755.2001.00742.x. [DOI] [PubMed] [Google Scholar]
- 10.Ross SD, Fahrbach K, Frame D, Scheye R, Connelly JE, Glaspy J. The effect of anemia treatment on selected health-related quality-of-life domains: a systematic review. Clin Ther. 2003;25:1786–1805. doi: 10.1016/s0149-2918(03)80170-4. [DOI] [PubMed] [Google Scholar]
- 11.Keown PA, Churchill DN, Poulin-Costello M, Lei L, Gantotti S, Agodoa I, Gitlin M, Gandra SR, Mayne TJ. Dialysis patients treated with Epoetin alfa show improved anemia symptoms: A new analysis of the Canadian Erythropoietin Study Group trial. Hemodial Int. 2010;14:168–173. doi: 10.1111/j.1542-4758.2009.00422.x. [DOI] [PubMed] [Google Scholar]
- 12.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3:329–338. doi: 10.1007/BF00451725. [DOI] [PubMed] [Google Scholar]
- 13.Wu AW, Fink NE, Cagney KA, Bass EB, Rubin HR, Meyer KB, Sadler JH, Powe NR. Developing a health-related quality-of-life measure for end-stage renal disease: The CHOICE Health Experience Questionnaire. Am J Kidney Dis. 2001;37:11–21. doi: 10.1053/ajkd.2001.20631. [DOI] [PubMed] [Google Scholar]
- 14.Churchill DN, Wallace JE, Ludwin D, Beecroft ML, Taylor DW. A comparison of evaluative indices of quality of life and cognitive function in hemodialysis patients. Control Clin Trials. 1991;12:159S–167S. doi: 10.1016/s0197-2456(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 15.Laupacis A, Muirhead N, Keown P, Wong C. A disease-specific questionnaire for assessing quality of life in patients on hemodialysis. Nephron. 1992;60:302–306. doi: 10.1159/000186769. [DOI] [PubMed] [Google Scholar]
- 16.Evans R, Manninen D, Garrison L. Healthcare Financing Special Report: Findings from the National Kidney Dialysis and Kidney Transplantation Study. Baltimore, MD: U.S. Department of Health and Human Services, Health Care Financing Administration; 1987. [Google Scholar]
- 17.Parfrey PS, Vavasour H, Bullock M, Henry S, Harnett JD, Gault MH. Development of a health questionnaire specific for end-stage renal disease. Nephron. 1989;52:20–28. doi: 10.1159/000185577. [DOI] [PubMed] [Google Scholar]
- 18.Cohen SR, Mount BM, Strobel MG, Bui F. The McGill Quality of Life Questionnaire: a measure of quality of life appropriate for people with advanced disease A preliminary study of validity and acceptability. Palliat Med. 1995;9:207–219. doi: 10.1177/026921639500900306. [DOI] [PubMed] [Google Scholar]
- 19.Weisbord SD, Fried LF, Arnold RM, Rotondi AJ, Fine MJ, Levenson DJ, Switzer GE. Development of a symptom assessment instrument for chronic hemodialysis patients: the Dialysis Symptom Index. J Pain Symptom Manage. 2004;27:226–240. doi: 10.1016/j.jpainsymman.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 21.Hunt SM, McKenna SP, McEwen J, Backett EM, Williams J, Papp E. A quantitative approach to perceived health status: a validation study. J Epidemiol Community Health. 1980;34:281–286. doi: 10.1136/jech.34.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Haes JC, van Knippenberg FC, Neijt JP. Measuring psychological and physical distress in cancer patients: structure and application of the Rotterdam Symptom Checklist. Br J Cancer. 1990;62:1034–1038. doi: 10.1038/bjc.1990.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E, Sobel K, Coyle N, Kemeny N, Norton L. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A:1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 24.Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Jan, [PubMed] [Google Scholar]
- 25.Whitlock EP, Lopez SA, Chang S, Helfand M, Eder M, Floyd N. AHRQ series paper 3: identifying, selecting, and refining topics for comparative effectiveness systematic reviews: AHRQ and the effective health-care program. J Clin Epidemiol. 2010;63:491–501. doi: 10.1016/j.jclinepi.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 27.Counsell C. Formulating questions and locating primary studies for inclusion in systematic reviews. Ann Intern Med. 1997;127:380–387. doi: 10.7326/0003-4819-127-5-199709010-00008. [DOI] [PubMed] [Google Scholar]
- 28.Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, Bouter LM, de Vet HC. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Nunnally J, Berstein I. Psychometric theory. New York: Mcgraw-Hill; 1994. [Google Scholar]
- 30.Aaronson N, Alonso J, Burnam A, Lohr KN, Patrick DL, Perrin E, Stein RE. Assessing health status and quality-of-life instruments: attributes and review criteria. Qual Life Res. 2002;11:193–205. doi: 10.1023/a:1015291021312. [DOI] [PubMed] [Google Scholar]
- 31.Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, Bouter LM, de Vet HC. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63:737–745. doi: 10.1016/j.jclinepi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Rothman M, Burke L, Erickson P, Leidy NK, Patrick DL, Petrie CD. Use of existing patient-reported outcome (PRO) instruments and their modification: the ISPOR Good Research Practices for Evaluating and Documenting Content Validity for the Use of Existing Instruments and Their Modification PRO Task Force Report. Value Health. 2009;12:1075–1083. doi: 10.1111/j.1524-4733.2009.00603.x. [DOI] [PubMed] [Google Scholar]
- 33.Schrama YC, Krediet RT, de Rooy-Roggekamp MC, Arisz L. [Relation between clinical condition and quality of life in patients on hemodialysis, a clinimetric study] Ned Tijdschr Geneeskd. 1991;135:1182–1185. [PubMed] [Google Scholar]
- 34.Brennan F, Siva B, Crail S. Appropriate Assessment of Symptom Burden and Provision of Patient Informatio. Nephrology (Carlton) 2013 doi: 10.1111/nep.12075. [DOI] [PubMed] [Google Scholar]
- 35.Micozkadioglu H, Ozdemir FN, Kut A, Sezer S, Saatci U, Haberal M. Gabapentin versus levodopa for the treatment of Restless Legs Syndrome in hemodialysis patients: an open-label study. Ren Fail. 2004;26:393–397. doi: 10.1081/jdi-120039823. [DOI] [PubMed] [Google Scholar]
- 36.Lee J, Nicholl DD, Ahmed SB, Loewen AH, Hemmelgarn BR, Beecroft JM, Turin TC, Hanly PJ. The prevalence of restless legs syndrome across the full spectrum of kidney disease. J Clin Sleep Med. 2013;9:455–459. doi: 10.5664/jcsm.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barakzoy AS, Moss AH. Efficacy of the world health organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol. 2006;17:3198–3203. doi: 10.1681/ASN.2006050477. [DOI] [PubMed] [Google Scholar]
- 38.Aitken E, McLellan A, Glen J, Serpell M, Mactier R, Clancy M. Pain resulting from arteriovenous fistulae: prevalence and impact. Clin Nephrol. 2013;80:328–333. doi: 10.5414/CN107917. [DOI] [PubMed] [Google Scholar]
- 39.Bots CP, Brand HS, Veerman EC, Korevaar JC, Valentijn-Benz M, Bezemer PD, Valentijn RM, Vos PF, Bijlsma JA, ter Wee PM, Van Amerongen BM, Nieuw Amerongen AV. Chewing gum and a saliva substitute alleviate thirst and xerostomia in patients on haemodialysis. Nephrol Dial Transplant. 2005;20:578–584. doi: 10.1093/ndt/gfh675. [DOI] [PubMed] [Google Scholar]
- 40.Fan WF, Zhang Q, Luo LH, Niu JY, Gu Y. Study on the clinical significance and related factors of thirst and xerostomia in maintenance hemodialysis patients. Kidney Blood Press Res. 2013;37:464–474. doi: 10.1159/000355717. [DOI] [PubMed] [Google Scholar]
- 41.Malekmakan L, Shakeri S, Haghpanah S, Pakfetrat M, Sarvestani AS, Malekmakan A. Epidemiology of erectile dysfunction in hemodialysis patients using IIEF questionnaire. Saudi J Kidney Dis Transpl. 2011;22:232–236. [PubMed] [Google Scholar]
- 42.Bonner A, Wellard S, Caltabiano M. Levels of fatigue in people with ESRD living in far North Queensland. J Clin Nurs. 2008;17:90–98. doi: 10.1111/j.1365-2702.2007.02042.x. [DOI] [PubMed] [Google Scholar]
- 43.Bonner A, Caltabiano M, Berlund L. Quality of life, fatigue, and activity in Australians with chronic kidney disease: a longitudinal study. Nurs Health Sci. 2013;15:360–367. doi: 10.1111/nhs.12038. [DOI] [PubMed] [Google Scholar]
- 44.McCorkle R, Cooley M, Shea J. A User's Manual for the Symptom Distress Scale. New Haven, CT: Yale University School of Nursing; 2000. pp. 1–99. [Google Scholar]
- 45.Kutlay S, Nergizoglu G, Keven K, Erturk S, Ates K, Duman N, Karatan O, Atli T. General or disease specific questionnaire? A comparative study in hemodialysis patients. Ren Fail. 2003;25:95–103. doi: 10.1081/jdi-120017472. [DOI] [PubMed] [Google Scholar]
- 46.Edgell ET, Coons SJ, Carter WB, Kallich JD, Mapes D, Damush TM, Hays RD. A review of health-related quality-of-life measures used in end-stage renal disease. Clin Ther. 1996;18:887–938. doi: 10.1016/s0149-2918(96)80049-x. [DOI] [PubMed] [Google Scholar]
- 47.Malindretos P, Sarafidis P, Spaia S, Sioulis A, Zeggos N, Raptis V, Kitos V, Koronis C, Kabouris C, Zili S, Grekas D. Adaptation and validation of the Kidney Disease Quality of Life-Short Form questionnaire in the Greek language. Am J Nephrol. 2010;31:9–14. doi: 10.1159/000252926. [DOI] [PubMed] [Google Scholar]
- 48.Chow E, Fan G, Hadi S, Filipczak L. Symptom clusters in cancer patients with bone metastases. Support Care Cancer. 2007;15:1035–1043. doi: 10.1007/s00520-007-0241-z. [DOI] [PubMed] [Google Scholar]
- 49.Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88:2164–2171. doi: 10.1002/(sici)1097-0142(20000501)88:9<2164::aid-cncr24>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 50.Steinhauser KE, Christakis NA, Clipp EC, McNeilly M, McIntyre L, Tulsky JA. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA. 2000;284:2476–2482. doi: 10.1001/jama.284.19.2476. [DOI] [PubMed] [Google Scholar]
- 51.Ramkumar N, Beddhu S, Eggers P, Pappas LM, Cheung AK. Patient preferences for in-center intense hemodialysis. Hemodial Int. 2005;9:281–295. doi: 10.1111/j.1492-7535.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- 52.Manns B, Hemmelgarn B, Lillie E, Dip SC, Cyr A, Gladish M, Large C, Silverman H, Toth B, Wolfs W, Laupacis A. Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol. 2014;9:1813–1821. doi: 10.2215/CJN.01610214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flythe JE, Mangione TW, Brunelli SM, Curhan GC. Patient-Stated Preferences Regarding Volume-Related Risk Mitigation Strategies for Hemodialysis. Clin J Am Soc Nephrol. 2014 doi: 10.2215/CJN.03280314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Magasi S, Ryan G, Revicki D, Lenderking W, Hays RD, Brod M, Snyder C, Boers M, Cella D. Content validity of patient-reported outcome measures: perspectives from a PROMIS meeting. Qual Life Res. 2012;21:739–746. doi: 10.1007/s11136-011-9990-8. [DOI] [PubMed] [Google Scholar]
- 55.Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, Ring L. Content validity--establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1--eliciting concepts for a new PRO instrument. Value Health. 2011;14:967–977. doi: 10.1016/j.jval.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 56.Jobe JB, Mingay DJ. Cognitive research improves questionnaires. Am J Public Health. 1989;79:1053–1055. doi: 10.2105/ajph.79.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campanelli P, Martin E, Rothgeb JM. The use of respondent and interviewer debriefing studies as a way to study response error in survey data. The Statistician. 1991:253–264. [Google Scholar]
- 58.Cagney KA, Wu AW, Fink NE, Jenckes MW, Meyer KB, Bass EB, Powe NR. Formal literature review of quality-of-life instruments used in end-stage renal disease. Am J Kidney Dis. 2000;36:327–336. doi: 10.1053/ajkd.2000.8982. [DOI] [PubMed] [Google Scholar]
- 59.Reeve BB, Wyrwich KW, Wu AW, Velikova G, Terwee CB, Snyder CF, Schwartz C, Revicki DA, Moinpour CM, McLeod LD, Lyons JC, Lenderking WR, Hinds PS, Hays RD, Greenhalgh J, Gershon R, Feeny D, Fayers PM, Cella D, Brundage M, Ahmed S, Aaronson NK, Butt Z. ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Qual Life Res. 2013;22:1889–1905. doi: 10.1007/s11136-012-0344-y. [DOI] [PubMed] [Google Scholar]
- 60.Frost MH, Reeve BB, Liepa AM, Stauffer JW, Hays RD Group. MFP-ROCM: What is sufficient evidence for the reliability and validity of patient-reported outcome measures? Value Health. 2007;(10 Suppl 2):S94–S105. doi: 10.1111/j.1524-4733.2007.00272.x. [DOI] [PubMed] [Google Scholar]
- 61.Tsutsui H, Koike T, Yamazaki C, Ito A, Kato F, Sato H, Tawada H, Oshida Y. Identification of hemodialysis patients' common problems using the International Classification of Functioning, Disability and Health. Ther Apher Dial. 2009;13:186–192. doi: 10.1111/j.1744-9987.2009.00683.x. [DOI] [PubMed] [Google Scholar]
- 62.Tsutsui H, Ohkubo T, Tsuruta Y, Kato S, Yasuda Y, Oshida Y. Development and validation of a short-version checklist for patients undergoing hemodialysis based on the International Classification of Functioning, Disability and Health. Clin Exp Nephrol. 2014 doi: 10.1007/s10157-014-1075-x. [DOI] [PubMed] [Google Scholar]
- 63.Curtin RB, Bultman DC, Thomas-Hawkins C, Walters BA, Schatell D. Hemodialysis patients' symptom experiences: effects on physical and mental functioning. Nephrol Nurs J. 2002;29 562, 567–574; discussion 575, 598. [PubMed] [Google Scholar]
- 64.Churchill DN, Bird DR, Taylor DW, Beecroft ML, Gorman J, Wallace JE. Effect of high-flux hemodialysis on quality of life and neuropsychological function in chronic hemodialysis patients. Am J Nephrol. 1992;12:412–418. doi: 10.1159/000168491. [DOI] [PubMed] [Google Scholar]
- 65.Laupacis A, Wong C, Churchill D. The use of generic and specific quality-of-life measures in hemodialysis patients treated with erythropoietin The Canadian Erythropoietin Study Group. Control Clin Trials. 1991;12:168S–179S. doi: 10.1016/s0197-2456(05)80021-2. [DOI] [PubMed] [Google Scholar]
- 66.Hughes C, Al-Ghadeer A, McClean M, McElnay J. Validity and reliability of the kidney disease questionnaire in patients receiving continuous ambulatory peritoneal dialysis. Int J Pharm Pract. 2001;9:R61. [Google Scholar]
- 67.Spiegel DM, Evans RW, Gitlin M, Mayne TJ. Psychometric evaluation of the National Kidney Dialysis and Kidney Transplantation Study symptom checklist: reliability and validity. Nephrol Dial Transplant. 2009;24:619–625. doi: 10.1093/ndt/gfn523. [DOI] [PubMed] [Google Scholar]
- 68.Chiou CP. Development and psychometric assessment of the physical symptom distress scale. J Pain Symptom Manage. 1998;16:87–95. doi: 10.1016/s0885-3924(98)00036-0. [DOI] [PubMed] [Google Scholar]
- 69.Hearn J, Higginson IJ. Development validation of a core outcome measure for palliative care: the palliative care outcome scale Palliative Care Core Audit Project Advisory Group. Qual Health Care. 1999;8:219–227. doi: 10.1136/qshc.8.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andresen EM, Rothenberg BM, Kaplan RM. Performance of a self-administered mailed version of the Quality of Well-Being (QWB-SA) questionnaire among older adults. Med Care. 1998;36:1349–1360. doi: 10.1097/00005650-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 71.de Haes J, Olschewski M, Fayers P, Visser M, Cull A, Hopwood P, Sanderman R. Measuring the quality of life of cancer patients with The Rotterdam Symptom Checklist: a manual. 2nd ed. Gronigen, the Netherlands, Research Institute SHARE, UMCG/ University of Groningen; 2012. [Google Scholar]
- 72.Tsutsui H, Ojima T, Tsuruta Y, Kato S, Yasuda Y, Oshida Y. Validity of a checklist for hemodialysis patients based on the International Classification Of Functioning, Disability and Health. Ther Apher Dial. 2014;18:473–480. doi: 10.1111/1744-9987.12163. [DOI] [PubMed] [Google Scholar]
- 73.Wu AW, Fink NE, Marsh-Manzi JV, Meyer KB, Finkelstein FO, Chapman MM, Powe NR. Changes in quality of life during hemodialysis and peritoneal dialysis treatment: generic and disease specific measures. J Am Soc Nephrol. 2004;15:743–753. doi: 10.1097/01.asn.0000113315.81448.ca. [DOI] [PubMed] [Google Scholar]
- 74.Korevaar JC, Merkus MP, Jansen MA, Dekker FW, Boeschoten EW, Krediet RT, group N-s. Validation of the KDQOL-SF: a dialysis-targeted health measure. Qual Life Res. 2002;11:437–447. doi: 10.1023/a:1015631411960. [DOI] [PubMed] [Google Scholar]
- 75.Rao S, Carter WB, Mapes DL, Kallich JD, Kamberg CJ, Spritzer KL, Hays RD. Development of subscales from the symptoms/problems and effects of kidney disease scales of the kidney disease quality of life instrument. Clin Ther. 2000;22:1099–1111. doi: 10.1016/S0149-2918(00)80087-9. [DOI] [PubMed] [Google Scholar]
- 76.Bass A, Ahmed SB, Klarenbach S, Culleton B, Hemmelgarn BR, Manns B. The impact of nocturnal hemodialysis on sexual function. BMC Nephrol. 2012;13:67. doi: 10.1186/1471-2369-13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim KH, Kim TH, Kang JW, Sul JU, Lee MS, Kim JI, Shin MS, Jung SY, Kim AR, Kang KW, Choi SM. Acupuncture for symptom management in hemodialysis patients: a prospective, observational pilot study. J Altern Complement Med. 2011;17:741–748. doi: 10.1089/acm.2010.0206. [DOI] [PubMed] [Google Scholar]
- 78.Hays R, Kallich J, Mapes D, Coons S, Amin N, Carter W, Kamberg C. Kidney Disease Quality of Life Short Form (KDQOL-SF), Version 1.3 A Manual for Use and Scoring. RAND. 1995 [Google Scholar]
- 79.Fainsinger RL, Davison SN, Brenneis C. A supportive care model for dialysis patients. Palliat Med. 2003;17:81–82. doi: 10.1191/0269216303pm666xx. [DOI] [PubMed] [Google Scholar]
- 80.Davison SN, Jhangri GS, Johnson JA. Longitudinal validation of a modified Edmonton symptom assessment system (ESAS) in haemodialysis patients. Nephrol Dial Transplant. 2006;21:3189–3195. doi: 10.1093/ndt/gfl380. [DOI] [PubMed] [Google Scholar]
- 81.Talley NJ, Phillips SF, Wiltgen CM, Zinsmeister AR, Melton LJ. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–1479. doi: 10.1016/s0025-6196(12)62169-7. [DOI] [PubMed] [Google Scholar]
- 82.Talley NJ, Phillips SF, Melton J, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111:671–674. doi: 10.7326/0003-4819-111-8-671. [DOI] [PubMed] [Google Scholar]
- 83.Aaronson NK, Bullinger M, Ahmedzai S. A modular approach to quality-of-life assessment in cancer clinical trials. Recent Results Cancer Res. 1988;111:231–249. doi: 10.1007/978-3-642-83419-6_27. [DOI] [PubMed] [Google Scholar]
- 84.Majkowicz M, Afeltowicz Z, Lichodziejewska-Niemierko M, Debska-Slizien A, Rutkowski B. Comparison of the quality of life in hemodialysed (HD) and peritoneally dialysed (CAPD) patients using the EORTC QLQ-C30 questionnaire. Int J Artif Organs. 2000;23:423–428. [PubMed] [Google Scholar]
- 85.Cohen SR, Mount BM, Bruera E, Provost M, Rowe J, Tong K. Validity of the McGill Quality of Life Questionnaire in the palliative care setting: a multi-centre Canadian study demonstrating the importance of the existential domain. Palliat Med. 1997;11:3–20. doi: 10.1177/026921639701100102. [DOI] [PubMed] [Google Scholar]
- 86.Cohen SR, Mount BM. Living with cancer: "good" days and "bad" days--what produces them? Can the McGill quality of life questionnaire distinguish between them? Cancer. 2000;89:1854–1865. doi: 10.1002/1097-0142(20001015)89:8<1854::aid-cncr28>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 87.Tasmoc A, Nistor I, Donciu M, Voroneanu L, Volvat C, Covic A. Validation and evaluation of Memorial Symptom Assessment Scale Short-Form in a Romanian Cohort of Hemodialysis Patients. 51st ERA-EDTA Congress; Amsterdam, The Netherlands: Nephrol Dial Transplant; 2014. pp. ii272–iii286. [Google Scholar]
- 88.Evans RW, Rader B, Manninen DL. The quality of life of hemodialysis recipients treated with recombinant human erythropoietin Cooperative Multicenter EPO Clinical Trial Group. JAMA. 1990;263:825–830. [PubMed] [Google Scholar]
- 89.Steinhauser KE, Bosworth HB, Clipp EC, McNeilly M, Christakis NA, Parker J, Tulsky JA. Initial assessment of a new instrument to measure quality of life at the end of life. J Palliat Med. 2002;5:829–841. doi: 10.1089/10966210260499014. [DOI] [PubMed] [Google Scholar]
- 90.Steinhauser KE, Clipp EC, Bosworth HB, McNeilly M, Christakis NA, Voils CI, Tulsky JA. Measuring quality of life at the end of life: validation of the QUAL-E. Palliat Support Care. 2004;2:3–14. doi: 10.1017/s1478951504040027. [DOI] [PubMed] [Google Scholar]
- 91.Kaplan R, Anderson JTGG. The quality of well-being scale: rationale for a single quality of life index. In: Walker S, Rosser R, editors. Quality of life assessment: Key issues in the 1990s. London: Kluwer Academic Publishers; 1993. pp. 65–94. [Google Scholar]
- 92.Kaplan R, Sieber W, Ganiats T. The quality of well-being scale: Comparison of the interviewer-administered version with a self-administered questionnaire. Psychology and Health. 2007;12:783–791. [Google Scholar]
- 93.Orenstein DM, Nixon PA, Ross EA, Kaplan RM. The quality of well-being in cystic fibrosis. Chest. 1989;95:344–347. doi: 10.1378/chest.95.2.344. [DOI] [PubMed] [Google Scholar]
- 94.Anderson JP, Kaplan RM, Berry CC, Bush JW, Rumbaut RG. Interday reliability of function assessment for a health status measure The Quality of Well-Being scale. Med Care. 1989;27:1076–1083. doi: 10.1097/00005650-198911000-00008. [DOI] [PubMed] [Google Scholar]
- 95.de Haes JC, Olschewski M. Quality of life assessment in a cross-cultural context: use of the Rotterdam Symptom Checklist in a multinational randomised trial comparing CMF and Zoladex (Goserlin) treatment in early breast cancer. Ann Oncol. 1998;9:745–750. doi: 10.1023/a:1008282806910. [DOI] [PubMed] [Google Scholar]
- 96.Richards MA, Hopwood P, Ramirez AJ, Twelves CJ, Ferguson J, Gregory WM, Swindell R, Scrivener W, Miller J, Howell A. Doxorubicin in advanced breast cancer: influence of schedule on response, survival and quality of life. Eur J Cancer. 1992;28A:1023–1028. doi: 10.1016/0959-8049(92)90447-a. [DOI] [PubMed] [Google Scholar]
- 97.McCorkle R, Young K. Development of a symptom distress scale. Cancer Nurs. 1978;1:373–378. [PubMed] [Google Scholar]
- 98.McCorkle R, Benoliel JQ, Donaldson G, Georgiadou F, Moinpour C, Goodell B. A randomized clinical trial of home nursing care for lung cancer patients. Cancer. 1989;64:1375–1382. doi: 10.1002/1097-0142(19890915)64:6<1375::aid-cncr2820640634>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 99.Frederickson K, Jackson BS, Strauman T, Strauman J. Testing hypotheses derived from the Roy Adaptation Model. Nurs Sci Q. 1991;4:168–174. doi: 10.1177/089431849100400409. [DOI] [PubMed] [Google Scholar]
- 100.Sarna L. Women with lung cancer: impact on quality of life. Qual Life Res. 1993;2:13–22. doi: 10.1007/BF00642885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.