Abstract
The technique of microneurography and the assessment of muscle sympathetic nerve activity (MSNA) are used in laboratories throughout the world. The variables used to describe MSNA, and the criteria by which these variables are quantified from the integrated neurogram, vary amongst studies and laboratories and, therefore, can become confusing to those starting to learn the technique. Therefore, the purpose of this educational review is to discuss guidelines and standards for the assessment of sympathetic nervous activity through the collection and analysis of MSNA. This review will reiterate common practices in the collection of MSNA, but will also introduce considerations for the evaluation and physiological inference using MSNA.
Keywords: Muscle Sympathetic Nerve Activity, MSNA, Microneurography, Standardization, Quantification
1 Introduction
For half a century, scientists have been measuring muscle sympathetic nerve activity in humans. The technique of microneurography in humans was developed by Hagbarth and Vallbo at the Academic Hospital in Uppsala, Sweden in 1965 (Vallbo et al., 2004). Through years of meticulous experimentation, the analysis of afferent responses to stimuli were characterized and along the way Gunner Wallin led the charge in studying the efferent signals of the sympathetic nervous system (Vallbo et al., 2004). As the technique became more recognized, investigators such as Allyn Mark M.D. and Dwain Eckberg M.D. traveled to Sweden to learn and bring back the technique to their own laboratories in the United States. Equipment was designed specifically for the study of nerve activity, and analysis techniques were further developed. Currently, many groups have successfully recorded and reported sympathetic nerve activity and sympathetic nerve responses in a myriad of experiments with relatively minor physical complications. However, a recurring issue, associated with the increasing number of groups using microneurography, is how we quantify changes in MSNA and statistically compare across groups involving age, disease, sex and ethnicity.
Historically, the method of acquisition and analysis of MSNA has relied upon the bequeathed knowledge of the mentor to the student, which has worked well for signal acquisition but small variations in quantifying the MSNA have started to add confusion to the interpretation of the data. These small variations include differences in terminology and opinions on which measurements are the most reliable and valid. Furthermore, the use of absolute change and relative change without consideration for equality of baseline values when quantifying and making conclusions can be misleading and is potentially mathematically inappropriate. This review is intended to start a conversation between research groups measuring MSNA to standardize the acquisition, quantification, analysis and interpretation of the MSNA recordings.
2 Acquisition of a Post-ganglionic Efferent Muscle Sympathetic Nerve Signal
2.1 Equipment for Acquiring MSNA
Equipment needed for recording MSNA: 1) electrode with which to impale a nerve; 2) amplifier; 3) signal integrator; and 4) output. The electrodes need to be made from a non-bioreactive material which is conductive and can remain stiff when very thin without being brittle. Tungsten is the preferred material and the active electrode is electrically insulated except for a few μM at the tip. The electrodes are attached to a grounding unit and preamplifier placed close to the recording electrode, which improves signal-to-noise and enables the recording unit to be electrically equal to the subject. The main amplifier is used to increase the raw nerve activity many thousand times before it is filtered. An analog or digital band pass filter is applied and the signal is rectified and integrated using capacitors and resistors or digital logic. The output may be routed through a computer software setup or as an analog signal to an oscilloscope, an acquisition system, or chart recorder. There are two well known commercially available systems: Absolute Design NTA (Solon, Iowa; formerly Iowa Biosystems) and ADI NeuroAmp EX module (NSW, Australia).
2.2 Choosing a nerve to record
Theoretically, any peripheral nerve can serve as a target for acquisition of MSNA as all include some sympathetic efferent axons; but only a few nerves are popularly used due to practical considerations. Firstly, a nerve must be large enough to identify using one of the techniques below; the nerve must be large enough to support and stabilize the microelectrode tip in place once an MSNA signal is acquired. Also, the nerve must be close enough to the surface of the skin for identification using methods in the next section.
Probably the most recorded peripheral nerve is the peroneal (fibular) nerve at or proximal to the fibular head. It is at this point where the common peroneal splits into the superficial and deep branches. Though a satisfactory MSNA signal can be obtained through the common, superficial, or deep peroneal nerve, the deep appears to offer a greater success rate of locating bundles of MSNA axons from which to record. Advantages of using the peroneal nerve are: 1) easy to identify; 2) located close to the skin; 3) easy to stabilize the limb for long periods of time; and 4) can be performed in the supine, semi-recumbent, or seated-upright position. Disadvantages are: 1) limits studies using exercise to a single-leg; and/or 2) upper body exercise only.
Just proximal to the fibular head at the popliteal fossa, the common peroneal (popliteal) nerve can be acquired. This location of nerve recording is less commonly used because it either requires the subject to lie in the prone position or for the investigators to support the leg and acquire the nerve from beneath. However, the advantages and disadvantages of the common peroneal (fibular) or popliteal nerves are similar to those identified above for the peroneal nerve. Advantages of using the popliteal nerve: 1) easy to identify; 2) close to the skin; 3) easy to stabilize the limb for long periods of time; and 4) allows easy access when a protocol calls for a prone position. Disadvantages: 1) much harder to acquire in the supine, semi-recumbent, or upright seated position than the peroneal nerve at the fibular head; and 2) limits studies using exercise to single-leg or upper body exercise.
In the arms, the radial, median, and ulnar nerves have all been used for acquisition of MSNA. These nerves have been used when a protocol calls for lower body exercise using both legs, except for a few cases comparing arm to leg MSNA, or to explore arm MSNA and forearm blood flow during lower body negative pressure (LBNP).
The radial nerve traverses the lateral surface of the humerus to the cubital fossa. This nerve is acquired 2 to 6 cm proximal to the cubital fossa. Because of its depth beneath the skin, ultrasound guidance is normally used to acquire this nerve. Advantages of using the radial nerve are: 1) easy to stabilize the limb for long periods of time; 2) can be performed in the supine, semi-recumbent, seated-upright or prone position; and 3) enables one to perform two-legged exercise. Disadvantages are: 1) typically requires ultrasound guidance; and 2) microelectrode inserted near or through the muscle, thereby, making any movement, or twitch, in the muscle a potential hazard to the quality of the MSNA signal.
The median nerve traverses the medial surface of the humerus to the cubital fossa where it is acquired and depending on the size of the arm, may require ultrasound guidance. Advantages are: 1) easy to stabilize the limb for long periods of time; 2) available to be performed in the supine, semi-recumbent, or seated-upright positions; 3) ability to perform two-legged exercise; and 4) not inserted through muscle. Disadvantages: 1) may require ultrasound guidance; and 2) near brachial artery so caution must be used to not puncture the artery.
The ulnar nerve enters the forearm between the ulnar and humeral heads and is unprotected by bone or muscle at the elbow. This makes it a very easy target for microneurography though it is rarely used. Advantages to using the ulnar nerve are: 1) easy to stabilize the limb for long periods of time; 2) available to be performed in the supine, semi-recumbent, seated-upright or prone position; 3) able to be performed during two-legged exercise; and are 4) easy to locate. Disadvantages include that: 1) they are associated with “funny bone” paresthesia; and 2) they have very little tissue to secure the electrode.
The appropriate nerve should be selected to match the goals of the protocol and to address the research question (i.e. use a nerve in the leg to study neural control of leg vasculature).
2.3 Positioning the limb
Position of the right leg for a peroneal nerve recording at the fibular head will be discussed in this section. First the subject must be comfortably supine or seated with the leg extended. The thigh is then elevated using foam wedges or moldable pillows so that when the lower leg is relaxed, the angle of the knee joint is between 30 and 45 degrees. This allows the nerve to relax within the leg and become separated from the fibular head. This also allows access to the preferential angles for electrode placement. After elevating the limb, it is recommended that the subject rotate the right hip inward so that when relaxed, the fibular head is further exposed to the microneurographer. Finally, the foot should be supported at a 90 degree angle and slightly rotated inward using foam wedges or moldable pillows. The goal of the leg position is to maintain subject comfort while providing a stable limb with access to the point of nerve acquisition. The same concepts can be applied to the left leg or to either arm.
2.4 Identifying the path of the nerve
The acquisition of MSNA requires that post ganglionic efferent sympathetic neurons innervating the vascular smooth muscle of the vessels within the skeletal muscle be located. This can be accomplished in various ways. Many groups use cutaneous electrical stimulation at the suspected location of the nerve’s path (Vallbo et al., 2004; Macefield, 2013), while others palpate (Steinback et al., 2010) or use ultrasound imaging (Curry & Charkoudian, 2011).
Cutaneous electrical stimulation has historically been the most popular method of identification (Vallbo et al., 1979). This method is ideal for identifying the path of a nerve close to the surface of the skin. This is accomplished by using a blunt tipped stimulator probe and applying a short duration (2mSec) electrical current intermittently to the area of skin above where the nerve is expected to lie. The voltage of the electrical current is adjusted to evoke muscle twitches in the muscles innervated by the neurons. When stimulating near the fibular head you will observe dorsiflexion and lateral deflection of the foot. This is due to the muscles innervated by the deep and superficial branches of the peroneal nerve. Lateral deflection occurs with stimulation of the superficial branch, and dorsiflexion occurs with stimulation of the deep branch (Vallbo et al., 1979).
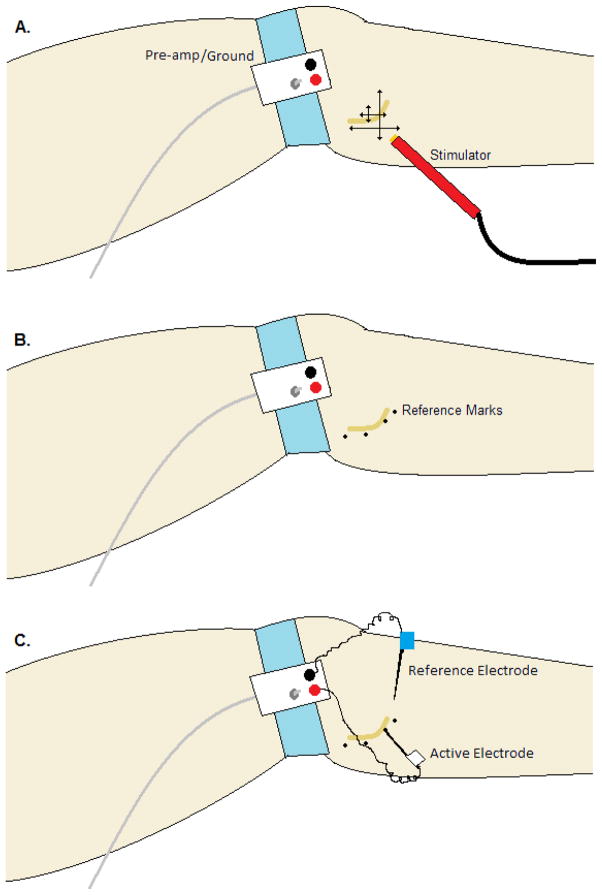
When targeting the deep peroneal nerve, electrical stimulation voltage should start low and be increased gradually until a deflection is detected visually. The stimulation probe should then be moved vertically and laterally in a systematic manner to find the location of the greatest twitch deflection (Figure 1A). Once dorsiflexion is observed, the stimulator voltage should be decreased and the systematic sweeping continued until the lowest voltage is found in which a dorsiflexion is still present. This spot should be marked and the areas to either side should be stimulated to establish the path of the nerve (Figure 1B). One thing to note, while stimulating, is the deformation of the cutaneous tissue with the moving of the probe, because the skin moves independent of the tissue beneath. Because of this property the investigator needs to allow for the tissue movement in marking the locus of the nerve.
Figure 1. Typical layout and procedure for a peroneal (fibular) nerve recording.
The pre-amplifier and ground are attached to the skin on a flat surface either at the lateral knee joint (shown) or distal to the fibular head on the lateral shin. A) The systematic pattern of cutaneous electrical stimulation to locate and map the path of the nerve. B) The dots are placed on the skin following the path of the nerve and act as a reference for insertion of the recording electrode. 3) The reference electrode (blue) is placed beneath the skin and into the tissue within 2 cm of the expected recording site. The active electrode (white) is inserted through the skin and manipulated until a satisfactory nerve signal is acquired.
2.5 Placing the electrodes
After the path of the nerve is located, an un-insulated tungsten microelectrode is inserted through the skin within 1 to 3 cm of the expected site of measurement (Figure 1C). This electrode acts as an electrical reference so that changes in the electrical potential within the nerve can be detected. Next, an insulated electrode with a 5 – 8 μM un-insulated tip is inserted through the skin and into the nerve bundle. The position of the electrode is manipulated until a satisfactory signal is acquired based on the following criteria: 1) bursting occurs pulse synchronously; 2) bursting becomes more frequent during breath hold; and 3) bursting is not evoked by startle stimuli (Hagbarth & Vallbo, 1968; Delius et al., 1972b; Vallbo et al., 1979; Fagius & Wallin, 1980). The presence of all three features demarks efferent sympathetic nerve activity that is directed to the vasculature in the skeletal muscle (MSNA) rather than the skin. Another potential indicator of muscle and not skin sympathetic activity is the assessment of afferent activity. Nerve discharge when applying pressure to the tendons of the foot indicates muscle afferent innervation and potential efferent activity, but does not guarantee a high quality efferent signal. Furthermore, discharge with light touching or stroking of the skin on the foot or leg indicates skin afferent activity and likely skin efferent interference. A less common method of locating where to place the microelectrode is to use an intraneural electrical stimulation, where a very small current is applied directly through a microelectrode in order to identify electrode position within the nerve bundle. Confirmation of accurate placement of the microelectrode in the nerve bundle is when an electrical stimulation causes a muscle twitch but not paresthesia (Jordan, 1997).
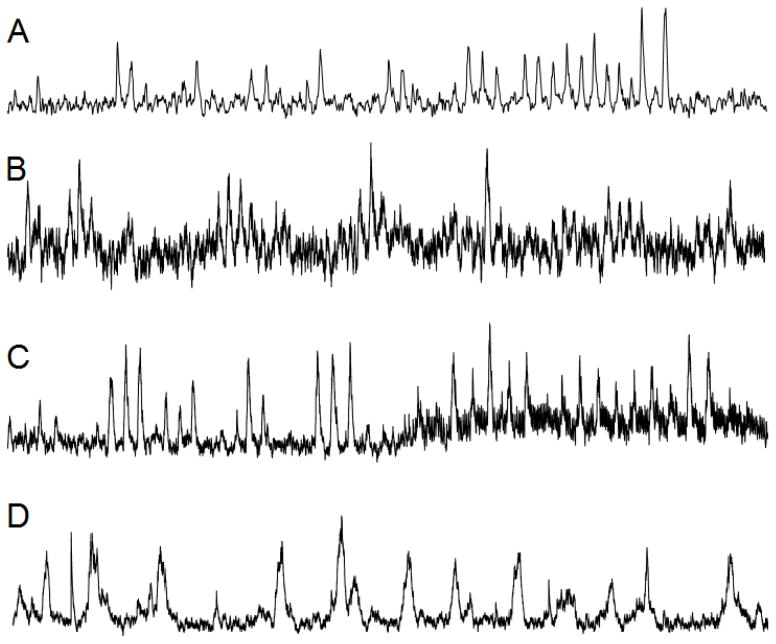
Because both afferent and efferent neurons innervating muscle and skin traverse the same nerve, it is common to receive interference from one or the other while attempting to establish a high quality signal. It is important to be able to differentiate between skin and muscle sympathetic nerve activity and this can be done by assessing the bursting characteristics. Muscle sympathetic nerves burst as described above but skin sympathetics have long, non-pulse synchronous discharges which may appear to be a wondering baseline or lengthy sympathetic bursts (Figure 2D). Skin sympathetic nerves are sensitive to startle reflexes and changes in skin temperature (Hagbarth et al., 1972; Normell & Wallin, 1974). Interference of skin sympathetic nerve activity while recording MSNA will lead to difficulties detecting and quantifying bursting. Hence, it is important to attempt to identify the absence of a startle reflex record before accepting that the MSNA being recorded is valid.
Figure 2. One minute segments of representative neurograms.
A) High quality neurogram
B) Low quality neurogram
C) Neurogram which degrades in quality due to motor unit activation
D) Mixed Muscle and Skin sympathetic nerve recording
It is important to keep in mind that acquiring a high quality nerve signal takes practice, patience, and persistence. The most important part of measuring MSNA is the quality of the nerve signal. All topics discussed above are involved in obtaining a high quality nerve signal and the success of a study using microneurography. Figure 2 shows actual neurograms with high quality (2A) and low quality (2B) signals along with a signal showing the change in signal quality with activation of motor units resulting from muscle tension (2C) and a mixed skin and muscle sympathetic nerve (2D). With a decrease in signal quality, you lose the ability to perform advanced quantification of MSNA which is discussed below. Poor signal quality also reduces the likelihood of visually detecting a burst and will lead to error in quantification.
2.6 Processing the signal
Normally, the raw MSNA signal is amplified, filtered, rectified and integrated either using an analog capacitor resistance systems or digital processing. Typical amplification of the raw signal is between 50,000 and 100,000 times (Delius et al., 1972b; Macefield, 2013). The amplified signal is then band pass filtered between 700Hz and 2000Hz for many investigators, while a few established investigators choose to filter from 400Hz to 3000Hz (Saito et al., 1990). The high frequency filtering threshold is based on the Nyquist rate dictating that a signal be analyzed twice as fast as it is expected to occur and the lower frequency threshold limits the amount of noise interference from outside sources (Nyquist, 2002). The amplified-filtered signal is then rectified and integrated using either a resistance-capacitance circuit (Absolute Design NTA) with a time constant of 100 msec or by digital integration with computer algorithms (ADI NeuroAmp EX). Often, the band-pass filtered signal can confirm the presence of action potentials in any selected bursts and enhances confidence when assessing small bursts.
Acquiring a high quality, clear signal (Figure 2A) makes quantification and analysis less difficult. A clear signal can be characterized by: 1) a steady baseline that does not fluctuate; 2) bursting that can be identified above the baseline noise; and 3) baseline noise amplitude that does not change over the period of the recording. Any of these problems can be avoided by proper placement of the recording electrode and assurance of subject comfort and relaxation.
Several challenges exist in finding and sustaining quality MSNA recording sites even for experienced microneurographers. A microneurographer has to know when to change insertion points of the electrode and when to attempt acquisition in the other arm or leg when a protocol allows it. A microneurographer also needs to be aware that after a while the subject may become fatigued due to the need for the limb to remain perfectly still for a high quality recording. If the subject can no longer maintain the limb relaxed, the quality of nerve will deteriorate and the quantification will be limited. Sometimes, a subject may move during a protocol causing the signal characteristics to change. A change in signal can constitute a small increase or decrease in baseline noise, up to a total loss of signal and the need to reacquire the nerve. In either situation, a new steady state resting period should be measured before using the quantification methods discussed below. Finally, a major concern is to prevent injury and inflammation at the site of interrogation (Eckberg et al., 1989). This is why time for acquiring a microneurographic signal is limited to 60 minutes per session and should not be attempted without proper training.
3 Normalization of Signal
3.1 Baseline
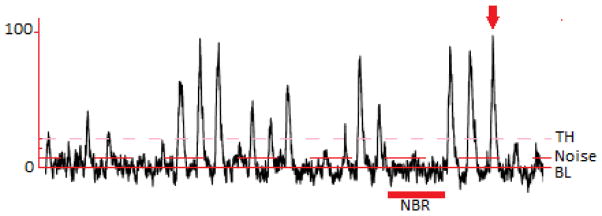
When analyzing an integrated neurogram, identifying the correct baseline is important for quantifying the nerve traffic (Figure 3). If the identification of a baseline is inconsistent, application of the rules for identification of a burst become biased by individual preference. Because the signal is rectified and then integrated, every point on the signal is a positive voltage above zero; but it would be impractical to analyze as such because the position of the neurogram during non-bursting periods just represents background noise. To account for the background noise, it is common practice to find a long non-bursting period and calculate its mean voltage, subsequently resetting the mean non-bursting voltage to 0 arbitrary units (au). Setting the baseline to 0au insures that any quantification will represent a deviation from the background noise. After identifying the baseline, the signal height can be normalized.
Figure 3. Thirty second neurogram with normalization labeling.
Shown is a neurogram with a non-bursting region (NBR) labeled, the mean voltage of which is used to calculate the baseline (BL) and noise. The arrow is pointing at the tallest spontaneous burst which is set to 100 arbitrary units. The threshold (TH) is calculated as 3 times the noise above BL. Only bursting occurring above the threshold should be counted.
3.2 Burst Height
Typically the height of the integrated neurogram is set to arbitrary 100 or 1000 units. The method many investigators use to set this height is by identifying the tallest spontaneous burst, not occurring with any provocation or outside stimulation, and setting it as the “maximum” (Figure 3). Another method of normalizing to a maximum voltage is to take the average peak height of multiple large bursts (usually 3) and use this value as 100 or 1000 units. Through the analysis of bursting associated with premature ventricular contractions (PVC), it is shown that decreased diastolic pressure-induced maximum burst height is consistent within a recording (Grassi et al., 2002; Maslov et al., 2012).
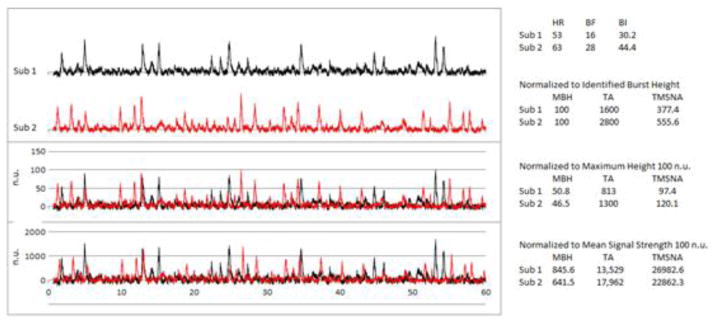
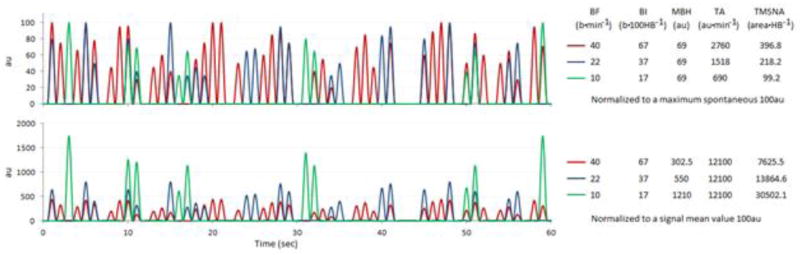
Other investigators calculate the mean strength of the signal and set it to 100au (Figure 5C). The bursts are then identified and the burst heights are normalized to the average area of all detected bursts (Cooke et al., 2004). In the potential case where the neurogram signal is normalized to the raw signal mean and not detected burst means, severe alteration of quantification will occur as shown in Figures 5C and 6. The two methods (maximum vs. mean) potentially yield different conclusions, the explanation of which is discussed below and in the figures.
Figure 5. Comparative neurograms showing overlapping signals after using different forms of normalization.
A) Mean neurogram from two different subjects before normalization. B) Overlapping of the two neurograms after normalization of each signal to the largest spontaneous burst. C) Overlapping of the two neurograms after normalizing to the mean signal strength. Subject 1 (Sub1) is in black and Subject 2 (Sub 2) is in red throughout the diagram. Descriptive quantifications corresponding to the two subjects are to the right. HR, Heart Rate; BF, Burst Frequency (b·min−1); BI, Burst Incidence (b·100HB−1); MBH, Mean Burst Height (nu); TA, Total Activity (nu·min−1); TMSNA, Total MSNA (n area•HB−1).
Figure 6. Comparative simulated tracings of nerve traffic normalized by maximum spontaneous burst and mean signal strength.
The top tracing shows each line having a different burst frequency (BF) but all lines have the same heart rate (HR). The simulated data are normalized to the highest burst given a value of 100au. The bottom tracing shows the same simulated data normalized to a mean signal strength of 100au. A conclusion that would be drawn from the top signals is that in a subject in which BF or BI is elevated, so are TA and TMSNA in a directly proportional manner. An alternative conclusion would be drawn from the bottom tracings in which BF and BI are the same as the top tracing, but TA shows all subjects being equal and TMSNA would conclude that the subject with the lowest bursting frequency has the highest activity. Simulated data such as these are helpful for illustrating the point that the method of signal normalization is extremely important for comparing data between subjects.
3.3 Digital Filtering
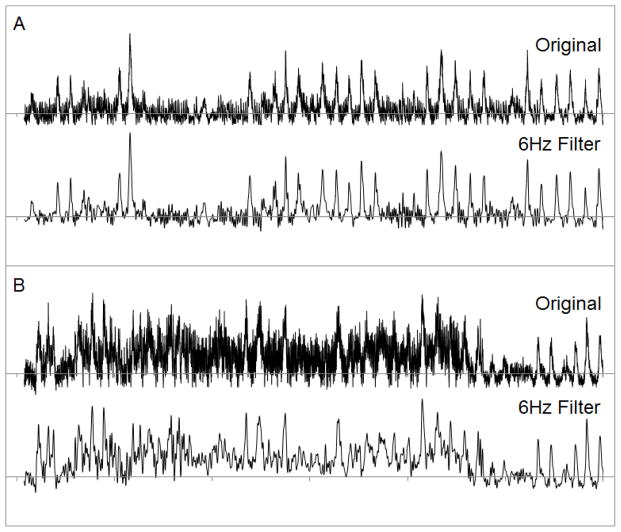
Typically, MSNA expresses a very poor signal-to-noise aspect. Digital filtering of the integrated neurogram can be used to account for some of the unwanted noise in the signal. This is an automatic component of the ADI NeuroAmp EX which constantly samples and filters ambient noise. A common practice used in processing electroencephalogram (EEG) signals includes application of low pass digital filters (Niedermeyer & Lopes da Silva, 2005), which is sometimes used in peripheral nerve analysis (Macefield, 2013). Bursting of postganglionic MSNA occurs pulse-synchronously; therefore, bursting can only occur as fast as the subject’s maximum heart rate (HR). If it is assumed that the maximum HR of a human is 220 beats·min−1, then it can be assumed that the Nyquist rate is about 6Hz (actual 7.33Hz @ 220 beats·min−1); therefore a lowpass filter can be set at 6Hz. This effectively reduces noise within the integrated neurogram usually associated with mild motor unit activity and/or 50/60Hz A/C cycling from a power source. In the case of moderate to severe ambient noise or motor unit activity, it would be inappropriate to use a digital filter as the neurogram would be severely altered (Figure 4B).
Figure 4. The use of a digital filter to eliminate excess noise.
The top tracing in each panel is unfiltered, the bottom tracing has had a 6Hz digital low pass filter applied. Notice the difference between the peaks and the shape of the signal after applying the filter. In panel A the noise coming from the motor unit activity is low compared to the sympathetic bursting, so a digital filter can be applied without affecting the quality of the signal. In panel B, the motor unit noise is almost equal to the sympathetic signal so the filter alters the signal characteristics greatly. When dealing with a signal that has this much noise, attempts should be made at relaxing the subject or changing the electrode position. Notice that the end of signal B is a high quality sympathetic nerve signal once the subject is relaxed. This example is to show that digital filtering cannot fix all noise; it is not an example of a way to bypass acquiring a high quality nerve signal.
4 Quantification of the Integrated Neurogram
4.1 Identification of Bursts
Identification of nerve bursts is a subjective measurement, which can be made less subjective by adhering to a strict set of standards. Most bursts of MSNA are easily identifiable, but a good acquisition and high quality signal (Figure 2A) can make this process much easier. First, the signal’s noise height must be identified. This is the difference between the baseline zero and the upper limit of the signal during a non-bursting region (Figure 3). This difference should be multiplied by three and the product is the threshold for identifying a burst in the neurogram. This is the “3:1 Signal to Noise Ratio” identified in most publications reporting the quantification of MSNA (Macefield, 2013). The use of 2:1 signal to noise ratio and also scanning for the presence of action potentials in the filtered raw neurogram are used by some laboratories, but these practices potentially lead to as much as a 30% difference in results. Data do not exist for the potential impact of differential identification of MSNA bursts.
Muscle sympathetic nerve activity is conducted through the body via small C-fibers which have an overall conduction velocity between the brainstem and leg recording site of about 1 m·sec−1 (Fagius & Wallin, 1980). Knowledge of conduction through nerves allows the accurate estimation of a time delay between a contraction of the heart and a burst of nerve traffic. The point of burst initiation corresponds with the diastolic period of the cardiac cycle; therefore, the time between the R-wave on an ECG signal and the nerve burst should correspond with the distance from the brainstem to the site of measurement. This signal time is not a direct calculation of distance at 1m·sec−1 because there is some delay caused by integration of the baroreflex through the central nervous system synapses, but the time is proportional to distance. A typical nerve burst occurs between 1 and 1.4 seconds post R-wave for a peroneal nerve recording and between 0.8 and 1.1 seconds for a typical radial or median nerve recording. The times are variable even within a subject based on the magnitude of the activation which is theorized to be due to recruitment of larger nerve fibers and/or synaptic delay variations (Kienbaum et al., 2001).
4.2 Common Quantification Methods and Considerations
4.2.1 Burst Occurrence Quantification
Quantification of the integrated neurogram is a topic of debate, with varying views on the utility and accuracy of different methods. Originally, MSNA was quantified as the number of integrated bursts per minute (burst frequency; BF). Some investigators questioned the BF measure because in comparing subjects with different resting heart rates, the higher the HR the higher the BF. Hence, a compromise of measuring bursts per 100 heart beats (burst incidence; BI) was used as a means of normalizing the measure between subjects with different HRs. Either of these quantification methods can be useful for making conclusions within subjects across a protocol where some treatment is given, especially in the absence of a HR change; but when the HR does change, certain cases will lead to divergent conclusions. For example, an increase in HR without a change in burst incidence, will lead to the conclusion that BF increased but BI did not; whereas, an increase in HR without a change in burst frequency will lead to the conclusion that BI decreased (Table 1). The usual case will be an increase in HR and an increase in burst frequency, but the proportion of the respective increases will influence the conclusion. Passive heat stress is a great example of the ratios of HR and MSNA increasing at different rates, which could lead to conservative conclusions depending on how you report the variables and develop your conclusions (Gagnon et al., 2015). If a treatment is given and resting HR is increased but burst frequency is not increased to the same degree, burst incidence will decrease. Therefore, conclusions will be negative concerning the treatment even if there was more bursting. Investigators typically report both measures of burst occurrence and allow the reader to develop conclusions from either measure. This is reasonable when there is no change in HR over a protocol, but can cause conclusions to potentially be conservative if using BI. BI should only be used when HR remains steady between conditions, therefore, BF will give the same answer.
Table 1.
Series of scenarios highlighting discrepancies in physiological conclusions based upon the burst occurrence quantification method and response to stimuli.
In the baseline, subject 1 and 2 have different HRs, and if quantifying SNA based on BF the 2 subjects are equal, but based on BI, subject 1 has higher SNA.
In Trial 1, each subject has an increase in HR of 10 beats min-1 but no change in BF. If drawing conclusions from BI, subject 1 had a decrease in SNA more than double the decrease of subject 2.
In Trial 2, there is no change in HR, but an equal increase in BF for both subjects. A conclusion from BI would indicate that subject 1 had a much greater increase in SNA.
In Trial 3, there is an increase in both HR and BF equally in both subjects. A conclusion from BI would indicate that subject 2 had the greater increase in SNA.
Finally in Trial 4, where both subjects are exercised up to the same HR, and if BF were the same in both subjects, a conclusion from BI would indicate that subject 2 did not change SNA, while subject 1 had a decrease. Interestingly, assuming that mean burst height is equal for both subjects, total activity would be the same in both subjects in each trial.
| HR | BF | BI | Max Change in BF to Ceiling | Max Change in BI to Ceiling | ΔHR | ΔBF | ΔBI | %Δ HR | %Δ BF | %Δ BI | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Subject 1 | 40 | 30 | 75 | 10 | 25 | Changes from Baseline | |||||
| Subject 2 | 60 | 30 | 50 | 30 | 50 | |||||||
| Trial 1 | Subject 1 | 50 | 30 | 60 | 20 | 40 | 10 | 0 | −15 | 25 | 0 | −20 |
| Subject 2 | 70 | 30 | 43 | 40 | 57 | 10 | 0 | −7 | 17 | 0 | −14 | |
| Trial 2 | Subject 1 | 40 | 40 | 100 | 0 | 0 | 0 | 10 | 25 | 0 | 33 | 33 |
| Subject 2 | 60 | 40 | 67 | 20 | 33 | 0 | 10 | 17 | 0 | 33 | 33 | |
| Trial 3 | Subject 1 | 50 | 40 | 80 | 10 | 20 | 10 | 10 | 5 | 25 | 33 | 7 |
| Subject 2 | 70 | 40 | 57 | 30 | 43 | 10 | 10 | 7 | 17 | 33 | 14 | |
| Trial 4 | Subject 1 | 100 | 50 | 50 | 50 | 50 | 60 | 20 | −25 | 150 | 67 | −33 |
| Subject 2 | 100 | 50 | 50 | 50 | 50 | 40 | 20 | 0 | 67 | 67 | 0 | |
4.2.2 Burst Height Quantification
Quantification of the burst height can be assessed using different methods. When the signal height is normalized using one of the methods in section 3, quantification of height becomes relatively easy. First, if the signal quality is excellent and the baseline is stable, the largest spontaneous burst or mean peak height of multiple tall bursts can be assigned a value (typically 1, 10, 100 or 1000) and the rest of the signal can be scaled based on the assigned value. A second quantification method is to select the start and end point of every burst and measure the local height of each and subsequently scale each of the bursts based on absolute height to the absolute height of the tallest burst. This method works when the baseline is not stable, but bursting characteristics remain similar throughout a recording and there is no sign of electrode displacement. However, this approach may limit the ability to differentiate actual changes in burst height because an unstable signal indicates fluctuating background noise. The final quantification method is to assign the mean value of a non-stimulatory section of the integrated signal an arbitrary value of 100 or 1000 units. This method is dependent on the number of bursts within the normalization period, as a signal with more burst occurrences spends less time at zero, thus the mean signal value will be higher but the maximum height will be lower (Figure 6). Once the signal is normalized and bursts detected, the simplest quantification is mean burst height (MBH) where the sum of the height of every burst is divided by the number of bursts in the calculation. MBH is used to conclude whether a treatment changed the strength of the nerve traffic independent of a change in frequency. Mean burst height becomes overly conservative when mean normalization is used because the “maximum” burst height is not consistent between subjects.
4.2.3 Total Signal Quantification
The integrated neurogram can further be quantified by the product of various combinations of burst occurrences and heights. First, the product of BF and MBH is commonly referred to as total activity (TA)(Sanders et al., 1988). This provides a quantification of MSNA over time which is independent of total active burst time, but does account for the height of each burst. A second quantification method calculates total burst area or burst size (Σ (burst height*burst time/2)) (Maslov et al., 2012). This quantification accounts for total time that the nerve is active in addition to accounting for the height of the bursts. When summed over time, total burst area may give the most complete representation of MSNA for that time period. The last of the common total signal quantifications is the product of total burst area/HR within a measurement period, commonly referred to as total MSNA (TMSNA), which provides a quantification of MSNA relative to HR, while accounting for the height and duration of each burst. This method generalizes MSNA to the average amount of nerve activity for every heartbeat. However, when using this method of quantification, be aware that the higher the HR the lower the calculated burst activity.
4.2.4 Considerations for Signal Quantification
There is no absolute agreed-upon quantification method for assessing MSNA, but some methods are preferred when dealing with certain signal characteristics, HRs, and/or whether trying to detect a response to stimuli. When the baseline of a signal is fluctuating the only quantification methods that should be used are counts of occurrence (BF or BI). The choice between using BF or BI is dependent on what is being tested and how different are the steady-state HRs (most groups report both). Grouping individuals with significantly different HRs can add inherent variability to the averaged data making it difficult to observe effects due to a stimulus. A person with a HR of 40 beats·min−1 and a BI of 75 bursts·100hb−1 has a BF of 30 bursts·min−1. If you take an individual with the same 30 bursts·min−1 but a steady-state HR of 60 beats·min−1, their BI is 50 bursts·100hb−1. This is further complicated when quantifying responses to sympathoexcitatory stimuli, because the maximum value (100bursts·100hb−1; ceiling effect) in BI that can occur for the subject with the steady state HR of 40 beats·min−1 is 25 bursts·100hb−1, or 33%, whereas the other individual with a steady-state HR of 60 beats·min−1can increase their BI by 50 bursts·100hb−1, or 100%. Table 1 contains various scenarios where divergent conclusions would be made based on differences in quantification methods. It has been demonstrated that the longitudinal repeatability for BI is not statistically ideal due to the reliance on both burst occurrence and HR which adds a variable amount of error into the measurement and, therefore, needs to be taken into consideration when making longitudinal comparisons of BI or TMSNA (Kimmerly et al., 2004).
When the signal quality is strong and there are no changes in baseline or signal characteristics over the recording period, accounting for the height in the quantification increases the utility of the measurement. Whereas BF and BI count every burst equally, TA, TMSNA, and Total Burst Area (Burst Size) take into account the height of the burst so that each burst contributes to the total based on its relative size. The ability to accurately quantify height is dependent on the method of signal height normalization. If normalized by a minimum-maximum method in which the maximum spontaneous or evoked burst is given a value and the baseline given a value of 0, then multiple measurements in different individuals will adhere to the same relative scale (Figure 5B and 6). While it is debatable as to whether it is appropriate to use the relative scale to compare between individuals, it remains a common practice. However, it needs to be understood that when normalizing to the mean signal strength, an individual with a low burst occurrence at resting baseline has the potential to increase their nerve activity exponentially compared to someone that has a greater burst occurrence because this method assumes all baseline activity to be equal in all individuals. However, when normalizing to the mean, measures of burst occurrence will calculate to the same relative change in response to a given sympathoexcitatory stimuli within an individual as calculated by normalizing to the maximum height because the measure disregards burst height.
5 Standardization of Nerve Recordings
5.1 Sympathetic Burst Signal Types
During non-stimulatory periods, vascular resistance (conductance) is controlled by cyclic periods of bursting which are related to diastolic blood pressure via baroreflexes (Hagbarth & Vallbo, 1968; Delius et al., 1972b; Sundlof & Wallin, 1977; Vallbo et al., 1979; Fairfax et al., 2013). These are essentially “maintenance bursts,” which could be characterized as the normal spontaneous bursting that regulate and maintain blood pressure in the absence of abnormal physiological stimuli; but when the need arises, larger or more frequent bursts occur. This phenomenon is apparent in response to hypoxia where the burst heights increase above the “maximum resting spontaneous burst” (Hagbarth & Vallbo, 1968; Delius et al., 1972b; Cutler et al., 2004a; Vallbo et al., 2004). Maintenance bursts appear to be more related to minor fluctuations in blood pressure (spontaneous baroreflex), but include maximum spontaneous bursts representative of the maximal activation of all of the baroresponsive nerve fibers within a recording field. Evidence for this exists during an ectopic heart beat which fails to cause a pressure generating contraction and the corresponding long sympathetic burst has a burst height limit no different than the maximum spontaneous burst, but continuing for a substantially longer time (Grassi et al., 2002; Maslov et al., 2012). Therefore, bursting should be categorized as either maintenance bursting (spontaneous) or stimulus reflex bursting (investigator evoked). Differentiating between the two types of bursts may be pertinent to future research in which analysis of different stimuli may be able to be sub-typed based on frequency, height, and recruitment of single fibers within a bundle.
5.2 Calibration of the Signal
The variability of MSNA among individuals and also within individuals in repeated measurements remains a challenge when trying to make meaningful conclusions (Vallbo et al., 2004; Macefield, 2013). Some of this variability may come from differences in electrode placement within an individual or by not being able to be consistent with the number of nerve fibers detected in a recording between individuals; however, these concerns do not appear to affect conclusions in repeated studies (Kimmerly et al., 2004). In addition, the degree of variability of the MSNA burst size likely relates to the number and size of fibers within the recording field (Tompkins et al., 2013). When the magnitude or nature of the stimulus results in recruitment of sympathetic efferent nerve fibers within the recording field, silent fibers may also become active (Salmanpour et al., 2011). If there is a differential activation of the sympathetic nervous system based on the nature of the stimulus (i.e., baro-, chemo-, or metabo-responsive)(Delius et al., 1972a; Delius et al., 1972b; Wallin et al., 1975; Vallbo et al., 1979), then it would make sense to calibrate the signal to the type of stimulus which will occur during the protocol. A hypoxia protocol could be normalized to the reflex bursting of a standard hypoxic stimulus (Cutler et al., 2004a), a blood pressure protocol should be normalized to the reflex bursting of a baro-stimulus (Wallin & Eckberg, 1982; Eckberg et al., 1988) and a metabolic challenge should be normalized to the reflex bursting of a standard metabolic stimulus (Victor et al., 1988). Calibration of this nature could be crucial in determining the degree of change for a stimulus response burst above maintenance bursting. This may present a challenge when testing the interaction between multiple stimuli. For example, testing baroreflex control following hypoxic training stimuli is a challenge because long term potentiation of SNA following hypoxia training (Cutler et al., 2004a; Cutler et al., 2004b; Leuenberger et al., 2005) may increase baroreflex sensitivity. In the case of hypoxia training, normalizing to a standard sympathoexcitatory stimulus prior to the hypoxia training may be warranted. The investigator would also have to consider the possibility that the testing protocol would affect the signal to a greater degree than the hypoxia training alone. To solve this problem, one should normalize to the standard sympathoexcitatory stimulus prior to hypoxia training, then check the effect of hypoxia training on the standard sympathoexcitatory stimulus, followed by the outcome measurement protocol and a final check of the standard sympathoexcitatory stimulus at the end.
5.3 Body Position
Posture becomes important in the acquisition and analysis of nerve signals as shown in various studies which load and unload the cardiopulmonary baroreceptors (Ray et al., 1993; Mano, 2001; O’Leary et al., 2003; Charkoudian et al., 2004; Fu et al., 2006; Kuipers et al., 2008; Kamiya et al., 2009). Comparisons of MSNA responses from studies in which the body posture was different and not the focus of the study need to be made with caution. However, when the experimental design of a study involves changes in posture that increase or decrease the central blood volume, the investigator needs to account for concurrent deceases and increases, respectively, in baseline and response sensitivity of MSNA.
6 Conclusion
In summary, we have attempted to provide a comprehensive collation of means of standardizing the methods and quantification of MSNA measurements required to enable statistical analyses between subjects of different ages and gender and across and between experimental conditions. It is important to remember that relationships between MSNA and vascular resistance are age and sex dependent (Hart & Charkoudian, 2014) so interpretation of MSNA recordings must be made beyond the basic quantification. For many experienced investigators the normalization procedures we describe are a reiteration of what they have already documented but the standardization of nomenclature and procedures is needed. The growth in the number of laboratories that use MSNA measurements for quantifying sympathetic nerve activity has resulted in a number of potentially inaccurate conclusions because of the lack of the standardization of MSNA methods and quantification.
Normalizing by assigning a maximum burst height to a non-stimulus, baseline, steady-state section of any protocol appears to be the appropriate method when attempting to compare MSNA between subjects and studies. We suggest that caution be used when making conclusions about MSNA relative to HR as the transduction time of a nerve burst into a change in vascular resistance is not HR dependent but time dependant. The need to understand the cause and effect of burst size and burst frequency was highlighted in order to allow comparison between individuals. Development of a consensus regarding a standard method of calibrating a response would improve the ability to compare data amongst studies. Even so, the question would still remain whether the standardized stimulus is causing the same effect in all individuals and whether the effect is a maximal effect in all individuals. More definitive classifications of nerve activity and the use of single unit recording and analysis could allow for concepts once thought to be impossible or inappropriate (such as comparing TA or TMSNA between subjects), to be realized and implemented in future analysis and conclusions. The ability to compare between subjects and within populations could make the measurement of MSNA a clinically relevant measure, especially when it is known that treatment methods for disease (i.e. renal denervation for hypertension) may work better when a subset of the disease is caused by increased sympathetic nerve activity (Esler et al., 2010; Esler et al., 2012; Esler et al., 2014).
Highlights.
We provide a step by step instruction for acquisition of MSNA
We provide a collation of methods for quantification of MSNA
We address concerns with conclusions drawn from different MSNA quantifications
We discuss the need for standardization of MSNA practices among researchers
Acknowledgments
The authors thank all the subjects for the time and cooperation in the studies involved in collecting the original data. The authors also thank Gilbert Moralez, Kanokwan Bunsawat, and Wendy Eubank for assistance in collecting the original nerve recordings. The studies from which the data were reported were supported in part by funds from the National Heart, Lung and Blood Institute Grant HL-045547 to PBR; Texas ACSM Student Research Development Award 2012 to DWW; the Cardiovascular Research Institute and the Department of Integrative Physiology at UNTHSC in Fort Worth, TX, and the Department of Kinesiology & Nutrition at UIC in Chicago, IL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Charkoudian N, Martin EA, Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Influence of increased central venous pressure on baroreflex control of sympathetic activity in humans. Am J Physiol-Heart C. 2004;287:H1658–H1662. doi: 10.1152/ajpheart.00265.2004. [DOI] [PubMed] [Google Scholar]
- Cooke WH, Carter JR, Kuusela TA. Human cerebrovascular and autonomic rhythms during vestibular activation. Am J Physiol Regul Integr Comp Physiol. 2004;286:R838–843. doi: 10.1152/ajpregu.00562.2003. [DOI] [PubMed] [Google Scholar]
- Curry TB, Charkoudian N. The use of real-time ultrasound in microneurography. Auton Neurosci. 2011;162:89–93. doi: 10.1016/j.autneu.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler MJ, Swift NM, Keller DM, Wasmund WL, Burk JR, Smith ML. Periods of intermittent hypoxic apnea can alter chemoreflex control of sympathetic nerve activity in humans. Am J Physiol Heart Circ Physiol. 2004a;287:H2054–2060. doi: 10.1152/ajpheart.00377.2004. [DOI] [PubMed] [Google Scholar]
- Cutler MJ, Swift NM, Keller DM, Wasmund WL, Smith ML. Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxic apnea. J Appl Physiol (1985) 2004b;96:754–761. doi: 10.1152/japplphysiol.00506.2003. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol Scand. 1972a;84:82–94. doi: 10.1111/j.1748-1716.1972.tb05157.x. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Wallin BG, Hongell A. General Characteristics of Sympathetic Activity in Human Muscle Nerves. Acta Physiologica Scandinavica. 1972b;84:65. doi: 10.1111/j.1748-1716.1972.tb05158.x. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Rea RF, Andersson OK, Hedner T, Pernow J, Lundberg JM, Wallin BG. Baroreflex modulation of sympathetic activity and sympathetic neurotransmitters in humans. Acta Physiol Scand. 1988;133:221–231. doi: 10.1111/j.1748-1716.1988.tb08401.x. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Wallin BG, Fagius J, Lundberg L, Torebjork HE. Prospective study of symptoms after human microneurography. Acta Physiol Scand. 1989;137:567–569. doi: 10.1111/j.1748-1716.1989.tb08804.x. [DOI] [PubMed] [Google Scholar]
- Esler MD, Bohm M, Sievert H, Rump CL, Schmieder RE, Krum H, Mahfoud F, Schlaich MP. Catheter-based renal denervation for treatment of patients with treatment-resistant hypertension: 36 month results from the SYMPLICITY HTN-2 randomized clinical trial. Eur Heart J. 2014;35:1752–1759. doi: 10.1093/eurheartj/ehu209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler MD, Krum H, Schlaich M, Schmieder RE, Bohm M, Sobotka PA. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126:2976–2982. doi: 10.1161/CIRCULATIONAHA.112.130880. [DOI] [PubMed] [Google Scholar]
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- Fagius J, Wallin BG. Sympathetic Reflex Latencies and Conduction Velocities in Normal Man. J Neurol Sci. 1980;47:433–448. doi: 10.1016/0022-510x(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Fairfax ST, Padilla J, Vianna LC, Davis MJ, Fadel PJ. Spontaneous bursts of muscle sympathetic nerve activity decrease leg vascular conductance in resting humans. Am J Physiol Heart Circ Physiol. 2013;304:H759–766. doi: 10.1152/ajpheart.00842.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Shook RP, Okazaki K, Hastings JL, Shibata S, Conner CL, Palmer MD, Levine BD. Vasomotor sympathetic neural control is maintained during sustained upright posture in humans. J Physiol-London. 2006;577:679–687. doi: 10.1113/jphysiol.2006.118158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon D, Schlader ZJ, Crandall CG. Sympathetic activity during passive heat stress in healthy aged humans. J Physiol. 2015 doi: 10.1113/JP270162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Bertinieri G, Stella ML, Turri C, Mancia G. Sympathetic response to ventricular extrasystolic beats in hypertension and heart failure. Hypertension. 2002;39:886–891. doi: 10.1161/01.hyp.0000013265.48954.a5. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Hallin RG, Hongell A, Torebjork HE, Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand. 1972;84:164–176. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Vallbo AB. Pulse and respiratory grouping of sympathetic impulses in human muscle-nerves. Acta Physiol Scand. 1968;74:96–108. doi: 10.1111/j.1748-1716.1968.tb04218.x. [DOI] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N. Sympathetic neural regulation of blood pressure: influences of sex and aging. Physiology (Bethesda) 2014;29:8–15. doi: 10.1152/physiol.00031.2013. [DOI] [PubMed] [Google Scholar]
- Jordan D. Central nervous control of autonomic function. Harwood Academic; Amsterdam; United Kingdom: 1997. [Google Scholar]
- Kamiya A, Kawada T, Shimizu S, Iwase S, Sugimachi M, Mano T. Slow head-up tilt causes lower activation of muscle sympathetic nerve activity: loading speed dependence of orthostatic sympathetic activation in humans. Am J Physiol-Heart C. 2009;297:H53–H58. doi: 10.1152/ajpheart.00260.2009. [DOI] [PubMed] [Google Scholar]
- Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol. 2001;531:861–869. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerly DS, O’Leary DD, Shoemaker JK. Test-retest repeatability of muscle sympathetic nerve activity: influence of data analysis and head-up tilt. Auton Neurosci. 2004;114:61–71. doi: 10.1016/j.autneu.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Kuipers NT, Sauder CL, Carter JR, Ray CA. Neurovascular responses to mental stress in the supine and upright postures. J Appl Physiol. 2008;104:1129–1136. doi: 10.1152/japplphysiol.01285.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuenberger UA, Brubaker D, Quraishi S, Hogeman CS, Imadojemu VA, Gray KS. Effects of intermittent hypoxia on sympathetic activity and blood pressure in humans. Auton Neurosci. 2005;121:87–93. doi: 10.1016/j.autneu.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Macefield VG. Sympathetic microneurography. Handb Clin Neurol. 2013;117:353–364. doi: 10.1016/B978-0-444-53491-0.00028-6. [DOI] [PubMed] [Google Scholar]
- Mano T. Muscle sympathetic nerve activity in blood pressure control against gravitational stress. J Cardiovasc Pharm. 2001;38:S7–S11. doi: 10.1097/00005344-200110001-00003. [DOI] [PubMed] [Google Scholar]
- Maslov PZ, Breskovic T, Brewer DN, Shoemaker JK, Dujic Z. Recruitment pattern of sympathetic muscle neurons during premature ventricular contractions in heart failure patients and controls. Am J Physiol Regul Integr Comp Physiol. 2012;303:R1157–1164. doi: 10.1152/ajpregu.00323.2012. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E, Lopes da Silva FH. Electroencephalography: basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins; Philadelphia: 2005. [Google Scholar]
- Normell LA, Wallin BG. Sympathetic skin nerve activity and skin temperature changes in man. Acta Physiol Scand. 1974;91:417–426. doi: 10.1111/j.1748-1716.1974.tb05696.x. [DOI] [PubMed] [Google Scholar]
- Nyquist H. Certain topics in telegraph transmission theory (Reprinted from Transactions of the A. I. E. E., February, pg 617–644, 1928) P Ieee. 2002;90:280–305. [Google Scholar]
- O’Leary DD, Kimmerly DS, Cechetto AD, Shoemaker JK. Differential effect of head-up tilt on cardiovagal and sympathetic baroreflex sensitivity in humans. Experimental Physiology. 2003;88:769–774. doi: 10.1113/eph8802632. [DOI] [PubMed] [Google Scholar]
- Ray CA, Rea RF, Clary MP, Mark AL. Muscle Sympathetic-Nerve Responses to Dynamic One-Legged Exercise - Effect of Body Posture. American Journal of Physiology. 1993;264:H1–H7. doi: 10.1152/ajpheart.1993.264.1.H1. [DOI] [PubMed] [Google Scholar]
- Saito M, Mano T, Iwase S. Changes in Muscle Sympathetic-Nerve Activity and Calf Blood-Flow during Static Handgrip Exercise. Eur J Appl Physiol O. 1990;60:277–281. doi: 10.1007/BF00379396. [DOI] [PubMed] [Google Scholar]
- Salmanpour A, Brown LJ, Steinback CD, Usselman CW, Goswami R, Shoemaker JK. Relationship between size and latency of action potentials in human muscle sympathetic nerve activity. J Neurophysiol. 2011;105:2830–2842. doi: 10.1152/jn.00814.2010. [DOI] [PubMed] [Google Scholar]
- Sanders JS, Ferguson DW, Mark AL. Arterial baroreflex control of sympathetic nerve activity during elevation of blood pressure in normal man: dominance of aortic baroreflexes. Circulation. 1988;77:279–288. doi: 10.1161/01.cir.77.2.279. [DOI] [PubMed] [Google Scholar]
- Steinback CD, Salmanpour A, Breskovic T, Dujic Z, Shoemaker JK. Sympathetic neural activation: an ordered affair. J Physiol. 2010;588:4825–4836. doi: 10.1113/jphysiol.2010.195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins RP, Melling CW, Wilson TD, Bates BD, Shoemaker JK. Arrangement of sympathetic fibers within the human common peroneal nerve: implications for microneurography. J Appl Physiol (1985) 2013;115:1553–1561. doi: 10.1152/japplphysiol.00273.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, Proprioceptive, and Sympathetic Activity in Human Peripheral-Nerves. Physiological Reviews. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Wallin BG. Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. J Appl Physiol (1985) 2004;96:1262–1269. doi: 10.1152/japplphysiol.00470.2003. [DOI] [PubMed] [Google Scholar]
- Victor RG, Bertocci LA, Pryor SL, Nunnally RL. Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest. 1988;82:1301–1305. doi: 10.1172/JCI113730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Eckberg DL. Sympathetic transients caused by abrupt alterations of carotid baroreceptor activity in humans. Am J Physiol. 1982;242:H185–190. doi: 10.1152/ajpheart.1982.242.2.H185. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Sundlof G, Delius W. The effect of carotid sinus nerve stimulation on muscle and skin nerve sympathetic activity in man. Pflugers Arch. 1975;358:101–110. doi: 10.1007/BF00583921. [DOI] [PubMed] [Google Scholar]