Abstract
Drug overdose now exceeds car accidents as the leading cause of accidental death in the U.S. Of those drug overdoses, a large percentage of the deaths are due to heroin and/or pharmaceutical overdose, specifically misuse of prescription opioid analgesics. It is imperative, then, that we understand the mechanisms that lead to opioid abuse and addiction. The rewarding actions of opioids are mediated largely by the mu opioid receptor (MOR), and signaling by this receptor is modulated by various interacting proteins. The neurotransmitter dopamine also contributes to opioid reward, and opioid addiction has been linked to reduced expression of dopamine D2 receptors (D2R) in brain. That said, it is not known if alterations in the expression of these proteins relates to drug exposure and/or to the “addiction-like” behavior exhibited for drug. Here, we held total drug self-administration constant across acquisition and showed that reduced expression of the D2R and the MOR interacting protein, Wntless, in the medial prefrontal cortex was associated with greater “addiction-like” behavior for heroin, in general, and with a greater willingness to work for drug, in particular. In contrast, reduced expression of the D2R in the nucleus accumbens and hippocampus was correlated with greater seeking during signaled non-availability of drug. Taken together, these data link reduced expression of both the D2R and Wntless to the explicit motivation for drug, rather than to differences in total drug intake, per se.
Keywords: opiate, heroin, Wntless, D2 receptor, motivation
One of the primary goals of drug abuse research is to elucidate the basic mechanisms involved in the development of addiction. Previous research has examined the neurobiological effects of opioid use, identifying changes in brain physiology and morphology that occur with exposure to heroin and other opioid drugs (Angelucci et al., 2007; Guitart, Beitner-Johnson, Marby, Kosten, & Nestler, 1992; Upadhyay et al., 2010). It remains unclear, however, which drug-induced modifications underlie the development of opioid addiction. Opioids activate the reward pathway by binding to the mu-opioid receptor (MOR). The MOR, a G protein-coupled receptor (GPCR), is part of a signaling complex in which protein-protein interactions regulate signal transduction following opioid exposure (Alfaras-Melainis, Gomes, Rozenfeld, Zachariou, & Devi, 2009; Petko et al., 2013; Rodriguez-Munoz, Bermudez, Sanchez-Blazquez, & Garzon, 2007). It is our hypothesis that identifying and characterizing novel MOR interacting proteins may help to elucidate the biological mechanisms involved in the development of opioid addiction and relapse.
Addiction, however, is defined not simply by the taking of drugs, but by compulsive use despite its inherent negative consequences (American Psychological American Psychiatric Association, 2013). The progression from recreational use to harmful abuse occurs in about 15–17% of human drug takers (Anthony & Petronis, 1995; Deroche-Gamonet, Belin, & Piazza, 2004), though the effect reportedly can be even higher for heroin self-administration (SAMHSA, 2012). Like other addictions, opioid addiction also has been linked to hypofunction of the dopaminergic system, specifically to low D2 dopamine receptor (D2R) expression in rats and man (Hou et al., 2012; Zhou, Leri, Cummins, & Kreek, 2015). That said, it has been difficult to determine whether changes in the expression of D2R, for example, or the MOR interacting proteins, relate to “addiction-like” behaviors (e.g., increased seeking and working for drug) or simply to mere drug exposure. Moreover, the relationship between the expression of D2R and MOR interacting proteins in addiction has not yet been explored.
Here, we examine “addiction-like” behavior for heroin using a rodent model that allows for ample experience, but holds drug exposure relatively equal across groups. In so doing, this paradigm provided a platform to examine potential molecular mechanisms underlying the transition to heroin addiction on a pharmacologically level playing field where subjects exhibited different degrees of “addiction-like” behaviors, despite having “consumed” the same amount of drug overall. Particular attention was paid to protein expression in the hippocampus (HPC), nucleus accumbens (NAc), and medial prefrontal cortex (mPFC). Electrophysiological and behavioral evidence suggests that the HPC plays a role in context learning and in the formation of drug-related memories (Floresco, Blaha, Yang, & Phillips, 2001; Robbins, Ersche, & Everitt, 2008). The NAc is important as a site for the initial (Breiter et al., 1997; Grigson & Hajnal, 2007) and longer term (Wheeler et al., 2011; Wheeler et al., 2008) reinforcing effects of drug and blockade of MORs in the NAc attenuates approach to morphine-paired cues (Bals-Kubik, Ableitner, Herz, & Shippenberg, 1993; Stinus, Le Moal, & Koob, 1990). Finally, the mPFC is important for decision making behavior (Schoenbaum & Shaham, 2008) and expression of drug-seeking (Chen et al., 2013; Kuntz, Patel, Grigson, Freeman, & Vrana, 2008). Thus, a subset of the subjects from the behavioral study was used to test whether “addiction-like” behavior for heroin was correlated with the expression of several MOR interacting proteins, as well as D2R, in the HPC, NAc, and the mPFC.
Methods
Subjects
All of the animals that provided data for this manuscript were treated in compliance with the ethical standards set forth by the APA and the Institutional Animal Care and Use Committee of The Pennsylvania State University. The subjects were forty-eight naïve male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), 90 days of age at the start of testing. This study was conducted in 2 replications (n=24 per experiment). Five subjects were eliminated during the course of the study due to lost catheter patency or unexpected death. Behavioral data from the forty-three rats that completed the study in full are reported. Rats were housed individually in hanging wire mesh cages in a colony room with automatically controlled temperature, humidity, and ventilation. Subjects were allowed ad lib access to a nutritionally complete commercial laboratory rodent chow (Laboratory Rodent Diet 5001, PMI Feeds, Richmond, IN) and water, except where noted otherwise. The rats were maintained on a 12 hour light-dark cycle.
Materials and Procedure
Apparatus
Each rat was trained in one of 12 identical operant chambers (MED Associates, St. Albans, VT), as described previously (Puhl, Cason, Wojnicki, Corwin, & Grigson, 2011; Twining, Bolan, & Grigson, 2009). Each chamber was equipped with two retractable sipper spouts that entered through 1.3 cm diameter holes, spaced 16.4 cm apart (center to center). A stimulus light was located 6.0 cm above each hole. Each chamber also was equipped with a houselight (25 W), a tone generator (Sonalert Time Generator, 2900 Hz, Mallory, Indianapolis, IN), and a speaker for white noise (75 dB). Heroin reinforcement was controlled by a lickometer circuit that monitored empty spout licking to operate a syringe pump (Model A, Razel Scientific Instruments, Stamford, CT). A coupling assembly attached the syringe pump to the catheter assembly on the back of each rat. The assembly consisted of Tygon tubing threaded through the center of a metal spacer attached to a metal spring, which were designed to protect the tubing from rat interference. The tubing was attached to a counterbalanced swivel assembly (Instech, Plymouth Meeting, PA) that, in turn, was attached to the syringe pump. The assembly entered the chamber through a 5.0 cm diameter hole in the top of the chamber. Events in the chamber and collection of data were controlled online with a Pentium computer that used programs written in the Medstate notation language (MED Associates).
Catheter Construction and Implantation
Intrajugular catheters were custom-made, as described previously (Twining et al., 2009). Rats were anesthetized using an intraperitoneal injection of 70 mg/kg ketamine/10 mg/kg xylazine and catheters were implanted into the jugular vein. After surgery, rats were allowed at least 7 days to recover. General maintenance of catheter patency involved daily examination and flushing of catheters with heparinized saline (0.2 mL of 30 IU/mL heparin). Catheter patency was verified, as needed, using 0.2 mL of propofol (Diprivan 1%) administered intravenously.
Drug Preparation
Heroin HCl was generously provided by the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC). Drug was dissolved in sterile, physiological (0.9%) saline at a concentration of 0.3 mg/ml. Each infusion consisted of an intravenous (iv) injection of 0.06 mg/0.2 ml of heroin delivered over 6 sec (Kuntz et al., 2008).
Habituation
Rats were habituated to the operant chambers 15-min/day for two days just prior to the start of self-administration training. At the beginning of each habituation session, the house light and white noise were turned on and the rats were allowed to freely explore the operant chambers. At the end of the 15 min session, rats were immediately returned to their home cages.
Intravenous Self-administration (SA) Training Protocol
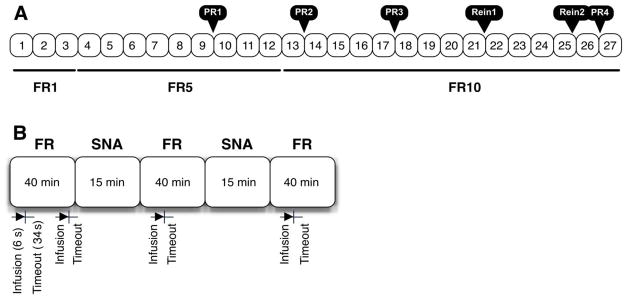
Following habituation, each rat was trained 5 days/week in 150 minute sessions, as described previously (Belin, Balado, Piazza, & Deroche-Gamonet, 2009; Deroche-Gamonet et al., 2004), for 27 sessions (see Figure 1). Each 150 minute session included three 40 minute fixed ratio (FR) self-administration periods alternated with 15 minute periods of signaled non-availability (SNA) in the following pattern: FR SNA FR SNA FR.
Figure 1.
A. Schematic overview of the SA training schedule which consisted of 27 sessions of a fixed ratio (FR) schedule of reinforcement, interspersed with 4 progressive-ratio (PR1–4) tests and 2 extinction/reinstatement tests (Rein1–2). B. Each SA session consisted of three 40 minute drug periods on a fixed ratio schedule, separated by two 15 minute periods of signaled non-availability (SNA). Each drug infusion was delivered intravenously over a period of 6 seconds, followed by 34 additional seconds for a total 40 second timeout period. During the timeout, additional licks would not elicit another infusion of heroin.
Rats were placed in the operant chambers in darkness. Immediately upon initiation of the 150 minute session, the white noise was turned on, the right and left empty spouts advanced into the chamber, and the cue light above the right spout was illuminated. Rats were then allowed to self-administer heroin (0.06 mg/0.2 mL infusion dose) for 40 minutes. The right spout was termed the “active” spout, while the left spout was termed the “inactive” spout. A fixed ratio (FR) 1 schedule of reinforcement was implemented initially (Sessions 1–3). During this time, completion of a single lick on the “active” empty spout was followed by a single intravenous infusion of heroin over 6 seconds. Drug delivery was signaled by offset of the stimulus light and onset of the houselight and tone. The tone and houselight remained on during an additional 34 seconds of a 40 second timeout (TO) period during which time “active” spout responses would not elicit additional drug infusions. Responding on the “inactive” spout served as a control for general activity and was without consequence throughout the entirety of each 150 minute session, including the TO periods. The reinforcement schedule was increased to FR5 (Sessions 4–12) and then to FR10 (Sessions 13–27) to fully distinguish between active and inactive responding. As alluded to, a 15 minute SNA period elapsed between each 40 minute drug period. During this time, the cue light above the right spout was turned off, a light on the chamber wall opposite the spouts was illuminated, and the infusion pump was turned off so no drug was delivered. Responding on the “active” spout, as well as on the “inactive spout,” was without consequence during SNA periods. Following each self-administration training session, the rats were returned to their home cages. The number of infusions self-administered during drug periods was recorded throughout self-administration training. In addition, active and inactive spout responding was compared across FR sessions (i.e., responding during Sessions 9, 13, 17 was compared to responding during Session 25). Finally, active and inactive spout responding was assessed independently during the 40 second TO periods and across the intervening SNA periods.
Progressive Ratio Schedule
To test the rats’ willingness to work for drug, a progressive ratio (PR) schedule of reinforcement was implemented as described previously (Belin et al., 2009; Deroche-Gamonet et al., 2004). PR testing was conducted between the 9th and 10th trial, between the 13th and 14th trial, between the 17th and 18th trial, and between the 26th and 27th trail. During PR testing, the ratio of responses required per infusion was increased after each infusion according to one of two PR testing schedules. For PR sessions conducted between the 9th and 10th, 13th and 14th, 17th and 18th SA sessions, PR Testing Schedule 1 was used in which the number of active responses required to receive the 1st infusion started at 10 and then progressively increased by 10. Due to relatively low response rates for heroin (e.g., compared to our experience with responding for cocaine), PR Testing Schedule 2 was used for the last PR session. For this PR session, conducted between the 26th and 27th SA sessions, the number of active responses required to receive the 1st infusion started at 10, followed by 12, 14, 16, 20, 24, 30, 36, and so on. In both PR schedules, both “active” and “inactive” spouts were retracted during the 6 sec drug infusion period and during the additional 14 sec of a 20 sec timeout period. During PR sessions, rats were allowed to self-administer heroin (0.06 mg/0.2 mL infusion) until a period of 30 minutes elapsed without receipt of an infusion. Terminal break point (i.e., the highest ratio completed during the final PR test) was assessed for each animal.
Extinction/Reinstatement
Between the 21st and 22nd and 25th and 26th SA sessions, a 90 minute extinction-reinstatement (REIN) session was conducted in the same chambers in which rats had previously received heroin. During the first 45 minutes of the Extinction/REIN session, completion of 10 active responses caused the cue light above the active spout to turn off, the spouts to retract, and a tone to sound for a total of 20 seconds, however no drug was infused. The subjects were primed with one computer controlled iv infusion of heroin (0.06 mg/0.2 mL infusion dose). Following this “priming” dose of heroin, the spouts retracted, the cue light turned off, and the tone sounded for 20 seconds. The final 45 minutes of the session proceeded as described during the 45 min extinction period. Responding during the initial 45 minutes and the 45 minutes following the heroin priming dose was assessed. Unlike our cocaine studies (Puhl et al., 2011), this extinction/reinstatement regimen did not produce orderly data for heroin, and as such, the data are not presented here.
Sacrifice and Tissue Dissection
Twenty-four hours after the final FR session, rats were transported to a wet lab for sacrifice by rapid decapitation without anesthesia. Brains were immediately removed and the prefrontal cortex, hippocampus, and nucleus accumbens were rapidly dissected on ice as described previously (Heffner, Hartman, & Seiden, 1980). Tissue samples were stored at −80°C until assayed for protein expression via Western blotting.
Western Blotting
Brain tissue samples were solubilized on ice by dounce-homogenization in a detergent-based lysis buffer (100 mM NaCl, 20 mM HEPES, 1 mM EDTA, 1 mM dithiothreitol, 1.0% Tween20, 1 mM Na3VO4 with 1 Complete Mini EDTA-free Protease Inhibitor Cocktail Tablet (Roche Applied Science, Indianapolis, IN) for every 10 mL lysis buffer prepared, incubating with rocking for 15 minutes at 4°C, and centrifuging at 10,000 ×g for 12 minutes at 4°C). Supernatant protein concentrations were determined using the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL). 8 μg of each sample (n=14 per gel) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 4–20% Mini-Protean TGX precast gels (BioRad). For immunoblotting, proteins were transferred using the iBlot dry transfer system (P3, 20 V for 4:50 min) to polyvinylidene difluoride (PDVF) membranes within the iBlot transfer stack (PVDF, Regular; Invitrogen, Carlsbad, CA) and membranes stained with Ponceau S (Sigma, St. Louis, MO) as a loading control. After destaining, membranes were incubated at room temperature for 1 hour with the following antibodies: rabbit anti-D2 dopamine receptor (1:1,000, Millipore, Bedford, MA); mouse anti-dynamin (1:10,000, Oncogene Research Products, Cambridge, MA); rabbit anti-GPR177/WLS (1:20,000; Sigma); rabbit anti-VAP33/VAPA antisera (1:10,000, generous gift of Dr. Paul Skehel, Centre for Integrative Physiology, University of Edinburgh, Scotland). Proteins were visualized using HRP-conjugated goat anti-mouse or anti-rabbit secondary antibodies (1:20,000, Jackson ImmunoResearch, West Grove, PA). Immunoreactivity was detected with enhanced chemiluminescence (ECL) using an ECL Plus kit (GE Healthcare, Piscataway, NJ), imaged on BioMax film (Kodak, Rochester, NY), and quantitated using the ImageJ software package (US National Institutes of Health, Bethesda, MD). Protein expression data was analyzed using Stata12 (StataCorp LP, College Station, TX).
Data and Statistical Analysis
Persistence of Drug-Seeking in the Absence of Heroin
Persistence of drug-seeking in the absence of heroin was assessed daily by measuring the number of responses made on the active spout during the signaled non-availability periods of SA training. For analysis, active spout responses were examined during the signaled non-availability periods of self-administration Sessions 9, 13, 17, and 25. Deroche-Gamonet et al. (2004) also examined “addiction-like” behavior at various time points across testing. In the present case, sessions 9, 13, 17, and 25 were chosen because they occurred just prior to PR test days.
Willingness to Work for Heroin
The last ratio completed, or breakpoint, during a progressive ratio schedule of testing was taken as an index of motivation. The progressive ratio breakpoint was assessed during 4 independent tests occurring between SA sessions: 9 and 10, 13 and 14, 17 and 18, and 26 and 27.
Persistence of Heroin-Seeking Behavior during SA sessions
Persistence in heroin-seeking behavior was examined daily by measuring the responses at the active spout during the timeout periods of SA training. For analysis, active responses were examined during timeout periods on self-administration sessions 9, 13, 17, and 25.
Onset Activity
Onset activity was quantified as the sum of the normalized scores (Z-scores) for the active responses during the timeout periods of the initial 40 min drug period and the active responses during the initial signaled non-availability period across self-administration training. Onset activity for self-administration Sessions 9, 13, 17, and 25 was examined.
Addiction-like Criteria Classification
Subjects were ranked for each “addiction-like” behavior including (1) Persistence of drug-seeking during signaled non-availability, (2) willingness to work for heroin on the 4th and final progressive ratio test, and (3) Persistence in drug-seeking (i.e., “active spout responses) during timeout periods across Sessions 23–25. A rat was considered positive for an addiction-like behavior when its score was in the 75th–99th percentile. The rats were then divided into four groups according to the number of positive criteria met (i.e., 0 criteria, 1 criterion, 2 criteria, or 3 criteria).
Addiction Severity Score
Addiction Severity Scores were generated as the sum of normalized scores (Z-scores) for each of the 3 “addiction-like” behaviors assessed on the 9th, 13th, 17th, and 25th self-administration Sessions and PR tests 1–4 (Belin et al., 2009). The Z-score for each behavior transforms the raw behavioral measurement into units of standard deviation above or below the mean, with a mean of zero and a variance of one standard deviation. The addiction-like severity score is thus distributed along a scale from −7 to 7.
Statistical Analysis
All data were analyzed with Stata12 (StataCorp LP, College Station, TX). Three types of analyses were conducted: analysis of variance (ANOVA), nonparametric Spearman’s correlation analysis, and linear regression. The p-values for correlational and regression analyses were adjusted using a Bonferroni multiple test correction where appropriate. Newman-Keuls post hoc tests were conducted on significant ANOVAs with α set at 0.05.
Results
Individual differences in terminal “addiction-like” Behavior
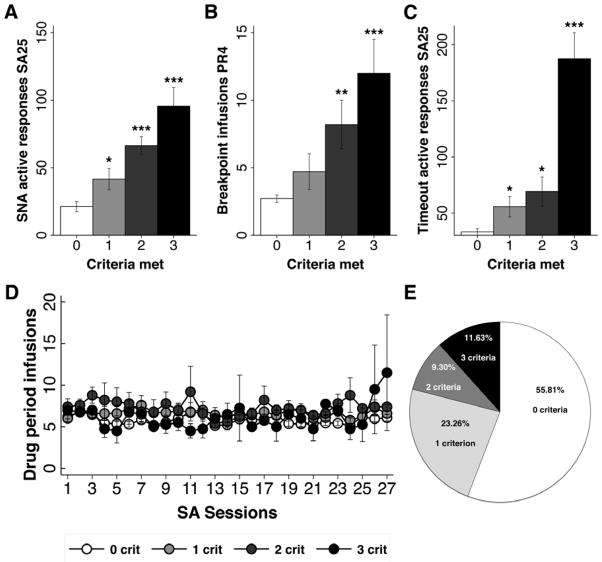
The results revealed large individual differences in the expression of “addiction-like” behavior and the magnitude of each addiction criteria achieved at the end of testing was proportional to the number of addiction-like criteria met by the subjects, i.e. the greater the overall addiction-like behavior score (0, 1, 2, or 3), the greater the performance on each individual criterion (see Figure 2).
Figure 2.
Panels A – C. Mean number of active spout responses (+/− SEM) during signaled non-availability (SNA) on SA trial 25 (Panel A), mean number of breakpoint infusions (+/− SEM) as assessed on the 4th progressive ratio (PR) trial (Panel B), and mean number of active spout responses (+/− SEM) emitted during timeout periods on SA trial 25 (Panel C) as a function of the number of criteria met for the three “addiction-like” behaviors. Each rat was positive for an addiction criterion when its metric fell within the 75–99th percentile for that behavior. Panel D. Heroin intake (+/− SEM) across SA sessions 1 – 27 as a function of the number of addiction criteria met. Panel E. Pie chart showing the percentage of the total population (n=43) of rats positive for 0 (n=24), 1 (n=10), 2 (n=4), or 3 (n=5) criteria (E). ***p<0.001, **p<0.01, *p<0.05 compared to zero criteria rats.
Our data indicate that seeking (i.e., the number of active responses emitted during signaled non-availability in SA session 25) increased as a function of the number of criteria met (Figure 2A). Thus, post-hoc tests of a significant main effect of criteria, F(3,39)=18.69, p<0.0001, revealed greater seeking behavior by rats scoring points on 1, 2, or 3 criteria, with 0<1<2=3, ps < 0.05. The data further showed that the willingness to work for drug (i.e., the number of breakpoint infusions during the 4th progressive ratio test) also increased as a function of the number of criteria met (Figure 2B). Post-hoc tests of a significant main effect of criteria, F(3,39)=14.05, p<0.0001, revealed greater working behavior by rats scoring points on 2 or 3 criteria with 0=1<2<3, ps < 0.05. This analysis also showed that persistence of drug seeking immediately following an infusion (i.e., the number of responses emitted during timeout periods as assessed on SA session 25) increased as a function of the number of criteria met (Figure 2C). Post-hoc tests of a significant main effect of criteria, F(3,39)=48.37, p<0.0001, revealed greater persistence in seeking behavior by rats scoring points on 1 to 3 criteria, with 0<1=2<3, ps < 0.05. Overall, the group that met all three criteria represented 11.63% of the entire sample (n=5). The group that met two criteria comprised 9.3% of the sample (n=4), while the group that met 1 criteria made up 23.26% of the sample (n=10). A large portion of the sample, 55.81% (n=24), did not meet any of the “addiction-like” criteria (Figure 2E). Finally, despite profound differences in “addiction-like” behavior scores, rats showing 0, 1, 2, or 3 “addiction-like” behaviors, when challenged, did not differ on intake of heroin during FR responding when examined across the entire SA period (Figure 2D, F<1). That said, they did as a group exhibit clear goal-directed behavior by making significantly more licks on the ‘active’ vs. the ‘inactive’ empty spout beginning with trial 4 and continuing through trial 27, ps < 0.05 (data not shown).
“Addiction” develops with experience over time
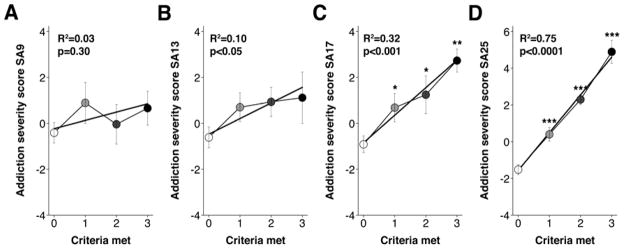
As described previously for cocaine (Belin et al., 2009), an Addiction Severity Score was computed for the heroin self-administering rats on self-administration sessions 9, 13, 17, and 25. This score was normally distributed and centered on 0, with a variance of one standard deviation (Figure 3).
Figure 3.
Regression analysis of the relationship between the terminal number of criteria met by SA session 25 and the Addiction Severity Score measured at (A) SA session 9, (B) SA session 13 (R2s<0.10), (C) SA session 17 (R2=0.32, p<0.001), and (D) SA session 25 (R2=0.75, p<0.0001). ***p<0.001, ** p<0.01, *p<0.05 compared to 0 criteria rats.
The relationship between this Addiction Severity Score, as assessed at each time-point, and the terminal number of criterion met for addiction on SA session 25 was then examined. Linear regression analysis demonstrated that there was not a significant relationship between the number of criteria met for “addiction-like” behavior as determined during SA session 25 and the Addiction Severity Score as measured at SA session 9 or 13 (R2s<0.1; see Figure 3A & 3B). However, as shown in Figure 3C, a significant relationship was found between the number of criteria met for “addiction-like” behavior determined during SA session 25 and the Addiction Severity Score when determined at SA session 17 (R2=0.32, p<0.001). This pattern of data reveals that “addiction” develops with experience over time. Specifically, 17 sessions of self-administration experience were required for performance on the Addiction Severity Score to approach the terminal classification of low (0) to high (3) “addiction-like” behaviors for heroin. By the end of the study (i.e., SA session 25), the Addiction Severity Score and the number of criteria met were highly correlated (R2=0.75, p< 0.0001).
Early persistence predicts later “addiction-like” behavior
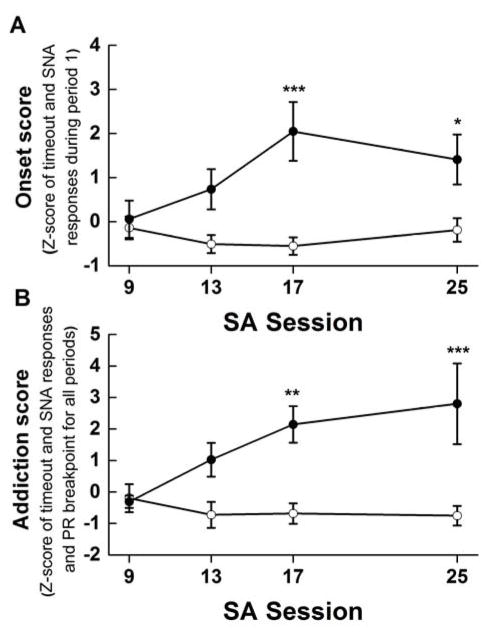
Although “addiction-like” behaviors were not evident until SA session 17, we were interested in determining if any other behaviors could be used to predict, at an earlier time point, which rats ultimately would meet three criteria versus zero criteria. To this end, correlational analyses were conducted to determine if any early behaviors were associated with the number of criteria met at the end of the study. The early behaviors considered were “active” responses exhibited during the timeout periods of the initial 40 min drug period and during the initial 15 min signaled non-availability period. Specifically, we analyzed “Onset Activity” defined here as the sum of the normalized scores for the “active” responses during the timeout periods of the initial 40 min drug period and the “active” responses during the initial signaled non-availability period during SA training. Onset Activity was measured for group 0 and group 3 for self-administration sessions 9, 13, 17, and 25. Onset Activity was significantly greater for group 3 vs. group 0 beginning on SA session 17 (Figure 4).
Figure 4.
Panel A. Onset score, defined as the sum of the normalized scores of active licks emitted during the initial timeout and signaled drug non-availability periods for rats having met 3 (filled circles) or zero (open circles) criteria for “addiction-like” behavior across SA sessions 9, 13, 17, and 25. Panel B. The addiction score, defined as the sum of the normalized scores of active licks emitted during the timeout and signaled drug non-availability periods and the progressive ratio breakpoint infusions for three criteria rats (solid circles) compared to zero criteria rats (open circles) at SA sessions 9, 13, 17 and 25. ***p<0.001, **p<0.01, *p<0.05 compared to 0 criteria rats.
Post hoc tests of a significant 2 × 4 mixed factorial ANOVA varying group (0 vs 3) and session (9, 13, 17, and 25), F (3,81) = 3.48, p = 0.02, showed that, again at self-administration session 17, there was a significant difference in the Onset Activity of rats who ultimately would meet 0 vs. 3 criteria, ps < 0.05 (see Figure 4A). The same also was true for the Addiction Severity Score (see Figure 4B). Finally, while the Onset Activity Score did not reveal statistically significant differences in responding between group 0 and group 3 on session 13, follow up correlational analysis demonstrated a significant relationship between the Onset Activity Score at self-administration session 13 and the Addiction Severity Score on SA25 (Spearman Rs=0.54, p<0.001). This relationship between the Onset Activity Score and the Addiction Severity Score became stronger and more highly significant by self-administration session 25, Spearman Rs=0.67, p<0.001, as shown in Figure 5.
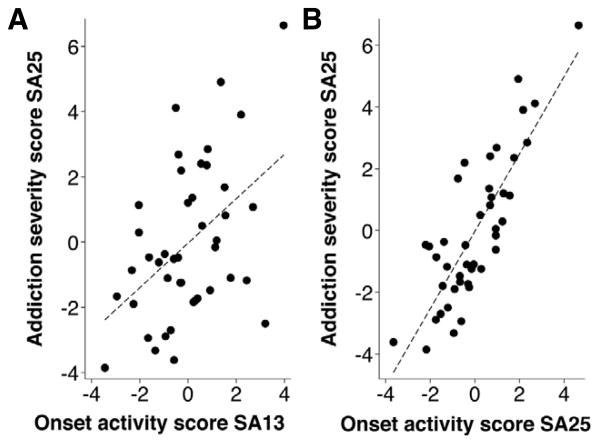
Figure 5.
Spearman ranked order correlation between Addiction Severity Score at SA session 25 and onset activity at SA session 13 (Panel A: Spearman Rs=0.54, p<0.001) and at SA session 25 (Panel B: Spearman Rs=0.67, p<0.001).
Thus, while neither the individual components that comprised the Addiction Severity Score, nor the composite Addiction Severity Score on session 13 could predict terminal “addiction-like” behaviors, onset activity (i.e., persistence in drug seeking during the first drug period and during the first signaled non-availability period) on session 13 was significantly correlated with the severity of terminal “addiction-like” behaviors for heroin in rats overall.
Underlying molecular mechanisms of “addiction-like” behavior
The hippocampus (including CA1, CA3, and dentate gyrus), nucleus accumbens (including both the core and the shell), and prefrontal cortex were used from 15 out of the 43 rats that had completed the behavioral portion of this study (one rat contributed to protein data for the HPC, but not for the NAc or mPFC). These rats self-administered heroin over 27 self-administration sessions, as described, and met a range of “addiction-like” behavior criteria including a “low” set (n=8 with a score of 0), a “middle” set (n=4 with a score of 1), and a “high” set (n=3 with a score of 3). Heroin self-administration was analyzed for this subset of rats using a 3 × 27 mixed factorial ANOVA varying criteria (0, 1, and 3) and sessions. The results found a significant main effect of session, F (26,312) = 3.43, p < 0.001, and a significant criteria x session interaction, F (52,312) = 2.59, p < 0.001. Post hoc Newman-Keuls tests of the two-way interaction revealed that rats in the subset having scored a 3 took more heroin on terminal sessions 26 and 27 than did rats that scored a 0 or a 1, ps < 0.05. That said, there were no such group differences in heroin self-administration on session 1 – 25, before or during which most addiction-like behavior measures were taken (save PR), and there were no group differences in the average number of heroin infusions self-administered/day across all 27 sessions for the 15 rats meeting 0 (5.26 +/− 0.31), 1 (6.35 +/− 0.42), or 3 (6.05 +/− 0.91) criteria for “addiction-like” behavior. No subjects were included having been positive for 2 addiction-like criteria. In accordance, the main effect of criteria was not significant, F (2,11) = 2.44, p = 0.133. Western blots were run on this subset of 15 subjects for each brain region, and blots were probed for candidate MOR interacting proteins and for expression of the D2R (see Figures 6 and 7).
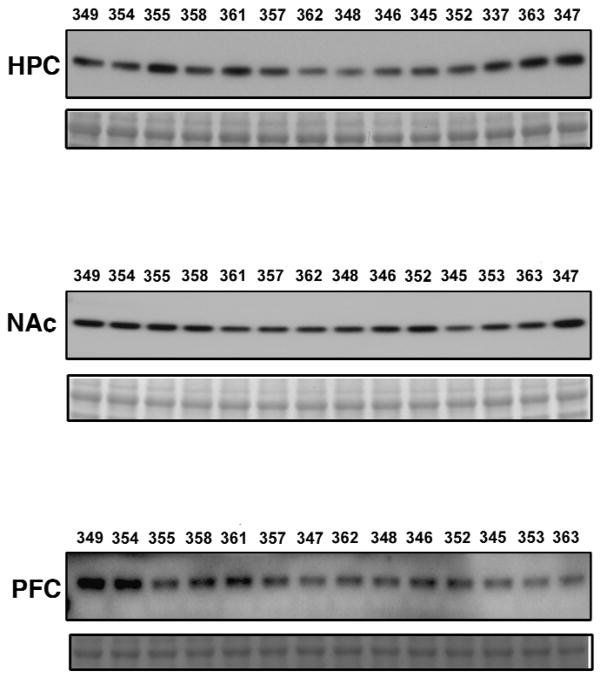
Figure 6. Western blots of D2 dopamine receptor expression.
Samples from 14 heroin self-administering rats were prepared from the hippocampus (HPC), nucleus accumbens (NAc), prefrontal cortex (PFC). Western blots were run for each brain region and probed for D2 dopamine receptor. Subject identification numbers mark each lane. Bottom bands show Ponceau loading control.
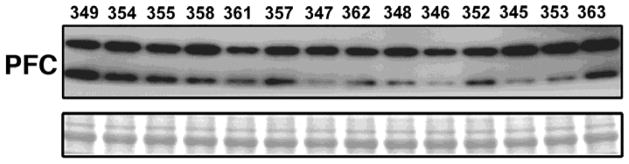
Figure 7.
Western blot of Wntless/GPR177 expression. Samples from 14 heroin self-administering rats were prepared from the prefrontal cortex (PFC). Western blots were run and probed for Wntless/GPR177. Subject identification numbers mark each lane. Bottom bands show Ponceau loading control.
As indicated, there was a significant pattern whereby the number of infusions increased on sessions 26 and 27 for rats in the subset having met 3 addiction criteria. Spearman rank correlations showed that a greater number of infusions on trial 27 was associated with reduced expression of D2R, R = −0.61, p=0.04, and Wntless, R = −0.61, p=0.04, in the PFC. That said, Spearman rank correlations found no relationship between the number of heroin infusions taken on session 25 (i.e., on the trial during which most of the “addiction-like” behaviors were measured) and the expression of either the D2R in the PFC, R = −0.24, p = 0.416, or Wntless in the PFC, R = −0.26, p = 0.367. Adjusted Spearman rank correlations also found no relationship between the total number of heroin infusions taken across the entire 27 session acquisition period and the expression of D2R in the PFC: R=−0.25, p>1.0, HPC: R=0.26, p>1.0, or NAc: R=0.18, p>1.0, or the expression of Wntless in the PFC, R=−0.47, p=0.74. Expression of the D2R and Wntless, then, was not correlated with total drug exposure.
High “addiction-like” behavior is associated with low expression of the D2R and Wntless
Where appropriate, adjusted Spearman rank order correlations were conducted to determine the relationship between each of the “addiction-like” behavior criteria and the protein expression levels in each brain region tested (Figure 8).
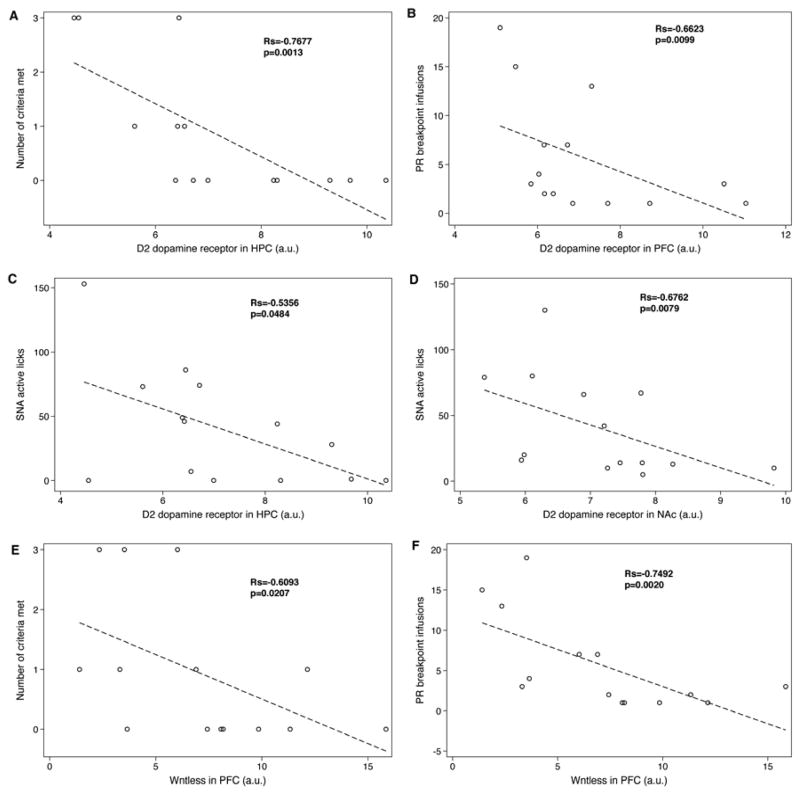
Figure 8.
Spearman correlations for (A) Number of criteria met and D2 dopamine receptor expression in the hippocampus, (B) PR breakpoint infusions and D2 dopamine receptor expression in the prefrontal cortex, (C) SNA active responses and D2 dopamine receptor expression in the hippocampus, (D) SNA active responses and D2 dopamine receptor expression in the nucleus accumbens, (E) Number of criteria met and Wntless expression in the prefrontal cortex, and (F) PR breakpoint infusions and Wntless expression in the prefrontal cortex. Figure shows unadjusted p values. See text for adjusted p values using a Bonferonni multiple comparision correction factor where appropriate.
In the HPC, the number of “addiction-like” behavior criteria met by subjects was negatively correlated with the expression of the D2R (Rs=−0.77, p=0.001), as shown in Figure 8A. Given the categorical nature of the variable, number of criteria met, the data also were analyzed using ordered logistic regression and Poisson regression. According to ordered logistic regression, the correlation between the number of criteria met and D2 dopamine receptor expression in the hippocampus approached significance (beta coefficient=−4.21, p=0.095) and Poisson regression, like Spearman rank correlation, found a significant association (IRR=0.412, p=0.004). When examining the behaviors individually, low expression of the D2R in the prefrontal cortex was associated with higher break point infusions during the final PR test (Rs= −0.66, p=.0099, adjusted p=0.018; Figure 8B). Thus, a greater willingness to work for heroin was associated with reduced expression of the D2R, specifically, in the prefrontal cortex. Low expression of D2R in the hippocampus and nucleus accumbens, on the other hand, was correlated with high seeking during SNA, Rs=−0.53, p=0.048 (Figure 8C), and, Rs=−0.68, p=0.008 (Figure 8D), respectively.
As with expression of the D2R, expression of the MOR interacting protein, Wntless, tended to vary as a function of “addiction-like” behavior (Figure 8E). Thus, in the prefrontal cortex, adjusted Spearman rank correlation found that the number of criteria met by subjects tended to be negatively correlated with the expression of Wntless (Rs=−0.61, p=0.021, adjusted p<.08). Again, because of the categorical nature of the “addiction score”, these data also were analyzed using ordered logistic regression and Poisson regression. The results showed that the correlation between the number of criteria met and Wntless expression in the prefrontal cortex approached significance with ordered logistic regression: beta coefficient=−0.84, unadjusted p=0.06, adjusted p=.12. The Poisson regression found a similar pattern: IRR=0.83, p=0.046, adjusted p=0.23. When these “addiction-like” behaviors were examined individually, lower expression of Wntless in the prefrontal cortex was correlated with a higher break point infusions during the final PR test, (Rs=−0.75, p=0.002, adjusted p=0.008; Figure 8F). Lower expression of Wntless within the prefrontal cortex, then, was associated with a greater willingness to work for drug and a tendency to exhibit a greater number of criteria met for “addiction-like” behavior. Finally, in the prefrontal cortex, the expression of Wntless was significantly and positively correlated with the expression of the D2R such that low Wntless expression was associated with low D2R expression (Rs=0.72, p<0.004). No changes in expression levels of dynamin, spinophilin, or VAPA were detected in any of the brain regions sampled (data not shown).
Discussion
Using this regimen of self-administration, all 43 rats took heroin on the FR schedule of reinforcement. There were no obvious high or low drug-takers and FR intake of heroin was fairly flat, across 27 trials. This finding is consistent with our earlier study with cocaine using a similar testing regimen across 38 trials (Puhl et al., 2011) and with the pattern of cocaine self-administration reported using a similar regimen across 55 – 60 trials (Deroche-Gamonet et al., 2004; Kasanetz et al., 2013). The flat acquisition function evidenced by the group as a whole may be due to the relatively short 2 h access period (i.e., three 40 min access periods), but more likely is related to the on/off/on/off/on daily regimen of drug availability. In our hands, self-administration of cocaine increases across 13 trials when rats are given 1 h (Grigson & Twining, 2002) or 1.5 h (Cason & Grigson, 2013) of daily access. A subset of rats also clearly increases intake of heroin across trials when given just 3 h of repeated daily access (Imperio & Grigson, 2015). Use of the present regimen, then, appears to clamp intake of drug and to minimize, or even eliminate, most individual differences in responding for drug on the FR schedule of reinforcement. Escalated intake in extended access models has been linked to an increase in tolerance to drug (Calipari, Ferris, & Jones, 2014). An interesting question for future consideration is whether this on/off/on/off/on regimen prevents the typical increase in intake of the drug over trials because it somehow prevents the development of tolerance.
Despite little variability in heroin self-administration among the 43 rats, individual differences clearly emerged in the same experimental subjects when challenged in progressive ratio testing or during periods of signaled non-availability. Thus, in spite of having taken the same amount of drug during FR trials 1 – 27 as a group, a subset of rats (nearly 12% here) exhibited the greatest willingness to work for drug, the greatest seeking during periods of signaled non-availability, and the greatest seeking during the brief mandatory timeout period that followed each self-administered infusion of drug. This finding with heroin closely parallels studies showing high “addiction-like” behavior in roughly 17% of the outbred rats tested using similar procedures with cocaine (Deroche-Gamonet et al., 2004; Kasanetz et al., 2010; Kasanetz et al., 2013). These findings in the rodent are of further note because, of those humans taking heroin, cocaine, or alcohol, about 15% are said to become addicted (Anthony, Warner, & Kessler, 1994).
Interestingly, and as is so often the case, evidence suggests that these numbers are not fixed in stone. Thus, in a separate experiment, the percentage of rats exhibiting high “addiction-like” behavior for cocaine increased from about 15% to 50% when the rats had a history of having binged on fat (Puhl et al., 2011). Likewise, 50% of rats exhibit high addiction-like behaviors for heroin when given extended access to drug (Imperio & Grigson, 2015). Finally, and very importantly, using the present regimen, about half of the subjects scored a zero. Thus, despite having taken the same amount of drug during acquisition, about half of the rats failed to exhibit high responding for drug when tested on any of the imposed challenges (See Figure 2 and Deroche-Gamonet et al., 2004). Clearly, some individuals are highly vulnerable to addiction, while others are highly resistant.
Using this on/off/on/off/on regimen, “addiction-like” behaviors developed in a subset of rats. Of relevance is when the behaviors develop over time and how early one can identify vulnerability and/or resilience. Here, differences in the number of criteria met for “addiction-like” behavior on terminal SA session 25 could be predicted by the Addiction Severity Score as determined on SA session 17. As discussed, the Addiction Severity Score reflects normalized performance during each of the three challenges (progressive ratio, signaled non-availability, and timeout). A different measure, the Onset Activity Score, reflects persistence in responding at the start of each daily session (i.e., during the first 40 min FR session and the first 15 min signaled non-availability period). While it was not possible on session 13 to significantly distinguish between rats that would later score a 0 vs. a 3 on “addiction-like” behavior, it was possible to use a correlational analysis and Onset Activity Scores on session 13 to predict Addiction Severity on session 25. Kasanetz et al. (2010) drew a similar conclusion by measuring persistence in responding during the latter part of the non-drug periods. Finally, Colechio et al. (2014) showed that early orofacial responses to a cocaine-paired taste cue could predict later differences in responding for cocaine following a single taste-drug pairing. Vulnerability for addiction, then, can potentially be evidenced very early on and the effect grows with experience, particularly for some rats.
As alluded to, greater avoidance of (or aversion to) a drug-paired taste cue predicts greater cocaine-seeking and taking behavior (Colechio & Grigson, 2014; Colechio et al., 2014; Grigson & Twining, 2002). As a consequence, in that paradigm, drug exposure ultimately differs between those found to be prone and those identified as resilient. A similar outcome is evident with the extended access model – by design, rats showing high and low motivation for drug have a history of having consumed high and low amounts of drug, respectively (Imperio & Grigson, 2015). While these models are useful, such differences in drug history can pose a confound when trying to link differences in motivation for drug to differences in brain. As discussed, despite a significant uptick in heroin self-administration on trials 26 and 27 by the subset of rats earning a 3, all rats for whom protein was analyzed (i.e., 0 criteria (n=8), 1 criteria (n=4), and 3 criteria (n=3)) took the same amount of drug across sessions 1 – 27 overall. Consequently, it was possible to hold total drug exposure constant and, thus, to examine changes in D2R and Wntless protein expression in brain as a function of the explicit motivation for drug, i.e., as a function of the willingness to work on the progressive ratio schedule, persistent seeking during signaled non-availability, and persistent seeking even immediately following the iv self-administration of drug. Results showed that reduced expression of the D2 dopamine receptor in the mPFC was associated with greater “addiction-like” behavior in the willingness to work for drug on the progressive ratio schedule of reinforcement. A similar pattern was obtained with Wntless in the mPFC. Interestingly lesions of the mPFC lead to perseverative responding on a progressive ratio schedule in the face of punishment (Allen & Leri, 2014). Reduced expression of the D2R in the NAc and HPC, on the other hand, was associated with an increase in persistent seeking during signaled non-availability. Changes in D2R expression, then, may impact different “addiction-like” behaviors (e.g., working or seeking), depending upon where in the brain the receptors are located.
It should be noted that, while the number of heroin infusions did not differ between groups, overall, the three rats found positive on 3 addiction criteria took more heroin infusions on sessions 26 and 27 than did rats in the 0 (n=8) or 1 (n=4) criteria conditions and a greater number of terminal infusions on session 27, but not sessions 26 or 25, was significantly correlated with reduced expression of D2Rs and Wntless in the PFC. This pattern of data suggests the possibility that reduced D2R and Wntless expression may be due to a proximal increase in heroin self-administration on the terminal acquisition sessions. While possible, this conclusion would seem unlikely because Briand et al. (2008) showed that the reduction in expression of D2R in the medial PFC required 3 weeks of daily Long 6 h Access to cocaine self-administration. Three weeks exposure to Short 1 h Access, on the other hand, was not sufficient.
The reduced expression of Wntless in the mPFC of heroin self-administering rats bears further consideration. We identified Wntless as a mu-opioid receptor (MOR) interacting protein in a membrane yeast two hybrid (MYTH) screen (Jin et al., 2010). Specifically, Jin et al. showed that Wntless plays a crucial role in the response to morphine in cultured cells. Wntless is a predicted transmembrane protein that is a component of the machinery required for Wnt secretion from Wnt-producing cells (Hausmann, Banziger, & Basler, 2007). Activation of MOR by morphine enhances MOR interaction with Wntless in tissue culture cells (Jin et al., 2010), as well as in rat brain (Jaremko et al., 2014; Reyes et al., 2012). These observations suggest that the enhanced MOR/Wntless interaction serves to “trap” Wntless at the plasma membrane, resulting in the inhibition of Wnt secretion. Wnts are secreted glycoproteins that bind Frizzled receptors, promoting trophic actions, including increased dendritic spines (Ciani & Salinas, 2005; Rosso, Sussman, Wynshaw-Boris, & Salinas, 2005) and increased hippocampal neurogenesis (Lie et al., 2005). These neuronal Wnt effects (increased dendritic spines and increased hippocampal neurogenesis) are opposite those produced by morphine, which causes loss of dendritic spines (Liao, Lin, Law, & Loh, 2005; Robinson & Kolb, 1999) and inhibition of hippocampal neurogenesis (Eisch, Barrot, Schad, Self, & Nestler, 2000; Harburg et al., 2007). Recently, we found that Wntless expression was significantly reduced in the midbrain and striatum of mice after chronic treatment with morphine (Petko et al., 2013). Thus, it is tempting to speculate that Wntless is a critical molecular component linking opioid agonist activation of the MOR and the downstream effects that result from the inhibition of Wnt secretion such as collapse of dendritic spines and decreased hippocampal neurogenesis that contribute to the development of opioid addiction.
Based on these results, it can be hypothesized that lower levels of the D2R and Wntless may increase an individual’s vulnerability to develop “addiction-like” behaviors. Alternatively, higher levels of the D2R and Wntless may be protective against the development of “addiction-like” behaviors. Either way, these relationships hold true regardless of the total amount of drug self-administered across all 27 sessions. That said, it remains unknown if the individual male Sprague-Dawley rats used in this study were predisposed to express lower levels of the D2R and Wntless, or if heroin self-administration elicited proteomic changes in vulnerable individuals. While the answer to this important question must await direct study, Kasanetz et al. (2010; 2013) showed that NMDAR-(N-methyl-D-aspartate receptor) long-term depression in the nucleus accumbens and mGluR2/3 long-term depression in the prelimbic prefrontal cortex was suppressed in cocaine addiction vulnerable rats following about 50, but not 17 days on this regimen. There may, then, be a functional significance for expression of the D2R and Wntless in the transition from heroin use to abuse and addiction. Indeed, a drug-induced reduction in the expression of mGluR2/3 (Kasanetz et al., 2013) and D2R may go hand-in-hand to increase motivation for drug (Kalivas, Volkow, & Seamans, 2005). Future experiments, in which the expression of D2R and Wntless is altered, either pharmacologically or genetically, will determine whether such manipulations are capable of attenuating the development of these “addiction-like” behaviors in this paradigm. Until such time, these data stand as the first to reveal individual differences in the motivation for heroin in this paradigm, to dissociate the impact of the motivation for heroin from overall heroin exposure, per se, and to link these differences in motivation (i.e., in “addiction-like” behaviors) for heroin to reduced expression of D2R and Wntless in brain.
Acknowledgments
The authors thank the National Institute on Drug Abuse for generously providing heroin. Support for this research was provided by NIH grants DA009815 (to PSG), DA025995 (to RL) and UL1 TR000127 (to AB). Support also was provided by a grant from the Pennsylvania Department of Health (PA DOH), Commonwealth Universal Research Enhancements SAP# 4100055576 (to PSG and RL) and by a second grant from the PA DOH Tobacco Settlement Funds SAP # 4100057673 (to RL). The State specifically disclaims responsibility for any analyses, interpretations, or conclusions. We thank Sarah Ballard for her technical assistance and Christopher Jenney for his comments on an earlier draft of the manuscript.
Footnotes
The authors have no conflicts of interest to report.
References
- Alfaras-Melainis K, Gomes I, Rozenfeld R, Zachariou V, Devi L. Modulation of opioid receptor function by protein-protein interactions. Frontiers in Bioscience. 2009;14:3594–3607. doi: 10.2741/3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CP, Leri F. Perseveration in the presence of punishment: the effects of chronic cocaine exposure and lesions to the prefrontal cortex. Behavioural Brain Research. 2014;261:185–192. doi: 10.1016/j.bbr.2013.12.025. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Angelucci F, Ricci V, Pomponi M, Conte G, Mathe AA, Attilio Tonali P, Bria P. Chronic heroin and cocaine abuse is associated with decreased serum concentrations of the nerve growth factor and brain-derived neurotrophic factor. Journal of Psychopharmacology. 2007;21(8):820–825. doi: 10.1177/0269881107078491. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug and Alcohol Dependence. 1995;40(1):9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Experimental and Clinical Psychopharmacology. 1994;2(3):244–268. doi: 10.1037/1064-1297.2.3.244. [DOI] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. The Journal of Pharmacology and Experimental Therapeutics. 1993;264(1):489–495. [PubMed] [Google Scholar]
- Belin D, Balado E, Piazza PV, Deroche-Gamonet V. Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biological Psychiatry. 2009;65(10):863–868. doi: 10.1016/j.biopsych.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19(3):591–611. doi: 10.1016/S0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, Robinson TE. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology. 2008;33(12):2969–2980. doi: 10.1038/npp.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Jones SR. Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine. Journal of Neurochemistry. 2014;128(2):224–232. doi: 10.1111/jnc.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Grigson PS. Prior access to a sweet is more protective against cocaine self-administration in female rats than in male rats. Physiology & Behavior, 112–113C. 2013:96–103. doi: 10.1016/j.physbeh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496(7445):359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nature Reviews Neuroscience. 2005;6(5):351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- Colechio EM, Grigson PS. Conditioned aversion for a cocaine-predictive cue is associated with cocaine seeking and taking in rats. International journal of comparative psychology/ISCP; sponsored by the International Society for Comparative Psychology and the University of Calabria. 2014;27(3):488–500. [PMC free article] [PubMed] [Google Scholar]
- Colechio EM, Imperio CG, Grigson PS. Once is too much: Conditioned aversion develops immediately and predicts future cocaine self-administration behavior in rats. Behavioral Neuroscience. 2014;128(2):207–216. doi: 10.1037/a0036264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305(5686):1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(13):7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. The Journal of Neuroscience. 2001;21(8):2851–2860. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS, Hajnal A. Once is too much: conditioned changes in accumbens dopamine following a single saccharin-morphine pairing. Behavioral Neuroscience. 2007;121(6):1234–1242. doi: 10.1037/0735-7044.121.6.1234. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behavioral Neuroscience. 2002;116(2):321–333. doi: 10.1037//0735-7044.116.2.321. [DOI] [PubMed] [Google Scholar]
- Guitart X, Beitner-Johnson D, Marby DW, Kosten TA, Nestler EJ. Fischer and Lewis rat strains differ in basal levels of neurofilament proteins and their regulation by chronic morphine in the mesolimbic dopamine system. Synapse. 1992;12(3):242–253. doi: 10.1002/syn.890120310. [DOI] [PubMed] [Google Scholar]
- Harburg GC, Hall FS, Harrist AV, Sora I, Uhl GR, Eisch AJ. Knockout of the mu opioid receptor enhances the survival of adult-generated hippocampal granule cell neurons. Neuroscience. 2007;144(1):77–87. doi: 10.1016/j.neuroscience.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann G, Banziger C, Basler K. Helping Wingless take flight: how WNT proteins are secreted. Nature Reviews Molecular Cell Biology. 2007;8(4):331–336. doi: 10.1038/nrm2141. [DOI] [PubMed] [Google Scholar]
- Heffner TG, Hartman JA, Seiden LS. A rapid method for the regional dissection of the rat brain. Pharmacology, Biochemistry, and Behavior. 1980;13(3):453–456. doi: 10.1016/0091-3057(80)90254-3. [DOI] [PubMed] [Google Scholar]
- Hou H, Jia S, Hu S, Fan R, Sun W, Sun T, Zhang H. Reduced striatal dopamine transporters in people with internet addiction disorder. Journal of Biomedicine & Biotechnology. 2012;2012:854524. doi: 10.1155/2012/854524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperio CG, Grigson PS. Greater avoidance of a heroin-paired taste cue is associated with greater escalation of heroin self-administration in rats. Behavioral Neuroscience. 2015;129(4):380–388. doi: 10.1037/bne0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaremko KM, Thompson NL, Jr, Reyes BA, Jin J, Ebersole B, Jenney CB, Van Bockstaele EJ. Morphine-induced trafficking of a mu-opioid receptor interacting protein in rat locus coeruleus neurons. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2014;50:53–65. doi: 10.1016/j.pnpbp.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Kittanakom S, Wong V, Reyes BA, Van Bockstaele EJ, Stagljar I, Levenson R. Interaction of the mu-opioid receptor with GPR177 (Wntless) inhibits Wnt secretion: potential implications for opioid dependence. BMC Neuroscience. 2010;11:33. doi: 10.1186/1471-2202-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45(5):647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, Piazza PV. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328(5986):1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Lafourcade M, Deroche-Gamonet V, Revest JM, Berson N, Balado E, Manzoni OJ. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Molecular psychiatry. 2013;18(6):729–737. doi: 10.1038/mp.2012.59. [DOI] [PubMed] [Google Scholar]
- Kuntz KL, Patel KM, Grigson PS, Freeman WM, Vrana KE. Heroin self-administration: II. CNS gene expression following withdrawal and cue-induced drug-seeking behavior. Pharmacology, Biochemistry and Behavior. 2008;90(3):349–356. doi: 10.1016/j.pbb.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Lin H, Law PY, Loh HH. Mu-opioid receptors modulate the stability of dendritic spines. Proceedings of the National Academy of Sciences U S A. 2005;102(5):1725–1730. doi: 10.1073/pnas.0406797102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Petko J, Justice-Bitner S, Jin J, Wong V, Kittanakom S, Ferraro TN, Levenson R. MOR is not enough: identification of novel mu-opioid receptor interacting proteins using traditional and modified membrane yeast two-hybrid screens. PLoS One. 2013;8(6):e67608. doi: 10.1371/journal.pone.0067608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, Cason AM, Wojnicki FH, Corwin RL, Grigson PS. A history of bingeing on fat enhances cocaine seeking and taking. Behavioral Neuroscience. 2011;125(6):930–942. doi: 10.1037/a0025759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Vakharia K, Ferraro TN, Levenson R, Berrettini WH, Van Bockstaele EJ. Opiate agonist-induced re-distribution of Wntless, a mu-opioid receptor interacting protein, in rat striatal neurons. Experimental Neurology. 2012;233(1):205–213. doi: 10.1016/j.expneurol.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Annals of the New York Academy of Sciences. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse. 1999;33(2):160–162. doi: 10.1002/(SICI)1098-2396(199908)33:2<160::AID-SYN6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Munoz M, Bermudez D, Sanchez-Blazquez P, Garzon J. Sumoylated RGS-Rz proteins act as scaffolds for Mu-opioid receptors and G-protein complexes in mouse brain. Neuropsychopharmacology. 2007;32(4):842–850. doi: 10.1038/sj.npp.1301184. [DOI] [PubMed] [Google Scholar]
- Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nature Neuroscience. 2005;8(1):34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- SAMHSA. NSDUH series H-44. Rockville, MD: 2012. Results from the 2011 national survey on drug use and health: Summary of national findings. [Google Scholar]
- Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biological Psychiatry. 2008;63(3):256–262. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinus L, Le Moal M, Koob GF. Nucleus accumbens and amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal. Neuroscience. 1990;37(3):767–773. doi: 10.1016/0306-4522(90)90106-E. [DOI] [PubMed] [Google Scholar]
- Twining RC, Bolan M, Grigson PS. Yoked delivery of cocaine is aversive and protects against the motivation for drug in rats. Behavioral Neuroscience. 2009;123:913–925. doi: 10.1037/a0016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Borsook D. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133(Pt 7):2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, Carelli RM. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biological Psychiatry. 2011;69(11):1067–1074. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57(5):774–785. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Leri F, Cummins E, Kreek MJ. Individual differences in gene expression of vasopressin, D2 receptor, POMC and orexin: vulnerability to relapse to heroin-seeking in rats. Physiology & Behavior. 2015;139:127–135. doi: 10.1016/j.physbeh.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]