Abstract
Using new biomarker data from the 2010 pilot round of the Longitudinal Aging Study in India (LASI), we investigate education, gender, and state-level disparities in health. We find that hemoglobin level, a marker for anemia, is lower for respondents with no schooling (0.7 g/dL less in the adjusted model) compared to those with some formal education and is also lower for females than for males (2.0 g/dL less in the adjusted model). In addition, we find that about one third of respondents in our sample aged 45 or older have high C-reaction protein (CRP) levels (>3 mg/L), an indicator of inflammation and a risk factor for cardiovascular disease. We find no evidence of educational or gender differences in CRP, but there are significant state-level disparities, with Kerala residents exhibiting the lowest CRP levels (a mean of 1.96 mg/L compared to 3.28 mg/L in Rajasthan, the state with the highest CRP). We use the Blinder-Oaxaca decomposition approach to explain group-level differences, and find that state-level disparities in CRP are mainly due to heterogeneity in the association of the observed characteristics of respondents with CRP, rather than differences in the distribution of endowments across the sampled state populations.
Keywords: Biomarkers, Health Disparities, Cardiovascular health, Anemia, Blinder-Oaxaca Decomposition, Aging
1. Introduction
Many developing countries are currently undergoing rapid demographic and economic transitions. In particular, the proportion of older individuals in lower- and middle-income nations is expected to rise rapidly in coming decades (Shetty, 2012). This is the result of an ongoing epidemiological transition in these countries in which life expectancy is rising and mortality is shifting towards later life (Prentice, 2006). The main risks for premature death are no longer solely the well-studied problems associated with poverty, such as malnutrition and poor sanitation. In India, non-communicable diseases (NCDs), such as cardiovascular disease, which had been largely limited to higher-income countries, are becoming the main causes of premature mortality (Kearney et al., 2005). For example, NCDs now account for 60% of all deaths in India (World Health Organization, 2014), and the contribution to mortality of non-communicable relative to communicable diseases worldwide is expected to rise substantially in coming years. At the same time, malnutrition and communicable diseases remain substantial health threats in lower- and middle-income countries (Narayan et al., 2010). For example, 70% of women and children in India currently suffer from anemia (Balarajan et al., 2011).
Such an epidemiological environment, where both cardiovascular disease and malnutrition are prevalent, creates a challenging environment for public health officials in India, and also in other lower- and middle-income countries. This challenge is exacerbated by difficulties in measuring the extent of health inequality and identifying the most at-risk populations. There are typically major differences in both health and access to health care by gender, region, level of education, and other socioeconomic measures within countries. However, there is an absence of objective data for certain parts of the population, particularly for older individuals in developing countries. Given that conditions such as cardiovascular disease and anemia can be expected to impact most severely on these cohorts, understanding group-level differences in health outcomes among older age groups is therefore important for establishing policy priorities and informing where resources should be targeted. In this paper we aim to shed light on these issues, using the 2010 pilot data from the Longitudinal Study of Aging in India (LASI), which recently collected information on a variety of biomarkers, including C-reactive protein (CRP), a marker of inflammation, and hemoglobin (Hb), a marker of anemia. The 2010 LASI pilot data collected data from four states: Punjab, Rajasthan, Kerala, and Karnataka, allowing us to examine regional differences. Using these biomarker data, we document the risk of cardiovascular disease and anemia among older Indians, and investigate health differences by gender, state of residence, and education. Our results indicate that there are substantial disparities in hemoglobin, with women and those with no formal education having lower levels. For CRP, we find that the oldest old are most at risk of inflammation, as are those living in urban areas. State-level differences are also apparent, with Kerala having the lowest levels of CRP, and Rajasthan the highest. These results confirm the existence of socioeconomic inequalities in anemia among an older sample of Indians, but imply that inflammation is more universal in that it is likely to affect those from all social backgrounds. Our Blinder-Oaxaca decomposition suggests that these state disparities are not driven by differences in endowments, but rather heterogeneity in the association between risk factors and the outcomes across states. Therefore, policies at the state level may be important for altering the association between risk factors and health outcomes.
The rest of this paper is structured as follows. In Section 2, we provide background information on health and health disparities in India, particularly cardiovascular disease and anemia. Section 3 discusses our data and analytic approach. In Section 4, we present our results. We conclude in Section 5.
2. Background
Cardiovascular disease and anemia in India
Cardiovascular disease (CVD) is now the second-most important contributor to mortality in India, accounting for 28% of deaths (World Health Organization, 2005). Yet, CVD has been a controversial subject in public discourse due to the perception that heart disease is mainly a problem of the urban upper middle class. The academic literature has debated this claim (Gwatkin, 2013; Lloyd-Sherlock et al., 2014; Subramanian et al., 2013). Given the increasing importance of heart disease and lack of adequate nutrition (Bentley and Griffiths, 2003), CVD and anemia are especially important public health issues in India, and these conditions are particularly likely to have the most severe impact on older individuals (Carmel, 2001; Chaves et al., 2005). However, good evidence on the prevalence of malnutrition and CVD in India is difficult to obtain, particularly for older age groups, and particularly at the regional level, where there is varying access to medical services and diagnosis.
There are two reasons for this. First, self-reporting on health in India is problematic due to differential state-level access to care, diagnosis, and treatment. This access affects the extent to which individuals are aware of their health status and the extent to which these reports are affected by recall bias, as well as how individuals perceive their health (Johnston et al., 2009). This can result in heterogeneity in the thresholds used by respondents for indicating that they suffer from a medical condition (Sen, 2002). For example, there are large differences in self-reported hypertension diagnosis and measured hypertension, and these discordances vary by state (Lee et al., 2012).
Second, among lower- and middle-income countries, there is a dearth of data on objective biological markers of malnutrition and CVD. In particular, there is little existing evidence from these countries about the risks for NCDs among persons aged 45 years and older, the population most likely to be affected by these conditions (Chaves et al., 2005). Even if health-service records are available, they may not provide a complete picture of population health because they only provide information on people seeking diagnosis or treatment. Such individuals may not be a representative sample of the population, especially if access to health care is low.
Biomarkers as objective measures of cardiovascular and anemia risk
Using biomarkers to directly assess risks for particular outcomes can help overcome the lack of good health information while also providing an immediate assessment of objective health disparities for both individuals and groups. Two attractive candidates for targeted biomarker data collection are CRP and Hb. CRP is a biomarker for inflammation, and higher levels of CRP are associated with increased risk of cardiovascular disease (Vikram et al., 2003). Elevated levels of CRP are also associated with diabetes and metabolic syndrome, with thresholds for high risk defined by the Center for Disease Control and Prevention (CDC) and the American Heart Association (AHA) (Myers et al., 2004).
Hb can be used to evaluate the prevalence of anemia (Balarajan et al., 2011). Anemia results from a lack of either red blood cells or hemoglobin, and leads to weakness or fatigue (Aguayo et al., 2003; Beghé et al., 2004; Denny et al., 2006). In developing countries, anemia is often associated with iron or vitamin deficiencies due to poor nutrition. Lower levels of Hb suggest a greater risk of anemia. Prior data collection on the prevalence of anemia has mainly focused on preschool-age children, pregnant women, and non-pregnant women of reproductive age, particularly in India (Balarajan et al., 2013; Bentley and Griffiths, 2003; Ghosh, 2009). For this reason, the World Health Organization (WHO) does not report country-level estimates for school-age children, men, and the elderly in India (Benoist et al., 2008).
Health Disparities
With economic development, CVD may increasingly affect all socioeconomic groups (Ezzati et al., 2005). However, the available data we have on women of child-bearing age suggests that inadequate nutrition certainly remains a problem for a substantial proportion of the less well-off in India (Bentley and Griffiths, 2003). India has the highest incidence of anemia in the world, with levels that have remained static for the past decade, despite economic growth (Balarajan et al., 2011).
India is a union of 30 states and 6 territories, which vary greatly in their economic development, cultures, education levels, and policies (Deaton and Dreze, 2002; Deaton and Kozel, 2005; Lee and Smith, 2014; Ravallion and Datt, 2002). These differences may lead to cross-state variation in risks for these health outcomes, and differential health and economic outcomes are indeed evident when comparing urban and rural groups, states, and genders. Table 1 illustrates some of these disparities in the four Indian states covered by the LASI pilot. While all four of these states saw urban consumption increase substantially between the 1960s and the 1990s, there was little improvement in rural consumption, except in Kerala. Kerala also had more favorable statistics for females, including a higher ratio of girls to boys and higher school attendance rates. These economic factors likely contribute to regional differences in health outcomes which have also been documented for India, including obesity, underweight, and infant mortality (Ackerson et al., 2008; Coffey, 2015).
Table 1.
Changes in Economic and Social Factors by State
| Punjab | Rajasthan | Karnataka | Kerala | |
|---|---|---|---|---|
| Economic Growth | ||||
| 1960-61 Mean Per Capita Expenditure Rs/Month1 | ||||
| Rural | 82 | 56 | 59 | 47 |
| Urban | 84 | 67 | 72 | 54 |
| 2011-12 Mean Per Capita Expenditure Rs/Month2 | ||||
| Rural | 2473 | 1681 | 1692 | 3186 |
| Urban | 2987 | 2508 | 2852 | 3742 |
| Male Preference | ||||
|
| ||||
| Child sex ratio (Girls per 1,000 boys aged 0 – 6)3 | 846 | 883 | 943 | 959 |
| Elementary School Attendance Rates (Ages 5 – 14) Per 1,0004 | ||||
| Boys | 897 | 847 | 898 | 968 |
| Girls | 882 | 710 | 866 | 985 |
Source:
Datt (1998)
National Social Survey 2011-2012
The Government of India 2011 Census
The Government of India, Ministry of Human Resource Development, Selected Educational Statistics: 2000-01
3. Data and Analytic Approach
Research Hypotheses
Using biomarker data on hemoglobin and CRP, we propose to empirically examine health disparities in anemia and cardiovascular risk among older Indians. First, we establish whether there is an education gradient among the 45 and above age group for hemoglobin. Given the existing literature, we hypothesize the existence of socioeconomic disparities for this outcome (Bentley and Griffiths, 2003). Second, we examine the risk factors associated with anemia separately for men and women, given the much higher incidence of anemia among women (Balarajan et al., 2011). Third, given the public discourse on socioeconomic status (SES) and cardiovascular disease in India, we assess whether there is an education gradient in CRP. We also examine differences between urban and rural areas. Previous research found no evidence of an SES gradient in CRP in Costa Rica (Rosero-Bixby and Dow, 2009), another country experiencing demographic, economic, and epidemiologic transitions. Therefore, we hypothesize that we will find no SES gradient in CRP in India. Finally, given the varying growth and social policies of Indian states in recent decades, we examine state-level variation in CRP. We aim to provide preliminary evidence on whether differences in economic growth and social policies contribute to state-level variation in CRP.
Data
The 2010 LASI pilot sample was drawn using a stratified, multistage, area probability sampling design based on the 2001 Indian Census. From each state, we randomly chose two Census districts. We then randomly selected eight primary sampling units (PSU) from each district to match the urban/rural share of the state population. Finally, we selected 25 community-residing households through random sampling from each PSU.
For external validity, it is important that the LASI pilot data provide a reasonable approximation to the population of interest. Arokiasamy et al. (2012) analyzed whether the LASI pilot data derived from two districts in each state was comparable to the older population in India as a whole, as well as whether the data in each of the four pilot states were comparable to the older population in those states. Overall, the characteristics of older respondents in the LASI pilot (aged 45 and over) closely matched the characteristics of older respondents in the National Sample Survey (NSS), the India Human Development Survey (IHDS), the World Health Survey (WHS), and the WHO Study on global AGEing and adult health (SAGE). The LASI pilot was also found to provide a good match with the age structure of the population of interest. Further details of this analysis are provided in Arokiasamy et al. (2012).
The LASI pilot provides survey weights, based on the 2011 Indian Census. One set of weights matches the biomarker sample with the population aged 45 and older in the four surveyed states (Punjab, Rajasthan, Kerala, and Karnataka) based on age, sex, and urban/rural place of residence. A second set of weights matches the biomarker sample to the population aged 45 and older in India as a whole. We use these latter weights in the analysis, but have verified that results are very similar when using state weights or when not weighting the data.
The LASI pilot has two main modules: the household and individual interview, and the biomarker collection component. The household interview asks about physical environment and household finances, while the individual interview asks about demographics, family, social activities, health and health behaviors, and work and pensions. For the collection of dried blood spots (DBS), respondents provided separate consent, permitting interviewers to prick their finger and place five drops of blood on a Whatman 903 Protein Saver card. The collected DBS cards were left to dry for at least 4 hours during the night, then sent to the National AIDS Research Institute (NARI) in Pune, India, where they were stored below −20°C and later assayed.
Both CRP concentration and hemoglobin levels in the DBS specimens were measured using validated methods. CRP concentration was measured using the enzyme-linked immunosorbent assay (ELISA) protocol developed by McDade et al. (2004). Hemoglobin levels were measured using the method developed by O’Broin and Gunter (1999). To ensure quality, all samples, standards, and controls were measured in duplicate. Internal quality controls were run on every plate, and plates with out-of-range quality-control values were re-run. We further ensured the quality of laboratory assay results through periodic use of external quality control samples prepared by the USC/UCLA Center on Biodemography and Population Health. We also externally validated work at the NARI laboratory. For CRP assays, we compared NARI’s results on 32 validation samples with DBS-based values from the reference laboratory in the United States (at the University of Washington); the correlation coefficient of these results was 0.95. For hemoglobin levels, 33 validation samples had DBS values from NARI and venous-based results from the UCLA Clinical Laboratory; the correlation coefficient of these results was 0.78. In general, NARI had higher Hb values than the corresponding venous-based results. The average difference was 0.55 g/dL (standard deviation: 0.86 g/dL). For measures of both Hb and CRP, DBS specimens were run in duplicate. The two values from duplicate measurements were very highly correlated. In our analysis, we combined the duplicate measures into an average. Using either the first or second measure on its own had little effect on the results. For further details of the LASI biomarker data collection see Bloom et al. (2014), for further details of the validation see Hu et al. (2015).
Table 2 presents descriptive statistics for the analysis of the two main dependent variables (hemoglobin, measured in grams per deciliter, g/dL, and CRP, measured in milligrams per liter, mg/L) and independent variables (age, gender, state, caste, urban/rural residency, and education). We focus on respondents over the age of 45, excluding a small number of spouses under this age. This left 1,150 observations in total. There were a small number of missing values for some covariates and outcomes, but these did not exceed 6% of observations. For example of the 1,150 total respondents, 1,077 had information on Hb. The mean Hb in the sample was 14.3 g/dL, and mean CRP was 2.7 mg/L. Table 2 also shows the proportion of respondents with anemia (20%) as determined by Hb levels (below 12 g/dL for women and below 13 g/dL for men), as well as those at high risk for cardiovascular disease (30%), as determined by CRP levels of more than 3 mg/L (Myers et al., 2004).
Table 2.
Descriptive Statistics for the Analysis Sample
| Median | Mean | SD | N | |
|---|---|---|---|---|
| Hemoglobin (g/dL) |
||||
| Overall | 14.3 | 14.2 | 2.5 | 1077 |
| Men | 15.4 | 15.3 | 2.4 | 517 |
| Women | 13.5 | 13.3 | 2.2 | 560 |
| Anemic (Men<13 g/dL; Women<12 g/dL) |
||||
| Overall | 0 | 0.2 | 0.4 | 1077 |
| Men | 0 | 0.1 | 0.4 | 517 |
| Women | 0 | 0.3 | 0.4 | 560 |
| C Reactive Protein (mg/L) |
||||
| Overall | 1.7 | 2.7 | 3.1 | 1106 |
| Men | 1.5 | 2.8 | 3.3 | 529 |
| Women | 1.8 | 2.6 | 2.8 | 577 |
| CRP High Risk (CRP>3 mg/L) |
||||
| Overall | 0 | 0.3 | 0.5 | 1106 |
| Men | 0 | 0.3 | 0.5 | 529 |
| Women | 0 | 0.3 | 0.5 | 577 |
| Age Group | No. | % | Residency | No. | % |
|---|---|---|---|---|---|
| 44-54 | 538 | 46.8 | Urban | 291 | 25.3 |
| 55-64 | 318 | 27.7 | Rural | 859 | 74.7 |
| 65-74 | 200 | 17.4 | Total | 1150 | 100 |
| 75+ | 93 | 8.1 | |||
| Total | 1149 | 100 | State | ||
| Punjab | 166 | 14.4 | |||
| Gender | Rajasthan | 353 | 30.7 | ||
| Male | 557 | 48.4 | Kerala | 272 | 23.6 |
| Female | 593 | 51.6 | Karnataka | 360 | 31.3 |
| Total | 1150 | 100 | Total | 1150 | 100 |
| Caste | |||||
| Scheduled Caste | 165 | 14.7 | Education | ||
| Scheduled Tribe | 177 | 15.8 | Some Schooling | 602 | 52.3 |
| Other Backward Class | 438 | 39.1 | No Schooling | 548 | 47.7 |
| None | 341 | 30.4 | Total | 1150 | 100 |
| Total | 1121 | 100 |
Source: LASI Pilot 2010 biomarker sample. Those under age 45 are excluded. The sample is weighted. SD=standard deviation.
Nearly half the sample (46%) was 45-54 years of age, nearly half (48%) were male, and more than half (55%) had received some formal schooling. There were roughly equal numbers of respondents in each state (ranging from 253 in Karnataka to 329 in Kerala). For covariates, we focus on pre-determined variables which are unlikely to be outcomes of socioeconomic status, health status, or health-care use. Nevertheless, as part of supplementary analysis we also considered controlling for smoking, body mass index (BMI), and chronic illness diagnosis, as these factors are correlated with Hb and CRP risk (Beghé et al., 2004; Carmel, 2001; Daly, 2013; Danesh et al., 2004). However, adding them to the model has little effect on our conclusions. Specifically, we find that there are educational and gender disparities in hemoglobin, and state-level disparities in CRP, even conditional on smoking and BMI.
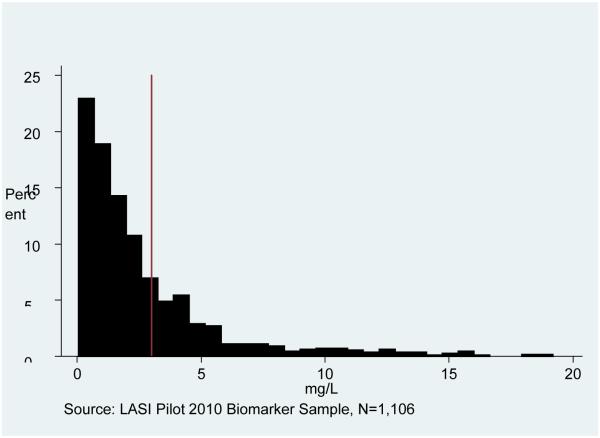
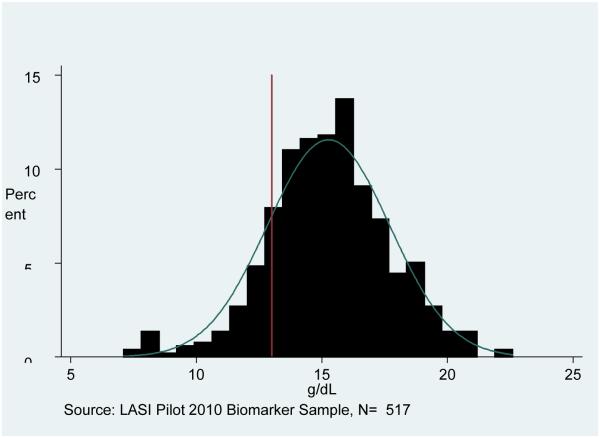
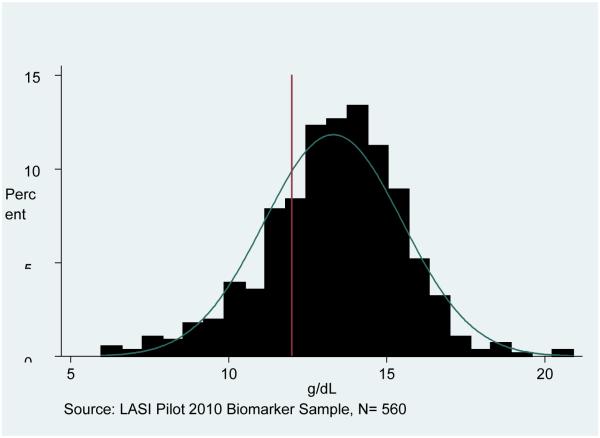
Figure 1 presents the distribution of CRP in the LASI pilot sample, showing the high risk cut-off at 3 mg/L. We show the combined sample, as we find no major gender differences for this outcome. The distribution is skewed to the right, although a substantial proportion has CRP above 3 mg/L (30%). Figures 2 and 3 show the Hb distributions stratified by gender, which in both cases approximates a normal distribution.
Figure 1. C-Reactive Protein Distribution for Men and Women.
Note: Sample is weighted. The cut-off for high cardiovascular risk is shown at 3 mg/L (above is at risk).
Figure 2. Hemoglobin Distribution for Men.
Note: Sample is weighted. The cut-off for anemia among men is shown at 13 g/dL (below is anemic).
Figure 3. Hemoglobin Distribution for Women.
Note: Sample is weighted. The cutoff for anemia among women is shown at 12 g/dL (below is anemic).
We then examine population-level differences in hemoglobin and CRP, by estimating bivariate associations between these biomarkers and the explanatory variables shown in Table 2. Table A1 in the appendix shows this analysis for Hb and Table A2 in the appendix shows it for CRP. We find that being female is significantly associated with a lower level of Hb, as is a lack of formal schooling. These results are consistent with a socioeconomic gradient in anemia, and with well-documented gender disparities (Rosero-Bixby and Dow, 2009). There are significant bivariate associations (at the 5% level) between CRP levels and being age 75 or older (higher for older respondents), rural location (lower for rural respondents), and the states of Kerala and Karnataka (lower).
These bivariate associations do not adjust for other variables, such as age and state, which are likely to be related to both the outcome and the covariates of interest. We seek to establish whether the findings above persist after adjusting for a number of relevant covariates. For example, the relationship between Hb and education may be explained by the fact that earlier birth cohorts have lower levels of educational attainment. Once we establish the relevance of the key covariates of interest in a multivariate analysis, we decompose CRP differences across groups using the Blinder-Oaxaca approach in order to understand the origin of these differences (Blinder, 1973; Jann, 2008; Liu et al., 2013; Maurer, 2011; Oaxaca, 1973; Powell et al., 2012; Sinning et al., 2008). Although our data do not allow us to interpret this decomposition in a causal manner, this analysis should still provide us with a preliminary indication as to whether the state-level differences in CRP we observe can be explained by differences in endowments of risk factors across states.
We begin by adopting the following regression model:
| (1) |
The biomarker outcomes (CRP and Hb) for individual t are modeled as a function of the covariates of interest in a linear regression model (OLS), which is adjusted for weighting and survey design. Xi is a matrix of control variables, including gender, state, education, age group, place of residence (urban or rural), and caste. β is the associated parameter vector. Our main coefficients of interest are the coefficients reflecting the adjusted association of the outcomes with gender, state, and education. As we explain above, we do not control for factors such as BMI, smoking, or chronic illness diagnosis, as these could potentially be outcomes of education or the biomarkers themselves, however it is important to note that doing so has little effect on our conclusions regarding the presence of education, state and gender disparities. We present results for the regression models in the following section. For each outcome, we present both the pooled and gender-stratified analyses.
Given that we observe state-level differences in CRP, we are interested in understanding the source of these differences. We focus on CRP rather than hemoglobin because the regression results in Table 3 are not as supportive of the hypothesis that there are systematic state-level disparities for the latter. We adopt the following decomposition approach, based on linear regression, first proposed by Blinder (1973) and Oaxaca (1973):
| (2) |
Table 3.
OLS Regression Results for Hemoglobin and CRP
| Variables | OLS All CRP |
OLS Men CRP |
OLS Women CRP |
OLS All Hb |
OLS Men Hb |
OLS Women Hb |
|---|---|---|---|---|---|---|
| Age Group: Omitted 45-54 | ||||||
| 55-64 | 0.266 | 0.17 | 0.397 | −0.314 | −0.416 | −0.198 |
| −0.215 | −0.362 | −0.283 | −0.237 | −0.343 | −0.239 | |
| 65-74 | 0.421 | 0.468 | 0.377 | −0.423* | −0.398 | −0.409 |
| −0.289 | −0.467 | −0.303 | −0.22 | −0.399 | −0.304 | |
| 75+ | 1.135** | 0.753 | 1.544*** | −0.399 | −0.852** | 0.023 |
| −0.485 | −0.878 | −0.516 | −0.287 | −0.364 | −0.407 | |
| Female | −0.099 | −1.969*** | ||||
| −0.207 | −0.154 | |||||
| Caste: Omitted None of them | ||||||
| Scheduled caste | −0.207 | −0.039 | −0.534 | 0.583 | 0.705 | 0.474 |
| −0.447 | −0.686 | −0.571 | −0.393 | −0.51 | −0.444 | |
| Scheduled tribe | −0.225 | −0.646 | 0.148 | 0.436 | 0.696* | 0.163 |
| −0.324 | −0.509 | −0.376 | −0.286 | −0.398 | −0.299 | |
| Other backward class (obc) | 0.025 | −0.295 | 0.3 | 0.03 | 0.389 | −0.3 |
| −0.378 | −0.55 | −0.454 | −0.311 | −0.413 | −0.34 | |
| No Formal Education | −0.216 | 0.02 | −0.558 | −0.707*** | −0.709* | −0.693** |
| −0.255 | −0.428 | −0.373 | −0.237 | −0.387 | −0.315 | |
| Rural | −0.772*** | −0.617** | −0.884** | −0.455 | −0.429 | −0.478 |
| −0.209 | −0.292 | −0.379 | −0.3 | −0.445 | −0.286 | |
| State: Omitted Rajasthan | ||||||
| Punjab | −0.472 (0.329) |
−0.331 (0.450) |
−0.632 (0.577) |
0.477 (0.417) |
0.356 (0.463) |
0.653 (0.509) |
| Kerala | −1.599***
(0.352) |
−1.431***
(0.458) |
−1.890***
(0.634) |
0.738**
(0.304) |
0.741*
(0.426) |
0.777*
(0.414) |
| Karnataka | −0.923***
(0.317) |
−0.862 (0.520) |
−0.979**
(0.466) |
0.389 (0.352) |
0.464 (0.461) |
0.382 (0.391) |
| Constant | 4.029***
(0.426) |
4.017***
(0.793) |
4.059***
(0.525) |
15.502***
(0.408) |
15.316***
(0.546) |
13.670***
(0.493) |
| Weighted | Y | Y | Y | Y | Y | Y |
| Number of Primary Sampling Units (PSU) |
63 | 63 | 63 | 63 | 63 | 63 |
| Observations | 1,077 | 517 | 560 | 1,048 | 505 | 543 |
| R-squared | 0.052 | 0.048 | 0.084 | 0.223 | 0.095 | 0.073 |
Robust standard errors in parentheses
p<0.01,
p<0.05,
p<0.1
Note: Sample is weighted. Standard errors are clustered to account for survey design. Hb is measured in g/dL. CRP is measured in mg/L.
For and we use the same variables contained in Xi from the regression in equation (1) with the exception of the state indicator variables as the decomposition models involve stratification by state. Therefore, the endowments in equation (2) are gender, education, age group, place of residence (urban or rural), and caste. Conceptually, there are three possible reasons for differences in mean CRP levels between two states (State 1 and State 2): either a difference in the observed covariates (XState 1, XState 2), which is the first term on the right hand side of equation (2), or a difference in the estimated association between the covariates and the outcomes , which is the second term on the right hand side of equation (2), or an interaction between the two, which is the last term on the right hand side of equation (2) (Daymont and Andrisani, 1984). We can use this approach to decompose differences in mean CRP levels between State 1 and State 2 into differences in endowments (i.e., establishing what State 2 outcomes would be if State 2 had State 1’s endowments), differences in the relevant estimated coefficients (i.e., establishing what State 2 outcomes would be if State 2 had State 1’s coefficients), and an interaction between the two. This method assumes that the state-level coefficients are unbiased (E(βState 1) = βState 1, E(βState 2) = βState 2), and that the state-level error terms have a mean expectation of 0 (E(μState 1) = 0, E(μState 2) = 0). Our data do not allow us to account for omitted variables, so we cannot be certain that this assumption holds in our analysis. We are careful to interpret our results with this limitation in mind. Nevertheless, we argue that this analysis is still useful because it provides initial evidence on why the linear predictions for the four states from a standard OLS regression model differ. These findings may be useful for further causal analysis when additional data becomes available.
We expect there to be state-level policies which at least partly determine health status which we hope to measure using this biomarker data. For example, Kerala is one of the best performing Indian states economically, but also has a history of implementing programs which invest in public health. Using the decomposition approach, we are able to explicitly test the hypothesis about whether there are factors (potentially unobserved) in states such as Kerala which mediate the relationship between known risk factors and health outcomes. For example, if we find that endowments mostly explain state-level differences in the biomarkers, this suggests that those in the better performing states are simply healthier, whereas if we find that differences in the association between risk factors and outcomes mostly explain the state-level differences, this is preliminary evidence of mediating influence.
4. Results
Table 3 presents results for the multivariate analysis of the predictors of Hb. Column 1 shows the results for the pooled sample, controlling for age, gender, state, caste, urban/rural residence, and education. Columns 2 and 3 replicate these specifications for men and women. There is clear evidence for gender and education disparities in the pooled sample, with women having around 2g/dL less Hb than men, and with persons with no formal schooling having about 0.6 g/dL less Hb than those with some education. These results are similar to the bivariate estimates. There are also some gender differences in the coefficients in the stratified model. Older men (ages 75+) have reduced levels of Hb.
Columns 4, 5 and 6 of Table 3 present the corresponding analysis for CRP. The overall results are also similar to the bivariate analysis. In the pooled sample, the oldest old (those aged 75 and older) have higher CRP levels, while those in rural areas have lower levels, as do those in Kerala. Stratification by gender indicates some differences in the association between the covariates and CRP. For example, the age gradient is only present among women.
As a robustness check, we implemented binary regression models (logit) for the cut-offs for anemia and CRP high-risk, and found similar results. Finally, we considered a propensity score matching approach for education, and found similar conclusions regarding an SES gradient in Hb; those with no formal schooling have lower levels of Hb, with associations comparable to those shown in Table 3.
Finally, we implement the Blinder-Oaxaca decomposition approach to analyze state-level differences in CRP, given that we find substantial disparities in Table 3. We begin by presenting results with Rajasthan as the base state for comparisons, given that Rajasthan is the state with highest CRP level (and therefore the greatest CVD risk). By doing so, we attempt to improve our understanding of what contributes to the elevated risk in that state. However, we also present decompositions with the other states as the base comparison group.
Table 4 shows the relevant means for the first base state (Rajasthan), compared to each of the other states. We use the regression specification from the model in Table 3 and equation 1 above. As with the regression model, adding additional controls (such as BMI, smoking and chronic illness diagnosis) had little effect on the conclusions about the source of state-level disparities in CRP. The decomposition table shows the mean difference, the estimated contribution from endowments, the coefficients, and their interaction (as illustrated in equation 2). Overall, the difference in mean CRP level is statistically significant between Rajasthan and Kerala, and between Rajasthan and Karnataka. The decomposition indicates that these differences are mainly due to differences in the association between the explanatory variables and CRP, rather than the distribution of risk factor endowments across states.
Table 4.
Blinder-Oaxaca Decomposition for State Differences in C-Reactive Protein (Base=Rajasthan)
| Punjab | Kerala | Karnataka | |
|---|---|---|---|
| Rajasthan Mean | 3.274***
(0.257) |
3.274***
(0.278) |
3.274***
(0.257) |
| Comparison State Mean | 3.055***
(0.179) |
1.926***
(0.198) |
2.509***
(0.173) |
| Difference | 0.219 (0.313) |
1.348***
(0.341) |
0.765**
(0.310) |
| Endowments | −0.254 (0.266) |
0.346 (0.510) |
−0.353 (0.306) |
| Coefficients | 0.946*
(0.467) |
1.967**
(0.717) |
0.799*
(0.430) |
| Interaction | −0.473 (0.461) |
−0.965 (0.872) |
0.318 (0.493) |
| Additional Controls | N | N | N |
| 63 | 63 | ||
| Observations | 532 | 560 | 507 |
Robust standard errors in parentheses
p<0.01,
p<0.05,
p<0.1
Note: Sample is weighted. Standard errors are clustered to account for survey design. The table shows results from the Blinder-Oaxaca decomposition for each state compared to Rajasthan, using the control variables from the specification in table 3. CRP is measured in mg/L.
When we choose other states as the base category, we reach the same conclusion that state-level differences in CRP are mainly driven by associational differences in the covariates. In particular, when we adopt the best performing state (Kerala) as the base category, we find that coefficient differences are statistically significant for explaining mean differences in Kerala compared to the other three states (Tables A3 - 5 in the appendix).
5. Conclusions
Using new biomarker data from the 2010 LASI pilot sample of older Indians, we investigate the relationship between respondents’ characteristics and objective measures of their health. We demonstrate the feasibility and value of collecting population-based biomarkers among older adults in a developing country.
We find evidence for an education gradient in Hb, but there is no evidence of state-level differences. Despite recent economic growth, the risk of anemia, most likely associated with malnutrition is higher for women and for those without schooling. This is consistent with previous evidence on younger Indians (Subramanian et al., 2009).We find that about one third of Indians have a CRP level considered to be high risk (>3 mg/L), which is comparable to results from the English Longitudinal Study on Ageing (Hamer and Molloy, 2009). We also find that CRP is greater among the oldest old and among urban residents. Although there are substantial state-level differences, there is no evidence of an education gradient for CRP, which is consistent with existing evidence from Costa Rica (Rosero-Bixby and Dow, 2009).
There are several policy relevant implications to these results (subject to the limitations we discuss below). First, our analysis shows that cardiovascular disease is likely to be an important detrimental factor for population health encompassing all socioeconomic groups in Indian society, and therefore interventions to improve cardiovascular health should not only be targeted at the better-off individuals living in urban areas. Second, gender and education disparities in hemoglobin (and therefore likely also in nutrition) persist among older Indians, also implying that nutrition programs should also be targeting this age group rather than just women of reproductive age and children, especially considering that the health of older individuals may be especially sensitive to these conditions (Carmel, 2001; Chaves et al., 2005). Third, when we decompose state-level differences, we find that these disparities are mainly due to differences in the association of risk factors with CRP rather than in the distribution of risk factors.
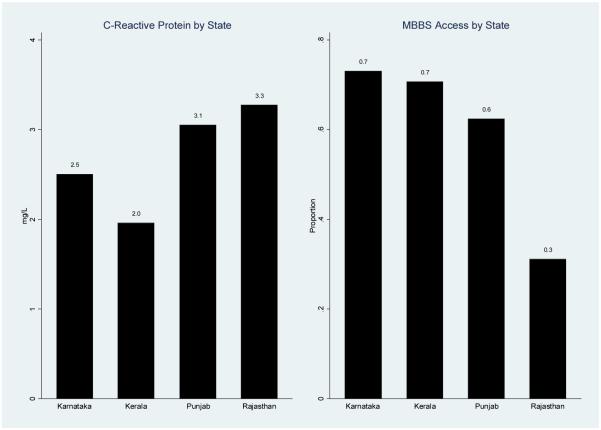
This result that the state-level differences in CRP risk can be explained by differential association of CRP with risk factors suggests that there might be some moderating factors that vary across states. We are cautious about drawing strong conclusions about the relationship between state level characteristics and CRP given that we only have four observations at this level of aggregation, however given that most policies are developed and implemented at state level, this finding is not surprising. Such differences in state policies can contribute to the variation in access to health care, and as a follow-up to this decomposition analysis, we therefore further examine heterogeneity in access to health care across states. In fact, we find some support for this hypothesis of state-level policy differences in health care in our data. As shown in figure 4, Kerala has a relatively high percentage of respondents (71%) who report having access to formal health care (a doctor with a recognized qualification), however, Karnataka also has a high percentage of respondents in this category (73%). Therefore, health care access is an example of a type of policy which could potentially alter the predictive power of risk factors for inflammation. If this relationship with CRP was found to hold in a larger sample of states, it would indicate the type of policy which could be pursued in order to better target cardiovascular disease in India. This is an important issue which we are unable to fully explore with the current data covering only four states, however this is another key direction for future research.
Figure 4. C-Reactive Protein and Health Care Access by State.
Note: Sample is weighted. The left figure shows the mean CRP level by state, while the right figure shows the proportion of respondents in each state who have ever visited a doctor with a formal qualification (e.g., an M.M.B.S. degree).
Robert Fogel’s path-breaking work on the relationship between health and economic development (Fogel, 2004a, 2004b, 1994) may also provide thoughtful guidance as to how to interpret the finding that the most economically advanced state in our sample (Kerala) also has the lowest CRP risk. Fogel’s work highlights the long-run gains which accrue to improvements in health, and Kerala is the state best-known for its policies promoting public health, and for financing health care publicly. Fogel (2004b) argues that policies which improve health and health care can be viewed as an investment in human capital, which in addition to enabling humans to live healthier lives, can also contribute to the acceleration of economic growth. Although the results we present here are consistent with this hypothesis, we do not yet have the data to test this theory in India. This will be an important line of inquiry for future research.
There are important limitations to this study. First, we are only able to document associations. Further data are required to establish, for example, whether the observed relationship between Hb and education is causal. In particular, we are careful to interpret the results of the Blinder-Oaxaca decomposition as being indicative only, because our estimates of the association between risk factors and CRP could be affected by omitted variables. Another area where causal analysis would be useful is in further investigating the gender differences we observe, not only in mean level differences, but also in terms of assessing gender differences in the relationship between risk factors and the outcomes of interest.
Second, a potentially important question which could be addressed using biomarker data is whether health assessments in survey data which are based on objective data differ from health assessments which are based on subjective data. Overall, biomarkers are likely to provide a useful complement to other health measures when determining the health status of older individuals in lower- and middle-income countries, as well as the health disparities between groups within these countries. The use of biomarkers may help to overcome the drawbacks associated with self-reported health measures. This measurement question has potential implications for our understanding of health disparities, as well as our understanding of the risk factors for health conditions, such as CVD.
Previous studies have found significant differences between objective and subjective measures among older individuals for some outcomes, for example in England using the English Longitudinal Study of Ageing (Johnston et al., 2009), and in India using SAGE (Vellakkal et al., 2013). However, the outcomes we consider in this paper are not well suited to analyzing this question. We do not have self-reported measures of nutrition in the data which we could compare to Hb levels. Although low-grade but persistent inflammation is associated with cardiovascular disease, and CRP is predictive of subsequent cardiovascular events and mortality, CRP is not considered as a direct objective measure of heart disease.
Finally, we are presently only able to consider the four pilot states, and it would be interesting to consider the relationships shown in this paper for all states. Future, nationally representative waves of LASI will enable us to examine whether these findings extend to other Indian states, and future iterations of the modules and questionnaires will provide valuable data for answering these and other important research questions.
Research Highlights for “Education, Gender, and State-Level Gradients in the Health of Older Indians: Evidence from Biomarker Data”.
Few studies have analyzed biomarker data on older populations in developing nations
Using new data for India, we explore levels of hemoglobin and C-reactive protein
Respondents with no formal education (compared to any) had 0.7 g/dL less hemoglobin
CRP is 1.1 mg/L higher at ages 75+ versus 45-54, and 0.8 mg/L higher in urban areas
Results indicate an educational gradient in anemia, but not cardiovascular risk
Acknowledgments
Funding: This project is funded by the National Institute on Aging/National Institutes of Health, [Grant No. R21AG034443 and R01 AG030153].
Role of the funding source: The funding agencies had no role in the study design, data collection, data analysis, data interpretation, writing of the study, or decision to submit for publication.
Appendix
Table A1.
Bivariate Association between Explanatory Variables and Hemoglobin
| Mean | SE | CI | N | T test P Value | |
|---|---|---|---|---|---|
| Total | 14.23 | 0.14 | [13.96,14.51] | 1,077 | |
| Gender | |||||
| Male | 15.27 | 0.16 | [14.94,15.60] | 517 | |
| Female | 13.27 | 0.14 | [12.98,13.55] | 560 | (0.000) |
| Age Group | |||||
| 44-54 | 14.41 | 0.14 | [14.12,14.70] | 506 | |
| 55-64 | 14.22 | 0.25 | [13.72,14.73] | 295 | (0.510) |
| 65-74 | 13.96 | 0.24 | [13.48,14.44] | 185 | (0.060) |
| 75+ | 13.81 | 0.32 | [13.17,14.46] | 90 | (0.089) |
| Caste | |||||
| Scheduled Caste | 13.79 | 0.25 | [13.29,14.30] | 179 | (0.159) |
| Scheduled Tribe | 13.99 | 0.32 | [13.34,14.65] | 137 | (0.573) |
| Other Backward Class | 14.54 | 0.18 | [14.18,14.91] | 367 | (0.161) |
| None | 14.20 | 0.19 | [13.81,14.59] | 366 | |
| Education | |||||
| Some Schooling | 14.88 | 0.16 | [14.56,15.19] | 580 | |
| No Schooling | 13.55 | 0.16 | [13.22,13.88] | 497 | (0.000) |
| Residency | |||||
| Urban | 14.69 | 0.33 | [14.02,15.36] | 257 | |
| Rural | 14.09 | 0.14 | [13.80,14.37] | 820 | (0.104) |
| State | |||||
| Punjab | 14.12 | 0.36 | [13.40,14.84] | 264 | |
| Rajasthan | 13.73 | 0.24 | [13.25,14.21] | 275 | (0.368) |
| Kerala | 14.69 | 0.13 | [14.43,14.94] | 306 | (0.143) |
| Karnataka | 14.46 | 0.29 | [13.87,15.05] | 232 | (0.472) |
Note: Sample is weighted. Confidence intervals and t-tests account for survey design. Hb is measured in g/dL.
Table A2.
Associations between Explanatory Variables and C-Reactive Protein
| Mean | SE | CI | N | T test P Value | |
|---|---|---|---|---|---|
| Total | 2.69 | 0.10 | [2.49,2.90] | 1,106 | |
| Gender | |||||
| Male | 2.78 | 0.15 | [2.48,3.08] | 529 | |
| Female | 2.61 | 0.15 | [2.32,2.90] | 577 | (0.432) |
| Age Group | |||||
| 44-54 | 2.47 | 0.16 | [2.15,2.79] | 513 | |
| 55-64 | 2.71 | 0.16 | [2.39,3.03] | 310 | (0.249) |
| 65-74 | 2.85 | 0.22 | [2.41,3.30] | 194 | (0.191) |
| 75+ | 3.61 | 0.49 | [2.61,4.60] | 88 | (0.030) |
| Caste | |||||
| Scheduled Caste | 2.78 | 0.23 | [2.31,3.25] | 180 | (0.971) |
| Scheduled Tribe | 2.94 | 0.41 | [2.12,3.76] | 130 | (0.779) |
| Other Backward Class | 2.48 | 0.16 | [2.16,2.80] | 390 | (0.298) |
| None | 2.79 | 0.23 | [2.33,3.26] | 378 | |
| Education | |||||
| Some Schooling | 2.53 | 0.11 | [2.30,2.75] | 499 | |
| No Schooling | 2.88 | 0.18 | [2.52,3.24] | 275 | (0.098) |
| Residency | |||||
| Urban | 3.16 | 0.19 | [2.78,3.53] | 290 | |
| Rural | 2.53 | 0.12 | [2.30,2.77] | 816 | (0.007) |
| State | |||||
| Punjab | 3.06 | 0.13 | [2.79,3.32] | 277 | |
| Rajasthan | 3.28 | 0.26 | [2.76,3.80] | 268 | (0.447) |
| Kerala | 1.96 | 0.17 | [1.63,2.30] | 314 | (0.000) |
| Karnataka | 2.51 | 0.15 | [2.20,2.81] | 247 | (0.009) |
Note: Sample is weighted. Confidence intervals and t-tests account for survey design. CRP is measured in mg/L.
Table A3.
Blinder-Oaxaca Decomposition for CRP (Base=Punjab)
| Rajasthan | Kerala | Karnataka | |
|---|---|---|---|
| Punjab Mean | 3.055***
(0.179) |
3.055***
(0.179) |
3.055***
(0.167) |
| Comparison State | |||
| Mean | 3.274***
(0.257) |
1.926***
(0.184) |
2.509***
(0.162) |
| Difference | −0.219 (0.313) |
1.129***
(0.256) |
0.545**
(0.233) |
| Endowments | 0.727*
(0.408) |
0.201 (0.364) |
−0.345 (0.235) |
| Coefficients | −0.474 (0.409) |
1.039***
(0.308) |
0.253 (0.235) |
| Interaction | −0.473 (0.461) |
−0.111 (0.458) |
0.637*
(0.328) |
| Additional Controls | N | N | N |
| PSUs | 63 | 63 | 63 |
| Observations | 532 | 570 | 517 |
Robust standard errors in parentheses
p<0.01,
p<0.05,
p<0.1
Note: Sample is weighted. Standard errors are clustered to account for survey design. The table shows results from the Blinder-Oaxaca decomposition for each state compared to Punjab, using the control variables from the specification in table 3. CRP is measured in mg/L.
Table A4.
Blinder-Oaxaca Decomposition for CRP (Base=Kerala)
| Rajasthan | Punjab | Karnataka | |
|---|---|---|---|
| Kerala Mean | 1.926***
(0.184) |
1.926***
(0.198) |
1.926***
(0.184) |
| Comparison State Mean | 3.055***
(0.179) |
3.274***
(0.278) |
2.509***
(0.173) |
| Difference | −1.129***
(0.256) |
−1.348***
(0.341) |
−0.584**
(0.252) |
| Endowments | −0.090 (0.279) |
0.619 (0.707) |
−0.336 (0.222) |
| Coefficients | −0.928**
(0.341) |
−1.002*
(0.502) |
−0.470*
(0.243) |
| Interaction | −0.111 (0.458) |
−0.965 (0.872) |
0.222 (0.317) |
| Additional Controls | N | N | N |
| PSUs | 63 | 63 | 63 |
| Observations | 570 | 560 | 545 |
Robust standard errors in parentheses
p<0.01,
p<0.05,
p<0.1
Note: Sample is weighted. Standard errors are clustered to account for survey design. The table shows results from the Blinder-Oaxaca decomposition for each state compared to Kerala, using the control variables from the specification in table 3. CRP is measured in mg/L.
Table A5.
Blinder-Oaxaca Decomposition for CRP (Base=Karnataka)
| Rajasthan | Punjab | Kerala | |
|---|---|---|---|
| Karnataka Mean | 2.509***
(0.162) |
2.509***
(0.173) |
2.509***
(0.173) |
| Comparison State Mean | 3.055***
(0.167) |
3.274***
(0.257) |
1.926***
(0.184) |
| Difference | −0.545**
(0.233) |
−0.765**
(0.310) |
0.584**
(0.252) |
| Endowments | −0.292 (0.205) |
0.035 (0.380) |
0.114 (0.227) |
| Coefficients | −0.891**
(0.349) |
−1.117**
(0.454) |
0.247 (0.253) |
| Interaction | 0.637*
(0.328) |
0.318 (0.493) |
0.222 (0.317) |
| Additional Controls | N | N | N |
| PSUs | 63 | 63 | 63 |
| Observations | 517 | 507 | 545 |
Robust standard errors in parentheses
p<0.01,
p<0.05,
p<0.1
Note: Sample is weighted. Standard errors are clustered to account for survey design. The table shows results from the Blinder-Oaxaca decomposition for each state compared to Karnataka, using the control variables from the specification in table 3. CRP is measured in mg/L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Authorship Statement: Jinkook Lee, Mark McGovern and Peifeng Hu conceived the study. Mark McGovern analysed the data. Jinkook Lee, Peifeng Hu and Mark McGovern wrote the first draft of the manuscript. Jinkook Lee, Mark McGovern, David Bloom, P. Arokiasamy, Arun Risbud, Jennifer O’Brien, Varsha Kale and Peifeng Hu revised the manuscript and prepared it for submission. All authors have approved the final article and the decision to submit.
References
- Ackerson LK, Kawachi I, Barbeau EM, Subramanian S. Geography of underweight and overweight among women in India: a multilevel analysis of 3204 neighborhoods in 26 states. Econ. Hum. Biol. 2008;6:264–280. doi: 10.1016/j.ehb.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo VM, Scott S, Ross J. Sierra Leone–investing in nutrition to reduce poverty: a call for action. Public Health Nutr. 2003;6:653–657. doi: 10.1079/phn2003484. [DOI] [PubMed] [Google Scholar]
- Arokiasamy P, Bloom D, Lee J, Feeney K, Ozolins M. Longitudinal aging study in India: vision, design, implementation, and some early results. In: Smith JP, Majmundar M, editors. Aging in Asia: Findings from New and Emerging Data Initiatives. National Academies Press; 2012. [PubMed] [Google Scholar]
- Balarajan Y, Ramakrishnan U, Özaltin E, Shankar AH, Subramanian S. Anaemia in low-income and middle-income countries. The Lancet. 2011;378:2123–2135. doi: 10.1016/S0140-6736(10)62304-5. doi:10.1016/S0140-6736(10)62304-5. [DOI] [PubMed] [Google Scholar]
- Balarajan YS, Fawzi WW, Subramanian S. Changing patterns of social inequalities in anaemia among women in India: cross-sectional study using nationally representative data. BMJ Open. 2013;3:e002233. doi: 10.1136/bmjopen-2012-002233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beghé C, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. Am. J. Med. 2004;116:3–10. doi: 10.1016/j.amjmed.2003.12.009. [DOI] [PubMed] [Google Scholar]
- de Benoist B, McLean E, Egll I, Cogswell M. Worldwide prevalence of anaemia 1993-2005: WHO global database on anaemia. World Health Organization; 2008. [Google Scholar]
- Bentley M, Griffiths P. The burden of anemia among women in India. Eur. J. Clin. Nutr. 2003;57:52–60. doi: 10.1038/sj.ejcn.1601504. [DOI] [PubMed] [Google Scholar]
- Blinder AS. Wage discrimination: reduced form and structural estimates. J. Hum. Resour. 1973;8:436–455. [Google Scholar]
- Bloom D, Hu P, Arokiasamy P, Risbud A, Sekher TV, Mohanty SK, Kale V, O’Brien J, Chien CS, Lee J. Longitudinal Aging Study In India: Biomarker Documentation. RAND Work. Pap. WR-1043. 2014.
- Carmel R. Anemia and aging: an overview of clinical, diagnostic and biological issues. Blood Rev. 2001;15:9–18. doi: 10.1054/blre.2001.0146. [DOI] [PubMed] [Google Scholar]
- Chaves PH, Semba RD, Leng SX, Woodman RC, Ferrucci L, Guralnik JM, Fried LP. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women’s Health and Aging Studies I and II. J. Gerontol. A. Biol. Sci. Med. Sci. 2005;60:729–735. doi: 10.1093/gerona/60.6.729. [DOI] [PubMed] [Google Scholar]
- Coffey D. Early life mortality and height in Indian states. Econ. Hum. Biol. 2015;17:177–189. doi: 10.1016/j.ehb.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M. The relationship of C-reactive protein to obesity-related depressive symptoms: A longitudinal study. Obesity. 2013;21:248–250. doi: 10.1002/oby.20051. [DOI] [PubMed] [Google Scholar]
- Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N. Engl. J. Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- Datt G. Poverty in India and Indian states: an update. Food Consum. Nutr. Div. Discuss. Pap. 47. 1998 [Google Scholar]
- Daymont TN, Andrisani PJ. Job preferences, college major, and the gender gap in earnings. J. Hum. Resour. 1984;19:408–428. [Google Scholar]
- Deaton A, Dreze J. Poverty and inequality in India: a re-examination. Econ. Polit. Wkly. 2002;37:3729–3748. [Google Scholar]
- Deaton A, Kozel V. Data and dogma: the great Indian poverty debate. World Bank Res. Obs. 2005;20:177–199. [Google Scholar]
- Denny SD, Kuchibhatla MN, Cohen HJ. Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am. J. Med. 2006;119:327–334. doi: 10.1016/j.amjmed.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Ezzati M, Vander Hoorn S, Lawes CM, Leach R, James WPT, Lopez AD, Rodgers A, Murray CJ. Rethinking the “diseases of affluence” paradigm: global patterns of nutritional risks in relation to economic development. PLoS Med. 2005;2:e133. doi: 10.1371/journal.pmed.0020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel R. Health, Nutrition, and Economic Growth. Econ. Dev. Cult. Change. 2004a;52:643–658. [Google Scholar]
- Fogel R. The escape from hunger and premature death, 1700-2100: Europe, America, and the Third World. Cambridge University Press; 2004b. [Google Scholar]
- Fogel R. Economic Growth, Population Theory, and Physiology: The Bearing of Long-Term Processes on the Making of Economic Policy. Am. Econ. Rev. 1994;84:369–395. [Google Scholar]
- Ghosh S. Exploring socioeconomic vulnerability of anaemia among women in eastern Indian States. J. Biosoc. Sci. 2009;41:763–787. doi: 10.1017/S0021932009990149. [DOI] [PubMed] [Google Scholar]
- Gwatkin DR. Metrics matter: the case of assessing the importance of non-communicable diseases for the poor. Int. J. Epidemiol. 2013;42:1211–1214. doi: 10.1093/ije/dyt167. [DOI] [PubMed] [Google Scholar]
- Hamer M, Molloy GJ. Association of C-reactive protein and muscle strength in the English Longitudinal Study of Ageing. Age. 2009;31:171–177. doi: 10.1007/s11357-009-9097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Herningtyas EH, Kale V, Crimmins EM, Risbud AR, McCreath H, Lee J, Strauss J, O’Brien JC, Bloom DE. External Quality Control for Dried Blood Spot-Based C-Reactive Protein Assay: Experience from the Indonesia Family Life Survey and the Longitudinal Aging Study in India. Biodemography Soc. Biol. 2015;61:111–120. doi: 10.1080/19485565.2014.1001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann B. A Stata implementation of the Blinder-Oaxaca decomposition. Stata J. 2008;8:453–479. [Google Scholar]
- Johnston DW, Propper C, Shields MA. Comparing subjective and objective measures of health: Evidence from hypertension for the income/health gradient. J. Health Econ. 2009;28:540–552. doi: 10.1016/j.jhealeco.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. The Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- Lee J, Arokiasamy P, Chandra A, Hu P, Liu J, Feeney K. Markers and drivers: Cardiovascular health of middle-aged and older Indians. In: Smith JP, Majmundar M, editors. Aging in Asia: Findings from New and Emerging Data Initiatives. National Academies Press; 2012. [PubMed] [Google Scholar]
- Lee J, Smith JP. Regional disparities in adult height, educational attainment, and late-life cognition: Findings from the Longitudinal Aging Study in India (LASI) J. Econ. Ageing. 2014;4:26–34. doi: 10.1016/j.jeoa.2014.02.002. doi:10.1016/j.jeoa.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fang H, Zhao Z. Urban–rural disparities of child health and nutritional status in China from 1989 to 2006. Econ. Hum. Biol. 2013;11:294–309. doi: 10.1016/j.ehb.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Sherlock P, Beard J, Minicuci N, Ebrahim S, Chatterji S. Hypertension among older adults in low-and middle-income countries: prevalence, awareness and control. Int. J. Epidemiol. 2014;43:116–128. doi: 10.1093/ije/dyt215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer J. Education and male-female differences in later-life cognition: International evidence from Latin America and the Caribbean. Demography. 2011;48:915–930. doi: 10.1007/s13524-011-0048-x. [DOI] [PubMed] [Google Scholar]
- McDade TW, Burhop J, Dohnal J. High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin. Chem. 2004;50:652–654. doi: 10.1373/clinchem.2003.029488. [DOI] [PubMed] [Google Scholar]
- Myers G, Rifai N, Tracy R, Roberts W, Alexander R, Biasucci L, Catravas J, Cole T, Cooper G, Khan B. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease-Application to Clinical and Public Health Practice-Report from the laboratory science discussion group. Circulation. 2004;110:E545–E549. doi: 10.1161/01.CIR.0000148980.87579.5E. [DOI] [PubMed] [Google Scholar]
- Narayan KV, Ali MK, Koplan JP. Global noncommunicable diseases—where worlds meet. N. Engl. J. Med. 2010;363:1196–1198. doi: 10.1056/NEJMp1002024. [DOI] [PubMed] [Google Scholar]
- Oaxaca R. Male-female wage differentials in urban labor markets. Int. Econ. Rev. 1973;14:693–709. [Google Scholar]
- O’Broin S, Gunter EW. Screening of folate status with use of dried blood spots on filter paper. Am. J. Clin. Nutr. 1999;70:359–367. doi: 10.1093/ajcn/70.3.359. [DOI] [PubMed] [Google Scholar]
- Powell LM, Wada R, Krauss RC, Wang Y. Ethnic disparities in adolescent body mass index in the United States: the role of parental socioeconomic status and economic contextual factors. Soc. Sci. Med. 2012;75:469–476. doi: 10.1016/j.socscimed.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice AM. The emerging epidemic of obesity in developing countries. Int. J. Epidemiol. 2006;35:93–99. doi: 10.1093/ije/dyi272. [DOI] [PubMed] [Google Scholar]
- Ravallion M, Datt G. Why has economic growth been more pro-poor in some states of India than others? J. Dev. Econ. 2002;68:381–400. [Google Scholar]
- Rosero-Bixby L, Dow WH. Surprising SES gradients in mortality, health, and biomarkers in a Latin American population of adults. J. Gerontol. B. Psychol. Sci. Soc. Sci. 2009;64:105–117. doi: 10.1093/geronb/gbn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A. Health: perception versus observation: Self reported morbidity has severe limitations and can be extremely misleading. BMJ. 2002;324:860. doi: 10.1136/bmj.324.7342.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty P. Grey matter: ageing in developing countries. The Lancet. 2012;379:1285–1287. doi: 10.1016/s0140-6736(12)60541-8. [DOI] [PubMed] [Google Scholar]
- Sinning M, Hahn M, Bauer TK. The Blinder-Oaxaca decomposition for nonlinear regression models. Stata J. 2008;8:480–492. [Google Scholar]
- Subramanian S, Corsi DJ, Subramanyam MA, Smith GD. Jumping the gun: the problematic discourse on socioeconomic status and cardiovascular health in India. Int. J. Epidemiol. 2013;42:1410–1426. doi: 10.1093/ije/dyt017. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Perkins JM, Khan KT. Do burdens of underweight and overweight coexist among lower socioeconomic groups in India? Am. J. Clin. Nutr. 2009;90:369–376. doi: 10.3945/ajcn.2009.27487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellakkal S, Subramanian S, Millett C, Basu S, Stuckler D, Ebrahim S. Socioeconomic inequalities in non-communicable diseases prevalence in India: disparities between self-reported diagnoses and standardized measures. PloS One. 2013;8:e68219. doi: 10.1371/journal.pone.0068219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikram NK, Misra A, Dwivedi M, Sharma R, Pandey R, Luthra K, Chatterjee A, Dhingra V, Jailkhani B, Talwar K. Correlations of C-reactive protein levels with anthropometric profile, percentage of body fat and lipids in healthy adolescents and young adults in urban North India. Atherosclerosis. 2003;168:305–313. doi: 10.1016/s0021-9150(03)00096-0. [DOI] [PubMed] [Google Scholar]
- World Health Organization India: Country profile, Noncommunicable diseases. 2014.
- World Health Organization Preventing chronic diseases: a vital investment. 2005.