Abstract
Depressive disorders are associated with significant economic and public health burdens as well as increased morbidity. Yet, perhaps due to the heterogeneous nature of the disease, prevention and intervention efforts are only moderately efficacious. A better understanding of core mechanisms of depressive disorders might aid in the development of more targeted intervention, and perhaps help identify individuals at risk. One mechanism that may be particularly important to depressive phenotypes is reward insensitivity. Examination of neurobiological correlates of reward-processing, which should relate more directly to the neuropathology of depression, may be helpful in identifying liability for the disorder. To that end, we used a family study design to examine whether a neural response to rewards is a familial risk factor for depression in a sample of probands with a wide range of internalizing psychopathology, as well as their biological siblings. Event-related potentials were recorded during a simple forced-choice gambling paradigm, in which participants could either win or lose small amounts of money. Lower levels of Positive Affect in probands predicted a reduced neural response to rewards in siblings, even over and above the sibling’s own level of positive and negative affect. Additionally, the neural response to rewards was familial (i.e., correlated among siblings). Combined, these analyses suggest that a blunted neural response to rewards may be useful in identifying individuals vulnerable to depressive illnesses.
Keywords: Reward-related Positivity, Feedback-related Negativity, positive affect, vulnerability, Depression
Major Depressive Disorder (MDD) is a leading cause of disability, morbidity, and diminished quality of life worldwide (Greenberg, Stiglin, Finkelstein, & Berndt, 1993; Mathers, Fat, & Boerma, 2008; Murray et al., 2013). Unfortunately, existing treatment and prevention efforts are only moderately efficacious (Bohlmeijer, Fledderus, Rokx, & Pieterse, 2011; Calear & Christensen, 2010; Kupfer et al., 1989; Reynolds et al., 2012). The Research Domain Criteria (RDoC) project aims to solve some of these persistent problems (Cuthbert, 2014; Cuthbert & Insel, 2010). RDoC seeks to move beyond the current diagnostic nomenclature, and instead identify transdiagnostic dimensional constructs—agnostic to disorder categories—that reflect abnormalities in fundamental functions of the central nervous system. These abnormalities are also presumed to index mechanisms for psychopathology. Identifying dysfunction in these core neural systems should help clarify pathogenesis in more homogenous groups of individuals, as well as identify more specific mechanisms and targets for intervention and prevention (Kujawa, Proudfit, & Klein, 2014; Nelson et al., 2013; Proudfit, 2014; Schmidt, Shelton, & Duman, 2011).
The ability to detect and respond to positive events in the environment likely represents one such fundamental neural function, and is well-represented in the Positive Valence Systems domain of the RDoC matrix. Reward responding relies heavily on a neural network responsible for the production and regulation of dopamine (DA; Heinz, Schmidt, & Reischies, 1994). This system, which includes neurons in the ventral tegmental area of the midbrain projecting to the striatum and the medial prefrontal cortex (Schultz, 2006), is thought to code the incentive properties of stimuli and events in the environment (Allman, Hakeem, Erwin, Nimchinsky, & Hof, 2001). Activity of this system increases, for example, following receipt of unexpected rewards, including primary (e.g., food) and secondary (e.g., money) reinforcers (O'Doherty et al., 2004).
Yet there is also enormous variation in the ability to respond adaptively to rewards (Pelizza & Ferrari, 2009; Shankman, Klein, Tenke, & Bruder, 2007; Shankman et al., 2013). In fact, many have proposed that low Positive Affect (PA), a trait which encompasses anhedonia and blunted reward response (Clark & Watson, 1991; Davidson, 1994; Depue & Iacono, 1989; Tellegen, 1985; Watson et al., 1999), constitutes a core deficit of unipolar mood disorders (Bogdan, Nikolova, & Pizzagalli, 2012; Nelson et al., 2013; Shankman et al., 2013; Treadway & Zald, 2011). Indeed, whereas high Negative Affect (NA) is thought to be a nonspecific vulnerability factor across multiple internalizing disorders, low PA is thought to be specific to depression (e.g., Mineka, Watson, & Clark, 1998; Shankman et al., 2013; Watson, Clark, et al., 1995; Watson et al., 1995). PA relies heavily on the activity of the ventral striatal dopamine system (e.g., Burgdorf & Panksepp, 2006), and there is evidence that blunted neural response to rewards relates specifically to low PA, and not symptoms of anxiety or broad NA (Foti, Carlson, et al., 2014; Keedwell, Andrew, Williams, Brammer, & Phillips, 2005; Shankman et al., 2013).
Low PA/ blunted response to rewards appears not only to be a pernicious characteristic of depression, but may also be a viable vulnerability marker for depression. In addition to evidence for familial aggregation of unipolar depressive disorders (Johnson, McGue, Gaist, Vaupel, & Christensen, 2002; Kendler, 1995), reward processing deficits appear to be subject to significant familial (e.g., Farmer et al., 2002) and perhaps genetic contributions (Bogdan & Pizzagalli, 2009). Moreover, children of depressed parents, who are themselves at elevated risk for depression (Gotlib, Joormann, & Foland-Ross, 2014; Hammen, 2009; Joormann, Eugène, & Gotlib, 2008; Weissman et al., 2006), tend to show blunted reward responding even in the absence of clinically-significant levels of depression (Gotlib et al., 2010). Reward-related impairments have also been shown to prospectively predict the onset of depression (Forbes, Shaw, & Dahl, 2007; Rawal, Collishaw, Thapar, & Rice, 2013). These deficits also appear to be relatively stable over time (Shankman et al., 2013), suggesting that neurobehavioral markers of this construct are also likely to be present in vulnerable individuals.
One classic way of identifying vulnerability markers is the family study method (Kendler, 2006; Robins & Guze, 1970). While family designs cannot disentangle environmental from genetic effects, they can examine familial vulnerability. Specifically, family studies can be used to examine: 1) whether reward sensitivity in probands predicts reward sensitivity in their first-degree relatives (i.e., if decreased reward sensitivity is a vulnerability marker, it should aggregate within families) and 2) whether reward sensitivity is abnormal in ‘healthy’ (or low symptom) relatives of probands with low levels of PA—even taking the current functioning of the relatives into account. That is, if blunted response to rewards is a stable familial vulnerability marker, it should be state-independent in the low-symptom relatives, and relate more strongly to family history of depression (e.g., Gottesman and Gould, 2003). To that end, the present study examined neural response to rewards in a sample of probands and siblings with a wide array of internalizing disorders and symptoms.
The index of neural response to rewards used in the present study was the Feedback Negativity (FN), an event-related potential (ERP) component which is increasingly used in research on reward processing (Carlson, Foti, Mujica-Parodi, Harmon-Jones, & Hajcak, 2011; Miltner, Braun, & Coles, 1997; Weinberg, Luhmann, Bress, & Hajcak, 2012; Weinberg, Riesel, & Proudfit, 2014). The FN peaks approximately 250-300 ms at frontocentral recording sites following the presentation of feedback (Holroyd & Coles, 2002; Miltner et al., 1997), and has typically been quantified and conceptualized as a negativity elicited by loss feedback that is absent following gain feedback. However, recent evidence suggests that the apparent differentiation in the ERP between gain and loss trials is driven by variation in a reward-related positivity (called the RewP; Proudfit, 2014) that is larger and more positive for rewards than non-rewards (Carlson et al., 2011; Foti, Carlson, et al., 2014; Foti & Hajcak, 2010; Foti, Weinberg, Dien, & Hajcak, 2011; Proudfit, 2014; Weinberg et al., 2012; Weinberg et al., 2014). The magnitude of the RewP is moderated by variation in genes governing the synaptic degradation of dopamine (Foti & Hajcak, 2012). Moreover, it appears that a larger RewP is associated with increased striatal response to rewards (Becker, Nitsch, Miltner, & Straube, 2014; Carlson, Foti, Harmon-Jones, & Proudfit, 2014; Carlson et al., 2011; Foti, Carlson, et al., 2014).
The magnitude of the RewP also reflects individual differences in reward sensitivity: a larger RewP is associated with both self-reported reward sensitivity (Bress, Smith, Foti, Klein, & Hajcak, 2012) and behavioral measures of reward-driven response biases (Bress & Hajcak, 2013). Additionally, there is evidence that depression is associated with a blunted RewP (Bress et al., 2012; Foti & Hajcak, 2009; Foti, Carlson, et al., 2014; Liu et al., 2014). This blunted RewP also appears to relate specifically to symptoms of depression, and not anxiety (Bress, Meyer, & Hajcak, 2013; Bress et al., 2012). Moreover, there is evidence that—even within a depressed sample—the blunted RewP relates most specifically to a decreased ability to respond to positive events in the environment, and is not observed across all symptoms of depression (Foti, Carlson, Sauder, & Proudfit, 2014).
A blunted RewP not only may be a correlate of depression but may also represent a neural marker of vulnerability for depressive disorders. For instance, a smaller RewP appears to prospectively predict the onset of depression, even accounting for other known risk factors (Bress, Foti, Kotov, Klein, & Hajcak, 2013). There is also emerging evidence that a family history of depression is associated with a blunted RewP in unaffected individuals (Foti, Kotov, Klein, & Hajcak, 2011; Kujawa et al., 2014). These two studies had similar aims to the present study, however, both only examined the RewP in the never-affected individual; thus, they were not able to examine or control for the familial nature of the RewP itself. Moreover, each of these studies used diagnoses of Major Depressive Disorder (MDD) as predictors of vulnerability. MDD and reward insensitivity are not synonymous (Foti, Carlson, et al., 2014; Pelizza & Ferrari, 2009) as not all individuals with MDD are reward insensitive, and low reward sensitivity may be present in individuals who fall below diagnostic cutoff for MDD as well as in other psychopathologies (e.g., generalized anxiety disorder and social anxiety disorder; DeVido et al., 2009; Guyer et al., 2012; Jazbec, McClure, Hardin, Pine, & Ernst, 2005; Kashdan, 2004).
The present study therefore used a family design and collected neural response to rewards in both probands and siblings. Rather than examining the transmission of MDD, we examined the familial nature of PA, measured dimensionally in a community sample selected to have a broad range of internalizing psychopathology. In addition, the present study examined whether the blunted RewP represents a familial vulnerability marker for low PA. In other family studies, this is done by looking at the risk factor in ‘healthy’ siblings (e.g., comparing eye-tracking dysfunction in healthy relatives of schizophrenics to relatives of controls; Holzman et al., 1974). However, in line with the RDoC initiative, any definition of ‘healthy siblings’ (and diagnostic group) would be arbitrary. We therefore identified probands on the basis of which sibling within the pair had higher self-reported symptoms of depression. Siblings in each instance were less symptomatic than the probands, but were still permitted to vary on their levels of internalizing symptomatology. We modeled sibling symptoms continuously and examined whether sibling symptoms or proband symptoms were better predictors of neural response in siblings.
Consistent with previous evidence for the familial nature of reward-processing deficits, we hypothesized that reward sensitivity (measured via self-reports of PA and the magnitude of the RewP) would be familial. We further predicted that the RewP in the low-symptom (i.e., “healthy”) siblings would relate specifically to probands’ self-report of PA, but not NA. And finally, we anticipated that proband PA may be a better predictor of sibling RewP than sibling PA (particularly given that siblings were selected to have low levels of symptoms). This pattern of results would indicate that the magnitude of neural response to rewards in unaffected individuals reflects vulnerability for depressive illness, rather than current symptom dysfunction (Kujawa et al., 2014).
Method
Participants
Participants were recruited from the community and area mental health clinics (via fliers, Internet postings, etc.), on the basis of symptoms of anxiety and depression. In keeping with the aims of RDoC, we did not use a cutoff to demarcate between normal and abnormal levels of internalizing symptoms (Cuthbert, 2014; Cuthbert & Insel, 2013). Instead, we used minimal symptom-based inclusion and exclusion criteria, and aimed to recruit a sample with a broad range of internalizing symptomatology. However, to ensure the clinical relevance of the sample, we also oversampled from individuals with severe psychopathology. Thus, the goal was to recruit a sample with normally distributed internalizing symptoms but with a mean more severe than the mean of the general population. Prior to their involvement in the study, participants were screened via telephone using the Depression, Anxiety, and Stress Scale (DASS; Lovibond & Lovibond, 1995), a brief (21-item) measure of broad internalizing psychopathology (the measure was used to ensure that the sample had the above-mentioned distribution on internalizing symptoms). As manic and psychotic symptoms have been shown to be separable from internalizing disorders (Watson, 2005), probands and siblings were excluded during screening if they had a personal or 1st degree family history of a manic/hypomanic episode or psychotic symptoms, assessed via items from the Structured Clinical Interview for DSM–IV (SCID; First, Spitzer, Gibbon, & Williams, 1996). Participants were also excluded if they were unable to read or write English, had a history of head trauma with loss of consciousness, or were left-handed. Potential participants were not excluded based on current psychotropic medication use, or current substance use.
Participants were eligible for the study if they were between the ages of 18 and 30, and had a full sibling between the ages of 18 and 30 who was also interested in participating. We opted to recruit siblings rather than other relatives because this approach allowed us to have siblings and probands matched on mean age. We restricted the age of the probands and siblings to 18-30 because we were interested in risk for internalizing psychopathology. It was therefore critical that ‘healthy’ (or low symptom) siblings were not out of the peak risk period for internalizing disorders (through age 45; Kessler et al., 2005). The premise of examining whether healthy or low-symptom siblings of symptomatic probands have abnormal neural responses is that even though siblings have not developed significant symptoms, they still may carry the vulnerability marker (Zubin & Spring, 1977). However, if a low symptom sibling was significantly past the peak risk period (e.g., age 50) and still had not developed symptoms, they may be less likely to carry the vulnerability marker, or may even be characterized by some resilience process that counteracted their vulnerability.
Participants were only included in the analyses that follow if complete ERP and self-report data were available from both members of the sibling pair (n = 160). Of these, 10 individuals (from 10 families) were excluded as a result of excessive noise in the ERP data, leaving a final sample of n = 140 individuals, from 70 sibling pairs. The final sample was 59% female, and was racially diverse (43.5% Caucasian-American, 28% Hispanic, 13.4% African-American, 7% Asian, 3.7 % Middle Eastern, 2.4% “Other”, and 2.4 % Mixed Race), well-educated (48.7% had completed at least some college education; 21.5% had completed four years of college) and relatively young (Age M = 22.54, SD = 3.15). All procedures were approved by the University of Illinois–Chicago Institutional Review Board.
Measures
Current depression and anxiety symptoms were assessed in all participants using the expanded Inventory of Depression and Anxiety Symptoms (IDAS-II; Watson et al., 2012). The IDAS-II is a 99-item factor-analytically derived self-report inventory of empirically distinct dimensions of depression and anxiety symptoms. Each item assesses symptoms over the past two weeks on a 5-point Likert scale ranging from 1 (Not at all) to 5 (Extremely). The IDAS-II has demonstrated good internal consistency, test-retest reliability, and convergent and discriminant validity with diagnoses and self-report measures in similar populations (Watson et al., 2007; Watson et al., 2012). The PA scale in the IDAS is called “well-being,” (8 items, α = .84), and is defined by items reflecting high energy and positive affect specifically relating to depression (Watson et al., 2007; Watson et al., 2012). In order to demonstrate the specificity of this association, we also include the NA scale, “dysphoria” (10 items, α = .88).
In addition to self-report, lifetime diagnoses of depression were assessed via the SCID (First, Spitzer, Gibbon, & Williams, 1996), during their visit to the lab. Diagnosticians were trained to criterion on the SCID and supervised by a licensed clinical psychologist. In addition to depression symptoms, interviewers dimensionally assessed functional impairment and subjective distress due to past depression. Interviewers made separate ratings for impairments in the domains of social, occupational, and daily life, as well as perceived distress, along a nine-point scale ranging from 0 (None) to 8 (Severe). The anchors for the scale were adopted from the Anxiety Disorders Interview Schedule (ADIS-IV; Brown, DiNardo, & Barlow, 1994) in which a 2 or higher was clinically significant distress or impairment.
Procedure
Both probands and siblings completed a battery of tasks and the order was counterbalanced across subjects. The present task was administered on a Pentium class computer, using the stimulus presentation software Presentation (Neurobehavioral Systems, Inc.).
Task and Materials
The reward task was a simple guessing task which has been used in other studies concerned with reward processing (Foti & Hajcak, 2010; Foti, Weinberg, Dien, & Hajcak, 2011). The task consisted of 60 trials, presented in three blocks of 20. At the beginning of each trial, participants were presented with an image of two doors and were instructed to choose one door by clicking the left or right mouse button. The doors remained on the screen until the participant responded. Next, a fixation mark (+) appeared for 1000 ms, and feedback was presented on the screen for 2000 ms. Participants were told that they could either win $0.50 or lose $0.25 on each trial. A win was indicated by a green “↑,” and a loss was indicated by a red “↓.” Next, a fixation mark appeared for 1500 ms and was followed by the message “Click for the next round,” which remained on the screen until the participant responded and the next trial began. Across the task, 30 win and 30 loss trials were presented in a random order.
Psychophysiological Recording, Data Reduction and Analysis
Continuous EEG recordings were collected using an elastic cap and the ActiveTwo BioSemi system (BioSemi, Amsterdam, Netherlands). Sixty-four electrodes were used, based on the 10/20 system, as well as two electrodes on the right and left mastoids. Electrooculogram (EOG) generated from eye movements and eyeblinks was recorded using four facial electrodes: horizontal eye movements (HEM) were measured via two electrodes located approximately 1 cm outside the outer edge of the right and left eyes. Vertical eye movements (VEM) and blinks were measured via one electrode placed approximately 1 cm below the left eye and electrode FP1. The data were digitized at a sampling rate of 1024 Hz, using a low-pass fifth order sinc filter with −3dB cutoff point at 208 Hz. Each active electrode was measured online with respect to a common mode sense (CMS) active electrode, located between PO3 and POz, producing a monopolar (non-differential) channel. CMS forms a feedback loop with a paired driven right leg (DRL) electrode, located between POz and PO4, reducing the potential of the participants and increasing the common mode rejection rate (CMRR). Offline, all data were analyzed in Brain Vision Analyzer (BVA) and were referenced to the average of the left and right mastoids, and band-pass filtered from 0.1 to 30 Hz; eye-blink and ocular corrections were conducted per an extension of the Gratton, Coles, and Donchin (1983) algorithm, which accounts for both vertical and horizontal eye movements.
A semi-automatic procedure was employed to detect and reject artifacts. The criteria applied were a voltage step of more than 50.0 µV between sample points, a voltage difference of 300.0 µV within a trial, and a maximum voltage difference of less than .50 µV within 100 ms intervals. Visual inspection of the data was then conducted to detect and reject any remaining artifacts.
We used Principal Components Analysis (PCA) to parse variability in the ERP related to gains versus loss. Traditional trial-averaged waveform measures of ERPs can conflate the activity of multiple components which overlap both spatially and temporally (e.g., Foti, Weinberg, et al., 2011; Luck, 2005, 2012). Variation in the magnitude of activity in the time-window of the RewP, for instance, could be driven by either reward- or loss-related processes, or both, but in component-scored methods it can be difficult to isolate the contribution each component makes to the observed ERP. PCA, which can empirically identify unique sources of systematic variance within the trial-averaged waveform, may allow for more accurate identification of these components. Therefore, we used a two-step temporal-spatial PCA to better isolate reward- from loss-related variance within the trial-averaged waveform. While PCA can be vulnerable to latency and topographic variability across subjects1, this two-step PCA has been shown to be particularly adept at addressing latency and topographic variability across subjects. As demonstrated by Dien (1998), the temporal and spatial PCAs have complementary—albeit counterintuitive—strengths: The temporal PCA is adept at identifying components that are spatially jittered, while the spatial PCA can characterize components that are temporally jittered (see Dien, 1998, for a more in-depth explanation of this).
The EEG was segmented into 1200 ms windows for each trial, beginning 200 ms before each feedback onset and continuing for 1,000 ms following feedback. A 200 ms window from −200 to 0 ms prior to feedback onset served as the baseline. Following this, two ERP averages for each participant were entered into the data matrix for the PCA (i.e., Reward and Non-reward). Using the Matlab ERP PCA Toolbox - Version 2 (Dien, 2010a), a temporal PCA was performed first in order to capture variance across time and to maximize the initial separation of ERP components (Dien & Frishkoff, 2005). A promax rotation was used to rotate to simple structure in the temporal domain (Dien, 2010b; Dien, Khoe, & Mangun, 2007). Following the first rotation, a parallel test (Horn, 1965) was conducted on the resulting Scree plot (Cattell, 1966), in which the Scree of the actual dataset is compared to a Scree plot derived from a fully random dataset. The number of factors retained is based on the largest number of factors that account for a greater proportion of variance than the fully random dataset (see Dien, 2010a for more information). Based on this criterion, 20 temporal factors were extracted for rotation, and the covariance matrix and Kaiser normalization were used (Dien, Beal, & Berg, 2005).
Following the temporal PCA, a spatial PCA was performed on each temporal factor retained in the previous step in order to reduce the spatial dimensions of the datasets. Infomax was used to rotate the spatial factors to independence (Dien, 2010b; Dien et al., 2007). Based on the results of the parallel test (Horn, 1965), four spatial factors were extracted from each temporal factor for Infomax rotation, yielding a total of 80 temporospatial factor combinations. To directly assess timing and spatial voltage distributions, we then translated the factors back into voltages (see, e.g., Foti, Weinberg, Dien, & Hajcak, 2011, for a more detailed account of the methods). Sixteen factor combinations accounted for more than 1% of the variance each. Of these, one factor combination resembled the RewP, both in terms of timing and scalp distribution, and significantly differentiated gains from non-reward (Temporal Factor 3, Spatial factor 1, TF3SF1). This factor combination accounted for 5.99% of the variance in the overall solution. We report the factor loadings for the non-reward and reward conditions separately.
Data were then statistically evaluated using SPSS (Version 20). Pearson’s r and intraclass correlations (ICC) were calculated for each measure the siblings had in common (e.g., proband PA associated with sibling PA). Pearson’s r was also calculated across measures (e.g., proband PA associated with sibling RewP) to examine the association between proband self-report and sibling neural response. Following this, two hierarchical linear regressions were conducted to examine the relative contribution of proband and sibling symptoms to sibling response.
Results
Participant characteristics
Table 1 displays demographic information for the proband and sibling groups, as well as means for subscales of the IDAS 2. As indicated, probands reported significantly more symptoms of depression than their siblings, as well as lower levels of PA. For the probands, means on each of the scales reported on here were within one standard deviation of the means for the outpatient clinical samples reported on in Watson and colleagues (2007).
Table 1.
Demographic information as well as means and standard deviations for subscales of the Inventory of Depression and Anxiety Symptoms (IDAS-II) for probands and siblings
| Proband |
Sibling |
|||
|---|---|---|---|---|
| Age | 22.62 (3.18) | 22.40 (3.10) | ||
| Sex (% female) | 62.2% | 57.3% | ||
| Psychotropic Medication Use | 12 (9.6%) | 6 (4.8%) | ||
| IDAS Scales | Range | Range | ||
| NA (Dysphoria) | 19.38 (7.71) ** | 10-42 | 14.12 (4.44) | 10-29 |
| PA (Well-Being) | 24.04 (6.35) ** | 8-35 | 27.68 (6.24) | 10-39 |
Note. * indicates p < .05;
indicates p < .001;
IDAS = Inventory of Depression and Anxiety Symptoms; PA = Positive Affect; NA = Negative Affect
Results of the PCA
The PCA-derived factor of interest (i.e., TF3SF1) was evident as a central positivity maximal at 330 ms. These results resemble the results of several previous PCA analyses of the RewP (Carlson et al., 2011; Foti, Weinberg, et al., 2011; Liu et al., 2014; Weinberg et al., 2014), in that the positivity is enhanced for rewards (M = 20.01, SD = 9.26) relative to non-rewards (M = 12.81, SD = 7.77), F(1, 128) = 144.16, p < .001, ηp2= .53. In what follows, we report results for factor loadings for non-reward and reward trials separately.
Within-Subject Association of Symptoms and ERPs
Table 2 displays Pearson’s correlations between ERP factor scores and the IDAS PA and NA subscales within the proband and sibling groups separately. In the probands, the magnitude of the response to gains correlated with PA in probands, such that greater PA predicted a larger response to gains. No such correlation was observed within the siblings.
Table 2.
Correlations between self-report and Event-Related Potential (ERP) variables for probands and siblings
| Probands | Siblings | |||
|---|---|---|---|---|
|
|
||||
| ERP to Non-Reward |
ERP to Reward | ERP to Non-Reward |
ERP to Reward | |
| NA | .07 | .04 | .24 | .01 |
| PA | .20 | .27* | −.04 | −.10 |
Note. **p < .01.
p < .05 ;
PA = Positive Affect; NA = Negative Affect
Familial Nature of ERPs and Symptoms
Table 3 displays Pearson’s correlations and ICC between sibling pairs for neural response to rewards and non-rewards, as well as symptoms reported on the IDAS. As shown in Table 3, both the neural response to rewards and non-rewards appear familial. However, the association was stronger for the response to rewards. The correlation between proband and sibling responses to non-rewards was only marginally significant. There was also evidence for familial transmission of current symptoms of NA, as well as trend-level associations for current PA.
Table 3.
Correlations of self-report and Event-Related Potential (ERP) measures between sibling Pairs.
| Sibling Pair r |
Sibling Pair ICC |
Proband IDAS and Sibling ERP to Non-Reward r |
Proband IDAS and Sibling ERP to Reward r |
|
|---|---|---|---|---|
| ERP to Non-Reward | .16 | .28† | -- | -- |
| ERP to Reward | .31* | .47** | -- | -- |
| NA | .57** | .66** | −.07 | −.12 |
| PA | .12 | .21 | .23 | .31* |
Note.
p < .01.
p < .05.
p =.10;
ERP = Event-Related Potential; ICC = Intraclass Correlation; r = Pearson’s Correlation; PA = Positive Affect; NA = Negative Affect
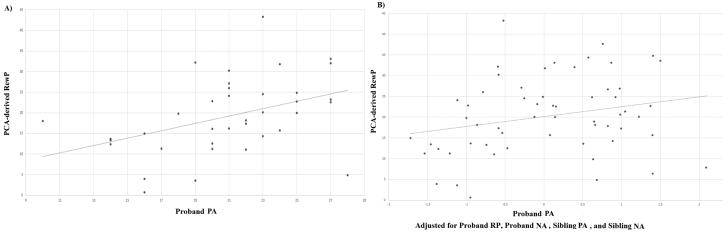
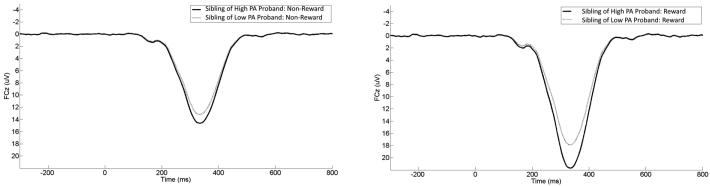
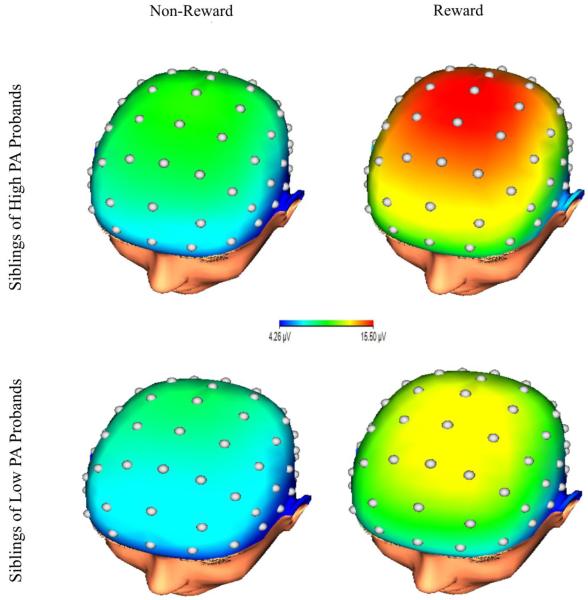
Because we were interested in predicting sibling neural response to rewards from proband symptoms, cross-measure correlations between sibling pairs are also presented in Table 3. As indicated, only proband PA was significantly related to sibling ERPs. This effect was significant and stronger for the response to rewards than to non-rewards. A scatterplot depicting this bivariate association with the response to rewards is presented in Figure 1 (A). For presentation purposes, a median split was conducted on proband PA (note: analyses were not conducted on this median split). Figure 2 displays grand average response-locked ERPs at FCz, displaying the ERPs in siblings of probands with high (i.e., above the median) levels of PA and low (i.e., below the median) levels of PA. Topographic maps are also presented in Figure 3 for siblings of probands high (top) and low (bottom) in PA. These maps depict voltage (in µV) across the scalp for non-reward (left) and reward (right) responses in the time window of the RewP.
Figure 1.
Scatterplots depicting the association between proband PA and sibling Event-Related Potential (ERP) to rewards. The zero-order correlation is depicted on the left. The right panel depicts the association controlling for the familial nature of the neural response to rewards, proband NA, and sibling PA and NA.
Figure 2.
Stimulus-locked Event-Related Potential (ERP) waveforms at electrode site FCz for siblings. For presentation purposes, a median split was conducted on proband PA to create groups of siblings of probands who were high in PA and siblings of probands who were low in PA. Responses to non-reward in siblings of high and low PA probands are on the left, corresponding responses to reward are on the right. For each panel, feedback onset occurred at 0 ms. Per ERP convention, negative voltages are plotted up.
Figure 3.
Scalp topographies representing the neural response to non-rewards (left) and neural response to rewards (right). These maps depict the average response in siblings of high PA probands (top) and low PA probands (bottom).
Specificity of effects (PA vs. NA and effect of sibling’s own symptoms)
Following this, two hierarchical multiple regression analyses were calculated to examine effects on sibling responses to rewards and non-rewards. In each instance, proband neural response to rewards or non-rewards was entered in the first step, followed by proband PA in the second step, and proband NA in the third step. Finally, sibling PA and NA were entered in the fourth step.
Results for neural response to rewards are presented in Table 4. In the final model, proband neural response to rewards continued to significantly predict sibling neural response to rewards, β=.27, t(57) = 2.00, p < .05. Additionally, the proband’s level of PA predicted the sibling’s neural response to rewards, β=.30, t(57) = 2.22, p < .05, even after accounting for proband NA, sibling PA, and sibling NA. Neither proband NA t(57) = .17, p = .87, sibling PA t(57) = .92, p = .36 nor sibling NA t(57) = .13, p = .90 significantly predicted the magnitude of the sibling neural response to rewards.
Table 4.
Hierarchical regression with proband Event-related Potential (ERP) to Rewards, proband Positive Affect (PA), proband Negative Affect (NA), sibling PA and sibling NA predicting sibling ERP to rewards.
| Predictor | Final b (SE) | Entry β | 95 % CI | Final β | 95 % CI |
|---|---|---|---|---|---|
| 1. Proband ERP to Rewards | .27 (.13) | .30* | .05−.56 | .27* | −.001−.54 |
| 2. Proband PA | .46 (.21) | .28* | .02−.83 | .30* | .02−.88 |
| 3. Proband NA | −.03 (.19) | −.04 | −.35−.25 | −.03 | −.42−.35 |
| 4. Sibling PA | −.17 (.19) | -- | −.12 | −.56−.20 | |
| 4. Sibling NA | −.04 (.32) | -- | −.02 | −.70−.59 | |
|
| |||||
| Total model R2 = .18 | |||||
Note.
**p < .01.
p < .05;
Results for neural response to non-rewards are presented in Table 5. After controlling for the effects of proband and sibling self-report, proband neural response to non-rewards did not significantly predict sibling neural response to non-rewards t(57) = .21, p = .84, nor did proband PA t(57) = 1.20, p = .24. In addition, neither sibling PA t(57) = .22, p = .83 nor sibling NA t(57) = 1.81, p = .07 significantly predicted the magnitude of the sibling neural response to non-rewards.
Table 5.
Hierarchical regression with proband event-related potential (ERP) response to non-reward, proband PA, proband NA, sibling PA and sibling NA predicting sibling ERP to non-rewards.
| Predictor | Final b (SE) | Entry β | 95 % CI | Final β | 95 % CI |
|---|---|---|---|---|---|
| 1. Proband ERP to Non-Rewards | .03 (.16) | .13 | −.11−.46 | .03 | −.28−.35 |
| 2. Proband PA | .22 (.18) | .17 | −.15−.58 | .17 | −.16−.60 |
| 3. Proband NA | −.15 (.16) | .03 | −.24−.29 | −.15 | −.48−.18 |
| 4. Sibling PA | −.04 (.16) | -- | −.03 | −.37−.29 | |
| 4. Sibling NA | .52 (.29) | -- | .32 | −.07−1.10 | |
|
| |||||
| Total model R2 = .10 | |||||
Note. **p < .01. *p < .05
Sibling history of Depression
The analyses above suggest that a sibling’s neural response to rewards is better predicted by proband symptoms than the siblings’ own current symptoms. In order to examine whether a blunting in siblings’ neural response to rewards might be a consequence of previous depressive episodes, we also compared the neural response to rewards in siblings with (n = 18) and without (n = 52) a prior diagnosis of MDD. The neural response to rewards t(69) = .46, p = .65 did not differentiate siblings with and without a history of MDD. Finally, we examined whether the neural response to rewards in siblings was related to past depression-related impairment. No measure of functional impairment (social, occupational, daily life, or perceived distress) was significantly correlated with the magnitude of the neural response to rewards in siblings (all ps > .20).
Discussion
The results of the present study demonstrate that neural response to rewards is related to symptoms of depression, and is familial. Consistent with previous studies, symptoms of depression (specifically low PA) were associated with a blunted neural response to rewards in the more-depressed proband group (Foti, Carlson, et al., 2014; Foti & Hajcak, 2009). Additionally, the data indicate that low PA in probands is associated with a reduced neural response to rewards in their less-ill siblings, even controlling for the sibling’s own current PA and NA. These results suggest that the RewP is familial, and that it may reflect a familial liability that is independent of current symptom expression. Combined, these analyses suggest that a blunted neural response to rewards may represent a stable and enduring vulnerability factor for the development of depression, and may therefore be a viable tool to aid in the identification of at-risk individuals.
These results are consistent with previous studies that have found that abnormal response to rewards may be a vulnerability marker for MDD (Foti et al., 2011; Gotlib et al., 2010; Kujawa et al., 2014; McCabe et al., 2012). For example, a prospective study found that blunted striatal response to rewards in adolescents predicted increased depression two years later (Morgan, Olino, McMakin, Ryan, & Forbes, 2013), and McCabe, Cowen, & Harmer (2009) found that abnormal striatal functioning persists even after the remission of an MDD episode. Combined with the present results, these studies indicate that abnormalities in the neural system supporting reward responding may represent a trait marker of vulnerability for depression.
However, the present findings extend previous results in several key ways. First, as discussed above, depressive disorders are heterogeneous and not all individuals with these conditions exhibit blunted reward response (Foti, Carlson, et al., 2014; Pelizza & Ferrari, 2009). Moreover, there are several non-depressive disorders that are also associated with impairments in PA and reward processing (Bernat, Nelson, Steele, Gehring, & Patrick, 2011; Bjork, Chen, Smith, & Hommer, 2010; Gatzke-Kopp et al., 2009; Volkow et al., 2009; Volkow et al., 2010). The present analyses (and study design) therefore took an RDoC approach and examined the dimension of positive affect as a predictor. These results may therefore be useful in further specifying a vulnerability for a transdiagnostic dimension that has been shown to have important predictive validity (Fawcett, Clark, Scheftner, & Hedeker, 1983; Pelizza & Ferrari, 2009).
Second, the majority of studies examining the association between reward processing and family history of depression have only collected neural response from the vulnerable individual, not the affected family member. In the present study, we collected neural response from both members of a sibling pair. This allowed us to examine and control for the significant correspondence between proband and sibling reward response.
Finally, in the present study, we used PCA to empirically identify a positivity that is enhanced for rewards and blunted for non-rewards. These results align with evidence from previous work suggesting that the trial-averaged ERPs in this time-window may depend more upon a positive-going response to rewards that is absent or reduced on non-reward trials (Foti, Weinberg, et al., 2011; Weinberg et al., 2012; Weinberg et al., 2014). Isolating this reward-related activity further allowed us to demonstrate specificity of the PA-reward associations.
Consistent with the aims of RDoC, the sibling group in the present study included individuals who were currently experiencing some level of depressive symptoms. This allowed us to examine whether proband PA was a better predictor of sibling reward response than the sibling’s own symptoms. Moreover, within the sibling group, the RewP did not differentiate those with and without a history of MDD, and was not related to past depression-related impairments, suggesting that the blunted RewP is not merely a consequence of having experienced depression. Within the sibling group, no association was observed between the RewP and level of PA, which may be due to the limited range (and thus limited variance) of symptoms in the Sibling group.
The age range selected in the present sample also ensured that the low-symptom siblings can still be considered at risk. In the general population, approximately 50% of first-onset episodes of MDD or dysthymia will occur after the age of 30 (Kessler et al., 2005), and more than 25% of all first-onset episodes will occur between the ages of 30 and 44 (Kessler et al., 2005). With a mean age of 22, our sample of siblings can therefore still be considered vulnerable to a first onset of depression, particularly considering that we did not select siblings on the basis of very low levels of depressive symptoms. Indeed, some individuals in the sibling group reported elevated levels of depression relative to normative young adult samples (Watson et al., 2007), and there is evidence that adults with observable depressive symptoms are four to five times more likely than those without symptoms to develop clinically-significant levels of depression in a one-year period (Horwath, Johnson, Klerman, & Weissman, 1992). Thus, it is also possible that the blunted reward processing that we have observed reflects risk for an exacerbation of symptoms and an increase in severity, rather than the first emergence of symptoms. This is still an important form of vulnerability.
Another possibility is that the siblings we observed are not vulnerable individuals, but rather resilient (Ingram & Luxton, 2005). That is, even though the siblings of probands exhibit the vulnerability marker of blunted neural response to reward, there could be other factors that protected them from developing depressive symptoms (or at least symptoms at the level of the proband). Longitudinal prospective studies will also be important in understanding this.
Because the present design was a family study drawing from a sample of siblings, we cannot draw conclusions as to whether the observed effects can be attributed to genetic or environmental influences—or some interaction of the two (Ingram & Luxton, 2005). Although there is some support for the activity of dopaminergic genes relating specifically to the magnitude of the RewP (e.g., Foti & Hajcak, 2012), there is also evidence that the experience of acute environmental stress can attenuate the RewP (Bogdan, Santesso, Fagerness, Perlis, & Pizzagalli, 2011). Prospective and genetically-informed (i.e., twin) designs will be necessary to evaluate the way that reward sensitivity relates to the association between familial vulnerabilities and the development of depression.
In sum, blunted neural response to rewards was apparent in unaffected individuals with a family history of low PA, and this family history of low PA was a better predictor of sibling reward response than the unaffected individual’s own current symptoms. It is important to note that reward-related disruptions may not be unique to depression and vulnerability to depression nor even to internalizing disorders (Bernat et al., 2011; Bjork et al., 2010; Gatzke-Kopp et al., 2009; Volkow et al., 2009; Volkow et al., 2010). It is possible that variation in the systems that support reward responding may play a role in externalizing or psychotic disorders as well (Kring & Elis, 2013). In light of RDoC’s mission, future research should continue to refine the specific phenotype to which the RewP relates, as well as whether and how abnormalities in the RewP cross diagnostic boundaries. Clearer explications of the transdiagnostic constructs described in RDoC should be useful in moving biological and psychological units of analysis closer to one another, and will be imperative to improving our understanding of the etiology of psychopathology (Shankman & Gorka, 2015).
GENERAL SCIENTIFIC SUMMARY.
The results of this study suggest that a blunted response to reward may connote vulnerability for certain features of depression. Additionally, neural markers of this vulnerability may be useful in identifying individuals at risk for depression.
Acknowledgments
This work was supported by National Institute of Mental Health Grants RO1 MH098093 to Stewart A. Shankman
Footnotes
This issue is not specific to PCA, however; mean activity in a time-window at a specific site or sites can also misrepresent a subject’s score.
Gender, age, and birth order were not significantly related to the self-report or ERP variables, either within or between probands and siblings (all ps > .20); they were therefore not included in the subsequent analyses.
References
- Allman J, Hakeem A, Erwin J, Nimchinsky E, Hof P. The anterior cingulate cortex: The evolution of an interface between emotion and cognition. Annals of the New York Academy of Sciences. 2001;935:107–117. doi: 10.1111/j.1749-6632.2001.tb03476.x. [PubMed] [Google Scholar]
- Becker MP, Nitsch AM, Miltner WH, Straube T. A single-trial estimation of the feedback-related negativity and its relation to BOLD responses in a time-estimation task. The Journal of Neuroscience. 2014;34:3005–3012. doi: 10.1523/JNEUROSCI.3684-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat E, Nelson L, Steele V, Gehring W, Patrick C. Externalizing psychopathology and gain–loss feedback in a simulated gambling task: Dissociable components of brain response revealed by time-frequency analysis. Journal of Abnormal Psychology. 2011;120:352. doi: 10.1037/a0022124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Chen G, Smith AR, Hommer DW. Incentive-elicited mesolimbic activation and externalizing symptomatology in adolescents. Journal of Child Psychology and Psychiatry. 2010;51:827–837. doi: 10.1111/j.1469-7610.2009.02201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Nikolova Y, Pizzagalli D. Neurogenetics of Depression: A Focus on Reward Processing and Stress Sensitivity. Neurobiology of Disease. 2012;52:12–23. doi: 10.1016/j.nbd.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli D. The heritability of hedonic capacity and perceived stress: a twin study evaluation of candidate depressive phenotypes. Psychological Medicine. 2009;39:211–218. doi: 10.1017/S0033291708003619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Santesso D, Fagerness J, Perlis R, Pizzagalli D. Corticotropin-releasing hormone receptor type 1 (CRHR1) genetic variation and stress interact to influence reward learning. The Journal of Neuroscience. 2011;31:13246–13254. doi: 10.1523/JNEUROSCI.2661-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlmeijer ET, Fledderus M, Rokx T, Pieterse ME. Efficacy of an early intervention based on acceptance and commitment therapy for adults with depressive symptomatology: Evaluation in a randomized controlled trial. Behaviour Research and Therapy. 2011;49:62–67. doi: 10.1016/j.brat.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Bress J, Foti D, Kotov R, Klein D, Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50:74–81. doi: 10.1111/j.1469-8986.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- Bress J, Hajcak G. Self-report and behavioral measures of reward sensitivity predict the feedback negativity. Psychophysiology. 2013;50:610–616. doi: 10.1111/psyp.12053. [DOI] [PubMed] [Google Scholar]
- Bress J, Meyer A, Hajcak G. Differentiating anxiety and depression in children and adolescents: evidence from event-related brain potentials. Journal of Clinical Child & Adolescent Psychology. 2013:1–12. doi: 10.1080/15374416.2013.814544. [DOI] [PubMed] [Google Scholar]
- Bress J, Smith E, Foti D, Klein D, Hajcak G. Neural response to reward and depressive symptoms in late childhood to early adolescence. Biological Psychology. 2012;89:156–162. doi: 10.1016/j.biopsycho.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J. The neurobiology of positive emotions. Neuroscience & Biobehavioral Reviews. 2006;30(2):173–187. doi: 10.1016/j.neubiorev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Calear AL, Christensen H. Systematic review of school-based prevention and early intervention programs for depression. Journal of Adolescence. 2010;33:429–438. doi: 10.1016/j.adolescence.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Carlson J, Foti D, Harmon-Jones E, Proudfit G. Midbrain volume predicts fMRI and ERP measures of reward reactivity. Brain Structure and Function. 2014:1–6. doi: 10.1007/s00429-014-0725-9. [DOI] [PubMed] [Google Scholar]
- Carlson J, Foti D, Mujica-Parodi L, Harmon-Jones E, Hajcak G. Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: A combined ERP and fMRI study. Neuroimage. 2011;57:1608–1616. doi: 10.1016/j.neuroimage.2011.05.037. doi: 10.1016/j.neuroimage.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Cattell R. The Scree test for the number of factors. Multivariate Behavioral Research. 1966;1:245–276. doi: 10.1207/s15327906mbr0102_10. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- Cuthbert B. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13(1):28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert B, Insel T. Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophrenia bulletin. 2010;36:1061–1062. doi: 10.1093/schbul/sbq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert B, Insel T. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC medicine. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVido J, Jones M, Geraci M, Hollon N, Blair R, Pine DS, Blair K. Stimulus-reinforcement-based decision making and anxiety: impairment in generalized anxiety disorder (GAD) but not in generalized social phobia (GSP) Psychological Medicine. 2009;39:1153–1161. doi: 10.1017/S003329170800487X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J. Addressing misallocation of variance in principal components analysis of event-related potentials. Brain Topography. 1998;11(1):43–55. doi: 10.1023/a:1022218503558. [DOI] [PubMed] [Google Scholar]
- Dien J. The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. Journal of Neuroscience Methods. 2010a;187:138–145. doi: 10.1016/j.jneumeth.2009.12.009. doi: 10.1016/j.jneumeth.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Dien J. Evaluating two-step PCA of ERP data with Geomin, Infomax, Oblimin, Promax, and Varimax rotations. Psychophysiology. 2010b;47:170–183. doi: 10.1111/j.1469-8986.2009.00885.x. doi: 10.1111/j.1469-8986.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- Dien J, Beal D, Berg P. Optimizing principal components analysis of event-related potentials: matrix type, factor loading weighting, extraction, and rotations. Clinical Neurophysiology. 2005;116:1808–1825. doi: 10.1016/j.clinph.2004.11.025. doi: 10.1016/j.clinph.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Dien J, Frishkoff G. Principal components analysis of event-related potential datasets. In: Handy TC, editor. Event-related potentials: A methods handbook. The MIT Press; Cambridge, MA: 2005. [Google Scholar]
- Dien J, Khoe W, Mangun G. Evaluation of PCA and ICA of simulated ERPs: Promax vs. Infomax rotations. Human Brain Mapping. 2007;28:742–763. doi: 10.1002/hbm.20304. doi: 10.1002/hbm.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer A, Redman K, Harris T, Mahmood A, Sadler S, Pickering A, McGUFFIN P. Neuroticism, extraversion, life events and depression The Cardiff Depression Study. The British Journal of Psychiatry. 2002;181:118–122. doi: 10.1017/s0007125000161823. [DOI] [PubMed] [Google Scholar]
- Forbes E, Christopher May J, Siegle G, Ladouceur C, Ryan N, Carter C, Dahl R. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. Journal of Child Psychology and Psychiatry. 2006;47:1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E, Dahl R. Neural systems of positive affect: relevance to understanding child and adolescent depression? Development and Psychopathology. 2005;17:827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E, Hariri A, Martin S, Silk J, Moyles D, Fisher P, Axelson D. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E, Shaw D, Dahl R. Alterations in reward-related decision making in boys with recent and future depression. Biological Psychiatry. 2007;61:633–639. doi: 10.1016/j.biopsych.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Foti D, Carlson J, Sauder C, Proudfit G. Reward dysfunction in major depression: Multimodal neuroimaging evidence for refining the melancholic phenotype. Neuroimage. 2014;101:50–58. doi: 10.1016/j.neuroimage.2014.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biological Psychology. 2009;81:1–8. doi: 10.1016/j.biopsycho.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G. State sadness reduces neural sensitivity to nonrewards versus rewards. Neuroreport. 2010;21:143. doi: 10.1097/WNR.0b013e3283356448. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Genetic variation in dopamine moderates neural response during reward anticipation and delivery: Evidence from event-related potentials. Psychophysiology. 2012;49:617–626. doi: 10.1111/j.1469-8986.2011.01343.x. [DOI] [PubMed] [Google Scholar]
- Foti D, Kotov R, Klein D, Hajcak G. Abnormal neural sensitivity to monetary gains versus losses among adolescents at risk for depression. Journal of Abnormal Child Psychology. 2011;39(7):913–924. doi: 10.1007/s10802-011-9503-9. [DOI] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Bernat E, Proudfit G. Anterior cingulate activity to monetary loss and basal ganglia activity to monetary gain uniquely contribute to the feedback negativity. Clinical Neurophysiology. 2015 doi: 10.1016/j.clinph.2014.08.025. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, Hajcak G. Event-related potential activity in the basal ganglia differentiates rewards from non-rewards: Temporospatial principal components analysis and source localization of the feedback negativity. Human Brain Mapping. 2011;32:2207–2216. doi: 10.1002/hbm.21182. doi: 10.1002/hbm.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzke-Kopp L, Beauchaine T, Shannon K, Chipman J, Fleming A, Crowell S, Aylward E. Neurological correlates of reward responding in adolescents with and without externalizing behavior disorders. Journal of Abnormal Psychology. 2009;118:203. doi: 10.1037/a0014378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Archives of General Psychiatry. 2010;67:380–387. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Foland-Ross LC. Understanding Familial Risk for Depression A 25-Year Perspective. Perspectives on Psychological Science. 2014;9:94–108. doi: 10.1177/1745691613513469. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Stiglin LE, Finkelstein SN, Berndt ER. The economic burden of depression in 1990. Journal of Clinical Psychiatry. 1993 [PubMed] [Google Scholar]
- Guyer A, Choate V, Detloff A, Benson B, Nelson E, Perez-Edgar K, Ernst M. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. American Journal of Psychiatry. 2012;169:205–212. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Adolescent depression, stressful interpersonal contexts, and risk for recurrence. Current Directions in Psychological Science. 2009;18:200–204. doi: 10.1111/j.1467-8721.2009.01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Schmidt L, Reischies F. Anhedonia in schizophrenic, depressed, or alcohol-dependent patients: neurobiological correlates. Pharmacopsychiatry. Supplement. 1994;27:7–10. doi: 10.1055/s-2007-1014317. [DOI] [PubMed] [Google Scholar]
- Holroyd C, Coles M. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. doi: 10.1037//0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holzman PS, Proctor LR, Levy DL, Yasillo NJ, Meltzer HY, Hurt SW. Eye-tracking dysfunctions in schizophrenic patients and their relatives. Archives of General Psychiatry. 1974;31:143–151. doi: 10.1001/archpsyc.1974.01760140005001. [DOI] [PubMed] [Google Scholar]
- Horn J. A rationale and test for the number of factors in factor analysis. Psychometrika. 1965;30:179–185. doi: 10.1007/BF02289447. doi: 10.1007/BF02289447. [DOI] [PubMed] [Google Scholar]
- Horwath E, Johnson J, Klerman GL, Weissman MM. Depressive symptoms as relative and attributable risk factors for first-onset major depression. Archives of General Psychiatry. 1992;49:817–823. doi: 10.1001/archpsyc.1992.01820100061011. [DOI] [PubMed] [Google Scholar]
- Ingram RE, Luxton DD. Vulnerability-stress models. Development of psychopathology: A vulnerability-stress perspective. 2005:32–46. [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine D, Quinn K, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jazbec S, McClure E, Hardin M, Pine D, Ernst M. Cognitive control under contingencies in anxious and depressed adolescents: an antisaccade task. Biological Psychiatry. 2005;58:632–639. doi: 10.1016/j.biopsych.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Johnson W, McGue M, Gaist D, Vaupel J, Christensen K. Frequency and heritability of depression symptomatology in the second half of life: evidence from Danish twins over 45. Psychological Medicine. 2002;32:1175–1185. doi: 10.1017/s0033291702006207. [DOI] [PubMed] [Google Scholar]
- Joormann J, Eugène F, Gotlib I. Parental depression: Impact on offspring and mechanisms underlying transmission of risk. In: Nolen-Hoeksema S, Hilt L, editors. Handbook of depression in adolescents. Routledge; New York: 2008. [Google Scholar]
- Kashdan T. The neglected relationship between social interaction anxiety and hedonic deficits: Differentiation from depressive symptoms. Journal of Anxiety Disorders. 2004;18:719–730. doi: 10.1016/j.janxdis.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kendler K. Is seeking treatment for depression predicted by a history of depression in relatives? Implications for family studies of affective disorder. Psychological Medicine. 1995;25:807–814. doi: 10.1017/s0033291700035054. [DOI] [PubMed] [Google Scholar]
- Kendler K. Reflections on the relationship between psychiatric genetics and psychiatric nosology. American Journal of Psychiatry. 2006;163:1138–1146. doi: 10.1176/ajp.2006.163.7.1138. [DOI] [PubMed] [Google Scholar]
- Kessler R, Berglund P, Demler O, Jin R, Merikangas K, Walters E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biological Psychiatry. 2008;63:686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring A, Elis O. Emotion deficits in people with schizophrenia. Annual Review of Clinical Psychology. 2013;9:409–433. doi: 10.1146/annurev-clinpsy-050212-185538. [DOI] [PubMed] [Google Scholar]
- Kujawa A, Proudfit G, Klein D. Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. Journal of Abnormal Psychology. 2014;123:287. doi: 10.1037/a0036285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, Perel JM. The advantage of early treatment intervention in recurrent depression. Archives of General Psychiatry. 1989;46:771. doi: 10.1001/archpsyc.1989.01810090013002. [DOI] [PubMed] [Google Scholar]
- Liu W.-h., Wang L.-z., Shang H.-r., Shen Y, Li Z, Cheung EF, Chan RC. The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia. 2014;53:213–220. doi: 10.1016/j.neuropsychologia.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Lovibond S, Lovibond P. Manual for the depression anxiety stress scales. The Psychology Foundation of Australia: Inc; Sydney: 1995. [Google Scholar]
- Luck S. An introduction to the event-related potential technique. MIT Press; Cambridge, MA: 2005. [Google Scholar]
- Luck S. Event-Related Potentials. In: Long D, editor. APA Handbook of Research Methods in Psychology. American Psychological Association; Washington, D.C.: 2012. [Google Scholar]
- Mathers CD, Fat DM, Boerma J. The global burden of disease: 2004 update. World Health Organization; 2008. [Google Scholar]
- Miltner W, Braun C, Coles M. Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a “generic” neural system for error detection. Journal of Cognitive Neuroscience. 1997;9:788–798. doi: 10.1162/jocn.1997.9.6.788. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Morgan J, Olino T, McMakin D, Ryan N, Forbes E. Neural response to reward as a predictor of increases in depressive symptoms in adolescence. Neurobiology of Disease. 2013;52:66–74. doi: 10.1016/j.nbd.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Abdalla S. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2013;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Nelson B, McGowan S, Sarapas C, Robison-Andew E, Altman S, Campbell M, Shankman S. Biomarkers of threat and reward sensitivity demonstrate unique associations with risk for psychopathology. Journal of Abnormal Psychology. 2013;122:662. doi: 10.1037/a0033982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Pelizza L, Ferrari A. Anhedonia in schizophrenia and major depression: state or trait. Annals of General Psychiatry. 2009;8:22. doi: 10.1186/1744-859X-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D, Holmes A, Dillon D, Goetz E, Birk J, Bogdan R, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated subjects with major depressive disorder. The American Journal of Psychiatry. 2009;166:702. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D, Jahn A, O’Shea J. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biological Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit GH. The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology. 2015 doi: 10.1111/psyp.12370. in press. [DOI] [PubMed] [Google Scholar]
- Rawal A, Collishaw S, Thapar A, Rice F. ‘The risks of playing it safe’: a prospective longitudinal study of response to reward in the adolescent offspring of depressed parents. Psychological Medicine. 2013;43:27–38. doi: 10.1017/S0033291712001158. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, Cuijpers P, Patel V, Cohen A, Dias A, Chowdhary N, Mazumdar S. Early intervention to reduce the global health and economic burden of major depression in older adults. Annual Review of Public Health. 2012;33:123. doi: 10.1146/annurev-publhealth-031811-124544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins E, Guze SB. Establishment of diagnostic validity in psychiatric illness: its application to schizophrenia. American Journal of Psychiatry. 1970;126:983–987. doi: 10.1176/ajp.126.7.983. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Shelton RC, Duman RS. Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology. 2011;36:2375–2394. doi: 10.1038/npp.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annual Review of Psychology. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Shankman S, Gorka S. Psychopathology research in the RDoC era: Unanswered questions and the importance of the psychophysiological unit of analysis. International Journal of Psychophysiology. 2015 doi: 10.1016/j.ijpsycho.2015.01.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman S, Klein D, Tenke C, Bruder G. Reward sensitivity in depression: A biobehavioral study. Journal of Abnormal Psychology. 2007;116:95. doi: 10.1037/0021-843X.116.1.95. [DOI] [PubMed] [Google Scholar]
- Shankman S, Nelson B, Sarapas C, Robison-Andrew E, Campbell M, Altman S, Gorka S. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology. 2013;122:322. doi: 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon DA, Keller MB, Leon AC, Mueller TI, Lavori PW, Shea MT, Maser JD. Multiple recurrences of major depressive disorder. American Journal of Psychiatry. 2000;157:229–233. doi: 10.1176/appi.ajp.157.2.229. [DOI] [PubMed] [Google Scholar]
- Steele J, Kumar P, Ebmeier KP. Blunted response to feedback information in depressive illness. Brain. 2007;130:2367–2374. doi: 10.1093/brain/awm150. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neuroscience & Biobehavioral Reviews. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Kollins SH, Wigal TL, Newcorn JH, Telang F, Ma Y. Evaluating dopamine reward pathway in ADHD: clinical implications. Jama. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Newcorn JH, Kollins SH, Wigal TL, Telang F, Logan J. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Molecular Psychiatry. 2010;16:1147–1154. doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: A quantitative hierarchical model for DSM-V. Journal of Abnormal Psychology. 2005;114:522. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Watson D, O'Hara M, Simms L, Kotov R, Chmielewski M, McDade-Montez E, Stuart S. Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS) Psychological Assessment. 2007;19:253. doi: 10.1037/1040-3590.19.3.253. [DOI] [PubMed] [Google Scholar]
- Watson D, O’Hara M, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, Ruggero C. Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II) Assessment. 2012;19:399–420. doi: 10.1177/1073191112449857. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Luhmann C, Bress J, Hajcak G. Better late than never? The effect of feedback delay on ERP indices of reward processing. Cognitive, Affective, & Behavioral Neuroscience. 2012;12:671–677. doi: 10.3758/s13415-012-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Riesel A, Proudfit G. Show me the Money: The impact of actual rewards and losses on the feedback negativity. Brain and Cognition. 2014;87:134–139. doi: 10.1016/j.bandc.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Pilowsky DJ, Wickramaratne PJ, Talati A, Wisniewski SR, Fava M, King CA. Remissions in maternal depression and child psychopathology: a STAR* D-child report. Jama. 2006;295:1389–1398. doi: 10.1001/jama.295.12.1389. [DOI] [PubMed] [Google Scholar]
- Zubin J, Spring B. Vulnerability: a new view of schizophrenia. Journal of Abnormal Psychology. 1977;86:103. doi: 10.1037//0021-843x.86.2.103. [DOI] [PubMed] [Google Scholar]