Abstract
Background
The approach to managing diabetic macular edema (DME) in eyes with prior vitrectomy is based on limited evidence. Therefore, an exploratory post-hoc assessment of 3-year data from eyes with and without vitrectomy prior to randomization in a DRCR.net trial that evaluated ranibizumab+prompt or deferred laser for DME is presented.
Methods
Visual acuity (VA) and ocular coherence tomography (OCT) outcomes were compared between eyes with and without prior vitrectomy.
Results
At baseline eyes with prior vitrectomy (n = 25) had longer duration of diabetes, worse VA, less thickened central subfield measurements on OCT, and were more apt to have worse diabetic retinopathy severity level or prior treatment for macular edema or cataract surgery than eyes without a history of vitrectomy (n = 335). Analyses adjusted for these baseline imbalances did not identify substantial differences between eyes with and without prior vitrectomy at each annual visit through 3 years for the favorable VA, OCT central subfield thickness or volume outcomes, although OCT improvement appeared slower in vitrectomy eyes during the first year.
Conclusion
This study provides little evidence that the beneficial clinical outcomes for patients with center-involved DME treated with anti-VEGF are affected in the long term by prior vitrectomy.
Keywords: Vitrectomy, diabetic macular edema, ranibizumab, anti-VEGF, OCT, visual acuity, macular thickness
Introduction
Several clinical trials have confirmed that intravitreal therapy with anti-vascular endothelial growth factor (anti-VEGF) drugs results in superior functional and anatomic outcomes for eyes with vision impairment and center-involved diabetic macular edema (DME) as compared with focal/grid photocoagulation through at least 2 years of management. 1-5
Independent of the management of DME, vitrectomy can play a critical role in the management of proliferative diabetic retinopathy (PDR) and vitreomacular interface disorders. The pharmacokinetics of drugs injected into the vitreous in animal eyes with and without vitrectomy suggests more rapid clearance of drugs post vitrectomy, particularly those of lower molecular weight.6-10 However, in humans, there is a paucity of pharmacokinetic studies that evaluate drug clearance in eyes with and without vitrectomy11 and none that investigate anti-VEGF drugs. If the drug half-life of a biologic agent, such as an anti-VEGF antibody, is shorter in the human eye post-vitrectomy, then there may be a shorter duration of action with less robust vision and anatomic outcomes and greater need for more frequent injections over an extended time period in eyes with DME when compared with eyes without vitrectomy. In part, these concerns may have led some trials evaluating anti-VEGF agents for DME to exclude eyes with prior vitrectomy.3
Since there is limited evidence regarding the effectiveness of intravitreal anti-VEGF on DME post vitrectomy, this exploratory post-hoc assessment of the three-year course of visual acuity, retinal thickness, and cumulative treatments was undertaken in eyes with vitrectomy prior to entry into a clinical trial of intravitreal anti-VEGF therapy administered to manage DME.
Methods and Materials
Data for this analysis were from a DRCR.net trial enrolling 854 eyes from 691 participants that compared sham+prompt focal/grid laser, intravitreal ranibizumab+prompt laser, intravitreal ranibizumab+deferred (≥24 weeks) laser, and intravitreal triamcinolone+prompt laser in the management of DME.2, 4, 12 The analysis included data from the baseline through the 3-year visits for 360 eyes (360 participants) assigned randomly to either of the 2 ranibizumab groups, with 25 eyes (7%) having vitrectomy prior to enrollment and 335 eyes (93%) without prior vitrectomy. Time since vitrectomy and reason for vitrectomy in the vitrectomy prior to enrollment group are reported in Table 1. The full protocol for this trial is available online (http://www.drcr.net); select pertinent components of the trial design are described below.
Table 1.
Baseline Study Participant and Ocular Characteristics
| Vitrectomy Status at Baseline | ||
|---|---|---|
| Vitrectomy N = 25 (7%) | No Vitrectomy N = 335 (93%) | |
| Participant Characteristic | ||
| Gender: Women, N (%) | 10 (40%) | 145 (43%) |
| Age (yrs) | ||
| Median (25th, 75th percentile) | 65 (55, 71) | 64 (57, 70) |
| Type of Diabetes Mellitus | ||
| Type 1 | 2 (8%) | 24 (7%) |
| Type 2 | 23 (92%) | 305 (91%) |
| Uncertain | 0 (0%) | 6 (2%) |
| Pre-existing cardiovascular condition | 7 (28%) | 115 (34%) |
| Pre-existing hypertension | 22 (88%) | 276 (82%) |
| Race/Ethnicity | ||
| White | 15 (60%) | 249 (74%) |
| African-American | 5 (20%) | 50 (15%) |
| Hispanic or Latino | 2 (8%) | 30 (9%) |
| Other | 3 (12%) | 6 (2%) |
| Duration of diabetes (yrs) | ||
| Median (25th, 75th percentile) | 23 (17, 27) | 17 (11, 23) |
| HbA1c (%)* | ||
| Median (25th, 75th percentile) | 7.5 (6.4, 8.5) | 7.4 (6.6, 8.4) |
| Ocular Characteristics | ||
| Prior PRP | 16 (64%) | 68 (20%) |
| Prior DME Treatment | 24 (96%) | 203 (61%) |
| Prior Treatment with Anti-VEGF for DME | 5 (20%) | 40 (12%) |
| Intraocular Pressure (mm Hg) | ||
| Median (25th, 75th percentile) | 17 (15, 18) | 16 (14, 18) |
| History of Glaucoma | 1 (4%) | 5 (1%) |
| Lens Status | ||
| Phakic | 9 (36%) | 242 (72%) |
| Posterior Chamber Intraocular Lens | 16 (64%) | 91 (27%) |
| Anterior Chamber Intraocular Lens | 0 (0%) | 2 (1%) |
| Visual Acuity (ETDRS letter score) | ||
| Median (25th, 75th percentile) | 60 (53, 67) | 65 (56, 72) |
| Snellen Equivalent | 20/63 (20/100, 20/50) | 20/50 (20/80, 20/40) |
| Mean (SD) | 59 (12) | 63 (12) |
| OCT Central Subfield Thickness† μm | ||
| Median (25th, 75th percentile) | 372 (290, 471) | 380 (311, 484) |
| Mean (SD) | 368 (115) | 408 (130) |
| OCT Volume‡ mm3 | ||
| Median (25th, 75th percentile) | 8.1 (7.5, 8.8) | 8.4 (7.5, 9.7) |
| Mean (SD) | 8.3 (1.4) | 8.9 (2.0) |
| Treatment Group Assignment | ||
| Anti-VEGF + prompt laser | 16 (64%) | 164 (49%) |
| Anti-VEGF + deferred laser | 9 (36%) | 171 (51%) |
| DR Severity Level (On Clinical Exam) | ||
| None | 0 (0%) | 1 (<1%) |
| Microaneurysms only | 2 (8%) | 9 (3%) |
| Mild/moderate NPDR | 4 (16%) | 184 (55%) |
| Severe NPDR | 1 (4%) | 70 (21%) |
| PDR and/or prior scatter laser | 18 (72%) | 71 (21%) |
| For eyes with prior vitrectomy: | ||
| Time since vitrectomy | - | |
| <1 year | 5 (20%) | - |
| 1 to <2 years | 9 (36%) | - |
| 2 to <5 years | 8 (32%) | - |
| ≥5 years | 3 (12%) | - |
| Reason for vitrectomy | - | |
| DME | 11 (44%) | - |
| Other§ | 13 (52%) | - |
| Both DME and other reason | 1 (4%) | - |
PRP = Panretinal photocoagulation, DME = Diabetic macular edema, VEGF = vascular endothelial growth factor, ETDRS = Eearly treatment diabetic retinopathy study, OCT = Optical coherence tomography, DR = Diabetic Retinopathy, NPDR = non-proliferative diabetic retinopathy, PDR = Proliferative diabetic retinopathy.
HbA1c was missing in 8 vitrectomy group and 1 no vitrectomy group participants;
OCT central subfield thickness was missing for 1 vitrectomy group subject;
OCT volume was missing for 4 vitrectomy group eyes and 75 no vitrectomy group eyes..
Other was not clarified further
The major trial eligibility criteria included (1) best-corrected Electronic ETDRS (E-ETDRS) visual acuity letter score of 78 to 24 (approximate Snellen equivalent, 20/32 to 20/320), (2) definite retinal thickening from DME involving the foveal center on clinical examination as the cause of vision loss, and (3) confirmation of foveal edema with a central subfield thickness (CST) of 250 μm or greater ascertained on time-domain optical coherence tomography (OCT). Prior vitrectomy surgery was not an exclusion criterion unless it was performed within 4 months of enrollment.
At baseline and at each follow-up visit, best-corrected visual acuity letter score was measured using the E-ETDRS Visual Acuity Test, and OCT images were obtained with a Zeiss Stratus OCT machine (Carl Zeiss Meditec, Inc.). After 52 weeks of study follow-up, spectral domain OCT (Cirrus, Carl Zeiss Meditec, or Spectralis, Heidelberg, Carlsbad, CA,) was allowed to replace Stratus OCT with instrument specific scan protocols. For analysis purposes, retinal thickness measurements from spectral domain instruments were converted into Stratus time domain equivalent values using validated conversion equations derived in an alternate DRCR.net protocol.13 Intravitreal ranibizumab injections were required every 4 weeks for the initial 12 weeks of the study, with the potential to receive ranibizumab at each subsequent visit if there was improvement in visual acuity or OCT from the previous visit and vision remained worse than 20/20 and central subfield thickness was ≥250 μm (Stratus equivalent). Re-injection was at investigator discretion if futility criteria were met or both vision and CST stabilized over several successive visits. Resumption of intravitreal treatment was encouraged if vision or CST worsened following treatment deferral. Additional details on the retreatment algorithm have been published previously.12, 14
Study participants assigned to the ranibizumab plus prompt laser group received focal/grid laser treatment within 3 to 10 days from their initial ranibizumab injection. Additional laser treatment was administered as often as every 13 weeks if persistent DME involved or threatened the fovea and if complete laser treatment had not been previously administered. Study participants assigned to the ranibizumab plus deferred laser group first became eligible for laser treatment at the 24-week visit; laser treatment could be applied at that visit or thereafter if DME persisted or threatened the fovea and successive intravitreal injections were not associated with incremental improvements in either visual acuity or CST. Vitrectomy was permitted at the discretion of the investigator to manage pathology other than DME or to manage DME if failure or futility criteria were met. During follow-up, 16 (5%) eyes in the no prior vitrectomy group underwent vitrectomy; data subsequent to vitrectomy from these individuals was censored. One eye in the vitrectomy group had repeat vitrectomy during study follow up with all data retained in analyses.
Statistical Methods
Data from the ranibizumab+prompt laser and ranibizumab+deferred laser groups were combined for the analysis. Observed means and mean changes from baseline in visual acuity letter score, OCT CST and volume were compared between vitrectomy status groups using the Student's t-test. In addition, these outcomes were compared between vitrectomy status groups using a linear mixed longitudinal model15 through the inclusion of a (categorical) visit and vitrectomy status interaction term, adjusting for baseline characteristics that differed between the vitrectomy and no vitrectomy groups and were potential confounders. These included baseline visual acuity, OCT CST, diabetic retinopathy severity level (as reported by the study ophthalmologist), lens status (phakic or pseudophakic), prior panretinal photocoagulation (PRP), prior DME treatment, duration of diabetes, and randomized treatment assignment. Baseline OCT volume also differed between vitrectomy status groups; however, only analyses of volume outcomes were adjusted for this due to the number of missing baseline volume data.
Binary outcomes of visual acuity improvement and improvement in CST, were compared between vitrectomy status groups using logistic regression adjusting for the imbalanced baseline characteristics. Improvement in CST was defined as a decrease of at least 20%.
Considering the number of outcomes tested, 99% confidence intervals were reported for the outcomes point estimates.
Results
Baseline Characteristics
At entry into the trial, there were no substantive differences identified between the individuals contributing eyes with or without prior vitrectomy with respect to gender, age, race/ethnicity distribution, diabetes type, medical co-morbidities, or glycemic control (Table 1). However, participants who had undergone prior vitrectomy differed from participants without prior vitrectomy on a number of baseline characteristics; in particular, they had longer duration of diabetes, higher rates of prior cataract surgery, and had worse diabetic retinopathy severity level (including greater prevalence of PDR), and greater prevalence of PRP or treatment for DME prior to enrollment. Furthermore, the initial visual acuity was slightly worse in the prior vitrectomy group relative to the no vitrectomy group, with mean letter scores of 59 versus 63 (approximate Snellen equivalent 20/63 vs. 20/63) respectively. The OCT CST and macular volume measurements were thinner in eyes with prior vitrectomy compared with eyes without prior vitrectomy (Table 1). By chance, there also was an imbalance in the randomized treatment assignment by prior vitrectomy.
Treatments Administered
Completion of follow-up, excluding deaths, at the 52- (1-year), 104- (2-year), and 156-week (3-year) study visits in the eyes with and without prior vitrectomy was 100% and 96%, 100% and 91%, and 91% and 87%, respectively. Between study entry and the 3-year visit, the cumulative number of intravitreal ranibizumab injections (mean and median) appeared similar between the vitrectomy (14,13) and the no vitrectomy groups (14,13) (Table 2). Only between the 24-week and 1 year study visits, when a maximum of 7 injections could have been given, there were more injections in the prior vitrectomy group relative to the no vitrectomy group (median [25th, 75th percentile] = 5 [2, 6] vs. 3 [1, 5]). Eyes with prior vitrectomy had a similar average total number of focal/grid laser sessions relative to those without prior vitrectomy within the respective ranibizumab+prompt laser or ranibizumab+deferred laser treatment arms (table 3). Among the 7 eyes with prior vitrectomy assigned to the ranibizumab+deferred laser arm, only 1 (14%) did not receive laser treatment whereas 75 of the 132 (57%) eyes without prior vitrectomy similarly assigned did not require any laser treatment during follow-up (P = 0.05).
Table 2.
Number of Intravitreal Ranibizumab Injections by Pre-Enrollment Vitrectomy Status
| Vitrectomy Status at Baseline |
||
|---|---|---|
| Follow Up Time | Vitrectomy | No Vitrectomy |
| Baseline up to 24-week visit | N = 25 | N = 317 |
| Mean (SD) | 5.4 (0.9) | 5.2 (1.0) |
| Median (25th, 75th percentile) | 6 (5, 6) | 6 (5, 6) |
| 24 up to 52-week visit | N = 24 | N = 312 |
| Mean (SD) | 4.3 (2.4) | 3.1 (2.1) |
| Median (25th, 75th percentile) | 5 (2, 6) | 3 (1, 5) |
| 52 up to 104 week visit | N = 24 | N = 284 |
| Mean (SD) | 3.9 (4.6) | 3.4 (3.4) |
| Median (25th, 75th percentile) | 2 (0, 8) | 2 (0, 5) |
| 104 up to 156-week visit | N = 20 | N = 258 |
| Mean (SD) | 1.4 (1.8) | 2.5 (2.9) |
| Median (25th, 75th percentile) | 1 (0, 2) | 1 (0, 4) |
| Baseline up to 156-week visit | N = 20 | N = 258 |
| Mean (SD) | 14.3 (7.4) | 14.3 (7.4) |
| Median (25th, 75th percentile) | 13 (9, 22) | 13 (8, 19) |
SD= standard deviation
Table 3.
Number of Laser Treatment Sessions by Treatment Arm and Pre-Enrollment Vitrectomy Status
| Ranibizumab + Prompt Laser Group | Ranibizumab + Deferred Laser Group | |||
|---|---|---|---|---|
| Follow Up Time | Vitrectomy | No Vitrectomy | Vitrectomy | No Vitrectomy |
| Baseline up to 52-wk visit | N = 15 | N = 150 | N = 9 | N = 162 |
| No. of eyes treated | 15 | 150 | 6 | 46 |
| Mean (SD) | 2.2 (1.0) | 2.2 (1.0) | 0.8 (0.7) | 0.4 (0.7) |
| Median (25th, 75th percentile) | 2 (1, 3) | 2 (1, 3) | 1 (0, 1) | 0 (0, 1) |
| Baseline up to 156-wk visit | N = 13 | N = 126 | N = 7 | N = 132 |
| No. of eyes treated | 13 | 126 | 6 | 57 |
| Mean (SD) | 2.5 (1.6) | 3.2 (1.9) | 1.3 (1.0) | 1.0 (1.6) |
| Median (25th, 75th percentile) | 2 (1, 3) | 3 (2, 4) | 1 (1, 2) | 0 (0, 2) |
SD=standard deviation
Other interventions, such as PRP, intravitreal bevacizumab or ranibizumab, and triamcinolone acetonide, aside from vitrectomy and treatments required by the study protocol, were given to manage DME, proliferative diabetic retinopathy, or other retinal diseases in 30 additional eyes (9%) in the no prior vitrectomy group, and no eyes in the prior vitrectomy group.
Vision Outcomes
At the one, two and three year visits, eyes with prior vitrectomy had a mean improvement of 7.7, 9.6, and 6.5 in the visual acuity letter score, while eyes without prior vitrectomy had mean improvement of 9.2, 9.4, and 9.4 letters. At the three year visit there was a 2.9 letter difference in visual acuity improvement favoring the eyes without prior vitrectomy (99% CI: −9.6 to 3.9 letters) (Table 4). After adjustment for potential confounders, the mean difference between groups in visual acuity change ranged from −1.1 to 2.0 letters at the annual visits (Table 4; Figure 1A), while the unadjusted differences were within 3 letters at each time point (Table 4; Figure 1B). Adjusted and unadjusted P-values on the mean difference in visual acuity at all time points were > 0.01. The proportion of eyes with improvement or worsening by 5 or more letters and by improvement of 10 or more letters also appeared similar in the two groups at each of the annual visits (Table 4).
Table 4.
Visual Acuity Outcomes by Pre-Enrollment Vitrectomy Status
| Vitrectomy Status at Baseline |
Difference Vitrectomy – No Vitrectomy* (99% CI) | P-value | ||
|---|---|---|---|---|
| Follow-up time | Vitrectomy | No Vitrectomy | ||
| 16-week visit | N = 19 | N = 306 | ||
| Visual acuity letter score | ||||
| Unadjusted Mean ± SE | 69±1.6 | 71±0.7 | −1.3 (−8.5, 5.9) | 0.45 |
| Snellen-equivalent Mean | 20/40 | 20/40 | ||
| Median (25th, 75th percentile) | 69 (65, 74) | 74 (65, 79) | ||
| Adjusted Mean ± SE | 71±2.0 | 71±0.5 | 0.9 (−4.5, 6.3) | 0.67 |
| Snellen-equivalent Mean | 20/40 | 20/40 | ||
| Visual acuity change from baseline, letter score | ||||
| Unadjusted Mean ± SE | 7.9±2.0 | 7.7±0.5 | 0.3 (−4.8, 5.3) | 0.89 |
| Median (25th, 75th percentile) | 5 (1,13) | 7 (2, 13) | ||
| Adjusted Mean ± SE | 8.5±2.0 | 7.6±0.5 | 0.9 (−4.2, 6.0) | 0.64 |
| 32-week visit | N=22 | N=294 | ||
| Visual acuity letter score | ||||
| Unadjusted Mean ± SE | 67±2.5 | 72±0.7 | −4.3 (−11, 2,6) | 0.11 |
| Snellen-equivalent Mean | 20/50 | 20/40 | ||
| Median (25th, 75th percentile) | 67 (58, 75) | 75 (65, 80) | ||
| Adjusted Mean ± SE | 73±2.0 | 71±0.5 | 2.0 (−3.3, 7.3) | 0.34 |
| Snellen equivalent | 20/40 | 20/40 | ||
| Visual acuity change from baseline, letter score | ||||
| Unadjusted Mean ± SE | 9.3±2.0 | 8.2±0.5 | 1.0 (−4.3, 6.4) | 0.62 |
| Median (25th, 75th percentile) | 12 (4, 17) | 8 (3, 14) | ||
| Adjusted Mean ± SE | 10.1±2.0 | 8.1±0.5 | 2.0 (−3.0, 7.0) | 0.31 |
| 52-week visit | N = 24 | N = 312 | ||
| Visual acuity | ||||
| Unadjusted Mean ± SE | 67±2.8 | 72±0.7 | −5.8 (−13, 0.9) | 0.06 |
| Snellen-equivalent Mean | 20/50 | 20/40 | ||
| Median (25th, 75th percentile) | 70 (64, 73) | 75 (67, 81) | ||
| Adjusted Mean ± (SE) | 72±2.0 | 72±0.5 | −0.2 (−5.5, 5.1) | 0.93 |
| Snellen-equivalent Mean | 20/40 | 20/40 | ||
| Visual acuity change from baseline | ||||
| Unadjusted Mean ± SE | 7.7±1.8 | 9.2±0.6 | −1.5 (−6.8, 3.9) | 0.45 |
| Median (25th, 75th percentile) | 8 (3, 15) | 9 (4, 15) | ||
| Adjusted Mean ± SE | 8.9±2.0 | 9.0±0.5 | −0.1 (−5.1, 4.8) | 0.94 |
| Categorical change in visual acuity from baseline, N (%)** | ||||
| ≥10 letter improvement | 11 (46%) | 152 (49%) | −3% (−29, 25%) | 0.77 |
| ≥5 letter improvement | 15 (63%) | 227 (73%) | −10% (−40, 14%) | 0.93 |
| ≥5 letter worsening | 2 (8%) | 27 (9%) | 0% (−10, 29%) | 0.59 |
| 104-week visit | N = 24 | N = 283 | ||
| Visual acuity letter score | ||||
| Unadjusted Mean ± SE | 69±3.0 | 73±0.8 | −4.1 (−11, 3.3) | 0.20 |
| Snellen equivalent | 20/40 | 20/40 | ||
| Median (25th, 75th percentile) | 71 (62, 78) | 76 (67, 82) | ||
| Adjusted Mean ± SE | 74±2.0 | 72±0.5 | 1.8 (−3.4, 7.1) | 0.37 |
| Snellen equivalent | 20/32 | 20/40 | ||
| Visual acuity change from baseline | ||||
| Unadjusted Mean ± SE | 9.6±1.9 | 9.4±0.7 | 0.2 (−5.8, 6.2) | 0.91 |
| Median (25th, 75th percentile) | 13 (4.5, 17) | 9 (4, 16) | ||
| Adjusted Mean ± SE | 10.8±2.0 | 8.9±0.5 | 2.0 (−3.0, 6.9) | 0.31 |
| Categorical change in visual acuity from baseline, N (%)** | ||||
| ≥10 letter improvement | 13 (54%) | 139 (49%) | 5% (−23, 31%) | 0.28 |
| ≥5 letter improvement | 18 (75%) | 198 (70%) | 5% (−26, 24%) | 0.21 |
| ≥5 letter worsening | 3 (13%) | 18 (6%) | 6% (−6, 36%) | 0.48 |
| 156-week visit | N = 20 | N = 255 | ||
| Visual acuity letter score | ||||
| Unadjusted Mean ± SE | 65±3.1 | 73±0.9 | −8.0 (−16, 0.4) | 0.02 |
| Snellen equivalent | 20/50 | 20/40 | ||
| Median (25th, 75th percentile) | 65 (56, 78) | 76 (69, 82) | ||
| Adjusted Mean ± SE | 71±2.1 | 72±0.6 | −1.2 (−6.7, 4.4) | 0.59 |
| Snellen equivalent | 20/40 | 20/40 | ||
| Visual acuity change from baseline | ||||
| Unadjusted Mean ± SE | 6.5±2.4 | 9.4±0.7 | −2.9 (−9.6, 3.9) | 0.27 |
| Median (25th, 75th percentile) | 8 (−1, 15) | 10 (4, 17) | ||
| Adjusted Mean ± SE | 7.6±2.0 | 8.7±0.5 | −1.1 (−6.3, 4.1) | 0.59 |
| Categorical change in visual acuity from baseline, N (%)† | ||||
| ≥10 letter improvement | 9 (45%) | 130 (51%) | −6% (−34, 25%) | 0.62 |
| ≥5 letter improvement | 12 (60%) | 176 (69%) | −9% (−41, 18%) | 0.46 |
| ≥5 letter worsening | 4 (20%) | 26 (10%) | 10% (−7, 43%) | 0.43 |
CI= confidence interval; SE = standard error
Unadjusted differences are the observed differences. Adjusted differences are from a linear mixed model with covariates for vitrectomy status and (categorical) follow up time by vitrectomy status interaction plus imbalanced baseline covariates (visual acuity, OCT CST, DR severity, lens status, prior PRP, prior DME treatment, duration of diabetes, and randomized treatment assignment).
Difference and 99% confidence interval are unadjusted for baseline imbalances; however, the P-value is adjusted using a logistic regression model.
Figure 1.
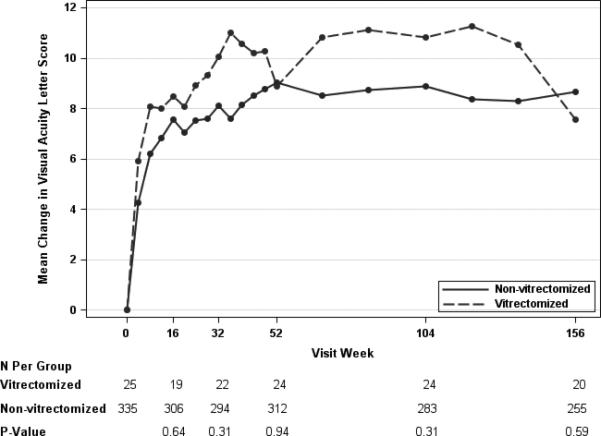
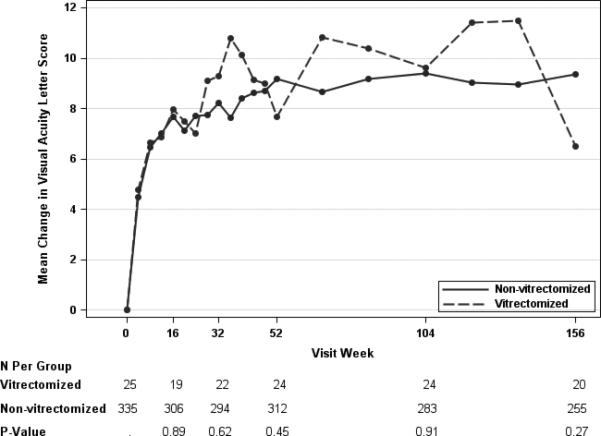
Mean Change in Visual Acuity Letter Score by Pre-Enrollment Vitrectomy Status, adjusted (Panel A)* and Unadjusted (Panel B)† for Baseline Cogvariates
Anatomic Outcomes
Anatomical parameters of CST and macular volume showed improvement over time in both vitrectomy status groups. The mean reduction in CST among eyes with prior vitrectomy was 83 μm, 90 μm, and 96 um at the one-, two- and three-year study visits versus 141 um, 155 um, and 165 um in the eyes without prior vitrectomy (Table 5). The observed (unadjusted) differences between the prior vitrectomy and no vitrectomy groups suggested that retinal thinning or reduction in edema in the vitrectomy group lagged behind the no vitrectomy group throughout the three years of study follow-up (Table 5, Figure 2B). When the analysis was adjusted for baseline confounders, results appeared to be similar to the unadjusted (Table 5, Figure 2A). At the one-year visit, 26% and 50% of the eyes had a CST less than 250 μm and at least a 10% reduction from baseline in CST, in the vitrectomy and no vitrectomy groups, respectively (observed difference = −24%, 99% CI: −44% to −9%, P = 0.03). At subsequent visits, the percentage of eyes with prior vitrectomy and normal thickness increased, and were similar to the eyes without prior vitrectomy. At the three year visit, 53% and 62% (difference = −10%, 99% CI:−40% to 20%, P = 0.43) of the eyes had a CST that was less than 250 um and at least a 10% reduction in CST from baseline in the vitrectomy and no vitrectomy subgroups, respectively. The volume data paralleled the CST data, although the difference noted between the vitrectomy groups early in the study for CST in the adjusted analysis was not reflected similarly in retinal volume at same time points (Table 5). The proportion of eyes with at least a 20% improvement in CST always favored the group without prior vitrectomy, but the magnitude of the difference between the groups decreased over time (Table 5).
Table 5.
OCT Central Subfield Thickness and Volume Outcomes by Pre-Enrollment Vitrectomy Status*
| Vitrectomy Status |
Difference Vitrectomy – No Vitrectomy† (99% CI) | P-value | ||
|---|---|---|---|---|
| Vitrectomy | No Vitrectomy | |||
| 16-week visit | ||||
| OCT Central Subfield Thickness, μm | ||||
| N | N = 19 | N = 306 | ||
| Unadjusted Mean ± SE | 318±15 | 274±5 | 45 (−5.3, 94) | 0.01 |
| Median (25th, 75th percentile) | 307 (256, 386) | 259 (213, 310) | ||
| Adjusted Mean ± SE | 325±19 | 274±5 | 51 (0.7, 102) | 0.009 |
| OCT Central Subfield Thickness Change from baseline, μm | ||||
| N | N = 19 | N = 305 | ||
| Unadjusted Mean ± SE | −53±21 | −134±7 | 81 (5, 157) | 0.001 |
| Median (25th, 75th percentile) | −41 (−91, −4) | −103 (−195, −53) | ||
| Adjusted Mean ± SE | −81±19 | −132±5 | 51 (0.7, 102) | 0.009 |
| OCT Volume (mm3) | ||||
| N | N =19 | N = 304 | ||
| Unadjusted Mean ± SE | 7.8±0.2 | 7.5±0.1 | 0.3 (−0.4, 1.0) | 0.21 |
| Median (25th, 75th percentile) | 7.6 (7.2, 8.2) | 7.3 (6.8, 7.9) | ||
| Adjusted Mean ± SE | 7.8±0.2 | 7.5±0.1 | 0.4 (−0.2, 1.0) | 0.094 |
| OCT Volume change from baseline (mm3) | ||||
| N | N = 16 | N = 235 | ||
| Unadjusted Mean ± SE | −0.6±0.2 | −1.4±0.1 | 0.8 (−0.1, 1.7) | 0.001 |
| Median (25th, 75th percentile) | −0.3 (−0.9, −0.1) | −1.0 (−1.8, −0.5) | ||
| Adjusted Mean ± SE | −1.0±0.2 | −1.4±0.1 | 0.4 (−0.2, 1.0) | 0.094 |
| 32-week visit | ||||
| OCT Central Subfield Thickness, μm | ||||
| N | N = 22 | N = 294 | ||
| Unadjusted Mean ± SE | 314±17 | 277±5 | 37 (−12, 86) | 0.05 |
| Median (25th, 75th percentile) | 276 (264, 380) | 258 (216, 308) | ||
| Adjusted Mean ± SE | 316±18 | 275±5 | 40 (−9, 90) | 0.035 |
| OCT Central Subfield Thickness Change from baseline, μm | ||||
| N | N = 22 | N = 293 | ||
| Unadjusted Mean ± SE | −52±26 | −136±8 | 84 (7.3, 161) | 0.004 |
| Median (25th, 75th percentile) | −42 (−100, 1) | −106 (−216, −45) | ||
| Adjusted Mean ± SE | −90±18 | −131±5 | 40 (−9, 90) | 0.035 |
| OCT Volume (mm3) | ||||
| N | N = 22 | N = 287 | ||
| Unadjusted Mean ± SE | 7.7±0.2 | 7.5±0.1 | 0.2 (−0.5, 1.0) | 0.29 |
| Median (25th, 75th percentile) | 7.5 (7.0, 8.1) | 7.2 (6.7, 7.9) | ||
| Adjusted Mean ± SE | 7.7±0.2 | 7.4±0.1 | 0.2 (−0.3, 0.8) | 0.26 |
| OCT Volume change from baseline (mm3) | ||||
| N | N = 19 | N = 225 | ||
| Unadjusted Mean ± SE | −0.8±0.2 | −1.5±0.1 | 0.6 (−0.3, 1.6) | 0.009 |
| Median (25th, 75th percentile) | −0.6 (−1.3, −0.2) | −1.1 (−2.0, −0.5) | ||
| Adjusted Mean ± SE | −1.2±0.2 | −1.4±0.1 | 0.2 (−0.3, 0.8) | 0.26 |
| 52-week visit | ||||
| OCT Central Subfield Thickness, μm | ||||
| N | N = 23 | N = 311 | ||
| Unadjusted Mean ± SE | 289±15 | 269±5 | 20 (−32, 72) | 0.22 |
| Median (25th, 75th percentile) | 276 (246, 342) | 244 (207, 301) | ||
| Adjusted Mean ± SE | 291±18 | 269±5 | 22 (−27, 71) | 0.24 |
| OCT Central Subfield Thickness Change from baseline, μm | ||||
| N | N = 23 | N = 310 | ||
| Unadjusted Mean ± SE | −83±26 | −141±8 | 58 (−16, 132) | 0.042 |
| Median (25th, 75th percentile) | −92 (−164, −18) | −120 (−210, −45) | ||
| Adjusted Mean ± SE | −115±18 | −138±5 | 22 (−27, 71) | 0.24 |
| Categorical Change in Central Subfield Thickness from Baseline, N (%)‡ | ||||
| ≥20% Reduction | 11 (48%) | 209 (67%) | −20% (−47, 9%) | 0.33 |
| OCT CST <250 μm and >10% reduction from baseline | 6 (26%) | 154 (50%) | −24% (−44, 9%) | 0.029 |
| OCT Volume (mm3) | ||||
| N | N = 22 | N = 286 | ||
| Unadjusted Mean ± SE | 7.5±0.2 | 7.4±0.1 | 0.1 (−0.6, 0.8) | 0.69 |
| Median (25th, 75th percentile) | 7.4 (6.8, 8.3) | 7.2 (6.6, 7.8) | ||
| Adjusted Mean ± SE | 7.5±0.2 | 7.3±0.1 | 0.2 (−0.4, 0.8) | 0.33 |
| OCT Volume change from baseline (mm3) | ||||
| N | N = 20 | N = 226 | ||
| Unadjusted Mean ± SE | −0.9±0.2 | −1.5±0.1 | 0.6 (−0.3, 1.5) | 0.019 |
| Median (25th, 75th percentile) | −0.6 (−1.3, −0.2) | −1.1 (−2.2, −0.5) | ||
| Adjusted Mean ± SE | −1.3±0.2 | −1.5±0.1 | 0.2 (−0.4 0.8) | 0.33 |
| 104-week visit | ||||
| OCT Central Subfield Thickness, μm | ||||
| N | N = 24 | N = 278 | ||
| Unadjusted Mean ± SE | 272±20 | 254±5 | 18 (−29, 66) | 0.37 |
| Median (25th, 75th percentile) | 253 (212, 325) | 234 (199, 284) | ||
| Adjusted Mean ± SE | 275±18 | 256±5 | 18 (−30, 67) | 0.33 |
| OCT CST Change from baseline, μm | ||||
| N | N = 24 | N = 278 | ||
| Unadjusted Mean ± SE | −90±30 | −155±9 | 65 (−16, 145) | 0.046 |
| Median (25th, 75th percentile) | −86 (−198, −11) | −131 (−234, −50) | ||
| Adjusted Mean ± SE | −131±18 | −150±5 | 18 (−30, 67) | 0.33 |
| Categorical Change in Central Subfield Thickness from Baseline, N (%)‡ | ||||
| ≥20% Reduction | 14 (58%) | 201 (72%) | −14% (−43, 11%) | 0.57 |
| OCT CST <250 μm and >10% reduction from baseline | 11 (46%) | 160 (58%) | −12% (−38, 17%) | 0.45 |
| OCT Volume (mm3) | ||||
| N | N = 20 | N = 239 | ||
| Unadjusted Mean ± SE | 7.1±0.2 | 7.3±0.1 | −0.1 (−0.8, 0.6) | 0.54 |
| Median (25th, 75th percentile) | 7.2 (6.6, 7.7) | 7.0 (6.6, 7.6) | ||
| Adjusted Mean ± SE | 7.2±0.2 | 7.2±0.1 | 0.0 (−0.5, 0.6) | 0.89 |
| OCT Volume change from baseline (mm3) | ||||
| N | N = 19 | N = 187 | ||
| Unadjusted Mean ± SE | −1.1±0.2 | −1.7±0.1 | 0.6 (−0.5, 1.6) | 0.042 |
| Median (25th, 75th percentile) | −0.7 (−1.6, −0.4) | −1.3 (−2.4, −0.6) | ||
| Adjusted Mean ± SE | −1.6±0.2 | −1.6±0.1 | 0.0 (−0.5, 0.6) | 0.89 |
| 156-week visit | ||||
| OCT Central Subfield Thickness, μm | ||||
| N | N = 19 | N = 251 | ||
| Unadjusted Mean ± SE | 281±32 | 246±6 | 35 (−23, 92) | 0.29 |
| Median (25th, 75th percentile) | 246 (199, 292) | 225 (191, 274) | ||
| Adjusted Mean ± SE | 282±19 | 249±5 | 33 (−19, 85) | 0.11 |
| OCT Central Subfield Thickness Change from baseline, μm | ||||
| N | N = 19 | N = 251 | ||
| Unadjusted Mean ± SE | −96±49 | −165±9 | 70 (−23, 163) | 0.18 |
| Median (25th, 75th percentile) | −110 (−219, −20) | −151 (−249, −65) | ||
| Adjusted Mean ± SE | −124±19 | −157±5 | 33 (−19, 85) | 0.11 |
| Categorical Change in Central Subfield Thickness from Baseline, N (%)‡ | ||||
| ≥20% Reduction | 13 (68%) | 188 (75%) | −6% (−40, 17%) | 0.81 |
| OCT CST <250 μm and >10% reduction from baseline | 10 (53%) | 156 (62%) | −10% (−40, 20%) | 0.43 |
| OCT Volume (mm3) | ||||
| N | N = 17 | N = 212 | ||
| Unadjusted Mean ± SE | 7.1±0.3 | 7.1±0.1 | 0 (−0.8, 0.7) | 0.98 |
| Median (25th, 75th percentile) | 6.9 (6.2, 7.6) | 6.8 (6.5, 7.5) | ||
| Adjusted Mean ± SE | 7.2±0.2 | 7.1±0.1 | 0.1 (−0.5, 0.7) | 0.65 |
| OCT Volume change from baseline (mm3) | ||||
| N | N = 15 | N = 173 | ||
| Unadjusted Mean ± SE | −1.1±0.2 | −1.7±0.1 | 0.7 (−0.5, 1.8) | 0.023 |
| Median (25th, 75th percentile) | −0.8 (−1.6, −0.4) | −1.3 (−2.6, −0.7) | ||
| Adjusted Mean ± SE | −1.6±0.2 | −1.7±0.1 | 0.1 (−0.5, 0.7) | 0.65 |
OCT=optical coherence tomography; CI=confidence interval; SE=standard error;
OCT CST conversion to Zeiss Stratus equivalent values was applied as follows: −43.12 +1.01×Zeiss Cirrus; −72.76 + 1.03×Spectralis. OCT volume conversion to Zeiss Stratus equivalent values was applied as follows: −1.21 + 1.02x((((CST×(4/9)+inner superior subfield thickness × (8/9)+ inner temporal subfield thickness× (8/9)+ inner inferior subfield thickness× (8/9)+ inner nasal subfield thickness × (8/9)+ outer superior subfield thickness×3 + outer temporal subfield thickness ×3 + outer inferior subfield thickness ×3 + outer nasal subfield thickness ×3) ×3×3×3.14)/16)/1000); −2.05 + 1.06×Spectralis. OCT CST and OCT volume values were converted to Stratus equivalent for 3 eyes at the 2 year visit and 23 eyes at the 3 year visit.
Unadjusted differences are the observed differences, and P values are from Student's t-test. Adjusted differences, and P values are from a linear mixed model with covariates for vitrectomy status and (categorical) follow up time by vitrectomy status interaction plus imbalanced baseline covariates (visual acuity, OCT CST, DR severity, lens status, prior PRP, prior DME treatment, duration of diabetes, and randomized treatment assignment). OCT volume change is also adjusted for baseline volume.
Difference and 99% confidence interval are unadjusted for baseline imbalances; however, the P-value is adjusted. Improvement in CST was defined as a decrease of at least 20%, calculated as the change in central subfield thickness from baseline divided by the baseline value and multiplied by 100.
Figure 2.
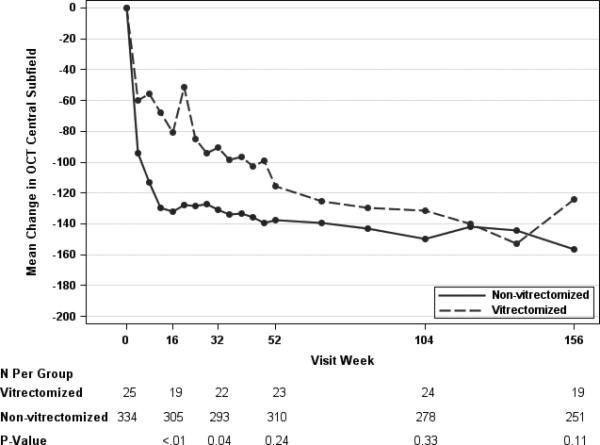
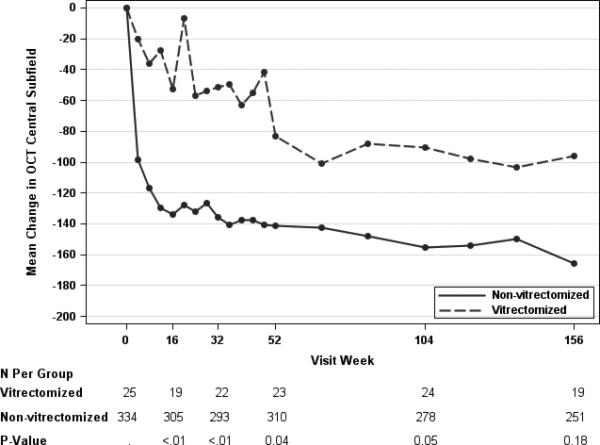
Mean Change in OCT Central Subfield Thickness by Pre-Enrollment Vitrectomy Status, Adjusted (Panel A)* B)† for Baseline Covariates.
Cataract Surgery and its Effects on Vision and Anatomic Outcomes
Eyes in the no vitrectomy group were twice as likely to be phakic at baseline compared with eyes in the vitrectomy group (72% vs. 36%). The visual acuity and OCT analyses were repeated censoring data from all eyes that underwent cataract surgery beginning with the date of cataract surgery, with no substantive differences seen in any of the results (data not shown).
Safety Outcomes
Twenty-nine eyes (9%, 99% CI: 5% to 13%) in the no vitrectomy group were reported as having at least one ocular adverse event, such as endophthalmitis, optic neuropathy, traction retinal detachment, venous occlusive disease, or vitreous hemorrhage, in contrast to no eyes (0%, 99% CI: 0% to 19%) in the prior vitrectomy group. The number of deaths, serious systemic adverse events, and Anti-platelet Trialists’ Collaboration (ATC) events did not differ by vitrectomy status (data not shown).
Discussion
Determining whether anti-VEGF therapy for DME can improve vision and anatomic outcomes in eyes post vitrectomy would be helpful to ophthalmologists managing such patients and when considering vitrectomy in eyes at risk of DME. Animal studies have suggested that drug clearance is more rapid once the vitreous has been removed.6-10 Pharmacokinetic studies evaluating vitreous clearance of commonly used anti-VEGF agents in humans are lacking, particularly post vitrectomy. Two small case series evaluating short term effects of bevacizumab for DME in eyes that had undergone vitrectomy led the authors to conclude that anti-VEGF therapy may have limited or no effectiveness in this setting, although there were no controlled comparisons.
Animals studies have suggested that VEGF levels decrease more rapidly in eyes that have undergone vitrectomy.16 Some authors hypothesize this leads to lower intravitreal VEGF levels following vitrectomy which may explain why some eyes with DME improve following vitrectomy. It is therefore plausible that anti-VEGF therapy, even if cleared more rapidly from the vitreous in eyes post vitrectomy, may reach sufficient levels with an adequate duration, to be an effective treatment for eyes with new or persistent DME following vitrectomy.
The present exploratory analysis of eyes assigned to ranibizumab with prompt or deferred laser in a DRCR.net trial of center-involved DME with vision impairment shows that the favorable functional and anatomic outcomes over the course of 3 years appeared similar in the small group of eyes with vitrectomy prior to study entry and the larger cohort of eyes that had not undergone prior vitrectomy. During the first year of DME management, eyes with prior vitrectomy appeared to have a slower rate of improvement in macular thickness, and appeared to require more injections in the second six months of management when compared with eyes without vitrectomy. However, the confidence that these differences represent true differences is limited by the small number of eyes evaluated post vitrectomy, the lack of persistence of these findings with longer follow-up, and the lack of a randomized comparison group to control for potential confounders that could affect these outcomes. Eyes in the vitrectomy group differed from eyes without a prior history of vitrectomy with respect to several baseline factors generally indicative of greater disease severity. This included poorer levels of initial visual acuity, thinner central subfield thicknesses, and greater prevalence of PDR, PRP, prior DME treatment, or cataract surgery, and longer duration of diabetes. The initial level of visual acuity and macular thickness are factors known to be associated with vision and OCT outcomes associated with anti-VEGF therapy.17 An analysis adjusting for these potential confounders narrowed the difference between groups in macular thickness outcomes, suggesting that the clinical impression of an inferior response of OCT outcome in eyes with vitrectomy to anti-VEGF therapy may be due partly to other characteristics associated with these eyes rather than the vitrectomy itself. It is impossible to know whether adjustment fully accounted for differences between the vitrectomy status groups; nevertheless, both adjusted and unadjusted analyses suggested a lag in response in macular thickness in eyes with vitrectomy during the early follow up period and this might be the result of differences in drug clearance. This might explain why clinicians have questioned the efficacy of anti-VEGF therapy in eyes post-vitrectomy, as they likely have focused on short-term observations. More importantly, the data through 3 years, did not demonstrate any long-term difference in the anatomic improvement between the two groups and did not show that the vitrectomy eyes required more injections, more sessions of focal/grid laser, or more procedures/interventions to manage their diabetic eye disease. However, in those that completed the 3 year visit, most (6 of 7) eyes with prior vitrectomy that were assigned to ranibizumab and deferred laser required at least one laser session during follow-up; whereas more than half of the eyes without vitrectomy (75 of 132) assigned to the same treatment strategy did not require focal/grid laser.
Strengths of this study include prospectively collected data, with standardized measures of disease outcome, in eyes all managed with a standardized retreatment protocol. There is a large comparison group, and follow-up through 3 years is evaluated. Limitations of the study include the small number of eyes with vitrectomy, lack of pharmacokinetic data, and imbalances between the vitrectomy and no vitrectomy groups at baseline on multiple factors, which might be related to the outcome. Such factors could be confounders for the relationship between vitrectomy and visual acuity and retinal thickness outcomes, or some could be mediators, i.e., factors that lie along the causal pathway through which vitrectomy affects visual acuity and retinal thickness. Potential mediating factors included prior DME treatment, lens status, and retinal thickness at baseline. Our data collection was not designed to capture the temporal relationship between vitrectomy and those factors, which leads to uncertainty as to whether analyses should be adjusted, as appropriate for confounders, or not adjusted, as appropriate for mediators.. Regardless, results were similar when potential mediators were removed from the adjusted models reported herein (data not shown).
In summary, early in the course of managing eyes with prior vitrectomy and DME, the rate of anatomic improvement may be slower than in eyes without vitrectomy, and may require more consistent monthly treatment within the first year of therapy. However, in contrast to previous studies, this exploratory analysis shows that eyes post-vitrecomy exhibit favorable functional and anatomic responses to anti-VEGF therapy. There was little evidence that eyes with DME and a history of prior vitrectomy, similar to those enrolled and treated in this trial, would have a clinically important different functional or anatomic result from those without vitrectomy, particularly when followed longer than one year. Given the small number of eyes with vitrectomy evaluated in this study, and the lack of randomization by vitrectomy status at baseline, these findings should be interpreted with caution, and should be taken into the context of disease severity when managing patients with prior vitrectomy and DME.
Summary Statement.
Prior vitrectomy did not appear to alter the favorable effect that ranibizumab has for visual acuity and OCT outcomes through 3 years in eyes with center-involved diabetic macular edema. However, OCT improvement may have been slower among the post-vitrectomy eyes during the first year.
Acknowledgments
Financial Support:
Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, U.S. Department of Health and Human Services EY14231, EY018817
Financial Disclosures:
The funding organization (National Institutes of Health) participated in oversight of the conduct of the study and review of the manuscript but not directly in the design or conduct of the study, nor in the collection, management, analysis, or interpretation of the data, or in the preparation of the manuscript.
Allergan, Inc. provided the triamcinolone and Genentech provided the ranibizumab. As per the DRCR.net Industry Collaboration Guidelines (available at www.drcr.net), the DRCR.net had complete control over the design of the protocol, ownership of the data, and all editorial content of presentations and publications related to the protocol. Allergan, Inc. has provided unrestricted funds to DRCR.net for its discretionary use and Genentech has provided funds restricted to DRCR.net clinical sites.
Dr N. Bressler is principal investigator of grants at The Johns Hopkins University sponsored by the Bayer; Genentech, Inc, Novartis Pharma AG, Regeneron, and The Emmes Corporation through the Office of Research Administration of the Johns Hopkins University School of Medicine, and has a contract agreement from the American Medical Association to the Johns Hopkins University School of Medicine.
Footnotes
Financial Conflicts of Interest:
A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net
References
- 1.Rajendram R, Fraser-Bell S, Kaines A, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol. 2012;130:972–9. doi: 10.1001/archophthalmol.2012.393. [DOI] [PubMed] [Google Scholar]
- 2.Elman MJ, Bressler NM, Qin H, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118:609–14. doi: 10.1016/j.ophtha.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for Diabetic Macular Edema: Results from 2 Phase III Randomized Trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 4.Elman MJ, Qin H, Aiello LP, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology. 2012;119:2312–8. doi: 10.1016/j.ophtha.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang GE, Berta A, Eldem BM, et al. Two-Year Safety and Efficacy of Ranibizumab 0.5 mg in Diabetic Macular Edema: Interim Analysis of the RESTORE Extension Study. Ophthalmology. 2013 doi: 10.1016/j.ophtha.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Schindler RH, Chandler D, Thresher R, et al. The clearance of intravitreal triamcinolone acetonide. Am J Ophthalmol. 1982;93:415–7. doi: 10.1016/0002-9394(82)90130-1. [DOI] [PubMed] [Google Scholar]
- 7.Doft BH, Weiskopf J, Nilsson-Ehle I, et al. Amphotericin clearance in vitrectomized versus nonvitrectomized eyes. Ophthalmology. 1985;92:1601–5. doi: 10.1016/s0161-6420(85)33838-1. [DOI] [PubMed] [Google Scholar]
- 8.Meredith TA. Antimicrobial pharmacokinetics in endophthalmitis treatment: studies of ceftazidime. Trans Am Ophthalmol Soc. 1993;91:653–99. [PMC free article] [PubMed] [Google Scholar]
- 9.Pearson PA, Hainsworth DP, Ashton P. Clearance and distribution of ciprofloxacin after intravitreal injection. Retina. 1993;13:326–30. doi: 10.1097/00006982-199313040-00010. [DOI] [PubMed] [Google Scholar]
- 10.Chin HS, Park TS, Moon YS, et al. Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina. 2005;25:556–60. doi: 10.1097/00006982-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Beer PM, Bakri SJ, Singh RJ, et al. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology. 2003;110:681–6. doi: 10.1016/S0161-6420(02)01969-3. [DOI] [PubMed] [Google Scholar]
- 12.Diabetic Retinopathy Clinical Research Network Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–77 e35. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bressler SB, Edwards AR, Chalam KV, et al. Reproducibility of Spectral-Domain Optical Coherence Tomography Retinal Thickness Measurements and Conversion to Equivalent Time-Domain Metrics in Diabetic Macular Edema. JAMA Ophthalmol. 2014 doi: 10.1001/jamaophthalmol.2014.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aiello LP, Beck RW, Bressler NM, et al. Rationale for the diabetic retinopathy clinical research network treatment protocol for center-involved diabetic macular edema. Ophthalmology. 2011;118:e5–14. doi: 10.1016/j.ophtha.2011.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verbeke G, Molenberghs G. Springer Series in Statistics. Springer; New York: 2000. Linear mixed models for longitudinal data. [Google Scholar]
- 16.Lee SS, Ghosn C, Yu Z, et al. Vitreous VEGF clearance is increased after vitrectomy. Invest Ophthalmol Vis Sci. 2010;51:2135–8. doi: 10.1167/iovs.09-3582. [DOI] [PubMed] [Google Scholar]
- 17.Bressler SB, Qin H, Beck RW, et al. Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol. 2012;130:1153–61. doi: 10.1001/archophthalmol.2012.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]


