Abstract
Background and Aim
Associations between pre-liver transplantation (pre-LT) BMI and post-LT survival are well described; however, there are few data assessing associations between the commonly observed post-LT BMI changes and survival. We investigated the impact of early post-LT BMI change on post-LT patient and graft survival.
Methods
Using UNOS data, we identified 2,968 adult primary LT recipients who were not overweight pre-LT, BMI >16 to ≤25 kg/m2, and who had BMI recorded at 2-years post-LT. Delta BMI was defined as the BMI difference from pre-LT and 2-years post-LT. Recipients were grouped into three categories: BMI Gain (increase >1 BMI point), BMI Loss (decrease >1 BMI point), and BMI Stable (maintained BMI within 1 point). Associations between Delta BMI and patient and graft survival were evaluated using Kaplan Meier and multivariable Cox regression analyses.
Results
BMI Gain was common (54%) and associated with significantly greater 5-year patient and graft survival (90% and 89%, respectively), compared to recipients who had either BMI Loss (77% and 74%, respectively, p<0.0001 for both) or BMI Stable (83%, p=0.04 and 82%, p=0.007, respectively). In multivariable analyses, increasing Delta BMI was inversely associated with risk for death and graft loss (HR 0.89 [95% CI 0.86–0.91], p<0.001; and HR 0.88 [95% CI 0.86–0.91], p<0.001, respectively).
Conclusion
This study of a large national liver transplant database demonstrated that post-LT BMI gain was associated with better patient and graft survival, whereas BMI loss was associated with reduced patient and graft survival.
Keywords: body mass index, outcomes, post-transplant, survival, weight gain, weight loss
Introduction
Data from the Centers for Disease Control and Prevention suggest that more than 35% of the U.S. adult population is obese. (1) Obesity is associated with increased mortality, and was linked to significant morbidity including diabetes, cardiovascular and chronic liver disease. (2, 3) The rise in prevalence of obesity in the general population has also had an impact on liver transplantation (LT) with more than 10% of new recipients having a BMI ≥35 kg/m2. (4)
Weight change post-LT is commonly observed, especially weight gain. (5–9) An analysis of a well-characterized multicenter LT database demonstrated that during the first and second year post-LT, mean weight gains were 6 kilograms (kg) and 3 kg, respectively. (6) Subsequent to 2-years post-LT, recipient weight did not change in a statistically or clinically significant manner. Pre-LT obesity was shown to be a risk factor for post-LT obesity, even after correcting for ascites weight. (10) And several studies have investigated the associations between pre-LT obesity and post-LT survival. (11–13) However, there is a paucity of data regarding how the frequently observed weight changes post-LT impact patient and graft survival. Therefore, we sought to analyze the impact of post-LT weight and BMI changes on patient and graft survival using a large, national liver transplant database.
Patients and methods
Data Source and Cohort
We utilized data from the United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research file. Adults (≥18 years) were identified who underwent primary LT from 3/2002 to 6/2009, had available pre-LT weight and height, and at least one recorded post-LT weight at two years from LT. We restricted our patient population by pre-LT BMI, as well, since a previously published report from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Liver Transplant Database demonstrated that the presence of ascites results in misclassification of approximately 11–20% of patients with large volume ascites as being overweight or obese. (12, 13) Therefore, our study cohort included only LT recipients who had pre-LT BMI >16 kg/m2 and ≤25 kg/m2 since we did not have data pertaining to ascites volume in the UNOS dataset.
BMI Calculations
Pre-LT BMI (kg/m2) was calculated using the body weight (kg) obtained at the time of LT and height (meters, m) recorded at any time pre-LT. BMI was calculated using the following formula: (weight, kg) /(height, m)2. Post-LT BMI was calculated using the post-LT recorded weight that was temporally closest to 2-years from transplantation. The rationale for the selection of this 2-year time point for calculating post-LT BMI was based on the published investigation using the NIDDK Liver Transplant Database demonstrating stabilization in post-LT weight by the second year post-LT. (12) The change in BMI from pre-LT to post-LT, defined as Delta BMI, was calculated by subtracting the pre-LT BMI from the post-LT BMI.
To account for outliers in the UNOS data, particularly with respect to the recording of weight data, we excluded the following: recipients with pre-LT BMI <16 kg/m2 (n=240) as it would be unlikely that these adult patients could safely undergo LT with that degree of malnutrition; recipients with donors under the age of 10 years (n=205) due to the low likelihood of such a young donor liver being suitable for an adult recipient; donors with recorded height <1.14 m (n=7) or recorded donor weight <36 kg (n=212) as these parameters correspond to 50th and <5th percentile for height and weight, respectively, for a 10-year-old American girl; donors weighing >114 kg (n=1,482) as it is unlikely such a large donor would be utilized.
Using Delta BMI, we categorized LT recipients into three groups: (1) BMI Gain, characterized by a gain of more than one BMI point from pre-LT to post-LT; (2) BMI Loss, characterized by a loss of more than one BMI point from pre-LT to post-LT; and (3) BMI Stable, characterized by a change of no more than one BMI point in either direction from pre-LT to post-LT. Substantial Delta BMI was defined as a gain or loss of greater than 3 BMI points between pre-LT and post-LT, which correlates with a clinically notable 13% change in body weight for an average-sized adult.
Covariates
Recipient characteristics analyzed in this study included the following: age, gender, race (White vs. non-white), height (m), weight (kg), BMI (kg/m2), MELD at LT, primary liver disease diagnosis (Hepatitis C [HCV], Alcohol, Cryptogenic/Non-alcoholic steatohepatitis [NASH], Hepatitis B [HBV], or Other), presence of hepatocellular carcinoma (HCC), diabetes, need for dialysis or mechanical ventilation at the time of LT, hospitalization within 90-days prior to LT, and treatment for acute rejection within the first post-LT year.
Donor characteristics analyzed in this study included the following: age, gender, race (White vs. non-white), weight, height, BMI, cold ischemia time, donation after cardiovascular death (DCD), partial or split graft, and living donor graft.
Statistical Analyses
Continuous data were summarized as mean (+/− standard deviation, SD) or median [interquartile range, IQR], as appropriate. Statistical comparisons were conducted using Student’s t-test, Wilcoxon rank sum, or chi-square testing, as appropriate.
Unadjusted patient and graft 5-year survivals were calculated using the Kaplan-Meier method and compared using a log rank test. Patient and graft survival were calculated for the overall cohort and stratified by categories of post-LT Delta BMI (BMI Gain, BMI Loss, and BMI Stable). Survival analyses were also stratified by recipient pre-LT BMI category (BMI < 19; 19 to < 22; and 22 to 25 kg/m2). And comparisons were made between recipients with and without substantial Delta BMI post-LT. Patient survival and graft survival were calculated from the time of LT until death, graft failure, or date of last follow-up.
Multivariable analyses to identify factors associated with patient and graft survival were performed using Cox proportional hazard regression models and data are presented as hazard ratios (HR) with 95% confidence intervals (95% CI). Covariates with p-values less than 0.10 in univariate analysis were entered into the multivariable model. Covariates with p-value less than 0.05 in multivariable analysis were considered statistically significant. All data analyses were performed using SAS ® software, version 9.3 (SAS Institute, Cary, NC).
Results
Liver Transplant Cohort Characteristics
We identified 31,254 primary adult LT recipients between March 2002 and June 2009. Of these, 9,243 LT recipients had follow-up weight data recorded at 2-years post-LT, and 2,968 of these recipients had a pre-LT BMI that was >16 and ≤25 kg/m2. These 2,968 LT recipients were included in our analyses and their characteristics are shown in Table 1. The mean age was 51 years (SD 11) and 63% (n= 1,856) were men. The median pre-LT BMI was 22.8 kg/m2 [IQR, 21.1–23.9] with hepatitis C (n=871, 29.4%) being the leading etiology of liver disease. Twenty percent (n=606) had a diagnosis of HCC pre-LT. The median survival was not reached in the cohort, but the 8-year overall survival was 70% and maximum post-LT follow-up time was 8 years.
Table 1.
Characteristics of Patient Population, Overall and Stratified by Delta BMI Category
| Variable | Overall (N=2968) |
BMI Loss (N=581) |
BMI Stable (N=775) |
BMI Gain (N=1612) |
p-value |
|---|---|---|---|---|---|
| RECIPIENT FACTORS | |||||
|
Age, years Mean (SD) |
51.4 (11.4) | 50.7 (11.7) | 51.5 (12.1) | 51.7 (10.9) | 0.20 |
| Gender (n, % male) | 1856 (62.5%) | 324 (55.8%) | 488 (63.0%) | 1044 (64.8%) | <0.001 |
| Ethnicity (n, % Caucasian) | 2086 (70.3%) | 409 (70.4%) | 542 (69.9%) | 1135 (70.4%) | 0.97 |
| Primary Liver Disease (n, %) | |||||
| HCV | 871 (29.3%) | 191 (32.9%) | 241 (31.1%) | 439 (27.2%) | <0.001 |
| Alcohol | 340 (11.5%) | 70 (12.1%) | 66 (8.5%) | 204 (12.7%) | |
| NASH/Cryptogenic | 175 (5.9%) | 20 (3.4%) | 36 (4.6%) | 119 (7.4%) | |
| HBV | 151 (5.1%) | 28 (4.8%) | 47 (6.1%) | 76 (4.7%) | |
| Other | 1431 (48.2%) | 272 (46.8%) | 385 (49.7%) | 774 (48.0%) | |
| HCC (n, %) | 606 (20.4%) | 141 (24.3%) | 205 (26.5%) | 260 (16.1%) | <0.001 |
|
BMI at LT, kg/m2 Median (IQR) |
22.8 (21.1–23.9) | 23.1 (21.9–24.2) | 22.9 (21.3–24.0) | 22.5 (20.7–23.8) | <0.001 |
|
BMI at 2-years post-LT, kg/m2 Median (IQR) |
23.8 (21.6–26.1) | 20.4 (19.1–21.6) | 22.9 (21.4–24.0) | 25.9 (24.1–27.6) | <0.001 |
|
Delta BMI, kg/m2 Median (IQR) |
1.4 (−0.5 to 3.5) | −2.3 (−3.4 to −1.6) | 0 (−0.4 to 0.5) | 3.3 (2.1 to 5.1) | <0.001 |
| Diabetes (n, %) | 524 (17.7%) | 91 (15.7%) | 129 (16.7%) | 304 (18.9%) | 0.15 |
|
Calculated MELD at LT Median (IQR) |
18 (13–24) | 17 (12–24) | 16 (11–23) | 19 (14–25) | <0.001 |
| Dialysis in week prior to LT (n, %) | 217 (7.3%) | 40 (6.9%) | 46 (5.9%) | 131 (8.1%) | 0.14 |
| Hospitalization within 90 days of LT (n, %) | 505 (17.0%) | 100 (17.2%) | 115 (14.8%) | 290 (18.0%) | 0.16 |
| Mechanical Ventilation at LT (n, %) | 100 (3.4%) | 27 (4.7%) | 25 (3.2%) | 48 (3.0%) | 0.16 |
| Rejection in 1st year post-LT (n, %) | 363 (12.2%) | 78 (13.4%) | 86 (11.1%) | 199 (12.3%) | 0.42 |
| DONOR FACTORS | |||||
|
Age, years Mean (SD) |
40.0 (17.5) | 40.7 (17.5) | 39.6 (17.4) | 39.9 (17.6) | 0.38 |
| Gender (n, % male) | 1583 (53.3%) | 306 (52.7%) | 414 (53.4%) | 863 (53.5%) | 0.94 |
| Ethnicity (n, % Caucasian) | 2079 (70.0%) | 395 (68.0%) | 545 (70.3%) | 1139 (70.7%) | 0.47 |
|
Height, cm Median (IQR) |
170 (163–178) | 170 (163–178) | 170 (163–178) | 170 (163–178) | 0.30 |
|
Weight, kg Median (IQR) |
72.8 (63.0–83.9) | 72.6 (63.0–83.5) | 71.2 (62.0–82.0) | 73.2 (63.5–85.0) | 0.004 |
|
BMI, kg/m2 Median (IQR) |
24.9 (22.0–28.2) | 24.8 (22.1–28.4) | 24.4 (21.7–27.7) | 25.1 (22.2–28.3) | 0.002 |
| DCD Allograft (n, %) | 147 (5.0%) | 29 (5.0%) | 35 (4.5%) | 83 (5.2%) | 0.80 |
|
Cold ischemia time, hours Mean (SD) |
7.0 (3.6) | 6.7 (2.9) | 7.0 (3.6) | 7.2 (3.9) | 0.027 |
| Partial or Split Liver Allograft (n, %) | 258 (8.7%) | 46 (7.9%) | 66 (8.5%) | 146 (9.1%) | 0.69 |
| Living Donor Allograft (n, %) | 195 (6.6%) | 37 (6.4%) | 57 (7.4%) | 101 (6.3%) | 0.59 |
Liver donor characteristics are shown in Table 1. The mean age was 40 years (SD 18) and 53.3% (n=1,583) were men. The median donor BMI was 24.9 kg/m2 [IQR, 22.0–28.2] and 147 donors (5%) donated after cardiac death.
Weight and BMI Change Post-Liver Transplant
The median post-LT BMI significantly increased from 22.8 [IQR, 21.1–23.9] pre-LT to 23.8 [IQR, 21.6–26.1], which correlates to an increase of 1.4 kg/m2 (p<0.001). Thirty-six percent (n=1,071) of LT recipients met BMI criteria for becoming either newly overweight (BMI ≥25 and <30) or obese (BMI ≥30) at post-LT year 2, with 139 of these individuals being obese. BMI Gain was demonstrated in 54% (n=1,612) of liver recipients, BMI Loss in 20% (n=581), and BMI Stable in 26% (n=775). Demographic comparisons of recipients in the three Delta BMI categories are shown in Table 1. The three groups were similar for most variables; however, LT recipients with BMI Loss at 2-years post-LT started with a higher pre-LT BMI (median 23.1, [IQR, 21.9 to 24.2]) compared to the other two groups (p<0.001 for both). The median calculated MELD at LT was higher among patients with BMI Gain (18 [IQR 13–24]), compared to BMI Loss (17 [IQR 12–24], p=0.005) and BMI Stable (16 [IQR 11–23], p<0.001). There was also a lower prevalence of HCC among patients with BMI Gain (16.1%) compared to those with BMI Loss (24.3%) or BMI Stable (26.5%), p<0.001 for both. Statistically significant differences were also seen in the following donor factors between the three Delta BMI groups: donor weight (p=0.004), donor BMI (p=0.002), and cold ischemia time (p=0.027).
Impact of Pre-LT BMI on Survival
Five-year patient survival in the overall cohort was 85.9%, and the 5-year patient survival was similar when stratified by pre-LT BMI: 90.5% for BMI <19 kg/m2 (n=201); 84.8% for BMI 19 to <22 kg/m2 (n=903); and 85.9% for BMI 22 to 25 kg/m2 (n=1,864) (p-values >0.10 for all comparisons). (Figure 1A). The 5-year graft survival for the overall cohort was 84.5%, and the 5-year graft survival stratified by pre-LT BMI were as follows: 90.5% for BMI <19 kg/m2; 83.4% for BMI 19 to <22 kg/m2; and 84.4% for BMI 22 to 25 kg/m2. (Figure 1B). Interestingly, LT recipients with pre-LT BMI <19 kg/m2 had a greater graft survival compared to LT recipients with pre-LT BMI 19 to <22 kg/m2, which was statistically significant by 8 years post-LT (p=0.032).
Figure 1.
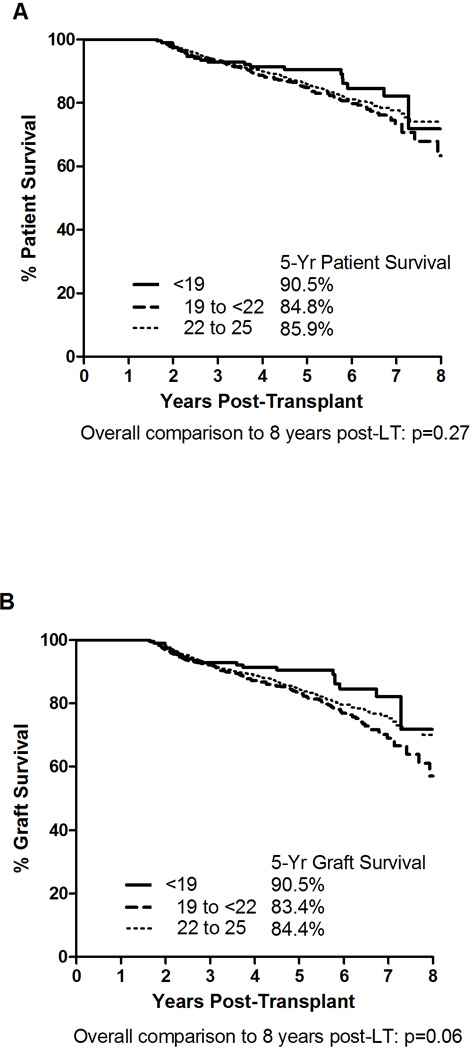
Unadjusted 5-Year Patient and Graft Survivals Stratified by Pre-LT BMI Category (<19, 19 to <22, and 22 to 25 kg/m2).. Panel A: Patient Survival; Panel B: Graft Survival
Impact of Delta BMI on Survival
Five-year patient and graft survival stratified by Delta BMI are demonstrated in Figures 2A and 2B, respectively. Patient survival was significantly better for recipients with BMI Gain (90.1%) compared to recipients with either BMI Loss (77.2%) or BMI Stable (83.3%), p<0.001 for both. Similarly, 5-year graft survival among recipients with BMI Gain (89.4%) was significantly greater than graft survival among recipients with either BMI Loss (74.3%) or BMI Stable (82.0%), p<0.001 for both.
Figure 2.
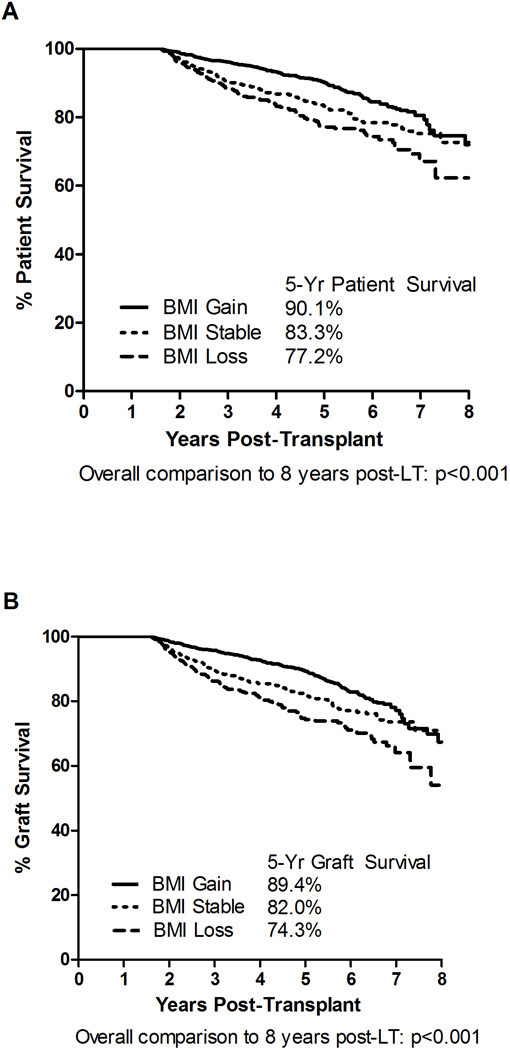
Unadjusted 5-Year Patient and Graft Survivals Stratified by Delta BMI Category (BMI Gain [>1 point increase in BMI], BMI Loss [>1 point decrease in BMI], and BMI Stable [≤1 BMI point change]). Panel A: Patient Survival; Panel B: Graft Survival
We further evaluated 5-year patient and graft survivals with specific attention to recipients with substantial Delta BMI, which are shown in Figures 3A and 3B, respectively. The 5-year patient survivals were: 89.7% for substantial BMI Gain (>3 kg/m2), 90.7% for BMI Gain (>1 to 3 kg/m), 83.3% for BMI Stable; 78.5% for BMI Loss (>1 to 3 kg/m2), and 74.5% for substantial BMI Loss (>3 kg/m2). The 5-year graft survivals were: 89.3% for substantial BMI Gain (>3 kg/m2), 89.5% for BMI Gain (>1 to 3 kg/m), 82.0% for BMI Stable; 77.1% for BMI Loss (<−1 to −3 kg/m2), and 68.7% for substantial BMI Loss (<−3 kg/m2). Recipients with substantial BMI Loss had the lowest patient (74.5%) and graft (68.7%) survival of the 5 groups analyzed. These changes were significantly lower compared to those recipients with substantial BMI gain (p<0.001 for patient and graft survival). There was no significant decrease in 5-year patient survival between recipients with substantial BMI Gain and recipients with lesser BMI gain (89.7% vs. 90.7%, respectively, p=0.43). Although the 5-year graft survival was similar (89.3% vs. 89.5%) between these two groups (substantial BMI gain and lesser BMI gain), graft survival was actually higher at 8-years post-LT for those recipients with substantial BMI Gain (74.1%) compared to recipients with lesser BMI gain (59.8%, p=0.041).
Figure 3.
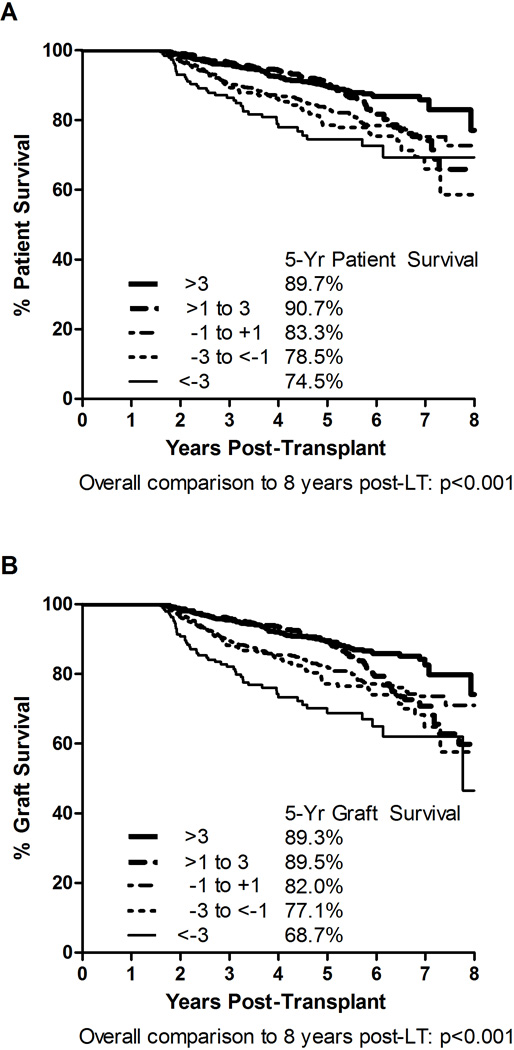
Impact of Substantial Delta BMI (greater than a 3 point change in BMI) on Patient and Graft 5-Year Survivals. Panel A: Patient Survival; Panel B: Graft Survival.
New-Onset Obesity Post-LT
We compared recipients who developed new-onset obesity (BMI ≥30 kg/m2) (n=139, 4.7%) by year 2 post-LT to recipients who maintained BMI Stable and found that recipients with new-onset obesity had significantly better 5-year patient (91.8% vs. 83.3%, p=0.020) and graft (91.8% vs. 82.0%, p=0.009) survivals. (Figure 4). Table 2 demonstrates the characteristics of LT recipients who developed new-onset obesity by 2 years post-LT compared to recipients whose BMI remained stable post-LT. The prevalence of HCV as the primary liver disease etiology and the prevalence of HCC were lower in the new-onset obesity group compared to recipients with BMI stable (25.2% vs. 31.1% for HCV; and 9.4% vs. 26.5% for HCC, p<0.001 for both comparisons). Recipients with new-onset obesity also had a statistically significantly higher prevalence of pre-LT diabetes (25.2% vs. 16.7%, p=0.016), were more likely to need dialysis pre-LT (11.5% vs. 5.9%, p=0.016), and had a higher calculated MELD score at the time of LT (22 [IQR 16–28] vs. 16 [IQR 11–23], p<0.001).
Figure 4.
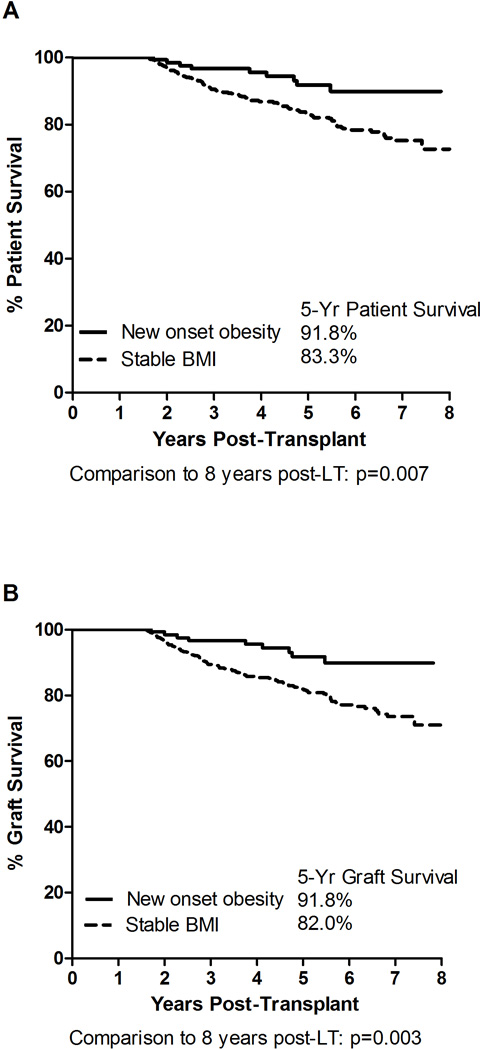
5-Year Survival Comparisons between LT Recipients with New-Onset Obesity and Stable BMI Post-LT. Panel A: Patient Survival; Panel B: Graft Survival.
Table 2.
Demographics at Baseline for Newly Obese (BMI ≥30) vs. BMI Stable at 2 years
| Variable | Overall (N=914) |
Obese (BMI ≥30) (N=139) |
Stable (BMI change −1 to +1) (N=775) |
p- value* |
|---|---|---|---|---|
| RECIPIENT FACTORS | ||||
|
Age, years Mean (SD) |
51.8 (11.7) | 53.1 (8.6) | 51.5 (12.1) | 0.54 |
| Gender (n, % male) | 569 (62.3%) | 81 (58.3%) | 488 (63.0%) | 0.29 |
| Ethnicity (n, % Caucasian) | 643 (70.4%) | 101 (72.7%) | 542 (69.9%) | 0.52 |
| Indication for LT (n, %) | ||||
| HCV | 276 (30.2%) | 35 (25.2%) | 241 (31.1%) | <0.001 |
| HBV | 51 (5.6%) | 4 (2.9%) | 47 (6.1%) | |
| Cryptogenic | 46 (5.0%) | 12 (8.6%) | 34 (4.4%) | |
| NASH | 10 (1.1%) | 8 (5.8%) | 2 (0.3%) | |
| ETOH alone | 95 (10.4%) | 29 (20.9%) | 66 (8.5%) | |
| Other | 436 (47.7%) | 51 (36.7%) | 385 (49.7%) | |
| HCC (n, %) | 218 (23.9%) | 13 (9.4%) | 205 (26.5%) | <0.001 |
|
BMI at LT, kg/m2 Median (IQR) |
23.1 (21.6–24.2) | 24.1 (23.2–24.7) | 22.9 (21.3–24.0) | <0.001 |
|
BMI at 2 years post-LT Median (IQR) |
23.4 (21.7–24.7) | 31.2 (30.5–32.1) | 22.9 (21.4–24.0) | <0.001 |
|
Delta BMI, kg/m2 Median (IQR) |
0.2 (−0.3 to 0.8) | 7.6 (6.5–8.8) | 0 (−0.4 to 0.5) | <0.001 |
| Diabetes (n, %) | 164 (17.9%) | 35 (25.2%) | 129 (16.7%) | 0.016 |
|
MELD at LT Median (IQR) |
17 (12–23) | 22 (16–28) | 16 (11–23) | <0.001 |
| Dialysis in week prior to LT (n, %) | 62 (6.8%) | 16 (11.5%) | 46 (5.9%) | 0.016 |
| Hospitalization within 90 days of LT (n, %) | 139 (15.2%) | 24 (17.3%) | 115 (14.8%) | 0.46 |
| Mechanical Ventilation at LT (n, %) | 26 (2.8%) | 1 (0.7%) | 25 (3.2%) | 0.10 |
| Rejection in 1st year post-LT (n, %) | 100 (10.9%) | 14 (10.1%) | 86 (11.1%) | 0.72 |
| DONOR FACTORS | ||||
|
Age, years Mean (SD) |
39.7 (17.5) | 40.0 (17.9) | 39.6 (17.4) | 0.74 |
| Gender (n, % male) | 476 (52.1%) | 62 (44.6%) | 414 (53.4%) | 0.055 |
| Ethnicity (n, % Caucasian) | 654 (71.6%) | 109 (78.4%) | 545 (70.3%) | 0.051 |
|
Height, cm Median (IQR) |
170 (163–178) | 168 (163–178) | 170 (163–178) | 0.15 |
|
Weight, kg Median (IQR) |
71.0 (62.0–82.0) | 70.3 (62.1–84.0) | 71.2 (62.0–82.0) | 0.60 |
|
BMI, kg/m2 Median (IQR) |
24.4 (21.7–27.8) | 24.8 (21.9–28.7) | 24.4 (21.7–27.7) | 0.15 |
| DCD Allograft (n, %) | 45 (4.9%) | 10 (7.2%) | 35 (4.5%) | 0.18 |
|
Cold ischemia time, hours Mean (SD) |
7.0 (3.7) | 7.5 (4.2) | 7.0 (3.6) | 0.21 |
| Partial or Split Liver Allograft (n, %) | 72 (7.9%) | 6 (4.3%) | 66 (8.5%) | 0.091 |
| Living Donor Allograft (n, %) | 61 (6.7%) | 4 (2.9%) | 57 (7.4%) | 0.052 |
Continuous variables compared with a Wilcoxon rank sum test, categorical variables compared with a chi-squared test
Multivariable Cox Regression Analyses
Four patients had missing data on their pre-LT MELD score. Therefore this analysis was based on 2964 patients. The median follow-up time for the overall cohort was 4.1 [IQR 2.8–5.9] years. For patient survival, Delta BMI (HR 0.89, 95% CI 0.86–0.92; p<0.001, per 1 kg/m2 change in BMI) was significantly and inversely associated with risk of death. Conversely, recipient and donor age, recipient male gender, and receipt of a deceased donor liver were all statistically significantly associated with increased risk of death post-LT (Table 3).
Table 3.
Univariate and Multivariable Patient Survival
| Variable [Reference group] |
Univariate patient survival HR (95% CI) |
p-value | Multivariable patient survival HR (95% CI) |
p-value | |
|---|---|---|---|---|---|
| RECIPIENT FACTORS | |||||
| Age | 1.03 (1.02–1.04) | <0.001 | 1.02 (1.01–1.03) | <0.001 | |
| Male [female] | 1.30 (1.05–1.61) | 0.015 | 1.34 (1.07–1.67) | 0.010 | |
| Non-caucasian [Caucasian] | 1.20 (0.97–1.49) | 0.093 | 1.08 (0.86–1.34) | 0.52 | |
| Indication for LT: | |||||
| HCC [non-HCC] | 1.67 (1.34–2.08) | <0.001 | 1.17 (0.90–1.51) | 0.23 | |
| HCV [non-HCV] | 1.24 (1.00–1.53) | 0.048 | 1.13 (0.92–1.40) | 0.25 | |
| HBV [non-HBV] | 0.73 (0.45–1.17) | 0.19 | |||
| BMI at LT: | |||||
| <19 [22 to 25] | 0.82 (0.53–1.27) | 0.36 | |||
| 19 to 21 [22 to 25] | 1.14 (0.92–1.41) | 0.25 | |||
| Delta BMI | 0.89 (0.87–0.93) | <0.001 | 0.89 (0.86–0.92) | <0.001 | |
| Diabetes | 1.36 (1.07–1.73) | 0.013 | 1.22 (0.96–1.56) | 0.11 | |
| MELD at LT | 0.98 (0.97–1.00) | 0.014 | 1.00 (0.98–1.01) | 0.50 | |
| Dialysis in week prior to LT | 1.01 (0.68–1.51) | 0.95 | |||
| Hospitalization within 90 days of LT | 1.03 (0.77–1.37) | 0.84 | |||
| Mechanical Ventilator | 0.57 (0.28–1.14) | 0.11 | |||
| Rejection in 1st year post-LT | 1.12 (0.84–1.50) | 0.44 | |||
| DONOR FACTORS | |||||
| Age | 1.02 (1.01–1.02) | <0.001 | 1.01 (1.01–1.02) | <0.001 | |
| Male [female] | 0.87 (0.72–1.07) | 0.19 | |||
| Non-caucasian [Caucasian] | 1.27 (1.03–1.57) | 0.028 | 1.24 (1.00–1.54) | 0.055 | |
| Height | 0.99 (0.98–1.00) | 0.061 | 0.99 (0.98–1.00) | 0.24 | |
| Weight | 1.00 (0.99–1.01) | 0.90 | |||
| BMI | 1.02 (0.99–1.04) | 0.17 | |||
| DCD Allograft | 1.13 (0.71–1.80) | 0.60 | |||
| Cold ischemia time | 1.02 (1.00–1.05) | 0.11 | |||
| Partial or Split Liver Allografta | 0.41 (0.25–0.68) | <0.001 | |||
| Deceased [Living Donor Allograft] | 2.64 (1.45–4.81) | 0.002 | 2.31 (1.25–4.24) | 0.007 | |
Correlated with living donor, so not included in the multivariable model
In multivariable Cox regression analysis of graft survival, Delta BMI (HR 0.89, 95% CI 0.86–0.91; p<0.001, per 1 kg/m2 change in BMI) was significantly and inversely associated with risk of graft loss. Conversely, recipient and donor age, recipient male gender, and receipt of a deceased donor liver were significantly associated with increased risk of graft loss (Table 4).
Table 4.
Univariate and Multivariable Graft Survival
| Variable [Reference group] |
Univariate Graft Survival HR (95% CI) |
p-value | Multivariable Graft Survival HR (95% CI) |
p-value | |
|---|---|---|---|---|---|
| RECIPIENT FACTORS | |||||
| Age | 1.02 (1.01–1.03) | <0.001 | 1.01 (1.00–1.02) | 0.046 | |
| Male [female] | 1.28 (1.05–1.57) | 0.016 | 1.34 (1.09–1.65) | 0.007 | |
| Non-caucasian [Caucasian] | 1.14 (0.93–1.40) | 0.20 | |||
| Indication for LT: | |||||
| HCC | 1.52 (1.23–1.88) | <0.001 | 1.12 (0.88–1.43) | 0.35 | |
| HCV | 1.26 (1.03–1.53) | 0.023 | 1.17 (0.96–1.44) | 0.12 | |
| HBV | 0.68 (0.43–1.07) | 0.10 | |||
| BMI at LT : | |||||
| <19 [22 to 25] | 0.73 (0.48–1.13) | 0.16 | |||
| 19–21 [22 to 25] | 1.19 (0.97–1.45) | 0.094 | |||
| Delta BMI | 0.89 (0.86–0.92) | <0.001 | 0.89 (0.86–0.91) | <0.001 | |
| Diabetes | 1.26 (1.00–1.59) | 0.051 | 1.19 (0.94–1.51) | 0.15 | |
| MELD at LT | 0.98 (0.97–1.00) | 0.006 | 0.99 (0.98–1.01) | 0.25 | |
| Dialysis in week prior to LT | 1.01 (0.69–1.47) | 0.97 | |||
| Hospitalization within 90 days of LT | 1.00 (0.76–1.31) | 0.97 | |||
| Mechanical Ventilator | 0.63 (0.34–1.18) | 0.15 | |||
| Rejection in 1st year post-LT | 1.13 (0.86–1.49) | 0.38 | |||
| DONOR FACTORS | |||||
| Age | 1.02 (1.01–1.02) | <0.001 | 1.02 (1.01–1.02) | <0.001 | |
| Male [female] | 0.83 (0.69–1.01) | 0.057 | 0.93 (0.72–1.21) | 0.60 | |
| Non-caucasian [Caucasian] | 1.22 (1.00–1.49) | 0.056 | 1.22 (0.99–1.50) | 0.07 | |
| Height | 0.99 (0.98–1.00) | 0.045 | 1.00 (0.98–1.01) | 0.53 | |
| Weight | 1.00 (0.99–1.01) | 0.98 | |||
| BMI | 1.02 (1.00–1.04) | 0.11 | |||
| DCD Allograft | 1.06 (0.68–1.66) | 0.80 | |||
| Cold ischemia time | 1.02 (1.00–1.04) | 0.10 | |||
| Partial or Split Liver Allografta | 0.48 (0.31–0.75) | 0.001 | |||
| Deceased [Living Donor Allograft] | 2.03 (1.23–3.33) | 0.006 | 1.98 (1.17–3.34) | 0.011 | |
Correlated with living donor, so not included in the multivariable model
Cause of Death
A total of 386 deaths occurred during the follow-up period. One hundred and six patients (18.2%) died in the BMI Loss group, 122 (15.7%) in the BMI Stable group, and 158 (9.8%) in the BMI Gain group. The most common identifiable causes of death included: malignancy (n=84), graft failure (n=49), infection (n=39), multi-organ failure (n=31), and cardiovascular (n=24). “Other” and “unknown” made up 36 and 81 deaths, respectively. Comparisons for cause of death stratified by Delta BMI category were not undertaken due to the small number of events in each group.
Discussion
Obesity is a prevalent disorder in the U.S. population that is associated with increased morbidity and mortality. (2) Similar to the general population, weight gain and propensity for obesity are commonly observed events among recipients of liver transplant, with post-LT weight gain demonstrated to stabilize in the majority of individuals by year 2 post-LT. (5–9) Although the impact of pre-LT weight and BMI on post-LT survival has been extensively examined in the literature, the impact of weight and BMI changes occurring in the post-LT setting is not well understood. Using the UNOS database, our study demonstrated that weight gain (BMI Gain) within the first 2 years post-LT is common, occurring in 54% of recipients, with 4.7% of non-overweight recipients achieving new-onset obesity. We also demonstrated that LT recipients who gained weight within 2 years post-LT had significantly greater patient and graft survival compared to recipients who maintained BMI Stable or who experienced BMI Loss. And LT recipients with new-onset obesity or who demonstrated substantial BMI Gain also had greater 5-year patient and graft survivals compared to recipients who maintained BMI Stable. Interestingly, LT recipients with normal pre-LT BMI who experienced weight loss had significantly reduced patient and graft survival compared to other groups. And this detrimental effect was more pronounced among LT recipients with substantial weight loss. Multivariable analysis identified increasing BMI as an independent predictor of improved patient and graft survival.
The baseline BMI used in this study limited the size of our cohort. However, it was chosen in order to mitigate the previously reported impact of pre-transplant BMI, which included unaccounted for ascites weight, on post-transplant survival using the UNOS database. (13) Indeed, our analysis of 5-year patient and graft survival stratified by baseline BMI did not reveal any such impact. Importantly, studies able to correct for ascites weight at LT have not reported a negative impact from pre-transplant weight on post-transplant survival. (11, 12) Therefore, we believe that limiting our analysis to patients with BMI ≤25 kg/m2 is warranted because we cannot correct for ascites weight in patients with high pre-transplant BMI using the UNOS database. At the same time, we recognize that this analysis may include selection bias.
Data from the renal transplantation literature demonstrates an association between weight gain post-transplant and features of the metabolic syndrome, and decreased patient and graft survival. (14–18) We hypothesized that similar negative effects would be noted in a post-LT cohort experiencing weight gain. However, our data demonstrate that weight gain, even substantial weight gain or new-onset obesity, at 2 years post-LT was associated with better patient and graft survivals compared to recipients who maintained BMI Stable. One potential explanation for the differences in the outcomes reported in the renal transplantation literature compared to the results of our investigation may be that baseline pre-transplant co-morbidities that contribute to patient mortality, such as cardiovascular and peripheral vascular diseases, are more prevalent among patients with end-stage renal disease undergoing renal transplantation compared to patients undergoing LT. We postulate that our LT BMI cohort had little ascites weight contributing to their pre-LT BMI and thus, early weight gain post-LT may serve as a marker of better prognosis out to 5-year post-LT. The analyzed BMI cohort likely included malnourished patients with cirrhosis who were still fit enough to undergo liver transplant surgery. Afterwards, these patients would have experienced an improvement in both the malnourishment and liver failure resulting in the observed increased BMI and better outcomes compared to the recipients who maintained a stable weight or who had post-LT weight loss. However, severely malnourished patients with normal BMI were likely few in this cohort as these patients were ultimately deemed of sufficient health to undergo surgery by their transplant teams and had robust enough health to survive at least two years post-LT. The impact of post-LT weight gain, especially substantial weight gain, on long-term (>5 years) patient and graft survivals is still not known. Furthermore, the impact of weight gain in pre-LT overweight or obese recipients, after accounting for ascites weight, is also not known. The negative effects on morbidity and mortality secondary to excessive weight and obesity typically take years or decades to manifest in the general population, and the same phenomenon may be true in this LT cohort.
Our study also demonstrated that weight loss was associated with significantly reduced patient and graft survival. A large study of renal transplantation recipients in Australia and New Zealand identified post-transplant weight loss of more than 5% as a marker of increased risk of death. (14) We do note that the BMI Loss group in our study had a greater prevalence of HCV and HCC diagnoses compared to LT recipients who experienced BMI Gain post-LT. However, multivariate analyses failed to identify diagnoses of HCV or HCC as independently associated with reduced patient or graft survival. A recent study of the SRTR dataset revealed that HCV infection was associated with increased risk of diabetes after LT. (19) We suspect that HCV infection may also associate with weight gain as diabetes is frequently associated with the metabolic syndrome and obesity. However, post-transplant weight changes were not analyzed in the study by Younossi et al, making our study novel. The higher prevalence of HCV in the BMI Loss group, compared to the BMI Gain group, likely represents a subset of patients that experience more severe HCV recurrence with resultant graft cirrhosis and failure within the first 5 years after transplant. Similarly, the increased prevalence of HCC in the BMI Loss group relative to the BMI Gain group also suggests a difference in tumor characteristics including HCC recurrence between these two groups. Malignancy, which included liver and non-liver tumors, graft failure, and infections were the most common causes of death in this cohort. These would be common causes of death in non-overweight recipients experiencing weight loss after LT and could explain our findings.
Our study has limitations that warrant further mention. Weight is not static. Previously published data using the NIDDK liver transplant database demonstrated that BMI post-LT stabilizes by year 2 post-LT. (6) Therefore, we specifically included cases that had documented BMI at year 2 and analyzed their survival with the assumption that the BMI would not change significantly after year 2. We also selected patients with a pre-LT BMI ≤25 (but > 16 kg/m2), which was undertaken in an effort to account for large volume ascites given the published data demonstrating that the negative associations between elevated pre-LT BMI and risk of death and graft failure post-LT are confounded by the presence of large volume ascites. (12) However, since the UNOS data do not include an accurate assessment of ascites volume, we used elevated BMI pre-LT above the normal BMI threshold (25 kg/m2) as a surrogate marker of ascites with the presumption that the included patients (BMI ≤ 25 kg/m2) did not have large volumes of ascites. We made this decision accepting the possibility of some degree of misclassification. Our survival analyses stratified by pre-LT BMI showed no impact on post-LT patient or graft survival suggesting that we selected a group of patients whose pre-LT weight, which may have also included a few individuals with otherwise unaccounted for ascites (i.e., misclassified), did not impact post-LT patient and graft survivals. Another surrogate marker for more severe liver disease, such as the albumin to globulin ratio, would allow us to classify patients with less or more severe liver disease. However, the albumin to globulin ratio and other surrogate serologic studies cannot accurately predict which patients have or do not have ascites. Furthermore, the UNOS dataset we used did not collect globulin data from the transplant centers. We note that NASH as the primary liver diagnosis was uncommon (5.9%) in this cohort, which likely reflects the selection of patients with normal pre-LT BMI. Finally, we recognize that post-transplant immunosuppressive medications can impact weight change as well as the risk of new-onset diabetes mellitus. (20) Corticosteroid use likely plays a large role in post-transplant weight changes as it remains a common post-transplant treatment. Newer immunosuppressive medications allow most centers to establish medication protocols with quickly taper off of corticosteroids such that most patients are no longer using corticosteroids within the first 6 months of transplant. Yet some post-transplant conditions, such as acute graft rejection, require reinstitution of these medications at very high doses. Hence, corticosteroid use fluctuates many times over the course of the first 2 years after transplant making statistical comparisons between steroid usage and weight changes very difficult. Furthermore, the UNOS dataset does not collect this granular level of information regarding dosage and duration of corticosteroid use. We could not assess the impact of immunosuppressive medications, particularly corticosteroids, on post-LT weight change due to the lack of granular data regarding corticosteroid use.
In conclusion, weight gain post-LT among non-overweight/non-obese recipients is not only common, but is also associated with excellent patient and graft survival. Within 8 years post-LT, there were no detrimental effects on survival from weight gain. We also demonstrated that LT recipients with weight loss within the first two years post-LT, particularly substantial weight loss, have a significantly increased risk for death and graft loss. These data suggest that recipients with weight loss post-LT require close monitoring for smoldering complications that may lead to poor post-LT outcomes. Although weight gain within the first two-years post-LT may be associated with favorable short-term prognoses, LT recipients should be discouraged from excessive weight gain until further research provides insight into the long-term impact of weight gain on patient and graft survival.
Acknowledgements
This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C (JG), and by research grant funding through the Department of Medicine at the University of Colorado Anschutz Medical Campus (KB). The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
This work was also supported in part by NIH/NCATS Colorado CTSI Grant Number UL1 TR000154. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- DCD
donation after cardiac death
- HR
hazard ratio
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IQR
interquartile range
- Kg
kilogram
- LT
liver transplantation
- NASH
nonalcoholic steatohepatitis
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- SD
standard deviation
- UNOS
United Network for Organ Sharing
Footnotes
Authorship: AMC: participated in study design, data collection, and wrote the manuscript. BEF: participated in study design, collected data, participated in data analysis, and provided critical revisions of the manuscript. JG: collected and analyzed data, provided critical revisions of the manuscript. LF: participated in study design. KB: participated in study design, analyzed data, and provided critical revisions of the manuscript.
Financial Disclosure: The authors have no financial disclosures to report related to this research.
Conflict of Interest: The authors have no conflicts of interest to report related to this research.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012 Jan;(82):1–8. PubMed PMID: 22617494. Epub 2012/05/24. eng. [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012 Jun;55(6):2005–2023. doi: 10.1002/hep.25762. PubMed PMID: 22488764. Epub 2012/04/11. eng. [DOI] [PubMed] [Google Scholar]
- 3.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006 Feb;43(2) Suppl 1:S99–S112. doi: 10.1002/hep.20973. PubMed PMID: 16447287. Epub 2006/02/01. eng. [DOI] [PubMed] [Google Scholar]
- 4.Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999–2008. Am J Transplant. 2010 Apr;10(4 Pt 2):1003–1019. doi: 10.1111/j.1600-6143.2010.03037.x. PubMed PMID: 20420649. Epub 2010/04/28. eng. [DOI] [PubMed] [Google Scholar]
- 5.Anastacio LR, Ferreira LG, de Sena Ribeiro H, Lima AS, Vilela EG, Toulson Davisson Correia MI. Body composition and overweight of liver transplant recipients. Transplantation. 2011 Oct 27;92(8):947–951. doi: 10.1097/TP.0b013e31822e0bee. PubMed PMID: 21869739. Epub 2011/08/27. eng. [DOI] [PubMed] [Google Scholar]
- 6.Everhart JE, Lombardero M, Lake JR, Wiesner RH, Zetterman RK, Hoofnagle JH. Weight change and obesity after liver transplantation: incidence and risk factors. Liver Transpl Surg. 1998 Jul;4(4):285–296. doi: 10.1002/lt.500040402. PubMed PMID: 9649642. Epub 1998/07/03. eng. [DOI] [PubMed] [Google Scholar]
- 7.Palmer M, Schaffner F, Thung SN. Excessive weight gain after liver transplantation. Transplantation. 1991 Apr;51(4):797–800. doi: 10.1097/00007890-199104000-00012. PubMed PMID: 2014532. Epub 1991/04/01. eng. [DOI] [PubMed] [Google Scholar]
- 8.Stegall MD, Everson G, Schroter G, Bilir B, Karrer F, Kam I. Metabolic complications after liver transplantation. Diabetes, hypercholesterolemia, hypertension, and obesity. Transplantation. 1995 Nov 15;60(9):1057–1060. PubMed PMID: 7491685. Epub 1995/11/15. eng. [PubMed] [Google Scholar]
- 9.Wawrzynowicz-Syczewska M, Karpinska E, Jurczyk K, Laurans L, Boron-Kaczmarska A. Risk factors and dynamics of weight gain in patients after liver transplantation. Ann Transplant. 2009 Jul-Sep;14(3):45–50. PubMed PMID: 19644159. Epub 2009/08/01. eng. [PubMed] [Google Scholar]
- 10.Richards J, Gunson B, Johnson J, Neuberger J. Weight gain and obesity after liver transplantation. Transplant international : official journal of the European Society for Organ Transplantation. 2005 Apr;18(4):461–466. doi: 10.1111/j.1432-2277.2004.00067.x. PubMed PMID: 15773968. Epub 2005/03/19. eng. [DOI] [PubMed] [Google Scholar]
- 11.LaMattina JC, Foley DP, Fernandez LA, Pirsch JD, Musat AI, D'Alessandro AM, et al. Complications associated with liver transplantation in the obese recipient. Clin Transplant. 2012 Nov;26(6):910–918. doi: 10.1111/j.1399-0012.2012.01669.x. PubMed PMID: 22694047. Pubmed Central PMCID: 3518672. Epub 2012/06/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leonard J, Heimbach JK, Malinchoc M, Watt K, Charlton M. The impact of obesity on long-term outcomes in liver transplant recipients-results of the NIDDK liver transplant database. Am J Transplant. 2008 Mar;8(3):667–672. doi: 10.1111/j.1600-6143.2007.02100.x. PubMed PMID: 18294163. Epub 2008/02/26. eng. [DOI] [PubMed] [Google Scholar]
- 13.Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology. 2002 Jan;35(1):105–109. doi: 10.1053/jhep.2002.30318. PubMed PMID: 11786965. Epub 2002/01/12. eng. [DOI] [PubMed] [Google Scholar]
- 14.Chang SH, McDonald SP. Post-kidney transplant weight change as marker of poor survival outcomes. Transplantation. 2008 May 27;85(10):1443–1448. doi: 10.1097/TP.0b013e31816f1cd3. PubMed PMID: 18497685. Epub 2008/05/24. eng. [DOI] [PubMed] [Google Scholar]
- 15.Diaz JM, Sainz Z, Oliver A, Guirado LI, Facundo C, Garcia-Maset R, et al. Post-renal transplantation weight gain: its causes and its consequences. Transplant Proc. 2005 Nov;37(9):3839–3841. doi: 10.1016/j.transproceed.2005.09.200. PubMed PMID: 16386557. Epub 2006/01/03. eng. [DOI] [PubMed] [Google Scholar]
- 16.Ducloux D, Kazory A, Simula-Faivre D, Chalopin JM. One-year post-transplant weight gain is a risk factor for graft loss. Am J Transplant. 2005 Dec;5(12):2922–2928. doi: 10.1111/j.1600-6143.2005.01104.x. PubMed PMID: 16303006. Epub 2005/11/24. eng. [DOI] [PubMed] [Google Scholar]
- 17.el-Agroudy AE, Wafa EW, Gheith OE, Shehab el-Dein AB, Ghoneim MA. Weight gain after renal transplantation is a risk factor for patient and graft outcome. Transplantation. 2004 May 15;77(9):1381–1385. doi: 10.1097/01.tp.0000120949.86038.62. PubMed PMID: 15167594. Epub 2004/05/29. eng. [DOI] [PubMed] [Google Scholar]
- 18.Micozkadioglu H, Ozdemir FN, Sezer S, Arat Z, Haberal M. Weight gain after living-related renal transplantation affects long-term graft function. Transplant Proc. 2005 Mar;37(2):1029–1032. doi: 10.1016/j.transproceed.2004.12.032. PubMed PMID: 15848613. Epub 2005/04/26. eng. [DOI] [PubMed] [Google Scholar]
- 19.Younossi Z, Stepanova M, Saab S, Trimble G, Mishra A, Henry L. The association of hepatitis C virus infection and post-liver transplant diabetes: data from 17 000 HCV-infected transplant recipients. Alimentary pharmacology & therapeutics. 2015 Jan;41(2):209–217. doi: 10.1111/apt.13027. PubMed PMID: 25413020. [DOI] [PubMed] [Google Scholar]
- 20.Bodziak KA, Hricik DE. New-onset diabetes mellitus after solid organ transplantation. Transplant international : official journal of the European Society for Organ Transplantation. 2009 May;22(5):519–530. doi: 10.1111/j.1432-2277.2008.00800.x. PubMed PMID: 19040489. [DOI] [PubMed] [Google Scholar]


