Abstract
Homeostatic T cell proliferation is more robust during human fetal development. In order to understand the relative effect of normal fetal homeostasis and perinatal exposures on CD8+ T cell behavior in PT infants, we characterized umbilical cord blood CD8+ T cells from infants born between 23–42 weeks gestation. Subjects were recruited as part of the NHLBI-sponsored Prematurity and Respiratory Outcomes Program. Cord blood from PT infants had fewer naïve CD8+ T cells and lower regulatory CD31 expression on both naïve and effector, independent of prenatal exposures. CD8+ T cell in vitro effector function was greater at younger gestational ages, an effect that was exaggerated in infants with prior inflammatory exposures. These results suggest that CD8+ T cells earlier in gestation have loss of regulatory co-receptor CD31 and greater effector differentiation, which may place PT neonates at unique risk for CD8+ T cell-mediated inflammation and impaired T cell memory formation.
Keywords: CD8+ T cell, neonatal immunity, inflammation, prematurity, fetus, immune dysregulation, bronchopulmonary dysplasia
1. Introduction
One in eight infants is born prematurely (PT, <37 weeks at birth) in the United States, and 60,000 are categorized very low birth weight (VLBW, <1500 grams)[1]. Although innovations in neonatology have increased survival of VLBW infants, many succumb to diseases related to severe recurrent viral infections and chronic inflammation. Unfortunately, without a more complete understanding of inflammatory mechanisms unique to premature infants, our therapies to prevent or treat these major morbidities are limited. Improving our understanding of cell populations initiating or propagating inflammation, as well as mechanisms limiting the formation of protective immune memory will make it possible to incisively target immunotherapies for PT infants.
PT host susceptibility to recurrent viral infection and chronic inflammation raise the suspicion that the adaptive immune system is involved in pathology. In fact, several previous reports demonstrate a correlation between T cell activation, as measured by CD45RO expression, and premature infants’ adverse outcomes, such as bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC) and periventricular leukomalacia (PVL)[2–4]. Although T cells from PT infants appear able to activate, their ability to either downregulate such a response or modulate it in favor of long-term memory over effector formation remains in question. CD8+ T cells are of particular interest in this scenario, in that they are largely responsible for clearing viral infection as well as killing infected target cells. There is very little known about PT infant CD8+ T cell development. Developmentally determined accelerated T cell activation may be beneficial by lowering the threshold of typically quiescent naïve T cells to respond to pathogen, but also places the PT infant at risk for immune dysregulation.
CD8+ T cell sensitivity to cytokine-supported (IL-2, IL-7 and IL15) homeostatic proliferation is inversely related to gestational age[5]. Homeostatic proliferation of T cells may be important during development when accelerated fetal growth outpaces thymic release. Rapid homeostatic expansion has been implicated in promoting T cell dysregulation in lymphopenic adults, resulting in a T cell pool that is hyper-responsive but poorly protective[6], similar to the clinical phenotype seen in PT infants. Full term (FT) neonatal CD8+ T cells, on the other hand, are able to maintain a naïve phenotype during homeostatic expansion[7, 8], which favors the establishment of a polyclonal, diverse repertoire. It is not known, however, if T cells released during earlier gestation are more or less permissive to CD8+ T cell differentiation during their accelerated growth period, or under conditions perturbing homeostasis such as premature delivery. It is also not clear if T cell activation occurs during normal fetal lymphocyte development, or if perinatal exposures, including in utero infection (chorioamnionitis), antenatal steroids, or vaginal delivery induce T cell differentiation. The purpose of the following study was to assess phenotypic and functional differences in umbilical cord blood CD8+ T cells across gestational ages that may be consistent with excessive homeostatic proliferation and CD8+ T cell dysregulation, and to determine the relative contribution of common prenatal exposures on changes observed across gestational ages.
2. Materials and Methods
2.1 Umbilical Cord Mononuclear Cell Collection and Isolation
Umbilical cord blood was collected in accordance with IRB-approved procedures, as part of the NHLBI-sponsored Prematurity and Respiratory Outcomes Program, and the University of Rochester Umbilical Cord Blood Biorepository. Samples from 82 PT (<36 0/7 weeks gestational age, GA) and 18 FT (≥37 weeks GA) subjects were selected based on gestational age at birth. The presence of congenital anomalies was an exclusion criterion. The need for NICU admission, including for sepsis evaluation due to signs of maternal chorioamnionitis, was an exclusion criteria for FT subjects. Clinical information was not available on a subset of subject samples due to the sample needing de-identification (Table 1). Study data were collected and managed using REDCap electronic data capture tools. Flow cytometry data were managed and integrated with clinical data using the BLIS (Bio-lab Informatics Server, URMC) system based on the open-source LabKey Server platform[9]. All sample processing was completed within 24 hours following sample collection, using a standardized operating procedure as previously published[10]. Briefly, blood was diluted with Dulbecco’s PBS and layered over Ficoll-Paque®PLUS (Lymphocyte Separation Medium, GE Healthcare, Cat#17-1440-03). The buffy coat was diluted with HBSS (Corning, Cat# 21-022-CV). Isolated cells were washed and centrifuged, then gently resuspended in HBSS. A small aliquot of resuspended cells were added to red cell lysis buffer (ACK Lysing Buffer, Lonza Biowhittaker, Cat#10-548E). Cell counts were determined by manual hemocytometer count using trypan blue exclusion. The remaining umbilical cord mononuclear cells (UCMC) were centrifuged, resuspended in freezing media and cryopreserved.
Table 1.
Distribution and demographics of subjects used for each flow cytometry analysis set.
| T Cell Maturation (n=45) | T Cell Phenotyping (n=86) | Intracellular Cytokine Staining (n=57) | ||||
|---|---|---|---|---|---|---|
| Preterm (<36 0/7) | Full term (≥37 0/7) | Preterm (<36 0/7) | Full term (≥37 0/7) | Preterm (<36 0/7) | Full term (≥37 0/7) | |
| Gestational Age (n) | 30 | 15 | 68 | 18 | 42 | 15 |
| Weeks (mean, SD) | 31.4, 2.6 | 39.2, 1.6 | 32.2, 2.7 | 39.0, 1.2 | 31.2, 2.6 | 39.0, 1.1 |
| Birth weight (n) | 30 | 4 | 37 | 10 | 40 | 9 |
| g (mean, SD) | 1701, 534 | 3596, 476 | 1686, 502 | 3414, 450 | 1692, 507 | 3420, 477 |
| APGAR score (n) | 26 | 4 | 37 | 10 | 40 | 9 |
| (mean, SD) | 8, 1.6 | 9.3, 0.6 | 7.9, 1.8 | 8.9, 0.8 | 8.1, 1.5 | 8.9, 0.83 |
| Gender (n) | 26 | 4 | 37 | 10 | 40 | 9 |
| Female % (CI) | 38.5 (20.2, 59.4) | 100 (0, 60.2) | 32.4 (18.0, 49.8) | 60.0 (26. 87.8) | 35.0 (20.6, 51.7) | 66.7 (29.9, 92.5) |
| Race (n) | 26 | 4 | 37 | 10 | 40 | 9 |
| White/Caucasian | 65.4 (44.3, 82.8) | 50.0 (6.8, 93.2) | 61.1 (43.5, 76.9) | 50.0 (18.7, 81.3) | 63.4 (46.9, 77.9) | 55.6 (21.2, 86.3) |
| Black/African-American | 23.1 (9.0, 43.6) | 25.0 (0.6, 80.6) | 30.6 (16.3, 48.1) | 30.0 (6.7, 65.2) | 24.4 (12.4, 40.3) | 33.3 (7.5, 70.1) |
| Other | 11.5 (2.4, 30.2) | 25.0 (0.6, 80.6) | 8.3 (1.8, 22.5) | 20.0 (2.5, 55.6) | 12.2 (4.1, 26.2) | 11.1 (0.3, 48.2) |
| Antenatal steroid exposure (n) | 26 | 4 | 37 | 10 | 40 | 9 |
| Exposed % (CI) | 80.8 (60.6, 93.4) | 0 (0, 60.2) | 70.3 (53.0, 84.1) | 0 (0, 30.8) | 72.5 (56.1, 85.4) | 0 (0, 33.6) |
| Clinical chorioamnionitis (n) | 26 | 4 | 37 | 10 | 40 | 9 |
| Exposed % (CI) | 19.2 (6.6, 39.4) | 0 (0, 60.2) | 17.1 (6.6, 33.6) | 0 (0, 30.8) | 17.5 (7.3, 32.8) | 0 (0, 33.6) |
| Membrane rupture > 18 hours (n) | 26 | 4 | 37 | 10 | 40 | 9 |
| Exposed % (CI) | 23.1 (9.0, 43.6) | 0 (0, 60.2) | 21.6 (9.8, 38.2) | 0 (0, 30.8) | 25.0 (12.7, 41.2) | 0 (0, 33.6) |
| Mode of delivery (n) | 26 | 4 | 37 | 10 | 40 | 9 |
| Vaginal % (CI) | 57.7 (36.9, 76.6) | 25.0 (6, 80.6) | 48.6 (31.9, 65.6) | 20.0 (2.5, 55.6) | 60.0 (43.3, 75.1) | 22.2 (2.8, 60.0) |
2.2 Umbilical Cord Blood Thawing and Preparation for Flow Cytometry
All aliquots tested were thawed and prepared for flow cytometry in batches of 5–10 subjects/experiment to reduce variation due to experimental conditions. Each run included samples from both preterm and full term subjects in order to minimize erroneous attribution of batch effect to group differences. UCMC recovery and viability were determined using manual count by trypan blue exclusion assay after RBC lysis. UCMC were rested overnight prior to in vitro stimulation.
Cells allocated for intracellular cytokine detection were washed with complete media, and 1–2 × 106 UCMC, as a 200 μL suspension, were added to each well of a 96-well v-bottom microtiter plate. Staphylococcal Enterotoxin-B (SEB, Sigma Cat#S4881), final concentration 2 μg/mL was added to stimulated wells. Samples were incubated at 37°C, 5% CO2 for 2 hours, after which Brefeldin A (BD Cat#555029) and Monensin (Sigma Cat#M5273) were added. Plates were again incubated for 8 hours, and then placed at 4° C overnight.
Immunocytochemistry was performed with fluorescently tagged antibodies using a micromethod as previously described[10]. Flow cytometry staining panel details are available in Supplemental Table 1. Briefly, rested UCMC were washed once in complete medium, counted and plated in PBS at approximately 1–2×106 UCMC/well of a 96-well v-bottom microtiter plate. UCMC were stained in Live/Dead cocktail (Invitrogen, Cat#L34959). After incubation, cold staining buffer (Biowhittaker Cat#17-512F, with 1% BSA, Fraction V ICN Cat#160069) was added and plates were centrifuged, then Fc-blocked with 5% normal mouse serum (Sigma, Cat#M5905). Following centrifugation, cells were resuspended in surface staining cocktail, and incubated in the dark at 4° C for 60 minutes.
Cells were then washed and centrifuged. Cytofix/Cytoperm reagent (BD Cat#554722) was added to each well and left to incubate at 4° C in the dark for 20 minutes. Permeabilization buffer (BD Cat#554723) was then added to wells and centrifuged as described above. UCMC were Fc-blocked, then centrifuged as described above. Intracellular cytokine staining cocktail was then to the cells, followed by incubation at 4° C in the dark for 60 minutes. Cells were again washed, resuspended in fixation buffer (2% paraformaldehyde in PBS) and incubated at room temperature for 20 minutes. Cells were then centrifuged and transferred to 5 mL FCS tubes in preparation for analysis.
Stained samples were evaluated on an 18-color LSR II (488, 633, 407 and 532 lasers) using BD FACS Diva software for collection and autocompensation. All flow cytometry analysis was performed manually using Flowjo® data analysis software (Version 8.8.6, Tree Star, Inc. Ashland, OR), adhering to published flow cytometry data interpretation and gating approaches[11]. Fluorescence-minus-one controls were used to accurately distinguish between fluorescence “spillover” and positive events. Healthy adult donor peripheral blood mononuclear cells (PBMC) were used for standardization across experiments, to test for batch effect, and to enable validation of marker expression based on published data from adults. Individuals performing manual gating were blinded to subject demographics, including gestational age and clinical covariates.
2.3 Statistical Analysis
Cell subpopulations were expressed as a percentage of the parent population, and counts (frequencies) were determined based on back calculation onto original cell count using the equation: Cell frequencies (cell subtype/mL umbilical cord blood)=[event count/(live, intact UCMC)] x [intact UCMC/mL counted at time of sample collection], then normalized to 1 million cells. Cell number calculation assumed that equal proportions of subpopulation were lost during processing, and that the debris field represented an equal distribution of cells between original count and cytometer collection. All final cell populations were assessed for batch effect prior to statistical analysis. Log transformation was applied to cell counts due to their positive skewness. Multivariate regression of both log scale counts and percentages was performed, using subjects with available clinical data, on gestational age clinical chorioamnionitis (determined based on prenatal diagnosis by Obstetric team), prolonged rupture of membranes (PROM>18 hours), 5-minute APGAR score, antenatal steroid exposure (any), mode of delivery (vaginal versus caesarean section), birth weight, gender and race. R squares are reported for the assessment of model fitting. Effects of clinical covariates, where significant, are described for each given cell population.
3. Results
3.1 Subjects and samples
For the purpose of our study, 82 PT and 18 FT subjects were selected based on gestational age, and represented a heterogeneous group of clinical presentations. Due to the need to distribute limited cell numbers across multiple flow cytometry panels, a minimum cell and subject number for each assay was determined based on expected robustness of markers and subject variability. Clinical data was available from 49/57 subjects used in intracellular cytokine detection assays, 47/86 used in T cell phenotyping and 34/45 used in T cell maturation assays. Details on subject number and demographics per staining panel are shown in Table 1. Viability and recovery post-thaw were consistent with our previously published study describing optimized umbilical cord blood processing procedures [10]. Overall median recovery was confounded by inclusion of nucleated red blood cells in pre-cryopreservation counts, with a median value of 68.4% (IQR 40.7, 90.5). The median viability, however, was high at 94.0% (IQR 90.0, 96.0). The effects of viability and recovery on frequencies and counts of cell populations were tested prior to formal statistical analysis, and did not confound our results.
3.2 Antenatal steroid exposure contributes to CD8+ T cell activation and reduction in naive CD8+ T cells at lower gestational ages
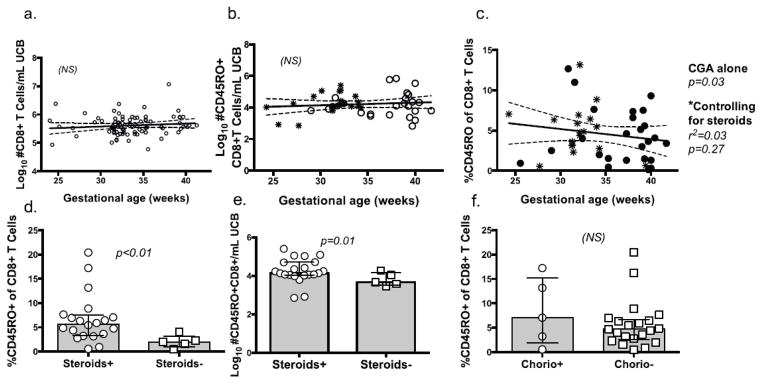
Previous studies have identified increased CD45RO+ CD8+ T cell frequencies in PT infants [12]. The relationships among CD8+ T cell activation, gestational age and antenatal exposures are poorly defined. To better understand the contributing factors to T cell activation, we compared the relative contribution of gestational age and factors associated with PT birth to CD45RO+ CD8+ T cell events in UCB. CD8+ T cells from isolated mononuclear cells were identified by flow cytometry using the following sequential gating strategy: intact lymphocytes/doublet-/live-dead negative/CD14-/CD235-/CD3+/CD4-/CD8+ events (Fig 1). No significant correlation was found between gestational age and CD8+ T cell frequencies (Fig 2a). CD8+ T cells were then analyzed to measure the distribution of activated (CD45RO+) cells present across gestational ages. Consistent with results obtained by other investigators, the proportion of CD45RO+ CD8+ T cells was higher in neonates born at lower CGA (p=0.03, Fig 2c). Similar frequencies of CD45RO+ were found, pointing to a reduction in numbers of naïve (CD45RO-) CD8+ T cells (Fig 2c). When controlling for antenatal steroid exposure, however, the correlation was no longer significant (Fig 2d, e). Antenatal steroids in preterm infants, therefore, are associated with increased proportion of activated CD45RO+ CD8+ T cells, an effect that is due to reduction in naïve counts of CD45RO- CD8+ T cells. In contrast, the presence of inflammatory stimuli, including chorioamnionitis did not contribute significantly to variation in CD8+ CD45RO expression in preterm infant cord blood (Fig 2f).
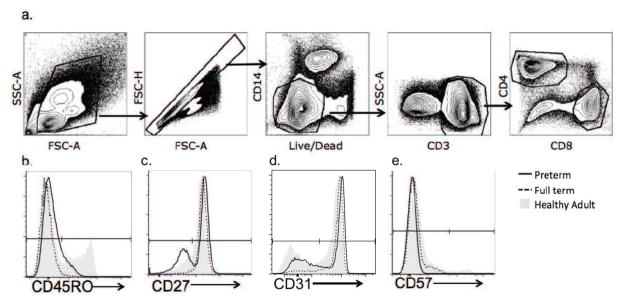
Fig 1.
Fig 1a–1f CD8+ T cells were sequentially identified by flow cytometry as intact lymphocyte/singlets/live/CD14-/CD3+/CD4-/CD8+ (a). CD8+ events were gated on CD45RO (b), CD27 (c), CD57 (d) and CD31 (e). Representative preterm (black solid line), full term (dotted line) and healthy adult donor (gray solid) subjects are shown in histogram overlays.
Fig 2.
Fig 2a–f CD8+ and CD45RO+ CD8+ T cells across gestational ages. Graph shows regression analysis (with 95% CI) for CD8+ count/mL blood (a), Log10 CD45RO+ CD8+ events/mL UCB (b) and CD45RO expression as a proportion of CD8+ T cells (c), based on gestational age at birth. Points represent individuals without (circles) and with (asterisks) antenatal steroid exposure. Bar graphs compare median percent, +/- IQR and Log10 event frequency/mL UCB of CD45RO+ of CD8+ T cells from PT subjects exposed or not exposed to either antenatal steroids (d, e) or chorioamnionitis (f).
3.3 Enrichment for more highly differentiated CD8+ T cells in earlier gestation is independent of prenatal exposures
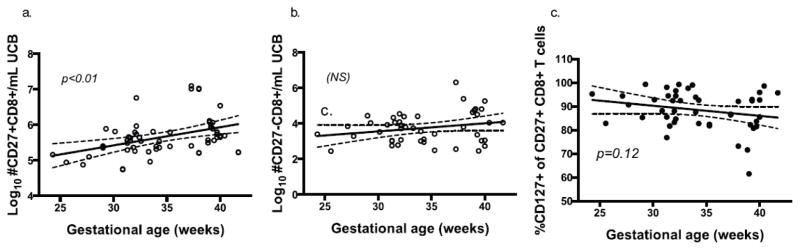
We found that the previously published association between gestational age and CD45RO expression was largely due to antenatal steroid exposure in our cohort. CD45RO isoform expression on CD8+ T cells can be transient, however, and may not reflect a differentiated memory or effector population. We were therefore interested in identifying CD8+ T cell differentiation using alternative, more durable biomarkers for cell differentiation. To pursue this objective, we measured CD27 co-receptor expression on CD8+ T cells. CD27 is highly expressed on naïve, central memory and early-activated CD8+ T cells, and is lost in effector differentiation following activation and homeostatic proliferation in adults[13]. Results showed a direct correlation between CD27+ CD8+ T cell frequencies and gestational age at birth, which remained significant when controlling for chorioamnionitis, antenatal steroids, prolonged rupture of membranes, mode of delivery, gender, race or APGAR score (p<0.001, r2=0.17, Fig 3a). CD8+ T cells negative for CD27 expression were similar across gestational ages (Fig 3b), suggesting a selective reduction in naïve CD8+ T cells and maintenance of post-replication and differentiation CD8+ T cell frequencies in earlier gestation. We then measured frequencies of CD127 (IL-7rα) on CD27+CD8+ T cells to determine if they retained potential to respond to homeostatic proliferation signal, IL-7. CD127 expression was similar in CD27+ CD8+ T cells across gestational ages (Fig 3c). Together these results suggest that there is a selective reduction in CD27+ T cells at earlier gestation, which is not due to a decreased potential to respond to homeostatic cytokine IL-7. Though CD8+ T cell numbers are similar across gestational ages, they are skewed towards a more highly differentiated effector state during earlier gestation, a phenotype that does not appear to be dependent on common perinatal exposures associated with PT delivery.
Fig 3.
Fig. 3a–d Regression analysis of Log10 CD27+ (a) and CD27- (b) CD8+ T cell events (with 95% CI) as a function of gestational age in weeks. Further analysis was performed on CD127+ CD27+ CD8+ events based on gestational age, controlling for perinatal factors.
3.4 CD8+ T Cells at Lower Gestational Ages Show Enhanced Cytokine Production When Stimulated In Vitro
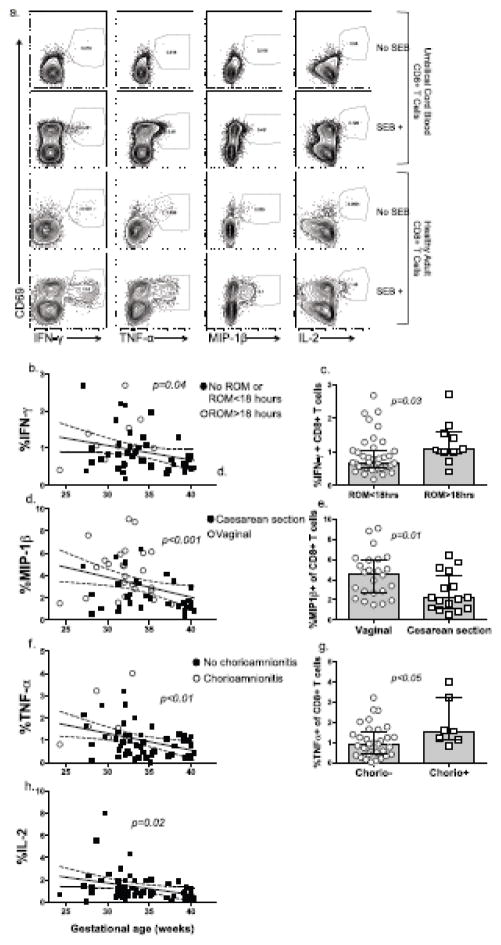
CD8+ T cell activation is often associated with enhanced effector function. Enhanced effector function would impute CD8+ T cells in PT inflammatory diseases. To determine if increased CD8+ T cell differentiation (loss of CD27) that we observed in PTs was accompanied by the acquisition of effector function, intracellular cytokine staining was performed following in vitro stimulation of UCMC with staphylococcus enterotoxin B. Gating strategy is shown in Fig 4a. All groups showed very low spontaneous CD8+ activation as measured by CD69 in unstimulated samples. Regression analysis showed higher proportions of CD8+ IFN-γ+ (p=0.04), MIP-1β+ (pα0.001), TNF-α (pα0.01) and IL-2+ (p=0.02) T cells at lower gestational ages (Fig 4b, d, f, h), following in vitro SEB stimulation. Elevation in IFN-γ+ and MIP-1β+ CD8+ T cells lost significance, however, when controlling for PROM>18 hours and vaginal delivery, respectively (Fig 4c, e). There were significant increases with TNF-α in PT infants exposed to chorioamnionitis (Fig 4g). The negative association between TNF-α and gestational age remained significant when controlling for clinical factors. Clinical variables did not contribute to variation of IL-2+ CD8+ T cell frequencies at lower gestational ages (Fig 4h). These data together suggest that there is a TNF-α, MIP-1β and IL-2 CD8+ T cell effector function bias in younger PT infants, which may be enhanced in an inflammatory environment. IFN-γ production in CD8+ T cells, however, is suppressed at all gestational ages, but can be induced in the context of prolonged inflammatory perturbation, such as in PROM >18 hours.
Fig 4.
Fig. 4a–h Cytokine+CD8+ T cells were sequentially identified by flow cytometry as intact lymphocyte/singlets/live/CD14-/CD235-/CD3+/CD4-/CD8+/CD69+/cytokine+, as shown using representative plots for umbilical cord mononuclear cells (top), and healthy adult donor PBMC (bottom) (a). Percent CD69+/IFN-γ+ (b), MIP-1β+ (d) TNF-α+ (f), and IL-2+ (h) CD8+ T cell were used for regression analysis (with 95% CI) as a function of gestational age in weeks, with p-values prior to controlling for clinical factors. Interacting clinical variables are displayed for each cytokine as an open circle on scatter plots. Bar graphs show comparisons between PT subjects with or without exposure to membrane rupture > 18 hours (ROM >18), mode of delivery (cesarean section or vaginal) and chorioamnionitis (chorio +/-) for IFN-γ (c), MIP-1β (e) and TNF-α (g) (Mann-Whitney test).
3.5 Cord blood CD8+ T cells do not acquire an exhausted phenotype in response to an inflammatory environment
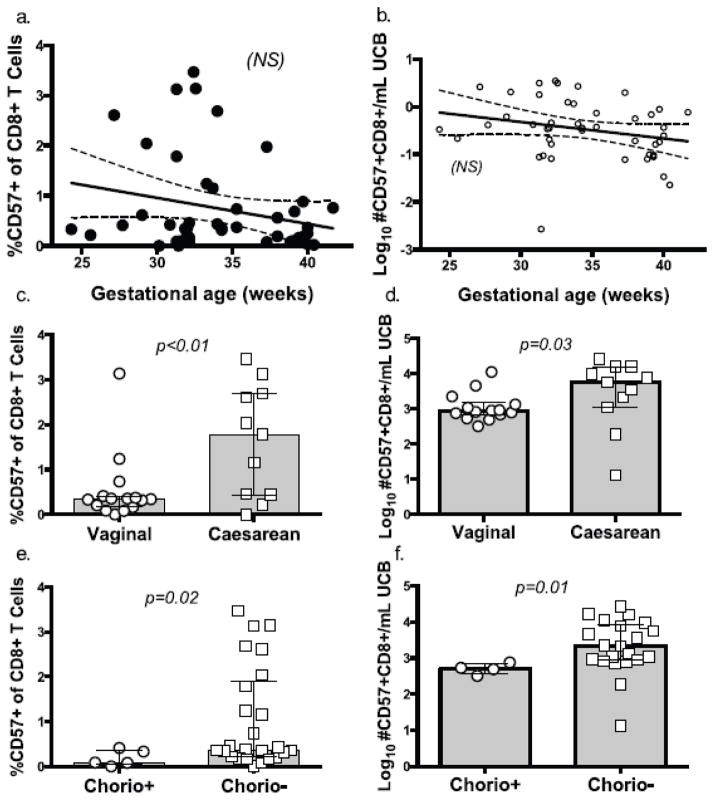
T cell activation and homeostatic proliferation are often associated with an “exhausted” T cell phenotype. CD57+ T cells are relatively resistant to proliferation signals and have shortened telomere length[14, 15]. We expected to find that reduced naïve T cell numbers would cause enhanced T cell replication under inflammatory conditions, resulting in increased CD57 expression on CD8+ T cells at lower gestational ages. We tested this hypothesis by measuring CD57 expression on UCB CD8+ T cells. Neither the proportion nor count of CD57+ CD8+ T cells correlated significantly with gestational age (Fig 5a, b), however, and any trend was lost when controlling for chorioamnionitis and mode of delivery. In direct contrast to our expectation that perinatal inflammatory stimuli would drive CD8+ T cells towards replicative senescence, vaginal delivery and chorioamnionitis were associated with a lower proportion (p=0.002 and 0.02, respectively) and frequency (p=0.03 and 0.014 respectively) of CD57+CD8+ T cells when comparing within the PT cohort (Fig 5c–f). These results indicate that while premature infant CD8+ T cells may have cycled into senescence during homeostasis, CD8+ T cells either downregulate CD57+ expression, are deleted, or undergo tissue migration under inflammatory conditions.
Fig 5.
Fig. 5a–f CD57+CD8+ T cells were gated on CD57 +/- expression. Plots show regression analysis (with 95% CI) for percent (b) and log-transformed number/mL UCB (c) of CD57+CD8+ T cells as a function of gestational age at birth in weeks. Bar graphs show comparison of percent and Log10 CD57+CD8+ event count/mL UCB for PT subjects by mode of delivery (d and e) and by exposed/not exposed to chorioamnionitis (f and g). (Mann-Whitney test).
3.6 CD31 expression on CD8+ T cells is developmentally determined
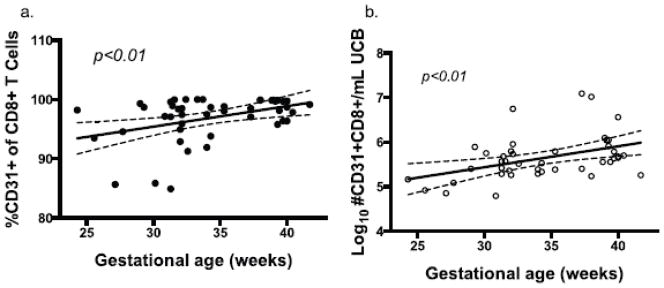
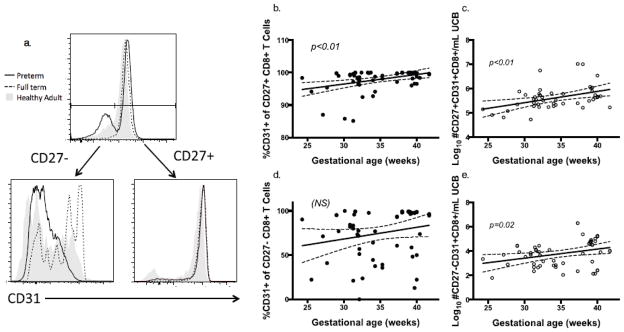
We observed an enhanced effector differentiation and function in CD8+ T cells from patients of lower gestational ages, which occurred independent of prenatal exposures. These findings suggest an intrinsic, developmental T cell process enabling escape from mechanisms designed to maintain quiescence during fetal growth. Though many potential mechanisms may be at play, we explored CD31, or PECAM-1, as a probable candidate molecule for contributing to age-dependent T cell behavior. CD31 is highly expressed on recent thymic emigrant CD8+ T cells, and dampens TCR signal strength, preventing excessive inflammation[16]. We measured CD31+CD8+ T cell populations in cord blood to test the hypothesis that enhanced phenotypic and functional CD8+ T cell differentiation in preterm infants was due, in part, to loss of CD31+ CD8+ T cells. After controlling for multiple clinical variables, we found significantly decreased proportion and frequency of CD8+ T cells expressing CD31 at lower gestational ages (pα0.01, Fig 6a, b). CD31 distribution was bimodal, and we detected no differences in mean fluorescence intensity of CD31+ CD8+ T cell populations based on gestational age or other clinical covariates (not shown). We then wanted to find if CD31 expression was differentially expressed based on CD27 expression (Fig 7a). CD31 was more highly expressed on CD27+CD8+ T cells when compared to CD27-CD8+ T cells. There was a greater reduction in CD31 at lower gestational ages in the CD27+ (naïve) T cell niche compared to CD27- (differentiated) CD8+ T cells (pα0.01 and p=0.02, respectively, Fig 7b–e). CD31+ CD8+ T cell frequency was not associated with any prenatal exposures tested, which suggests that their reduction is likely due to normal fetal development.
Fig 6.
Fig. 6a–f Regression analysis (with 95% CI) of CD31+CD8+ T cell percent (a) and Log10 CD31+ CD8+ event count/mL UCB (b) by gestational age at birth.
Fig 7.
Fig. 7a–e CD27+ and CD27- CD8+ T cell populations were gated on CD31 expression. Representative preterm (black solid line), full term (dotted line) and healthy adult donor (gray solid) subjects are shown in histogram overlays (a). CD31+ percent and number/mL UCB on CD27+ (b, c) and CD27- (d, e) CD8+ T cells were analyzed as a function of gestational age at birth.
3.7 Lower CD31+ CD8+ T cell frequencies in earlier gestation is not due to loss of IL-7rα expression
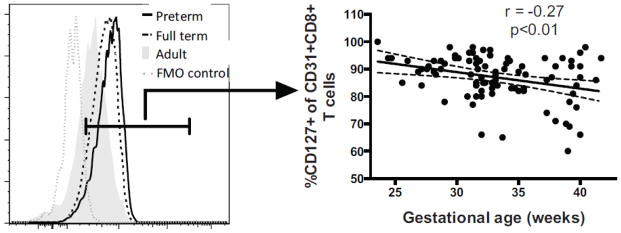
We identified a reduction of CD31+ CD8+ T cells in infants born earlier in gestation, which was not strongly associated with prenatal exposures. Maintenance of CD31+ CD4+ T cells is partly dependent on IL-7rα expression. We were interested in testing if a similar mechanism was acting in cord blood CD8+ CD31+ T cells. We therefore predicted that CD127+ expression would be lower on CD31+ CD8+ T cells from infants born younger. Contrary to our prediction based on available literature, CD127+ frequencies in the CD31+ CD8+ subpopulation were negatively correlated with gestational age (Fig 8, pα0.01). This finding demonstrates that the reduction in CD31+ CD8+ T cells seen in younger preterm infants is not secondary to lower IL-7rα expression.
Fig. 8.
CD31+ T cells were gated on CD8+ T cell events as shown in Figure 1d. CD31+ events were further subsetted on CD127 expression. Representative preterm (black solid line), full term (dotted black line), healthy adult donor (gray solid) subjects and fluorescence minus one negative control (FMO, dotted gray line) are shown in histogram overlays. Frequencies of CD127+ events within the CD31+CD8+ T cell subset are shown as a function of gestational age at birth.
4. Discussion
The relationship between CD8+ T cell homeostasis during earlier gestation, exposures associated with preterm birth, and T cell activation is poorly understood. T cells from FT infants retain a naïve phenotype and resist immune senescence despite rapid homeostatic expansion[5, 8], but whether or not this is true for PT infants has not been previously explored. Our study suggests that early fetal development supports loss of regulatory co-receptor CD31, T cell activation and effector differentiation, regardless of prenatal exposure to infection, steroids, vaginal delivery, prolonged membrane rupture or perinatal stress. Umbilical cord blood from PT infants, when compared to older neonates, shows a selective reduction in naïve (CD27+) CD8 T cells, while effector (CD27-) and activated (CD45RO+) CD8+ T cell frequencies are maintained. Phenotypic CD8+ T cell maturation in PT is accompanied by a gain of pro-inflammatory TNF-α, MIP-1β and IL-2 effector cytokine function, and enhanced IFN-γ following prolonged rupture of membranes. In addition, reduced CD31 expression at lower gestational ages is consistent with T cell-intrinsic difference that may support a T cell-mediated inflammatory response when homeostasis is perturbed. The results of this study go against the notion that CD8+ T cell activation found in UCB from PT infants is driven by prenatal inflammation. Rather, PT infant UCB CD8+ T cells, compared to those from FT infants, appear to be alternatively primed during normal homeostasis and development to support an inflammatory response in the absence of cognate antigen exposure.
The bias towards reduced CD27+ CD8+ T cells in earlier gestation, and maintenance of CD27- population, even without an inflammatory stimulus, is consistent with naïve CD8+ T cells having undergone differentiation as a result of homeostatic expansion. Several recent studies using murine models of lymphopenia have demonstrated the effect of homeostatic expansion on naïve CD8+ T cell phenotype and function. Using germ-free mice, Haluszczak et al showed that while homeostatically expanded CD27- effector CD8+ T cells bearing a “memory” phenotype do not support the formation of long-lasting memory, they confer a degree of transient protection to a naïve neonatal host[17]. Indeed, this speculation is supported by a recent study showing that CD27-/- mice had impaired ability to clonally expand in response to antigen-specific stimulation when compared to wild type, but were capable of exerting cytolytic and cytokine effector function[18]. It may be that reduced CD27 expression on CD8+ T cells during development benefits infants born preterm, by supplying a pool of easily recruited, low specificity T cells in response to novel pathogen. Alternatively, lower activation threshold in CD8+ T cells from PT infants may predispose to non-pathogen mediated inflammatory conditions, such as those resulting from lung or other tissue injury.
The phenomenon of immune dysregulation and non-specific inflammation secondary to rapid homeostatic expansion is well-established in lymphopenic mouse models, where T cell transfer in lymphopenic mice can lead to fatal colitis[19]. Goldrath et al demonstrated in a RAG−/−OT-I murine model, that “memory-like” naive CD8+ T cells retained the capacity to revert back to a naïve state once the host was lymphoreplete, a process that was highly dependent on intact thymic function[20]. Thymus-dependent homeostasis is also shown in humans during recovery from bone marrow ablation, in which older patients, who have lower thymic output, are at greater risk for T cell dysregulation[21, 22]. Recent newborn screening programs measuring TREC number to detect SCID have provided substantial evidence for lower thymic emigrants in PT infants [23]. During the vulnerable period preceding development of full thymic function, a PT infant’s bias towards lower CD27 and CD31 on CD8+ T cells could both support immune dysregulation and enhanced inflammation, as well as compromise the ability to generate a sustained memory population in the face of pathogen exposure.
Exogenous challenges imposed on a preterm infant’s ability to fill a growing T cell niche may further drive homeostatic expansion and result in CD8+ T cell activation. Luciano et al showed that while CD45RO isoform expression on CD4 T cells from FT infants required exposure to chorioamnionitis, PT infant CD4 T cells did not[12]. Our results showing an increased fraction of CD45RO+ CD8+ T cells from PT exposed to antenatal steroids supports this notion. Peripheral naïve and double-positive thymic T cells are highly sensitive to steroid-induced apoptosis, while effector and memory T cells are relatively resistant[24, 25]. It may be that the reduction of a diverse naïve population from steroids and diminished thymic function at lower gestation drives a compensatory maintenance, expansion and differentiation of peripheral T cells. This hypothesis is supported by our results showing that CD27- CD8+ numbers are similarly maintained across the age spectrum despite lower number of CD27+ CD8+ T cells from which a new supply of CD27- T cells could be generated. Further studies are needed to test if excessive expansion of a more pauciclonal repertoire leaves the PT host more susceptible to infection due to a loss of repertoire diversity.
Mechanisms for enhanced CD8+ T cell function and activation in PT infants to altered homeostasis during fetal development are not well studied. To our knowledge, this is the first report showing higher CD31 expression on T cells from full term infants. CD31 expression is one possible mechanism by which full term neonatal cord blood CD8+ T cells are maintained in a naïve, quiescent state during early activation. Loss of CD31, which may occur occur during rapid homeostatic expansion under lymphopenic conditions [26, 27], could lower activation threshold and enhanced effector function seen in CD8+ T cells from premature infants. CD31, or PECAM-1, is an ITIM-containing member of the Ig-superfamily, and inhibits early calcium mobilization during TCR signal-transduction, but not late, sustained effects from TCR ligation[28]. CD31 on T cells promotes telomerase activity and cell survival during activation[29], and loss of this integrin is associated with immune aging and autoimmune diseases[30, 31]. Exaggerated loss has also been demonstrated in lymphopenic hosts during rapid homeostatic expansion and is associated with excessive inflammation[28, 16, 32]. Developmentally determined lower expression of CD31 on fetal CD8+ T cells may partly explain their enhanced activation. Poorly regulated TCR signaling via reduced CD31 would also explain their diminished ability to establish memory following pathogen exposure. Indeed, recent murine models do show that neonatal mice, born with a less mature adaptive immune system than full term human neonates, support short-lived effector responses, but do not efficiently form memory during primary infection[33, 34]. It may be that CD31- CD8+ T cells are more prone to apoptosis during the contraction phase of an adaptive immune response. It is also possible that fetal T cell sensitivity to homeostatic expansion, in combination with a pro-inflammatory environment, synergize to promote a dysregulated effector T cell pool via loss of CD31-mediated regulatory function. The exclusive measurement of CD31 expression and intracellular cytokine production in our experiments limits the conclusions that can be made regarding functional consequences of CD31 loss on CD8+ T cell in premature infants. Although our data do not support a link between CD127 and CD31 expression, it is possible that reduced circulating IL-7 levels contribute to the observed reduction in CD31+ CD8+ T cells. Further studies directly testing the relationship between circulating cytokines, CD31 expression and T cell activation thresholds are warranted. If lower CD31 expression in early gestation indeed contributes to their immunodeficiency and immunopathology, CD31-targeted therapies could provide a novel therapeutic opportunity to gently modulate the cytotoxic T cell response, either to enhance protective function or reduce immunopathology.
One limitation of this study is the relatively low number and skewed distribution of subjects in whom clinical exposures, such as chorioamnionitis and prenatal steroids, were present. Although the overall sample size was adequate to detect CD8+ T cell characteristics associated with gestational age at birth, the sample size necessary for multivariate analysis may not have been sufficient to control for subtle changes based on low-frequency exposures. Variance data obtained in our study, however, can be used to power future studies to test the explicit effect of chorioamnionitis and antenatal steroid exposure on CD8+ T cell phenotype and function.
Though the clinical consequences of altered T cell homeostasis in PT infants cannot be determined based on these results, non-human primate studies provide some evidence linking inflammatory diseases in PT neonates and T cell dysregulation. Rosen et al demonstrated that chronic lung disease in prematurely delivered baboons was associated with early thymic involution. Consistent with our observed cord blood phenotype, authors found increased peripheral T cells carrying markers of maturation, poorly targeted pathogen-specific function, and robust non-specific cytokine secretion. Most remarkably, they identified an increase in lung-autoreactive CD4+ T cells, suggesting that unchecked T cell maturation was associated with a break in tolerance[35]. These findings are also consistent with several human studies that found strong direct correlations between pro-inflammatory T cell cytokine levels and poor outcomes in prematurity, such as BPD[2] and PVL[36]. Evaluation of long-term respiratory and neurologic outcomes in our longitudinal study cohort will elucidate both duration and clinical significance of altered CD8+ T cell homeostasis in PT infants.
5. Conclusion
Overall these results support the hypothesis that developmentally-determined fetal CD8+ T cell properties may be interacting with environmental stimuli to reinforce an “at risk” dysregulated phenotype, characterized by loss of CD27 and CD31, and enhanced CD8+ T cell effector differentiation. In addition to BPD, NEC or PVL, accelerated T cell maturation and hyper-responsiveness could leave the premature infant susceptible to immune dysregulation, poorly directed antigen-specific responses and clonal restriction prior to restoration of homeostasis. Further studies are indicated to clarify the role of CD31 in immune regulation during gestation as well as the downstream consequences of enhanced effector differentiation on clinical outcomes.
Supplementary Material
Highlights.
CD8+ T cell CD31 expression, which dampens T cell receptor signal strength, is reduced in preterm infants at birth
Cord blood is enriched for effector CD8+ T cells in preterm infants born at younger gestational ages
Cytokine production at younger gestational ages is enhanced following perinatal inflammatory exposure
Based on developmentally-determined T cell differences, preterm infants are at risk for immune dysregulation
Acknowledgments
The authors wish to acknowledge the Obstetrics and Neonatal ICU Nursing Teams, Ravi Misra, Dee Maffett, Elizabeth Werner, Tania Scalise, Shannon Castiglione, Valerie Lunger, Lisa Denmark, Jennifer Dutra and Theresa Banker for subject recruitment, sample management, study and data coordination. We thank Andrea Sant for her comments and suggestions in manuscript preparation. We also thank the University of Rochester Human Immunology Center and Flow Cytometry Core Facility for assistance in assay and LSR instrument performance optimization.
Statement of Financial Support:
CD8+ T Cell Dysregulation in Premature Infants (1K08AI108870-01A1)
Prematurity and Respiratory Outcomes Program (U01 HL101813-01)
Translational Molecular Pediatrics (1K12HD068373-01)
Respiratory Pathogens Research Center (HHSN272201200005C)
The project described in this publication was also supported by the University of Rochester CTSA award number UL1 TR000042 from the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- PT
preterm
- FT
full term
- VLBW
very low birth weight
- BPD
bronchopulmonary dysplasia
- NEC
necrotizing enterocolitis
- PVL
periventricular leukomalacia
- UCMC
umbilical cord blood mononuclear cell
- SEB
staphylococcal enterotoxin B
- PROM
prolonged rupture of membranes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Underwood MA, Danielsen B, Gilbert WM. Cost, causes and rates of rehospitalization of preterm infants. J Perinatol. 2007;27(10):614–9. doi: 10.1038/sj.jp.7211801. 7211801 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Ambalavanan N, Carlo WA, D’Angio CT, McDonald SA, Das A, Schendel D, et al. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123(4):1132–41. doi: 10.1542/peds.2008-0526. 123/4/1132 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballabh P, Simm M, Kumari J, Krauss AN, Jain A, Auld PA, et al. Lymphocyte subpopulations in bronchopulmonary dysplasia. Am J Perinatol. 2003;20(8):465–75. doi: 10.1055/s-2003-45387. [DOI] [PubMed] [Google Scholar]
- 4.Duggan PJ, Maalouf EF, Watts TL, Sullivan MH, Counsell SJ, Allsop J, et al. Intrauterine T-cell activation and increased proinflammatory cytokine concentrations in preterm infants with cerebral lesions. Lancet. 2001;358(9294):1699–700. doi: 10.1016/s0140-6736(01)06723-x. S014067360106723X [pii] [DOI] [PubMed] [Google Scholar]
- 5.Schonland SO, Zimmer JK, Lopez-Benitez CM, Widmann T, Ramin KD, Goronzy JJ, et al. Homeostatic control of T-cell generation in neonates. Blood. 2003;102(4):1428–34. doi: 10.1182/blood-2002-11-3591. 2002-11-3591 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Blaser BW, Roychowdhury S, Kim DJ, Schwind NR, Bhatt D, Yuan W, et al. Donor-derived IL-15 is critical for acute allogeneic graft-versus-host disease. Blood. 2005;105(2):894–901. doi: 10.1182/blood-2004-05-1687. 2004-05-1687 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Lin SJ, Cheng PJ. Effect of interleukin-7 and -15 on activation of purified umbilical cord blood and adult peripheral blood CD4+ T cells. Biol Neonate. 2004;85(1):3–10. doi: 10.1159/000074950. 74950 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Szabolcs P, Park KD, Reese M, Marti L, Broadwater G, Kurtzberg J. Coexistent naive phenotype and higher cycling rate of cord blood T cells as compared to adult peripheral blood. Exp Hematol. 2003;31(8):708–14. doi: 10.1016/s0301-472x(03)00160-7. S0301472X03001607 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Nelson EK, Piehler B, Eckels J, Rauch A, Bellew M, Hussey P, et al. LabKey Server: an open source platform for scientific data integration, analysis and collaboration. BMC bioinformatics. 2011;12:71. doi: 10.1186/1471-2105-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheible K, Secor-Socha S, Wightman T, Wang H, Mariani TJ, Topham DJ, et al. Stability of T cell phenotype and functional assays following heparinized umbilical cord blood collection. Cytometry A. 2012 doi: 10.1002/cyto.a.22203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herzenberg LA, Tung J, Moore WA, Parks DR. Interpreting flow cytometry data: a guide for the perplexed. Nat Immunol. 2006;7(7):681–5. doi: 10.1038/ni0706-681. ni0706-681 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Luciano AA, Yu H, Jackson LW, Wolfe LA, Bernstein HB. Preterm labor and chorioamnionitis are associated with neonatal T cell activation. PLoS One. 2011;6(2):e16698. doi: 10.1371/journal.pone.0016698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma CS, Hodgkin PD, Tangye SG. Automatic generation of lymphocyte heterogeneity: Division-dependent changes in the expression of CD27, CCR7 and CD45 by activated human naive CD4+ T cells are independently regulated. Immunol Cell Biol. 2004;82(1):67–74. doi: 10.1046/j.0818-9641.2003.01206.x. 1206 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Wood KL, Twigg HL, 3rd, Doseff AI. Dysregulation of CD8+ lymphocyte apoptosis, chronic disease, and immune regulation. Front Biosci. 2009;14:3771–81. doi: 10.2741/3487. 3487 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28- and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134(1):17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marelli-Berg FM, Clement M, Mauro C, Caligiuri G. An immunologist’s guide to CD31 function in T-cells. J Cell Sci. 2013;126(Pt 11):2343–52. doi: 10.1242/jcs.124099. [DOI] [PubMed] [Google Scholar]
- 17.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, et al. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206(2):435–48. doi: 10.1084/jem.20081829. jem.20081829 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1(5):433–40. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 19.Tajima M, Wakita D, Noguchi D, Chamoto K, Yue Z, Fugo K, et al. IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17-producing CD8+ T cells. J Exp Med. 2008;205(5):1019–27. doi: 10.1084/jem.20071133. jem.20071133 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192(4):557–64. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eyrich M, Wollny G, Tzaribaschev N, Dietz K, Brugger D, Bader P, et al. Onset of thymic recovery and plateau of thymic output are differentially regulated after stem cell transplantation in children. Biol Blood Marrow Transplant. 2005;11(3):194–205. doi: 10.1016/j.bbmt.2004.12.001. S1083879104006263 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Hakim FT, Memon SA, Cepeda R, Jones EC, Chow CK, Kasten-Sportes C, et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest. 2005;115(4):930–9. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verbsky JW, Baker MW, Grossman WJ, Hintermeyer M, Dasu T, Bonacci B, et al. Newborn screening for severe combined immunodeficiency; the Wisconsin experience (2008–2011) J Clin Immunol. 2012;32(1):82–8. doi: 10.1007/s10875-011-9609-4. [DOI] [PubMed] [Google Scholar]
- 24.Bianchini R, Nocentini G, Krausz LT, Fettucciari K, Coaccioli S, Ronchetti S, et al. Modulation of pro- and antiapoptotic molecules in double-positive (CD4+CD8+) thymocytes following dexamethasone treatment. J Pharmacol Exp Ther. 2006;319(2):887–97. doi: 10.1124/jpet.106.108480. jpet.106.108480 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Nijhuis EW, Hinloopen B, van Lier RA, Nagelkerken L. Differential sensitivity of human naive and memory CD4+ T cells for dexamethasone. Int Immunol. 1995;7(4):591–5. doi: 10.1093/intimm/7.4.591. [DOI] [PubMed] [Google Scholar]
- 26.Azevedo RI, Soares MV, Barata JT, Tendeiro R, Serra-Caetano A, Victorino RM, et al. IL-7 sustains CD31 expression in human naive CD4+ T cells and preferentially expands the CD31+ subset in a PI3K-dependent manner. Blood. 2009;113(13):2999–3007. doi: 10.1182/blood-2008-07-166223. blood-2008-07-166223 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Junge S, Kloeckener-Gruissem B, Zufferey R, Keisker A, Salgo B, Fauchere JC, et al. Correlation between recent thymic emigrants and CD31+ (PECAM-1) CD4+ T cells in normal individuals during aging and in lymphopenic children. Eur J Immunol. 2007;37(11):3270–80. doi: 10.1002/eji.200636976. [DOI] [PubMed] [Google Scholar]
- 28.Newton-Nash DK, Newman PJ. A new role for platelet-endothelial cell adhesion molecule-1 (CD31): inhibition of TCR-mediated signal transduction. J Immunol. 1999;163(2):682–8. [PubMed] [Google Scholar]
- 29.Kushner EJ, Weil BR, MacEneaney OJ, Morgan RG, Mestek ML, Van Guilder GP, et al. Human aging and CD31+ T-cell number, migration, apoptotic susceptibility, and telomere length. J Appl Physiol. 2010;109(6):1756–61. doi: 10.1152/japplphysiol.00601.2010. japplphysiol.00601.2010 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caligiuri G, Groyer E, Khallou-Laschet J, Al Haj Zen A, Sainz J, Urbain D, et al. Reduced immunoregulatory CD31+ T cells in the blood of atherosclerotic mice with plaque thrombosis. Arterioscler Thromb Vasc Biol. 2005;25(8):1659–64. doi: 10.1161/01.ATV.0000172660.24580.b4. 01.ATV.0000172660.24580.b4 [pii] [DOI] [PubMed] [Google Scholar]
- 31.Kohler S, Thiel A. Life after the thymus: CD31+ and CD31- human naive CD4+ T-cell subsets. Blood. 2009;113(4):769–74. doi: 10.1182/blood-2008-02-139154. blood-2008-02-139154 [pii] [DOI] [PubMed] [Google Scholar]
- 32.den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mogling R, de Boer AB, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36(2):288–97. doi: 10.1016/j.immuni.2012.02.006. S1074-7613(12)00055-6 [pii] [DOI] [PubMed] [Google Scholar]
- 33.Rudd BD, Venturi V, Smith NL, Nzingha K, Goldberg EL, Li G, et al. Acute neonatal infections ‘lock-in’ a suboptimal CD8+ T cell repertoire with impaired recall responses. PLoS Pathog. 2013;9(9):e1003572. doi: 10.1371/journal.ppat.1003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith NL, Wissink E, Wang J, Pinello JF, Davenport MP, Grimson A, et al. Rapid proliferation and differentiation impairs the development of memory CD8+ T cells in early life. J Immunol. 2014;193(1):177–84. doi: 10.4049/jimmunol.1400553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosen D, Lee JH, Cuttitta F, Rafiqi F, Degan S, Sunday ME. Accelerated thymic maturation and autoreactive T cells in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2006;174(1):75–83. doi: 10.1164/rccm.200511-1784OC. 200511-1784OC [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaukola T, Herva R, Perhomaa M, Paakko E, Kingsmore S, Vainionpaa L, et al. Population cohort associating chorioamnionitis, cord inflammatory cytokines and neurologic outcome in very preterm, extremely low birth weight infants. Pediatr Res. 2006;59(3):478–83. doi: 10.1203/01.pdr.0000182596.66175.ee. 59/3/478 [pii] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.