Abstract
Objective
To evaluate the association of baseline characteristics and early visual acuity (VA) response with visual outcomes at Years 1 or 2 in the Comparison of Age-related Macular Degeneration (AMD) Treatments Trials (CATT).
Design
Secondary analysis of a randomized clinical trial.
Participants
1185 patients with neovascular AMD and baseline VA 20/25 to 20/320.
Methods
Patients were assigned to ranibizumab or bevacizumab and to one of 3 dosing regimens. VA responses were classified as ≥3-lines gain/loss, 1–2 lines gain/loss, or within one-line change from baseline. Associations of baseline characteristics and early VA response (week 4 or 12) with VA response at Years 1 or 2 were assessed by R2 from linear regression analyses. Patients who had a poor initial response (VA 20/40 or worse with persistent fluid and without ≥1-line VA gain) were defined as candidates for “switching” based on estimates of current clinical practice.
Main Outcome Measures
VA change from baseline.
Results
Statistically significant (p<0.05) baseline predictors for less VA gain at Year 2 were older age, VA 20/40 or better, larger CNV area, presence of GA, total foveal thickness ≤325 or >425 microns, and elevation of retinal pigment epithelium. Among 176 eyes gaining ≥3 lines at week 12, 78% had a ≥3-line gain at Year 2, while among 113 eyes losing ≥1 line at week 12, 27% improved to a ≥1 line gain at Year 2. VA response at week 12 was more predictive of VA response at Year 2 (R2=0.30) than VA response at week 4 (R2=0.17) and baseline predictors (R2=0.13) (p<0.0001). Among 126 candidates for “switching” drug at week 12, mean VA improved 2.8 letters (p=0.050), mean total retinal thickness decreased 53u (p<0.0001), and fluid resolved in 33% (p<0.0001) between week 12 and Year 1 with continued use of the same drug and regimen. Similar improvements were observed among 83 candidates for “switching” at week 24.
Conclusion
VA response at week 12 is more predictive of two-year vision outcomes than either several baseline characteristics or week 4 response. Eyes with poor initial response may benefit from continued treatment without switching to another drug.
INTRODUCTION
Anti-vascular endothelial growth factor (anti-VEGF) treatments have revolutionized the treatment of choroidal neovascularization (CNV) secondary to age-related macular degeneration (AMD).1–7 Treatment with ranibizumab (Lucentis, Genentech, South San Francisco, CA), bevacizumab (Avastin, Genentech, South San Francisco, CA) or aflibercept (Eylea, Regeneron, Tarrytown, NY) has become standard care for the management of neovascular AMD. Despite the effectiveness of these drugs, there is large variation in the response across patients and response fluctuates over time within a patient. 8–10 In an attempt to better understand this variation, we previously investigated the baseline demographic, clinical and genetic predictors for VA response at Year 1 and found that age, baseline visual acuity, CNV lesion area and retinal pigment epithelium (RPE) elevation in OCT were predictors for VA response at 1 Year, 8 while genetic factors (either AMD-related SNPs or VEGF-related SNPs) did not predict VA response.11–12
The purpose of this paper is to evaluate how the early VA response (at week 4 or at week 12) to anti-VEGF treatment, baseline characteristics, and their combinations are associated with VA responses at Year 1 or at Year 2 by using the data collected for the Comparison of AMD Treatments Trials (CATT). This evaluation is important for several reasons. First, it may allow adjustment of expectations by ophthalmologists and patients about longer-term results from treatment after the first injections have been completed. Second, if Year 1 or Year 2 VA gain is predicted to be unlikely with the current treatment, switching to alternative treatments (e.g., different anti-VEGF agents or combination therapy) may be considered. Third, if early VA response is strongly associated with Year 1 or Year 2 VA response, early VA response might be considered as a surrogate outcome in future clinical trials of anti-VEGF agents or combination therapy. Finally, understanding the association of early poor vision response and VA response at Years 1 or 2 provides background information when evaluating the effects of switching to another drug.
METHODS
Details on the study design and methods have been reported in previous publications 5–6 and on ClinicalTrials.gov (NCT00593450). Only the major features related to this paper are described here.
Study Participants
The institutional review board associated with each clinical center approved the study protocol and informed consent was obtained from each patient. Patients were enrolled from 43 clinical centers in the United States and randomized to one of four treatment groups at baseline: (1) ranibizumab monthly; (2) bevacizumab monthly; (3) ranibizumab as needed (pro re nata, PRN); and (4) bevacizumab PRN. At the end of Year 1, patients initially assigned to monthly treatment retained their drug assignment but were reassigned randomly to either monthly or PRN treatment. Patients initially assigned to PRN treatment retained both their drug and regimen for Year 2.
The study enrollment criteria included age of 50 or older, the study eye (one eye per patient) having untreated active CNV due to AMD, and VA between 20/25 and 20/320 on electronic VA testing. Determination of active CNV required both leakage of dye on fluorescein angiography and fluid, located either within or below the retina or below the RPE, on time-domain OCT.
Study Procedures
During the initial visit, patients provided information on demographic characteristics and medical history. Certified photographers obtained stereoscopic, color fundus photographs and fluorescein angiograms at baseline, Year 1 and Year 2. Time domain optical coherence tomography (OCT) images were obtained throughout the first year, while 22.6% of scans were obtained on spectral domain OCT in the second year by certified OCT imagers.6 Both photographic and OCT images were evaluated at reading centers using standardized protocols.13,14
At baseline and at follow-up weeks 4, 12, 24, 36, 52 (Year 1), 64, 76, 88 and 104 (Year 2), certified visual acuity examiners, masked to the treatment assignment, measured visual acuity after refraction in both eyes using the Electronic Visual Acuity (EVA) Tester following the protocol used in the Diabetic Retinopathy Clinical Research Network.15 The VA at other follow-up visits, which occurred every 4 weeks after enrollment, was also measured but without refraction. The VA scores from EVA range from 0 to 100, corresponding to Snellen equivalents of worse than 20/800 to 20/10.
Statistical Analysis
We previously evaluated the baseline predictors for VA response at Year 1 using multiple linear regression analysis.8 Following the same analysis approach for the same candidate baseline predictors, we evaluated the baseline predictors for VA response at Year 2. Early VA response at weeks 4 and 12, and VA responses at Years 1 or 2 were calculated as the VA change from baseline. To facilitate the clinical results interpretation, we also divided the VA response into 5 categories including: ≥3 lines gained (i.e., ≥15 letters gained from baseline), 1–2 lines gained (5–14 letters gain from baseline), within 1 line change (i.e., lost or gained less than 5 letters from baseline), 1–2 lines lost, ≥3 lines lost. The agreement between VA response categories at early and at Years 1 or 2 was calculated.
To evaluate whether or not the baseline characteristics and/or early VA response predict vision outcomes at Year 1 and Year 2, we calculated R2 from linear regression models using various predictors including statistically significant baseline predictors alone, early VA response (i.e., VA change from baseline at week 4 or week 12 alone, or in combination). In these linear models, early VA response and VA response at Year 1 and Year 2 were represented as continuous variables. R2 was computed as the ratio of the variance of Year 1 or Year 2 VA response explained by predictors and the total variance in the VA response. R2 ranges from 0 to 1, with 0 meaning no prediction beyond random variation and 1 meaning perfect prediction. The comparisons of R2 from various prediction models were performed by the method of Meng.16
Patients in CATT maintained their randomly assigned drug for two years. In a subgroup of CATT patients who in clinical practice might have been considered candidates for switching drug due to a poor clinical response by week 12 or week 24, we evaluated the visual acuity and morphological results when the same drug and dosing regimen were continued through Year 1 and Year 2. We surveyed a variety of past reports of switching drugs to define criteria for candidates for switching drugs among CATT patients.17–22 Candidates had to have received all three initial monthly treatments (baseline, weeks 4, 8) for “switching” at week 12, and had to have received 5 of 6 initial monthly treatments (baseline, weeks 4, 8, 12, 16, 20) for “switching” at week 24. In addition, candidates also had to meet all three of the following criteria for poor clinical response at the week of switching: (1) visual acuity 20/40 or worse; (2) ≤1-line gain from baseline at week; and (3) persistent OCT fluid at the foveal center. We calculated the VA change and change in OCT total retinal thickness from the switching week and percentage with OCT foveal center fluid resolved at 4 weeks after switching and at Year 1 and Year 2) for all candidates eligible for “switching”. Statistical significance for mean changes from the switching week was assessed using the paired t-test. For the analyses in this paper, study participants were pooled across the ranibizumab and bevicizumab treatment groups because the treatment effects on visual acuity were similar for both the previously reported primary analyses and the analyses in this paper.5–6 All data analyses were performed in SAS v9.4 (SAS Institute Inc., Cary, NC) and two-sided p values <0.05 were considered to be statistically significant.
RESULTS
Visual acuity over time
Among all CATT patients (N=1185), the mean VA at baseline was 61 letters. The mean VA improved by 3.6 letters at week 4, 5.8 letters at week 12, 6.4 letters at week 24, and stabilized at approximately 6 to 7 letters gain through the end of Year 2 (Table 1, available at http://aaojournal.org). The percentages of eyes with VA gain or loss from baseline that were within one line, between 1–2 lines, and 3 lines or more in 2 years are also displayed in Table 1. Over time, the percentage with a gain of ≥3 lines increased from approximately 10% at week 4, to 27% at week 36 and stabilized at approximately 30% after week 36. The percentage with a loss of ≥3 lines was 2.6% at week 4 and gradually increased to 9.2% at Year 2.
Table 1 (online).
Visual acuity change over time
| Week | N | Mean visual acuity in letters (SD) | Mean change in visual acuity from baseline (SD) | Visual acuity change from baseline n (%) | ||||
|---|---|---|---|---|---|---|---|---|
| ≥ 3-lines gain | 1–2 lines gain | Within 1 line change | 1–2 lines loss | ≥ 3-lines loss | ||||
| 000 | 1185 | 60.6 (13.5) | ||||||
| 004 | 1157 | 64.1 (14.5) | 3.6 ( 9.0) | 114 ( 9.9) | 378 (32.7) | 509 (44.0) | 126 (10.9) | 30 (2.6) |
| 012 | 1085 | 66.6 (15.6) | 5.8 (11.3) | 198 (18.3) | 415 (38.3) | 336 (31.0) | 95 ( 8.8) | 41 (3.8) |
| 024 | 1061 | 67.2 (16.9) | 6.4 (12.9) | 235 (22.2) | 405 (38.2) | 287 (27.1) | 82 ( 7.7) | 52 (4.9) |
| 036 | 1026 | 68.4 (16.6) | 7.4 (13.7) | 277 (27.0) | 383 (37.3) | 236 (23.0) | 72 ( 7.0) | 58 (5.7) |
| 052 | 1106 | 68.0 (17.8) | 7.3 (14.7) | 327 (29.6) | 381 (34.5) | 246 (22.2) | 84 ( 7.6) | 68 (6.2) |
| 064 | 986 | 68.5 (17.6) | 7.7 (14.9) | 293 (29.7) | 330 (33.5) | 223 (22.6) | 79 ( 8.0) | 61 (6.2) |
| 076 | 984 | 67.5 (18.1) | 6.9 (15.7) | 284 (28.9) | 349 (35.5) | 193 (19.6) | 84 ( 8.5) | 74 (7.5) |
| 088 | 948 | 68.0 (17.5) | 7.0 (15.0) | 268 (28.3) | 321 (33.9) | 200 (21.1) | 85 ( 9.0) | 74 (7.8) |
| 104 | 1034 | 67.3 (18.3) | 6.3 (16.6) | 307 (29.7) | 322 (31.1) | 218 (21.1) | 92 ( 8.9) | 95 (9.2) |
Baseline predictors for VA response at Year 1 and Year 2
We previously reported 8 the baseline predictors for less VA gain at Year 1 (Table 2) including older age (p=0.003), baseline VA 20/40 or better in the study eye (p<0.0001), larger CNV area (p=0.02), absence of RAP lesion (p=0.03), and presence of RPE elevation in OCT (p=0.004). This analysis found that all the baseline predictors for less VA gain at Year 1 were significant at Year 2 including older age (p=0.02), baseline VA 20/40 or better in study eye (p<0.0001), larger CNV area (p=0.02), and presence of RPE elevation (p=0.001), with the except of RAP lesion. Additionally, the presence of GA in study eye (p=0.04), thicker (>425 microns) or thinner (≤325 microns) total foveal thickness (p=0.01) was significant predictors for less VA gain at Year 2 but not at Year 1 (Table 2).
Table 2.
Multivariate analysis for association baseline characteristics with visual acuity change from baseline at Year 1 and at Year 2
| VA score change (letters) from baseline at Year 1 (N=1069§) | VA score change (letters) from baseline at Year 2 (N=1014 §) | |||||
|---|---|---|---|---|---|---|
| Baseline Characteristics | N | Adjusted Mean (SE) | P-value | N | Adjusted Mean (SE) | P-value |
| Age (years) | ||||||
| 50–69 | 125 | 10.8 (1.3) | 0.003 | 127 | 9.3 (1.4) | 0.02 |
| 70–79 | 374 | 8.2 (0.8) | 357 | 7.1 (0.8) | ||
| 80–89 | 500 | 5.8 (0.6) | 465 | 5.0 (0.7) | ||
| ≥90 | 70 | 6.2 (1.7) | 65 | 4.7 (1.9) | ||
| Visual acuity in study eye | ||||||
| 68–82 letters, 20/25 – 20/40 | 382 | 3.3 (0.7) | <0.0001 | 378 | 0.7 (0.8) | <0.0001 |
| 53–67 letters, 20/50 – 20/80 | 401 | 8.4 (0.7) | 373 | 6.9 (0.8) | ||
| 38–52 letters, 20/100 – 20/160 | 218 | 11.9 (1.0) | 201 | 14.3 (1.1) | ||
| 23–37 letters, 20/200 – 20/320 | 68 | 7.9 (1.7) | 62 | 10.5 (2.0) | ||
| Area of CNV (mm2) | ||||||
| ≤2.54 | 435 | 8.7 (0.7) | 0.02 | 411 | 7.5 (0.8) | 0.004 |
| >2.54 to ≤5.08 | 214 | 7.5 (1.0) | 199 | 7.8 (1.1) | ||
| >5.08 to ≤10.2 | 207 | 6.7 (1.0) | 191 | 6.0 (1.1) | ||
| >10.2 | 102 | 4.2 (1.4) | 99 | 2.1 (1.6) | ||
| Can’t measure | 111 | 4.8 (1.4) | 114 | 3.4 (1.5) | ||
| Geographic atrophy | ||||||
| None/questionable | 948 | 6.5 (0.5) | 0.04 | |||
| Present | 66 | 2.4 (1.9) | ||||
| RAP lesion | ||||||
| No | 951 | 6.9 (0.5) | 0.03 | |||
| Yes | 118 | 10.1 (1.3) | ||||
| Total foveal thickness (μ) | ||||||
| 1st quartile (≤325) | 261 | 5.4 (1.0) | 0.01 | |||
| 2nd quartile (>325 to ≤425) | 259 | 8.9 (1.0) | ||||
| 3rd quartile (>425 to ≤550) | 232 | 6.0 (1.0) | ||||
| 4th quartile (>550) | 262 | 4.7 (1.0) | ||||
| RPE elevation | ||||||
| No | 139 | 10.5 (1.2) | 0.004 | 136 | 10.3 (1.4) | 0.001 |
| Yes | 930 | 6.8 (0.5) | 878 | 5.6 (0.5) | ||
| Treatment Group in Year 1 | ||||||
| Ranibizumab Monthly | 280 | 8.6 (0.9) | 0.07 | |||
| Bevacizumab Monthly | 251 | 7.9 (0.9) | ||||
| Ranibizumab PRN | 276 | 6.9 (0.9) | ||||
| Bevacizumab PRN | 262 | 5.5 (0.9) | ||||
| Treatment Group in 2 Years | ||||||
| Ranibizumab Monthly for 2 years | 135 | 8.0 (1.3) | 0.21 | |||
| Bevacizumab Monthly for 2 years | 124 | 7.7 (1.4) | ||||
| Ranibizumab Monthly year 1 PRN year 2 | 130 | 7.2 (1.4) | ||||
| Bevacizumab Monthly year 1 PRN year 2 | 122 | 4.4 (1.4) | ||||
| Ranibizumab PRN for 2 years | 256 | 6.5 (1.0) | ||||
| Bevacizumab PRN for 2 years | 247 | 4.8 (1.0) | ||||
SE = Standard Error; VA= Visual Acuity; CNV= choroidal neovascularization; RAP = retinal angiomatous proliferans; RPE=retinal pigment epithelium; PRN = pro re nata.
Number of subjects included in the multivariate model, 37 patients were excluded due to missing value in one or more predictors in multivariate model of Year 1 VA outcome, 20 patients were excluded due to missing value in one or more predictors for multivariate model of Year 2 VA outcome.
This analysis found that baseline predictors for less VA gain at Year 2 were similar to those at Year 1 and included older age (p=0.02), baseline VA 20/40 or better in study eye (p<0.0001), larger CNV area (p=0.02), presence of GA in study eye (p=0.04), thicker (>425 microns) or thinner (≤325 microns) total foveal thickness (p=0.01), and presence of RPE elevation (p=0.001) (Table 2).
Association of VA response at week 4 or 12 with response at Year 1
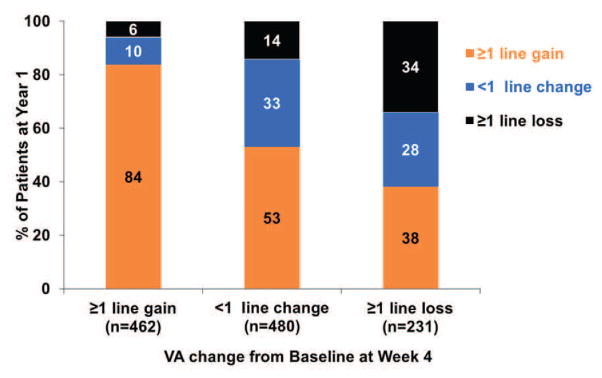
The association between VA response at week 4 and at Year 1 is shown in the top part of Table 3. Among 108 eyes with a gain of ≥3 lines at week 4, 90 (83%) had a similar gain of ≥3 lines, and only 2 (1.8%) eyes had ≥1 line loss at Year 1. Among 147 eyes with loss of ≥1 line at week 4, 56 (38%) gained ≥1 line from baseline, while 50 (34%) eyes had a similar loss of ≥1 line at Year 1 (Figure 1A). In particular, among 27 eyes with loss of ≥3 lines at week 4, 7 (26%) eyes gained ≥1 line from baseline at Year 1 (Table 3).
Table 3.
Association of visual acuity response at week 4 or 12 with visual acuity response at Year 1.
| VA change from baseline at Year 1 | ||||||
|---|---|---|---|---|---|---|
| VA change from baseline at week 4 | n | ≥3 lines gain (%) | 1–2 lines gain (%) | within 1 line change (%) | 1–2 lines loss (%) | ≥3 lines loss (%) |
| ≥3 lines gain | 108 | 90 (83.3) | 13 (12.0) | 3 ( 2.8) | 1 ( 0.9) | 1 ( 0.9) |
| 1–2 lines gain | 354 | 143 (40.4) | 141 (39.8) | 44 (12.4) | 12 ( 3.4) | 14 ( 4.0) |
| within 1 line change | 480 | 65 (13.5) | 190 (39.6) | 157 (32.7) | 42 ( 8.8) | 26 ( 5.4) |
| 1–2 lines loss | 120 | 19 (15.8) | 30 (25.0) | 34 (28.3) | 19 (15.8) | 18 (15.0) |
| ≥3 lines loss | 27 | 6 (22.2) | 1 ( 3.7) | 7 (25.9) | 7 (25.9) | 6 (22.2) |
| Total | 1089 | 323 (29.7) | 375 (34.4) | 245 (22.5) | 81 ( 7.4) | 65 ( 6.0) |
| VA change from baseline at week 12 | ||||||
| ≥3 lines gain | 187 | 152 (81.3) | 27 (14.4) | 7 ( 3.7) | 1 ( 0.5) | 0 ( 0.0) |
| 1–2 lines gain | 399 | 120 (30.1) | 202 (50.6) | 62 (15.5) | 11 ( 2.8) | 4 ( 1.0) |
| within 1 line change | 312 | 25 ( 8.0) | 115 (36.9) | 127 (40.7) | 32 (10.3) | 13 ( 4.2) |
| 1–2 lines loss | 88 | 6 ( 6.8) | 14 (15.9) | 26 (29.6) | 22 (25.0) | 20 (22.7) |
| ≥3 lines loss | 39 | 1 ( 2.6) | 1 ( 2.6) | 5 (12.8) | 8 (20.5) | 24 (61.5) |
| Total | 1025 | 304 (29.7) | 359 (35.0) | 227 (22.1) | 74 (7.2) | 61 (6.0) |
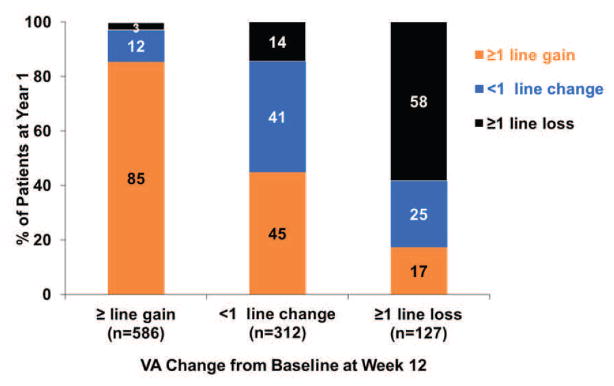
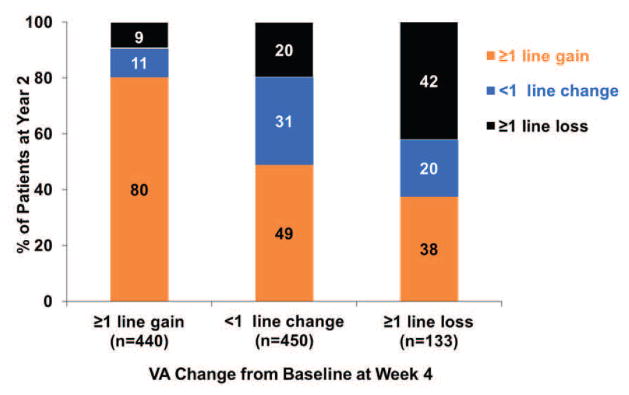
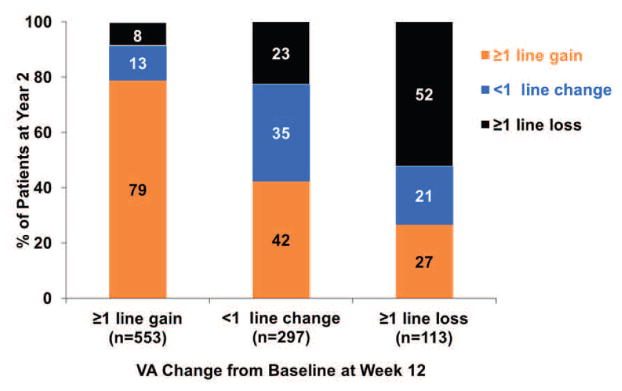
Figure 1. The VA response category (≥1 line gain, <1 line change, ≥1 line loss) at Year 1 or at Year 2 by the early VA response category at week 4 or at week 12.
(A) VA response category at Year 1 by VA response at Week 4; (B) VA response category at Year 1 by VA response at Week 12; (C) VA response category at Year 2 by VA response at Week 4; (D) VA response category at Year 2 by VA response at Week 12.
The association between VA response at week 12 and at Year 1 is shown in the bottom part of Table 3. Among the 187 eyes with gain of ≥3 lines at week 12, 152 (81%) eyes had a similar gain of ≥3 lines at Year 1, only 8 (4%) eyes had loss of ≥1 line at Year 1. In contrast, among 127 eyes with VA loss of ≥1 line at week 12, 22 (17%) eyes had gain of ≥1 line, while 58% had similar loss of ≥1 line at Year 1 (Figure 1B).
Association of VA response at week 4 or 12 with response at Year 2
The association between VA response at week 4 and at Year 2 is presented in the top part of Table 4. Among 103 eyes that had gain of ≥3 lines from baseline, 86 (84%) eyes had similar gain of ≥3 lines, only 5 (5%) eyes had loss of ≥1 line at Year 2. Among 133 eyes with loss ≥1 line at week 4, 50 (38%) eyes had gain of ≥1 line, while 56 (42%) eyes had similar loss of ≥1 line at Year 2 (Figure 1C).
Table 4.
Association of visual acuity response at week 4 or 12 with visual acuity response at Year 2
| VA change from baseline at Year 2 | ||||||
|---|---|---|---|---|---|---|
| VA change from baseline at week 4 | n | ≥3 lines gain (%) | 1–2 lines gain (%) | within 1 line change (%) | 1–2 lines loss (%) | ≥3 lines loss (%) |
| ≥3 lines gain | 103 | 86 (83.5) | 10 (9.7) | 2 ( 1.9) | 3 ( 2.9) | 2 ( 1.9) |
| 1–2 lines gain | 337 | 124 (36.8) | 133 (39.5) | 44 (13.1) | 16 ( 4.8) | 20 ( 5.9) |
| within 1 line change | 450 | 72 (16.0) | 148 (32.9) | 142 (31.6) | 50 (11.1) | 38 ( 8.4) |
| 1–2 lines loss | 107 | 16 (15.0) | 26 (24.3) | 21 (19.6) | 17 (15.9) | 27 (25.2) |
| ≥3 lines loss | 26 | 6 (23.1) | 2 (7.7) | 6 (23.1) | 6 (23.1) | 6 (23.1) |
| Total | 1023 | 304 (29.7) | 319 (31.2) | 215 (21.0) | 92 (9.0) | 93 (9.1) |
| VA change from baseline at week 12 | ||||||
| ≥3 lines gain | 176 | 137 (77.8) | 25 (14.2) | 5 ( 2.8) | 3 (1.7) | 6 ( 3.4) |
| 1–2 lines gain | 377 | 101 (26.8) | 173 (45.9) | 66 (17.5) | 22 (5.8) | 15 ( 4.0) |
| within 1 line change | 297 | 40 (13.5) | 86 (30.0) | 104 (35.0) | 37 (12.5) | 30 (10.1) |
| 1–2 lines loss | 79 | 6 ( 7.6) | 18 (22.8) | 19 (24.1) | 16 (20.3) | 20 (25.3) |
| ≥3 lines loss | 34 | 3 ( 8.8) | 3 ( 8.8) | 5 (14.7) | 7 (20.6) | 16 (47.1) |
| Total | 963 | 287 (29.8) | 305 (31.7) | 199 (20.7) | 85 (8.8) | 87 ( 9.0) |
The association between VA response at week 12 and at Year 2 is shown in the bottom part of Table 4. Among 176 eyes that had gain of ≥3 lines from baseline, 137 (78%) eyes had similar gain of ≥3 lines, while 9 (5%) eyes had loss of ≥1 line at Year 2. Among 113 eyes with loss of ≥1 line at week 12, 30 (27%) eyes had gain of ≥1 line, while 59 (52%) eyes had similar loss of ≥1 line at Year 2 (Figure 1D).
Prediction of Year 1 and Year 2 outcomes using baseline predictors and early VA response
The predictions of VA response at Year 1 and Year 2 using baseline predictors alone, early VA response alone, and their combinations are show in Table 5. Using the statistically significant baseline predictors for VA response at Year 1 and Year 2 respectively, the corresponding R2 for predicting VA change at Year 1 and Year 2 is 0.09 and 0.13, which are lower than those from using early VA response at week 4 alone (R2 = 0.22 for Year 1 and 0.17 for Year 2), and week 12 alone (0.47 for Year 1 and 0.30 for Year 2) (all p<0.001 for comparison with baseline predictors). Combining the baseline predictors with the week 12 VA response resulted in modest increases of R2 to 0.49 for Year 1 and 0.35 for Year 2 (p<0.001). Adding the VA response at week 4 to the regression models did not improve the models that already included VA response at week 12 (Table 5).
Table 5.
The Proportion of variance R2 in VA response at Year 1 and Year 2 explained by baseline predictors and early visual acuity response at week 4 or at week 12
| Predictors | R2 for visual acuity change from baseline at Year 1 (N=982§) | R2 for visual acuity change from baseline at Year 2 (N=937§) |
|---|---|---|
| Baseline predictors* | 0.09 | 0.13 |
| Visual acuity change at week 4 | 0.22 | 0.17 |
| Visual acuity change at week 12 | 0.47 | 0.30 |
| Visual acuity change at both week 4 and 12 | 0.47 | 0.31 |
| Baseline predictors + visual acuity change at week 4 | 0.26 | 0.25 |
| Baseline predictors + visual acuity change at week 12 | 0.49 | 0.35 |
| Baseline predictors + visual acuity change at week 4 and 12 | 0.49 | 0.36 |
Among those with complete data for baseline predictors, visual acuity at week 4 and at week 12.
Baseline predictors are: age, visual acuity in study eye, area of choroidal neovascularization, lesion of retinal angiomatous proliferans, elevation of retinal pigment epithelium, and treatment group for Year 1; age, visual acuity in study eye, area of CNV, geographic atrophy, total foveal thickness, elevation of retinal pigment epithelium and treatment group in Year 2.
Visual acuity and morphological change over time among “switching” eligible patients
Among 126 patients who were candidates for “switching” drug at week 12, the mean VA at week 12 was 53 letters (Snellen equivalent 20/80). There was a mean loss of 0.4 letters from week 12 to week 16 (p=0.57), a mean gain of 2.8 letters from week 12 at Year 1 (p=0.050) and 2.9 letters at Year 2 (p=0.11). The total retinal thickness decreased from week 12 with a mean decrease of 53u at Year 1 (p<0.0001) and 54u at Year 2 (p=0.0004). After week 12, fluid at the foveal center resolved in 33% of eyes at Year 1 and 54% at Year 2 (Table 6, top panel).
Table 6.
Visual acuity and OCT morphological outcomes for eyes meeting criteria for hypothetical switching drug outside of the clinical trial
| Switching at week 12 (n=126) | Week 12 (n=126) | Week 16 §£ (n=116) | Year 1 (n=117) | Year 2 (n=108) |
|---|---|---|---|---|
| VA in letters: mean (SD) | 52.5 (16.3) | 52.0 (18.6) | 55.5 (20.9) | 55.5 (23.0) |
| VA change in letters from week 12: mean (SD) | −0.4 (7.12) | 2.8 (15.5) | 2.9 (18.3) | |
| P-value for VA comparison with week 12 | 0.57 | 0.050 | 0.11 | |
| Fluid at foveal center: n(%) | 126 (100%) | -- | 77 (67.0%) | 49 (46.2%) |
| Total thickness (μ): mean (SD) | 401 (163) | -- | 357 (163) | 360 (176) |
| Change in total thickness (μ) from week 12: mean (SD) | -- | −53 (137) | −54 (153) | |
| P-value for total thickness comparison with week 12 | <0.0001 | 0.0004 | ||
| Switching at week 24 (n=83) | Week 24 (n=83) | Week 28 §£ (n=77) | Year 1 (n=82) | Year 2 (n=81) |
| VA in letters: mean (SD) | 50.0 (17.5) | 51.7 (18.3) | 53.6 (20.8) | 55.0 (20.3) |
| VA change in letters from week 24: mean (SD) | 1.9 (7.8) | 3.3 (13.8) | 4.9 (16.1) | |
| P-value for comparison with week 24 | 0.03 | 0.03 | 0.008 | |
| Fluid at foveal center: n(%) | 83 (100%) | -- | 54 (68.4%) | 38 (48.7%) |
| Total thickness (μ): mean (SD) | 427 (169) | -- | 399 (177) | 384 (188) |
| Change in total thickness (μ) from week 24: mean (SD) | -- | −26 (108) | −36 (137) | |
| P-value for total thickness comparison with week 12 | 0.04 | 0.02 |
Only PRN treated subjects had OCT measurements.
The visual acuity was measured without refraction.
--: Not calculated because OCT images were not evaluated in Reading Center in patients randomized to Monthly treatment.
Among 83 patients who were candidates for “switching” drug at week 24, the mean VA at week 24 was 50 letters (Snellen equivalent 20/100). There was a mean gain of 1.9 letters from week 24 at week 28 (p=0.03), 3.3 letters at Year 1 (p=0.03) and 4.9 letters at Year 2 (p=0.008). The total retinal thickness decreased from week 24 with a mean decrease of 26u at Year 1 (p=0.04) and 36u at Year 2 (p=0.02). After week 24, fluid at fovea center resolved in 32% of eyes at Year 1 and 51% at Year 2 (Table 6, bottom panel).
Among 10 patients who had progressive loss of vision (n=8) or progressive increase of total retinal thickness over the first three visits (n=2), there was a mean of 3.2 letters gain from week 12 at Year 1 (p=0.68) and 7 letters gain at Year 2 (p=0.41). The total retinal thickness decreased from week 12 with a mean decrease of 83u (p=0.03) at Year 1 and 100u decrease at Year 2 (p=0.01).
DISCUSSION
We evaluated the association of baseline predictors and early VA response on Year 1 and Year 2 vision outcomes among CATT patients treated with ranibizumab or bevacizumab on a monthly or PRN basis for neovascular AMD. Baseline predictors for Year 2 vision response were nearly identical to those that we previously reported for Year 1. 8 Age, baseline VA and CNV lesion size remain significant predictors, consistent with Year 1 and also consistent with findings in other treatment trials for neovascular AMD.23 Although a number of these baseline variables were highly significant in their association with Year 1 and Year 2 vision response, these predictors only explain a small portion of the variation in VA response, with R2 values of 0.09 for Year 1 and 0.13 for Year 2, indicating that their actual ability to predict vision outcome was quite modest.
The strongest predictor of vision outcome at Years 1 or 2 in our study was VA response at week 12. Other studies that have evaluated anti-VEGF drugs for neovascular AMD, have also demonstrated a rapid rise in VA in the first 12 weeks followed by a plateau that remains relatively flat throughout the remaining one or two years of study. 1,2,4,7,24,25 With only a small change in VA improvement between 12 weeks and two years, one might expect that the VA response at week 12 would be highly predictive of VA response at Year 1 or at Year 2. Instead, it was surprising to learn that the VA response at 12 weeks only predicted less than 50% of the variation in the VA responses at Years 1 or 2, with R2 values of 0.47 for Year 1 and 0.30 for Year 2 VA outcomes. Combining all baseline predictors with the week 12 VA response only increased the R2 (from 0.47 to 0.49 for Year 1 and 0.30 to 0.35 for Year 2). This fluctuation of VA during the course of anti-VEGF treatment makes it challenging to determine the beneficial effect on VA from switching to another treatment. Eyes that had a VA gain of at least one line at 12 weeks generally had a similar gain at Years 1 and 2. However, some eyes that initially had a loss of ≥1 line at 12 weeks were able to gain ≥1 line at Year 1 (17%) or at Year 2 (27%). This shift from early VA loss to later VA gain contributes to the lower than expected association between early VA response and later VA response at Years 1 or 2. While forecasting treatment response at Years 1 or 2 may not be exact, the response at 12 weeks does provide valuable information on the likely response at Years 1 or 2, as illustrated in Figure 1B and 1D. In addition, the fact that a meaningful percentage of eyes eventually had visual acuity gain despite early loss is encouraging and should prompt ophthalmologists and patients to not give up anti-VEGF treatment even if early VA response is not optimal or at the very least to be careful about the attribution of improvement in VA or retinal thickness after switching treatment.
Although the Week 4 VA response was a better predictor than other baseline variables for predicting Year 1 and 2 VA outcomes, it was considerably worse than the week 12 VA response for predicting the VA response at Year 1 or Year 2. The most likely explanation comes from consideration of the visual acuity response curve where there is continued improvement in many eyes through the first three monthly injections; in other words, some eyes will not have reached their optimal treatment benefit after a single injection and need additional injections to do so. Combining week 4 response with week 12 response did not improve the predictions beyond what was predicted by VA response at week 12, no matter whether baseline predictors were considered or not.
When a patient does not respond to treatment after a few injections, ophthalmologists may consider switching to another anti-VEGF drug. Several uncontrolled studies have investigated the effect of switching from one anti-VEGF drug to another on vision and morphological outcomes. 17–22 Despite the fact that different switching criteria were used among the studies, most found some improvement in morphological outcomes (decrease in retinal thickness, resolution of fluid in the retina) and stabilization or slight improvement in VA after switching.17,18,21,22 In the largest of these studies, Yonekawa et al evaluated 132 eyes that switched from ranibizumab or bevacizumab to aflibercept because of refractory or recurrent neovascular AMD, and found that central retinal thickness decreased by 30u (p<0.0001) and VA improved by approximately 3 letters (p=0.25) after an average of 4 aflibercept injections.17 The primary limitations in all of these studies are the absence of a group of similar patients who were not switched and the implicit assumption that vision and retinal thickness would not change with continued use of the same drug. These studies do not provide convincing evidence that switching from one anti-VEGF drug to another anti-VEGF drug has any long term benefit.
To date, there are no widely accepted prospectively defined criteria for switching anti-VEGF drugs. When we surveyed a variety of past reports of results after switching drugs, it was clear that the decision to switch anti-VEGF treatments was highly subjective, but always involved failure to achieve a desired result for vision or macular morphology. We, therefore, attempted to prospectively define the criteria by which switching would be considered at either 12 weeks or 24 weeks. Variables considered were visual acuity, macular morphology (mostly persistence of fluid on OCT), changes in vision over time, and the number of injections already given. We arrived at the following definition for hypothetical switching-eligible patients in CATT. First, patients had to have a visual acuity of 20/40 or worse. Second, patients had to have gained less than 1 line of vision. Third, patients had to have persistent fluid at the center of the fovea on OCT. Finally, patients had to have received all three initial monthly treatments of ranibizumab or bevacizumab up to the time of hypothetical switching (at baseline, week 4, and week 8) for “switching” at week 12, and had to have received 5 of 6 monthly treatments (baseline, weeks 4, 8, 12, 16, 20) for “switching” at week 24. Because there is no consensus on the number of injections that need to be received before considering switching drug, we considered two possible switching time points, one at week 12, another at week 24 after initiating treatments. Patients who met our hypothetical drug switching criteria at week 12 achieved on average an additional 3 letters VA improvement and 53 micron reduction in retinal thickness at Year 1. There was almost no additional VA gain or reduction in thickening between Year 1 and Year 2. Patients who met our hypothetical drug switching criteria at week 24 achieved on average an additional 3 letters VA improvement at Year 1 and 5 letters gain at Year 2, while the retinal thickness decreased by 26u and 36u at Year 1 and Year 2, respectively. This degree of VA gain and anatomical improvement is strikingly similar to the degree of improvement that has been reported when patients actually did switch drugs, such as the 3-letter and 30-micron improvements reported by Yonekawa et al.17 Caution must be exercised in comparing our cohort who remained on the same drug to patients who actually switched in other studies because of differences in patient populations and the exact criteria for switching. However, the results of “switching” at week 12 or “switching” at week 24 from our study establishes that outcomes can be improved when the same drug is continued and underscores the need for a control group when interpreting the changes observed after switching drugs in other studies.
In conclusion, we found that baseline predictors are similar for VA response at Year 1 and at Year 2. The more powerful predictor of visual acuity outcomes was the VA response at week 12; the majority of eyes with early VA gain had a similar VA gain at Year 1 or at Year 2. However, some eyes with an initial decline in VA had VA gains late even without switching to another drug, supporting the continuation of anti-VEGF therapy.
Supplementary Material
Acknowledgments
Supported by cooperative agreements U10 EY017823, U10 EY017825, U10 EY017826, U10 EY017828 and R21EY023689 from the National Eye Institute, National Institutes of Health, Department of Health and Human Services.
Footnotes
This article contains online-only material. The following should appear online-only: Table 1.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenfeld PJ, Brown DM, Heier JS, et al. for the MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 2.Brown DM, Kaiser PK, Michels M, et al. for the ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–5. [PubMed] [Google Scholar]
- 4.Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–72. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 5.CATT Research Group. Ranibizumab and bevacizumab for neovascular agerelated macular degeneration. N Engl J Med. 2011;364:1897–908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The CATT Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: 2-year results. Ophthalmology. 2012;119:1388–98. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heir JS, Brown DM, Chong V, et al. for theVIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Ying GS, Huang J, Maguire MG, et al. for the CATT Research Group. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120:122–9. doi: 10.1016/j.ophtha.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ying GS, Kim BJ, Maguire MG, et al. for the CATT Research Group. Sustained visual acuity loss in the in the Comparison of AMD Treatments Trials (CATT) JAMA Ophthalmology. 2014;132:915–21. doi: 10.1001/jamaophthalmol.2014.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim BJ, Ying GS, Huang J, et al. for the CATT Research Group. Sporadic visual acuity loss in the comparison of age-related macular degeneration treatments trials (CATT) Am J Ophthalmol. 2014;158:128–35. doi: 10.1016/j.ajo.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagstrom S, Ying GS, Pauer GJ, et al. for the CATT Research Group. Pharmacogentics of anti-VEGF therapy in the Comparison of AMD Treatments Trials (CATT) Ophthalmology. 2013;120:593–9. doi: 10.1016/j.ophtha.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagstrom S, Ying GS, Pauer GJ, et al. for the CATT Research Group. VEGF-A and VEGFR-2 gene polymorphisms and response to anti-VEGF therapy in the Comparison of AMD Treatments Trials (CATT) JAMA Ophthalmology. 2014;132:521–7. doi: 10.1016/j.ophtha.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grunwald JE, Daniel E, Ying GS, et al. for the CATT Research Group. Photographic assessment of baseline fundus morphologic features in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2012;119:1634–41. doi: 10.1016/j.ophtha.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeCroos FC, Toth CA, Stinnett SS, et al. for the CATT Research Group. Optical coherence tomography grading reproducibility during the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2012;119:2549–57. doi: 10.1016/j.ophtha.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the Early Treatment of Diabetic Retinopathy Study testing protocol. Am J Ophthalmol. 2003;135:194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 16.Meng XL, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Psychological Bulletin. 1992;111:172–5. [Google Scholar]
- 17.Yonekawa Y, Andreoli C, Miller JA, et al. Conversion to Aflibercept for chronic refractory or recurrent neovascular Age-related Macular Degeneration. Am J Ophthalmol. 2013;156:29–35. doi: 10.1016/j.ajo.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 18.Eadie JA, Gottlieb JL, Ip MS, et al. Response to Aflibercept in patients with persistent exudation despite prior treatment with bevacizumab or ranibizumab for age-related macular degeneration. Ophthalmic Surg Lasers Imaging Retina. 2014;9:1–4. doi: 10.3928/23258160-20140909-03. [DOI] [PubMed] [Google Scholar]
- 19.Ehlken C, Jungmann S, Bohringer D, et al. Switch of anti-VEGF agents is an option for nonresponders in the treatment of AMD. Eye. 2014;28:538–45. doi: 10.1038/eye.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aslankurt M, Aslan L, Aksoy A, et al. The results of switching between 2 anti-VEGF drugs bevacizumab and ranibizumab in the treatment of neovascular age-related macular degeneration. Eur J Ophthalmol. 2013;23:553–7. doi: 10.5301/ejo.5000268. [DOI] [PubMed] [Google Scholar]
- 21.Cho H, Shah CP, Weber M, Heier JS. Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol. 2013;97:1032–5. doi: 10.1136/bjophthalmol-2013-303344. [DOI] [PubMed] [Google Scholar]
- 22.Bakall B, Folk JC, Boldt HC, et al. Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol. 2013;156:15.e1–22.e1. doi: 10.1016/j.ajo.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Finger RP, Wickremasinghe SS, Baird PN, Guymer RH. Predictors of anti-VEGF treatment response in neovascular age-related macular degeneration. Survey of Ophthalmology. 2014;59:1–18. doi: 10.1016/j.survophthal.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 24.The IVAN Study Investigators. Ranibizumab versus Bevacizumab to treat neovascular age-related macular degeneration: one year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Busbee BG, Ho AC, Brown DM, et al. for the HARBOR Study Group. Twelve-month efficacy and safety of 0.5 mg or 2. 0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120:1046–56. doi: 10.1016/j.ophtha.2012.10.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.