Abstract
Previous studies in our laboratory have shown that the neuron-specific specificity protein 4 (Sp4) transcriptionally regulates many excitatory neurotransmitter receptor subunit genes, such as those for GluN1, GluN2A, and GluN2B of N-methyl-D-aspartate (NMDA) receptors and Gria2 of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. It also regulates Atp1a1 and Atp1b1 subunit genes of Na+/K+-ATPase, a major energy-consuming enzyme, as well as all 13 subunits of cytochrome c oxidase (COX), an important energy-generating enzyme. Thus, there is a tight coupling between energy consumption, energy production, and excitatory neuronal activity at the transcriptional level in neurons. The question is whether inhibitory neurotransmitter receptors are also regulated by Sp4. In the present study, we tested our hypothesis that Sp4 regulates receptor subunit genes of a major inhibitory neurotransmitter, GABA, specifically GABAA receptors. By means of multiple approaches, including in silico analysis, electrophoretic mobility shift and supershift assays, real-time quantitative PCR, chromatin immunoprecipitation, promoter mutational analysis, over-expression and shRNA of Sp4, functional assays, and western blots, we found that Sp4 functionally regulates the transcription of Gabra1 (GABAA-α1) and Gabra2 (GABAA-α2), but not Gabra3 (GABAA-α3) subunit genes. The binding sites of Sp4 are conserved among rats, humans, and mice. Thus, our results substantiate our hypothesis that Sp4 plays a key role in regulating the transcription of GABAA receptor subunit genes. They also indicate that Sp4 is in a position to transcriptionally regulate the balance between excitatory and inhibitory neurochemical expressions in neurons.
Keywords: Gene regulation, Sp4, GABAA receptors, Gabra1, Gabra2, Gabra3
1. Introduction
Neuronal activity and energy metabolism are tightly coupled processes [1]. Previously, we have shown that the neuron-specific specificity protein 4 (Sp4) transcriptionally regulates many excitatory neurotransmitter receptor subunit genes, such as Grin1 (GluN1), Grin2a (GluN2A), and Grin2b (GluN2B) of N-methyl-D-aspartate (NMDA) receptors [2] and Gria2 of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors [3]. It also regulates Atp1a1 and Atp1b1 subunit genes of Na+/K+-ATPase, a major energy-consuming enzyme [4], as well as all 13 subunits of cytochrome c oxidase (COX), an important energy-generating enzyme [5] in neurons. As neuronal activity involves both excitation and inhibition, the question naturally arises as to how the inhibitory neurotransmitter receptors are transcriptionally regulated, and if Sp4 plays a role in this regulation.
Gamma-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the central nervous system [6,7] and the fast-acting ionotropic type A receptors (GABAAR) are prevalent among neurons [8,9]. Functional alterations of GABAA receptors are often associated with a variety of disorders, such as epilepsy, anxiety, insomnia, and schizophrenia [10], linking frequently to an excitation/inhibition imbalance in specific populations of neurons [11–13]. Understanding the genetic mechanism underlying the synaptic balance at the cellular and molecular levels will lead to a better insight into normal and abnormal functioning of neurons, and will lay a foundation for new therapeutic tools for the prevention of a variety of neurological disorders. We have uncovered the transcriptional regulation of a number of excitatory neurotransmitter receptor genes [2,3,14–16]. Deciphering the transcriptional regulation of different inhibitory GABAA receptor subunit genes will be the next step towards such an understanding. The goal of the present study was to determine if the three major GABAA receptor subunit genes, Gabra1 (GABAA α1), Gabra2 (GABAA α2), and Gabra3 (GABAA α3) are transcriptionally regulated by the same transcription factor, Sp4, as some of the key excitatory neurotransmitter receptor subunit genes. Our hypothesis is that they are.
By means of multiple approaches, including in silico analysis, electrophoretic mobility shift and supershift assays, real-time quantitative PCR, chromatin immunoprecipitation, promoter mutational analysis, over-expression, shRNA, functional assays, and western blots, we found that Sp4 functionally regulates the transcription of Gabra1 and Gabra2, but not Gabra3 subunit genes in neurons.
2. Materials and Methods
All experiments involving rats were approved by and conducted in accordance with the Institutional Animal Care and Use Committee (IACUC) of the Medical College of Wisconsin (Milwaukee, WI). All efforts were made to minimize the number of animals used and their suffering.
2.1 Primary neuronal cultures
Rat or mouse primary visual cortical neurons were cultured as described previously [17]. In brief, neonatal one-to-two day old pups were euthanized by decapitation. The brains were detached from the skull, meninges were removed, and the visual cortex was dissected. Pieces of the visual cortex were treated with trypsin and suspended by pipetting. Neurons were then dissociated by trituration, and cells were seeded in a six-well plate (35 mm; pre-coated with poly-L-lysine) at a density of 1×106 cells/well. Cells were allowed to grow in Neurobasal-A media containing L-glutamine and B27 supplement (Life Technologies, Carlsbad, CA, USA) and maintained in a humidified incubator with 5% CO2 at 37°C. Cytosine arabinoside (Ara-C) (Sigma, St Louis, MO, USA) was added to the culture media to suppress the division of glial/non-neuronal cells.
2.2 In silico analysis of GABAA receptor subunit promoters
Using DNAStar Lasergene 8 Suite – Sequence Builder and Genequest software, sequences surrounding I kb upstream and 1 kb downstream of the transcription start points (TSPs) of mouse, rat, and human GABAA receptor α1, α2, and α3 subunit genes (Gabra1, Gabra2, and Gabra3) were derived from the genome database in GenBank and were aligned using Megalign. Computer-assisted search for the typical Sp binding motif (‘GGGCGG’), the atypical Sp binding motifs (‘GGGTGG’ and ‘CCCTCC), or their complements, was conducted to uncover the putative Sp binding sites.
2.3. Preparation of nuclear extracts and electrophoretic mobility shift and supershift assays
Nuclear extraction protocol was as reported previously [18]. In brief, the frozen brain tissue was rinsed in the PBS buffer, spun and transferred to a Dounce tissue homogenizer. Buffer A was added at 2.5 times per g of the tissue weight with a minimum volume of 2 ml. Five strokes of pestle were used to homogenize the tissue to a liquid mass. After induction with NP-40 (0.5%), five additional strokes were applied and the tissue was incubated for 10 min to lyse the cells with the detergent. The homogenate was transferred equally into fresh tubes and centrifuged at 13,000 rpm for 30 sec. The supernatant containing the cytoplasmic constituents was removed, and buffer C was added to the nuclear pellet. Tubes were mixed thoroughly, placed on a rotary shaker for 15 min, and centrifuged. The supernatant containing the protein from the nuclear extract was removed carefully and transferred to a fresh tube. The protein was measured, aliquotted, and stored at −80°C until use.
Electrophoretic mobility shift (EMSA) and supershift assays’ protocols were as described previously [3]. Based on the in silico analysis, oligonucleotide probes with putative Sp4 binding site on each promoter were synthesized (Table 1), annealed, and labeled by a fill-in reaction with Klenow fragment and [32P]dATP (50 μCi/200 ng; Perkin-Elmer, Shelton, CT, USA). Each labeled probe was incubated with 2 g of calf thymus DNA and 5 g of brain nuclear extract. For supershift experiments, the reactions were incubated with 2 μg of Sp4 polyclonal antibodies (Sp4, V-20, SC-645, Santa Cruz Biotechnology [SCBT], Santa Cruz, CA, USA) for 20 min at room temperature (RT). For competition, 100-fold excess of unlabeled oligonucleotides were incubated with brain nuclear extract before adding labeled or nonspecific oligonucleotides. Shift reactions were loaded onto 4% polyacrylamide gel and run at 200 V for 3 h in 0.25X TBE buffer. Gels were dried and exposed for 4–24 h at −80°C. Mouse GM3 synthase with a known Sp4 binding site was designed as previously described [5] and was used as a positive control. Sp4 mutants with mutated sequences were used as negative controls.
Table 1.
EMSA probes with putative Sp4 binding sites underlined.
| Gene Promoter | EMSA Sequence |
|---|---|
| Gabra1 | F: 5’ TTTTGCATATAAAAAATGGGCGGATTGGTG 3’ |
| R: 5’ TTTTCACCAATCCGCCCATTTTTTATATGC 3’ | |
| Gabra2 | F: 5’ TTTTGGCCAGGATTGGGGTGGGGGATAAGGGG 3’ |
| R: 5’ TTTTCCCCTTATCCCCCACCCCAATCCTGGCC 3’ | |
| Gabra3 | F: 5’ TTTTCTACTTCATTGGGGTGGGGATCAG 3’ |
| R: 5’ TTTTCTGATCCCCACCCCAATGAAGTAG 3’ | |
| GM3 Synthase | F: 5’ TTTTGCGCGACCCCGCCCCCGCCTA 3’ |
| R: 5’ TTTTTAGGCGGGGGCGGGGTCGCGC 3’ | |
| Mutant Sp Gabra1 | F: 5’ TTTTGCATATAAAAAATGGAAGGATTGGTG 3’ |
| R: 5’ TTTTCACCAATCCTTCCATTTTTTATATGC 3’ | |
| Mutant Sp Gabra2 | F: 5’ TTTTGGCCAGGATTGGGAAGGGGGATAAGGGG 3’ |
| R: 5’ TTTTCCCCTTATCCCCCTTCCCAATCCTGGCC 3’ |
2.4. Chromatin immunoprecipitation assays
The protocol for in vivo chromatin immunoprecipitation (ChIP) was as described previously [2] with a few modifications. Murine visual cortical tissue was kept on ice at all times to avoid protein degradation. Tissue was cut in a petri dish resting on a block of dry ice and then chopped into tiny pieces, fixed in 2% formaldehyde, and resuspended in the swelling buffer (85 mM KCl, 5 mM PIPES, pH 8.0, 1% Nonidet P-40, and protease inhibitors). The tissue was transferred to a Dounce homogenizer, homogenized and centrifuged to isolate the nuclei. The nuclei were resuspended and sonicated in SDS lysis buffer (1% SDS, 50 mM Tris–HCl, pH 8.1, 10 mM EDTA). Immunoprecipitation was performed with anti-Sp4 polyclonal antibodies (2 μg). Two μg of anti-nerve growth factor receptor (NGFR) antibodies (sc-6188, SCBT) or ‘no antibody’ blanks were used as negative controls. Based on our in silico analysis, putative Sp binding sites were identified and semi-quantitative PCR was performed by utilizing primers surrounding the Sp binding sites (Table 2). GM3 synthase was the positive control. Neurotrophin 3 [19] was also used (data not shown), and β-actin promoter (Actb) was the negative control. PCR additives and cycling parameters were used to improve the reproducibility and quality of ChIP. PCR products were visualized in 2% agarose gels.
Table 2.
Primers for ChIP analysis.
| Gene Promoter | Sequence | Amplicon Length |
|---|---|---|
| Gabra1 | F: 5’ GGAATGCTTGCAGCAGATTG 3’ | 216 bp |
| R: 5’ GTAGTAATACGTCCCAGCGC 3’ | ||
| Gabra2 | F: 5’ GCAGGGTGTGGGCTCCACTC 3’ | 290 bp |
| R: 5’ TAGCGATCGCAGGAGCCGG 3’ | ||
| Gabra3 | F: 5’ GACTGTTTTGCCTCCTTTGC 3’ | 336 bp |
| R: 5’ CCTTCCATCTCTATGGGTGTGC 3’ | ||
| GM3 Synthase | F: 5’ CACCTACTTCTCGGCTGGAG 3’ | 198 bp |
| R: 5’ AATTCAGCCCCGGACAGT 3’ | ||
| β-Actin | F: 5’ GCTCTTTTCCAGCCTTCCTT 3’ | 187 bp |
| R: 5’ CGGATGTCAACGTCACACTT 3’ |
2.5. Luciferase reporter assay
Luciferase reporter constructs of the three GABAA receptor subunit promoters (Gabra1, Gabra2, and Gabra3) were made by PCR cloning the proximal promoter sequences, using genomic DNA prepared from mouse N2a cells [15] as template. Digestion with restriction enzymes was performed, followed by ligation of the product directionally into pGL3 basic vector (Promega, Madison, WI). Sequences of primers used for PCR cloning are provided in Table 3. Site-directed mutations of putative Sp factor binding sites in each promoter were generated using QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). Primers used for mutagenesis are also listed in Table 3. All constructs were verified by sequencing.
Table 3.
Primers used for promoter cloning and mutagenesis analysis. Mutated Sp4 binding sites are underlined with mutated nucleotides in bold.
| Gene Promoter | Primer |
|---|---|
| Gabra1 | F: 5’ CAGACGCGTTGCTTCCTAGCTTGCGTTCA 3’ |
| R: 5’ CAGCTCGAGGTGCTCCTGCACTGGAGATT 3’ | |
| Mutant Sp Gabra1 | F: 5’ GCGCTGCATATAAAAAATGTGATGATTGGTGTCCAGATCCAGTGGCTG 3’ |
| R: 5’ CAGCCACTGGATCTGGACACCAATCATCACATTTTTTATATGCAGCGC 3’ | |
| Gabra2 | F: 5’ CAGGGTACCAAGGCGTGATTAGCCCCTTC 3’ |
| R: 5’ CAGAAGCTTAAGACCAGGCAGCAAACGAA 3’ | |
| Mutant of Gabra2 | F: 5’ GGCTTGGCCAGGATTGGGTTTTGGGATAAGGGGTCCCC 3’ |
| R: 5’ GGGGACCCCTTATCCCAAAACCCAATCCTGGCCAAGCC 3’ |
Primary neurons were cultured and plated into 24-well plates before transfection. Each promoter construct was transfected into the primary neurons using Neurofect (Genlantis, San Diego, CA). Each well received 0.6 μg of reporter construct and 0.06 μg of pRL-TK Renilla luciferase vector [20]. Firefly luciferase and Renilla luciferase activities were measured sequentially using the Dual Luciferase Reporter Assay System (Promega). The firefly luciferase activity was normalized according to Renilla and expressed as relative luciferase units to reflect the promoter activity.
2.6. Sp4 knockdown and KCl treatment
To knockdown Sp4 expression, a vector-based shRNA approach was used, and two target sequences were chosen from the RNAi Consortium’s Public TRC Cloning Database at the Broad Institute (Table 4) and each cloned into the pLKO.1 TRC cloning vector (Plasmid 10878; Addgene, Cambridge, MA, USA) as reported previously [5]. To rule out the non-specific consequences of shRNA, the pLKO.1 non-mammalian shRNA control vector SHC002 (Sigma), a scrambled negative control, was also used.
Table 4.
Sp4 shRNA sequences cloned into the pLKO.1 vector for silencing Sp4
| Gene | Hairpin Sequence | |
|---|---|---|
| Sp4 | 1 | 5’ CCGG—CCAGTAACAATCACTAGTGTT—CTCGAG—AACACTAGTGATTGTTACTGG—TTTTTG 3’ |
| 5’ AATTCAAAAA—CCAGTAACAATCACTAGTGTT—CTCGAG—AACACTAGTGATTGTTACTGG 3’ | ||
| 2 | 5’ CCGG—CTGGACAACAGCAGATTATTA—CTCGAG—TAATAATCTGCTGTTGTCCAG—TTTTTG 3’ | |
| 5’ AATTCAAAAA—CTGGACAACAGCAGATTATTA—CTCGAG—TAATAATCTGCTGTTGTCCAG 3’ |
Transfection of primary neurons was carried out 4 days post-plating with both Sp4 shRNA constructs (2 μg of each construct) or the pLKO.1 non-mammalian control (2 μg), using Neurofect transfection reagent per 6-well plate according to the manufacturer's instructions (Genlantis). TurboGFP (0.5 μg) vector was added to each well for transfection visualization and selection efficiency. Transfection efficiency was around 50–60% before selection. Puromycin selection, however, effectively yielded 100% transfected cells. Transfection efficiency was observed using green fluorescence. Primary neurons transfected with shRNA against Sp4 were further stimulated with KCl. A final concentration of 20 mM KCl was added to the culture medium for 5 h according to our published method [2]. After this period, cells were harvested for RNA and protein isolation.
2.7. Sp4 over-expression and TTX treatment
Using Gateway Multisite Cloning kit (Invitrogen), human Sp4 clones of cDNA (Open Biosystems Lafayette, CO, USA) were cloned into pcDNA Dest40 vectors according to the manufacturer's instructions and as described previously [21].
As mentioned above, the transfection protocol for over-expressing Sp4 in primary cultures was similar to that described for shRNA. Either 2 μg of Sp4 over-expression vector, or 1.5 μg of the pcDNA3.1 empty vector and 0.5 μg of turboGFP vector for green fluorescence were used for primary neuronal cultures. Transfected primary neurons were blocked for 3 days with tetrodotoxin (TTX) at a final concentration of 0.4 μM and starting on the day after transfection as previously described [20]. Two to four days after transfection, neurons were harvested for RNA and protein isolation.
2.8. Knockdown of Sp4 followed by over-expression of Sp4 with or without KCl treatment
Two sets of experiments were performed. In the first set, primary neurons in culture were transfected with Sp4 shRNA as described in section 2.6 above for one day, then they were transfected with Sp4 over-expression vectors as described in 2.7 above for another day. In the second set of experiments, primary neurons were also transfected with Sp4 shRNA followed by transfection with Sp4 over-expression vectors; however, in between the two procedures, they were treated with KCl for 5 h as described in section 2.6 above.
2.9. RNA isolation and cDNA synthesis
Cultured and transfected neurons were washed with PBS, followed by the extraction of total RNA by the TRIzol (Life Technologies, Carlsbad, CA, USA) method according to the manufacturer's instructions. Any residual genomic DNA was digested with DNase I on total RNA, and cDNA synthesis was carried out using iScript cDNA synthesis kit (170-8891, BioRad, Hercules, CA, USA).
2.10. Quantification of gene expression by real-time PCR analysis
mRNA levels of various genes were determined in a BioRad iCycler using IQ SYBR Green Supermix and/or Cepheid Smart Cycler Detection system (Cepheid, Sunnyvale, CA, USA) following manufacturer’s protocols. Primers for real-time are shown in Table 5. PCR runs: hot start 2 min at 95 C, denaturation 10 s at 95 C, annealing 15 s according to the Tm of each primer, and extension 10 s at 72 C for 15–30 cycles. Melt curve analyses verify the formation of single desired PCR product. Mouse/rat compatible Actb (β-actin) and Gapdh were used as internal controls, and the 2−Δ ΔCT method [22] was performed for the relative amount of transcripts.
Table 5.
Real-time qPCR primers
| Gene | Primer |
|---|---|
| Gabra1 | F: 5’ ACTGTCTTTGGAGTGACGACCGTT 3’ |
| R: 5’ AATAAACCAGTCCATGGCCGTTGC 3’ | |
| Gabra2 | F: 5’ TCAGTGCTCGAAATTCCCTTCCCA 3’ |
| R: 5’ TACACTCTTTCCATCCCAAGCCCA 3’ | |
| Gabra3 | F: 5’ AGGAAGCTCACACTTTGGTCCTGA 3’ |
| R: 5’ TTGGCCAGTGGTTCCAGGTAGAAT 3’ | |
| Sp4 | F: 5’ TTGCAGCAAGGCCAGCAGACC 3’ |
| R: 5’ GCTTCTTCTTTCCTGGTTCACTGCT 3’ | |
| Actb | F: 5’ GTGACGTTGACATCCGTAAAGA 3’ |
| R: 5’ GCCGGACTCATCGTACTCC 3’ | |
| Gapdh | F: 5’ AGGTCGGTGTGAACGGATTTG 3’ |
| R: 5’ GGGGTCGTTGATGGCAACA 3’ |
2.11. Western blots
Control, Sp4 shRNA, and Sp4 over-expression protein samples were harvested using the sample buffer (12.5 mM EDTA, 50 mM Tris–HCl pH 6.8, 1% SDS, 10% glycerol). Equal protein amounts of each sample were electrophoresed in SDS gels and transferred onto polyvinylidene difluoride membranes. Membranes were blocked with 5% non-fat dry milk in TBS-T (150 mM NaCl, 50 mM Tris–HCl, pH 8.0, and 0.05% Tween 20) at RT for 1h, then incubated with primary antibodies against Sp4 (1:1000; SCBT), GABAA α1, GABAA α2 , or GABAA α3 (all at 1:1000; all from Phosphosolutions, Aurora, CO, USA). The loading control was β-actin (1:5000; Sigma) followed by an incubation with horseradish peroxidase-conjugated secondary antibodies from Vector Laboratories (Burlingame, CA, USA). ECL reagent was used to visualize protein position and intensity on blots, which were exposed to autoradiographic films (RPI, Mount Prospect, IL, USA). Gel Doc system (BioRad) was used to perform quantitative analyses of relative changes.
2.12. Statistical Analysis
Data were analyzed using ANOVA. Significance between two groups was analyzed by student’s t test. P values of 0.05 or less were considered significant.
3. Results
3.1. In silico promoter analysis of GABAA receptor subunit genes
Proximal promoters of mouse and rat GABAA subunit genes Gabra1, Gabra2, and Gabra3 with DNA sequence 1 kb 5’ upstream and 1 kb 3’ beyond TSP were analyzed using in silico analysis. The α1-subunit gene is strongly expressed in all brain regions [23], and we found a GC-rich promoter with many typical Sp binding motifs, the GC-box (GGGCGG), as well as a TATA box upstream of the TSP. The Gabra2 promoter also contained more than one typical binding motif, ‘CCCGCC’ as well as atypical binding sites, whereas the Gabra3 promoter showed atypical Sp binding sites, such as ‘GGGTGG’ or ‘CCCTCC’ and were conserved only between mouse and rat, but not in humans.
3.2. In vitro Sp4 bound to GC-box region of GABAA subunits α1 and α2
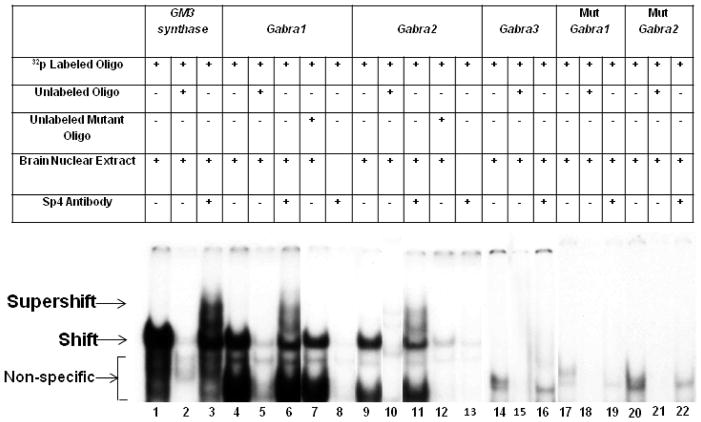
We performed EMSA to determine if Sp4 would bind to the GC boxes of Gabra 1, 2, and 3 subunit promoters. Based on our previous finding that GM3 synthase formed specific DNA/Sp4 shift and supershift complexes with mouse visual cortical nuclear extract but almost fade or absent with HeLa cell nuclear extract [3], we used GM3 synthase with murine visual cortical nuclear extract as our positive control. Indeed, when incubated with mouse visual cortical tissue nuclear extract, GM3 synthase formed specific DNA/ Sp4 shift and supershift complexes (Fig. 1, lanes 1 and 3 for Sp4 shift and supershift, respectively).
Fig. 1.
In vitro binding of Sp4 transcription factor to putative binding sites on GABAA receptor subunit promoters using EMSA and supershift assays. All lanes contain 32P-labeled oligonucleotides and are indicated by a “+” or a “−” sign depending on whether they also contain mouse brain nuclear extract, excess unlabeled oligos, unlabeled mutant oligos, or Sp4 antibody. Sp4 shift, supershift, and non-specific complexes are indicated by arrows. The positive control for Sp4 factor binding was GM3 Synthase. Specific Sp4 shift bands were revealed upon incubation with cortical nuclear extract (lane 1). Excess unlabeled competitor competed out the shift band (lane 2). The addition of Sp4 antibody yielded a strong supershift band (lane 3) corresponding to the presence of tandem Sp binding. Incubation of cortical nuclear extract with the Gabra1 and Gabra2 probes yielded specific Sp4 shift bands (lane 4 and 9, respectively) that were competed out with the addition of excess cold probes (lanes 5 and 10, respectively). The addition of Sp4 antibody yielded fainter but specific supershift bands (lanes 6 and 11, respectively). The addition of excess unlabeled mutant Gabra1 and Gabra2 probes did not compete out the shift reaction (lanes 7 and 12, respectively). Labeled Gabra1 and Gabra2 with Sp4 antibody without any nuclear extract was used as a positive control, to check for any antibody-oligo interaction, and they did not show any shift or supershift bands (lanes 8 and 13). Labeled Gabra3 probes did not yield specific Sp4 shift (lane 14) or supershift bands (lane 16), and cold competitor was also blank (lane 15). Labeled Gabra1 and Gabra2 probes with mutant Sp sites did not yield specific Sp4 shift or supershift bands (lanes 17–19 and 20–22, respectively).
When murine visual cortical nuclear extract was incubated with GABAA receptor subunit probes containing putative Sp4 sites, Gabra 1 and Gabra2 gave positive shift bands (Fig.1, lanes 4 and 9, respectively) that were competed out with the addition of unlabeled competitors (Fig. 1, lanes 5 and 10, respectively). A supershift band against Sp4 was present when Sp4 antibody was added to either Gabra1 or Gabra2 (Fig.1, lanes 6 and 11). The addition of unlabeled probes with mutated Sp4 binding sites did not compete out the Sp4 shift bands (Fig. 1, lanes 7 and 12). Labeled Gabra1 and Gabra2 with Sp4 antibody without any nuclear extract was also run to rule out antibody-oligo interaction, and they did not show any shift or supershift bands (Fig. 1, lanes 8 and 13). Gabra3 did not give a positive shift (Fig. 1, lane 14) or a Sp4 supershift band (Fig.1, lane 16). Shift, competitor, and supershift reactions with mutated Gabra1 and Gabra2 did not show any binding to the Sp4 sites (Fig. 1, lanes 17–22).
3.3. In vivo interaction verification through ChIP assay
To provide further corroboration of the physical association of Sp4 with its binding sites on the GABAA subunit promoters, we performed a ChIP assay using mouse visual cortical tissue (Fig. 2). A 0.5% dilution of input chromatin was used as a standard to indicate the efficiency of the PCR. As Sp factors are known to regulate GM3 Synthase [24], the promoter of these genes were used as positive controls, whereas β-actin exon 5 (Actb) served as a negative control.
Fig. 2.
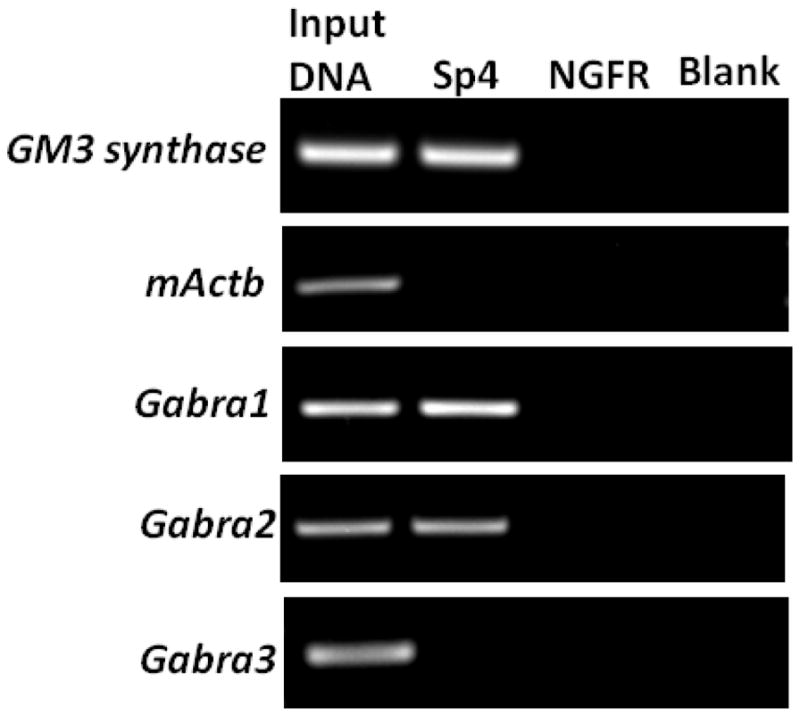
In vivo chromatin immunoprecipitation showing Sp4’s interactions with GABAA receptor subunit promoters in mouse visual cortical nuclear extract. Immunoprecipitation was carried out with anti-Sp4 antibodies (Sp4 lane), anti-nerve growth factor receptor p75 antibody (negative control, NGFR lane), or no antibody (negative control, blank lane). 0.5% of input chromatin (Input DNA lane) served as the control for PCR reaction. The positive control for Sp4 binding was GM3 synthase, whereas β-Actin was the negative control. Sp4 interacted with Gabra1 and Gabra2, but not with Gabra3.
When the sonicated nuclear lysates were immunoprecipitated with Sp4 polyclonal antibodies and the resultant DNA was subjected to PCR analysis, the anti-Sp4 antibody specifically co-precipitated the Gabra1 and Gabra2 promoter fragments, but failed to do so with the Gabra3 promoter. The appearance of a distinct band with Sp4 antibody verified the in vivo interaction of GABAA receptor subunits Gabra1 and Gabra2 with Sp4. Nerve growth factor receptor (NGFR) antibody was used as a control for the immunoprecipitation reaction and it did not yield any PCR product. To rule out the possibility of a bead-DNA interaction, an additional “no antibody” or blank control was used, which did not yield any PCR product.
3.4. Effect of mutated Sp4 binding sites on the Gabra1 and Gabra2 promoters
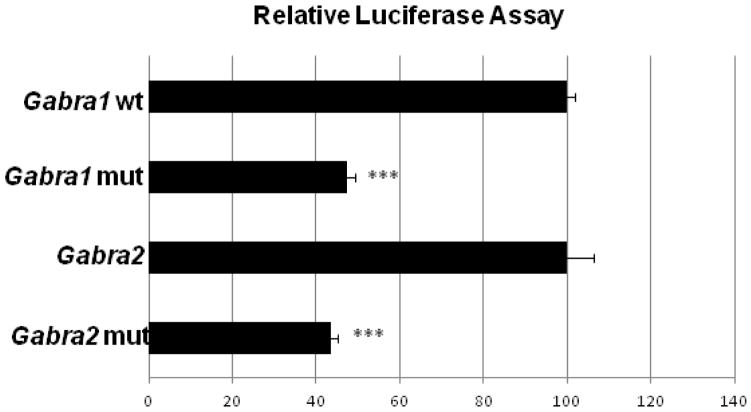
Gabra1 and Gabra2 promoters that bound to Sp4 in vitro and in vivo were each cloned into pGL3 basic luciferase vectors. Site-directed mutations of the putative Sp4 binding sites were constructed. Transfection of control or mutated Sp promoter regions into cultured primary neurons resulted in a significant fall of 53% and 61% in the activity of Gabra1 and Gabra2 promoters, respectively, containing the mutated Sp4 motif (P < 0.001 for all, Fig. 3).
Fig. 3.
Relative luciferase activity of the wild type Gabra1 and Gabra2 promoters (wt) and the Gabra1 and Gabra2 promoters with mutated Sp binding site (mut). Mutating the Sp binding site on Gabra1 and Gabra2 resulted in a significant decrease in luciferase activity as compared to the wild type controls. N = 3 for each construct. ***= P < 0.001 as compared to the wild type.
3.5. Knock down of Sp4 decreased mRNA levels of GABAA receptors and the effect of KCl depolarization in primary neurons
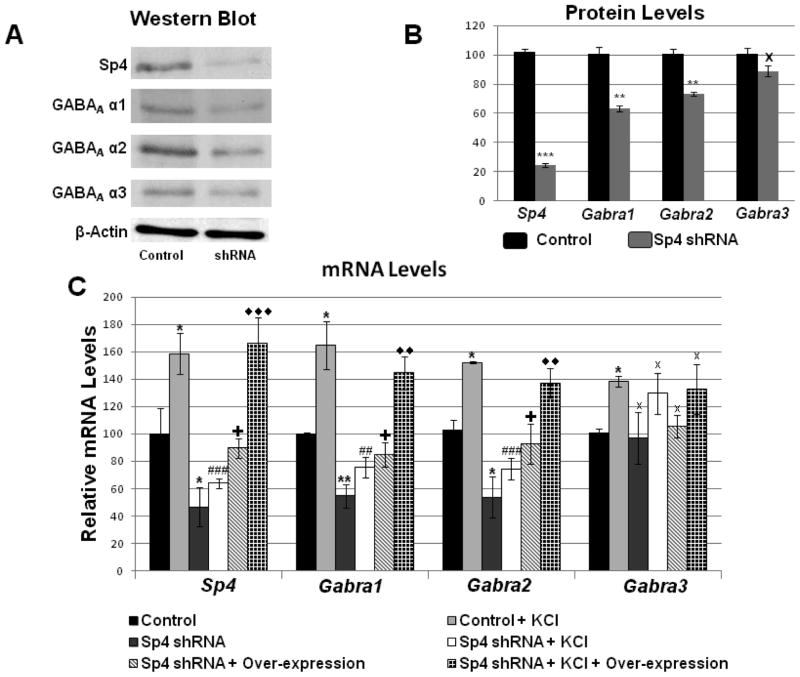
To provide further confirmation to our findings, we employed shRNA constructs targeted to Sp4 mRNA in cultured primary neurons. β-Actin was used as an internal control. Sp4 protein showed a significant decline of 76% (P < 0.001). GABAA α1 and GABAA α2 were decreased by 36.77% and 26.65%, respectively (P < 0.01, Fig. 4, A–B), whereas GABAA α3 did not show any remarkable change (Fig. 4, A–B). With silencing, mRNA levels were also quantified, and β-Actin and Gapdh were used as internal PCR controls. For transfection controls, neurons received scrambled shRNA, which failed to bind to any known mRNA. Silencing of Sp4 resulted in a significant decrease of 44% in Sp4 mRNA (P < 0.001), and significant decreases of 56% and 54% in Gabra1 and Gabra2 mRNA levels, respectively (P < 0.01 for both, Fig. 4C). On the other hand, mRNA levels of GABAA α3 did not show any significant change with silencing (Fig. 4C).
Fig. 4.
Effect of silencing Sp4, depolarizing stimulation, and Sp4 overexpressing. (A– B) Western blots revealed a down-regulation of Sp4, GABAA α1, and GABAA α2, but not GABAA α3 protein levels in Sp4 shRNA-transfected primary neurons. β-actin served as a loading control (N = 3). Using scrambled vector transfection as a control, primary neurons exposed to KCl up-regulated their mRNA levels for Sp4, Gabra1, Gabra2, and Gabra3 (* P < 0.05 when compared with controls; N = 3). When transfected with Sp4 shRNA, transcript levels of Sp4, Gabra1, and Gabra2 were significantly down-regulated, but not that of Gabra3 (* P < 0.05; ** P < 0.01; X = non-significant when compared to controls; N = 3). In the presence of Sp4 shRNA, KCl was no longer able to up-regulate mRNA levels of Sp4, Gabra1, and Gabra2 to that of control + KCl, though it was still able to do so for Gaba3 (## P < 0.01; ### P < 0.001; X = non-significant when compared to KCl alone). Transfection of neurons with Sp4 shRNA followed by Sp4 over-expression rescued their Sp4, Gabra1, and Gabra2 transcripts from being down-regulated by Sp4 shRNA, but this treatment had no effect on Gaba3 (+ P < 0.05; X = non-significant when compared to shRNA alone; N = 3). Neurons transfected with Sp4 shRNA followed by Sp4 over-expression as well as KCl treatment showed a level of Sp4, Gabra1, and Gabra2 transcripts that was significantly higher than that of shRNA + KCl, although it had no effect on that of Gabra3 (◇◇ = P < 0.01; ◇◇◇ = P < 0.001; X = non-significant when compared to shRNA + KCl; N = 3). All four transcript levels were not significantly different from those of control + KCl.
Primary cultured neurons (control and Sp4 transfected) were then subjected to 20 mM KCl for 5 h. As shown in Figure 4C, KCl depolarization significantly increased Sp4 itself, Gabra1, and Gabra2 transcript levels to 158%, 164%, and 152%, respectively (P < 0.05 for all). In the presence of Sp4 shRNA, KCl could no longer increase transcripts of Sp4, Gabra1 and Gabra2 to levels of control-plus-KCl (P < 0.001, P < 0.01, and P < 0.001, respectively, when compared to control-plus-KCl). The values of shRNA-plus-KCl were also not significantly different from those of shRNA alone (P = 0.218, P = 0.120, and P = 0.202, respectively, when compared to shRNA alone). The transcript level of Gabra3 was also significantly increased with KCl treatment (P < 0.05), but was not changed in the presence of Sp4 shRNA.
3.6. Sp4 silencing followed by over-expression rescued the effects of the knockdown
Cultured primary neurons were transfected first with Sp4 shRNA for one day and then with Sp4 over-expression vectors for another day. Over-expression of Sp4 was able to increase Sp4, Gabra1, and Gabra2 transcript levels significantly above those with Sp4 shRNA alone (P < 0.05 for all), and these values were not significantly different from those of controls (Fig. 4C). This indicates that the shRNA-induced down-regulation of transcripts was rescued by Sp4 over-expression. However, this combination had no effect on Gabra3 transcripts (Fig. 4C). Sp4-silenced primary neurons treated with KCl for 5 hours and then transfected with Sp4 over-expression vectors showed increases in their mRNA levels of Sp4, Gabra1, and Gabra2 that were significantly above those treated with shRNA and KCl but without over-expression (P < 0.001, P < 0.01, and P < 0.01, respectively, when compared with shRNA-plus-KCl). Again, Sp4 over-expression was able to counter the effect of shRNA and allowed the neurons to be activated by KCl. Thus, the values of transcripts for Sp4, Gabra1, and Gabra2 were not significantly different between shRNA-plus-KCl-plus-over-expression and control-plus-KCl. Again, this combination had no effect on Gabra3 (Fig. 4C).
3.7. Over-expressing Sp4 increased mRNA levels of GABAA receptors and the effect of TTX blockade in primary neurons
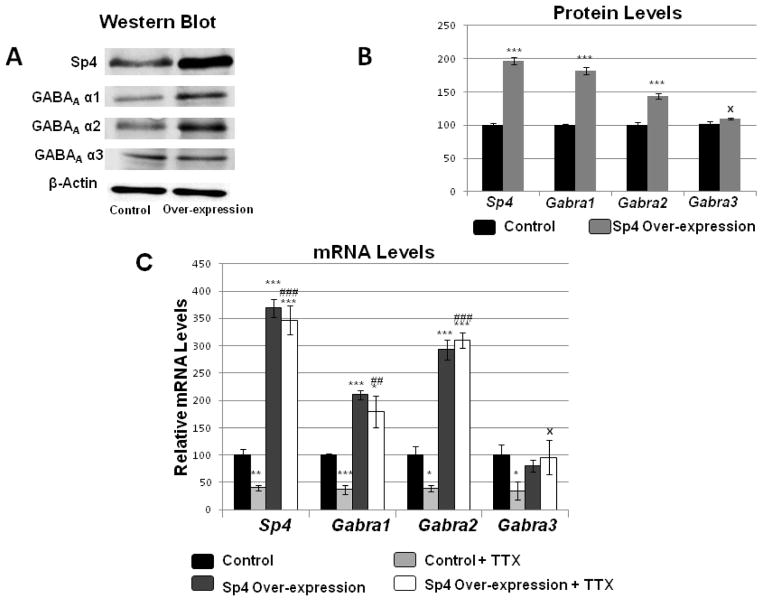
To determine the effect of over-expressing Sp4 on transcript levels of GABAA receptor subunits Gabra1 and Gabra2, Sp4 over-expression plasmids were transfected into primary cultured neurons. β-actin was used as the internal control. Sp4 over-expression increased the protein levels of Sp4 to 196% (P < 0.001, Fig. 5A–B), and those of GABAA α1 and GABAA α2 to 182% and 144%, respectively (P < 0.001 for both, Fig. 5A–B). Likewise, mRNA levels showed a similar pattern of change. Over-expressing Sp4 significantly increased the mRNA levels of Sp4 to 369%, and those of Gabra1 and Gabra2 to 210% and 293%, respectively (P < 0.001 for all, Fig. 5C). On the other hand, neither GABAA α3 protein nor Gabra3 mRNA exhibited any significant change with Sp4 over-expression (Fig. 5A–C).
Fig. 5.
Effect of over-expressing Sp4 on the transcript levels of GABAA receptor subunit genes. (A-B) Primary neurons transfected with Sp4 over-expression vectors revealed an up-regulation of Sp4, GABAA α1, and GABAA α2 but not GABAA α3 protein levels using western blots (N = 3). (B) Sp4 over-expression also increased Sp4, Gabra1 and Gabra2 transcript levels but not that of Gabra3. (N = 3). (C) Over-expression of Sp4 in primary neurons increased mRNA levels of Sp4, Gabra1, and Gabra2 mRNA levels in primary neurons, but not of Gabra3. N = 3. *= P < 0.05, **= P < 0.01, and ***= P < 0.001 when compared to controls. Transcript levels of Sp4 and all three GABAA receptor subunit genes were decreased with 3 days of 0.4 μM TTX treatment. Sp4 over-expression rescued the TTX-mediated down-regulation of Sp4, Gabra1, and Gabra2 transcript levels, but not that of Gabra3. N =3. *= P < 0.05, **= P < 0.01, and ***= P < 0.001 when compared to controls. ##= P < 0.01, ### = P < 0.001 and X = non-significant when compared to TTX treatment alone.
To determine if Sp4 over-expression could rescue GABAA transcript levels suppressed by TTX-induced impulse blockade, primary cultured neurons (controls and Sp4 transfected) were subjected to 0.4 μM TTX treatments for 3 days. Control neurons transfected with empty vectors were exposed to TTX showed a significant decrease of 60%, 63%, and 62% in transcript levels of Sp4, Gabra1, and Gabra2, respectively (P < 0.01, P < 0.001, and P < 0.05, respectively; Fig. 5C). However, neurons that were transfected with Sp4 over-expression vectors did not exhibit any down-regulation of their transcript levels of Sp4, Gabra1, and Gabra2 with TTX. Rather, these levels were 346%, 179% and 309% respectively, as compared to controls (P < 0.001 for Sp4 and Gabra2, and P < 0.01 for Gabra1 as compared to TTX alone; Fig. 5C). Gabra3 transcript level decreased significantly with TTX treatment (P < 0.05) but was not rescued by Sp4 over-expression (Fig. 5C).
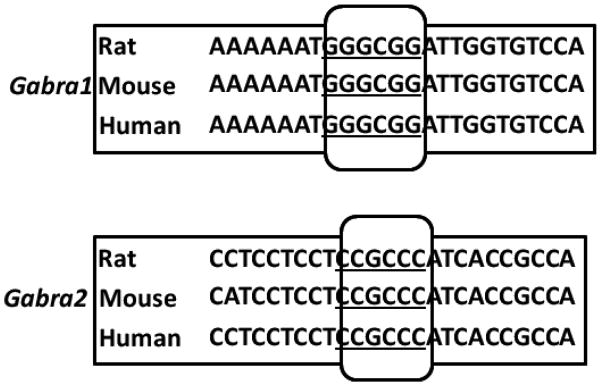
3.8. Homology
The functional Sp4 binding sites are conserved among mice, rats, and humans for Gabra1 and Gabra2 (Fig. 6).
Fig. 6.
Aligned partial sequences of the Gabra1 and Gabra2 promoters from mice, rats, and humans showed conserved Sp binding sites (boxed and underlined).
4. Discussion
The present study analyzed the proximal promoter regions of GABAA receptor subunit genes Gabra1, Gabra2, and Gabra3 by means of multiple molecular and biochemical approaches, including in silico analysis, electrophoretic mobility shift and supershift assays, ChIP, RNA interference, over-expression, functional assays, and western blots in rodent primary cortical neurons. We found that Gabra1 and Gabra2, but not Gabra3, subunit genes are regulated by the neuron-specific transcription factor Sp4. The typical Sp binding motifs on the Gabra1 and Gabra2 promoters are conserved among mice, rats, and humans.
Specificity protein (Sp) is a family of zinc finger transcription factors that bind to GC-rich DNA motifs and regulate the expression of a large number of genes implicated in various biological functions [25]. The Sp binding site, also known as the GC box, contains an asymmetric hexanucleotide core ‘GGGCGG’ sequence with high affinity, or the GT (‘GGGTGG’) or CT (‘CCCTCC’) boxes with significantly lower affinities, and is recognized by members of the Sp/KLF family of transcription factors with zinc finger motifs [25,26]. Increasing evidence indicate that Sp4 is amply expressed in the brain, with high levels in neurons and low levels in astrocytes [5,27,28]. Sp4 reportedly regulates developmental cerebellar dendritic patterning, postnatal development of hippocampal dentate granule cells, postnatal hippocampal cell proliferation, hippocampal long-term potentiation, and animal behaviors including contextual and spatial memory, sensorimotor gating, and prepulse inhibition, some of which have been associated with psychiatric disorders [29–31]. Although Sp-cis motif has been reported in the promoter regions of Gabra1 and Gabra2 [32], it has not been characterized previously. The present study provides experimental evidence from multiple approaches that Sp4 functionally regulates the expression of Gabra1 and Gabra2 in neurons.
GABAA receptors are the dominant inhibitory neurotransmitter receptors in the brain, and their kinetics is known to be faster than that of GABAB by roughly ten-fold [33]. Binding of GABA to ionotropic GABAA receptors opens the intrinsic ion channels, enabling the influx of chloride through the channels and subsequent hyperpolarization of neurons [34]. At least 19 GABAA receptor subunit isoforms (α1–6, β1–3, γ1–3, ρ1–3, π, θ, δ ε) have been characterized in mammals [35,36]. The most typical heteropentameric GABAA receptors contain two α, two β, and one γ subunits [37,38].
Our current study indicates that Sp4 regulates the Gabra1 and Gabra2 but not Gabra3 subunit genes of GABAA receptors. We have focused on these three subunits because they are developmentally regulated [39–43], vary in receptor kinetics [44,45], and the α subunit provides the interface for binding GABA [46] and benzodiazepine [47].
GABAA α1 expression increases with age, reaching a stable level in the adult [44,48] as well as in the aging brain [49]. In the immature cortex, the kinetics of the ionotropic GABAA receptors is relatively slow [45], but it accelerates three-fold during development until GABAA α1 dominates the receptor complex [50]. GABAA α1 subunit is responsible for sedative, amnestic, and anticonvulsant actions [51] and is also known to drive ocular dominance plasticity in the visual cortex [52]. The α1 subunit also has a high-affinity for binding GABA and benzodiazepine receptor ligands [53]. It is now clear that this important subunit is transcriptionally regulated by Sp4 (the present study).
The distribution and expression of the GABAA α2 subunit are reportedly strong in the superficial layers of neocortex and become abundant and widespread within the whole cortex during development; they then show a stable expression only in the superficial layers during adulthood and aging [49]. In the rat brain stem, this subunit is maintained at a steady level during the first three postnatal weeks [42,43]. It is thought to mediate anxiolytic actions [51,54] and is involved in modulating neuronal firing [52]. The persistence of α2 into adulthood underscores the importance of Sp4 in its regulation.
In contrast, the GABAA α3 subunit is abundantly expressed only during the early postnatal weeks and is decreased or absent in the adult and aging brain [49]. The inclusion of GABAA α3 in the receptor is reportedly the reason for the slow kinetics during early postnatal development, and a developmental switch from α3 to α1 is essential for a faster kinetics [44]. This developmental switch in GABAA subunits occurs in rats [41–42,43], cats [40], and macaque monkeys [55]. Such apparent subunit switches have been suggested to underlie the transition from depolarizing/excitatory to a hyperpolarizing/inhibitory mode of GABA action during development [56]. Our present data indicate that Sp4 does not play an important role in regulating this neonatal subunit of GABAA receptors.
The ratio of the different subunits is a more accurate predictor of GABAA receptor function than the expression of a single subunit [57,58] . Yu and colleagues [49] have revealed a continuously low α3 subunit and a relatively stable and high α1 and α2 subunit expression in the cortex during normal aging, and suggested that subunit composition may modulate the binding of GABA and benzodiazepine to the receptors. The present study documents that the two key GABAA receptor subunit genes, Gabra1 and Gabra2, are transcriptionally regulated by Sp4 in neurons, indicating that Sp4’s control over GABAA receptor expression is sustained throughout the postnatal life of the animal.
The fact that Sp4 plays an important role in regulating mediators of inhibitory transmission (GABAA receptors; the present study) is significant, as we have found that it also regulates many excitatory neurotransmitter receptor subunit genes, such as Grin1, Grin2a, and Grin2b of NMDA receptors [2] and Gria2 of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors [3]. Thus, Sp4 is in an ideal position to regulate the balance between excitation and inhibition to achieve a homeostatic state of neuronal functioning. Normal neuronal functioning is dependent on a proper balance between excitation and inhibition, both at the single cell and neuronal network levels. Neuronal firing rate is thought to be maintained around a homeostatic set point, and the maintenance of that stability is dependent on a number of factors, including synaptic scaling and the balancing of excitation and inhibition [reviewed in 59]. To date, the mechanism underlying such a homeostatic balance has not be fully elucidated. We propose that one such mechanism is via transcriptional regulation, and that Sp4 is capable of regulating mediators of both excitatory glutamatergic [2, 3] and inhibitory GABAergic [present study] neurotransmission. In fact, we have shown that over-expressing Sp4 up-regulates the mRNA and protein levels of both glutamatergic receptors [2,3] and GABAergic receptors (the present study), and knocking down Sp4 down-regulates both. Transcriptional regulation is a viable mechanism that allows for an exquisite orchestration of the delicate balance between excitation and inhibition.
In addition to regulating mediators of neuronal activity, Sp4 also transcriptionally regulates mediators of energy generation (cytochrome c oxidase or COX) as well as energy consumption (Na+/K+-ATPase) in neurons [4,5]. COX is the terminal enzyme of the mitochondrial electron transport chain critical in generating ATP, and it has proven to be a sensitive and reliable indicator of neuronal activity [1]. Its13 subunits are derived either from the mitochondrial genome or the nuclear DNA across 9 different chromosomes, and we found that Sp4 regulates all 13 subunits from the two genomes [5]. Na+/K+-ATPase is a plasma membrane protein that maintains the ionic imbalance across the membrane by actively extruding Na+ in exchange for the influx of K+ [60]. It is a major energy consumer in neurons because of the constant need for membrane repolarization after depolarizing activities, and its distribution is highly correlated with that of COX, a key energy generator [1]. Thus, Sp4 is a master regulator that tightly couples energy metabolism (both generation and consumption) with neuronal activity (both excitation and inhibition) at the transcriptional level. This ensures that the energy requirement of neuronal activity is met with an adequate production of energy. The present study also highlights Sp4’s role in mediating a homeostatic balance between excitation and inhibition in the nervous system.
Highlights.
Sp4 regulates GABAA receptor subunits Gabra1 and Gabra2, but not Gabra3.
Over-expression of Sp4 enhances Gabra1 and Gabra2 mRNA and protein levels.
Knocking down Sp4 reduces Gabra1 and Gabra2 mRNA and protein levels.
Sp4 regulates inhibitory and the previously reported excitatory receptor genes.
Acknowledgments
This work is supported by NIH Grant R01 EY018441 and NIH/NEI Training Grant 1- T32-EY14537.
Abbreviations
- COX
cytochrome c oxidase
- Gabra
gene name for GABAA receptor subunit
- Sp4
specificity protein 4
Footnotes
Conflict of Interest: The authors declare no competing financial interest.
All authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wong-Riley MT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989;12:94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
- 2.Priya A, Johar K, Wong-Riley MT. Specificity Protein 4 functionally regulates the transcription of NMDA receptor subunits GluN1, GluN2A, and GluN2B. Biochim Biophys Acta. 2013b;1833:2745–2756. doi: 10.1016/j.bbamcr.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Priya A, Johar K, Nair B, Wong-Riley MT. Specificity Protein 4 (Sp4) regulates the transcription of AMPA receptor subunit GluA2 (Gria2) Biochim Biophys Acta. 2014a;1843:1196–1206. doi: 10.1016/j.bbamcr.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johar K, Priya A, Wong-Riley MT. Regulation of Na(+)/K(+)-ATPase by neuron-specific transcription factor Sp4: implication in the tight coupling of energy production, neuronal activity and energy consumption in neurons. Eur J Neurosci. 2014;39:566–578. doi: 10.1111/ejn.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johar K, Priya A, Dhar S, Liu Q, Wong-Riley MT. Neuron-specific specificity protein 4 biogenomically regulates the transcription of all mitochondria- and nucleus-encoded cytochrome c oxidase subunit genes in neurons. J Neurochem. 2013;127:496–508. doi: 10.1111/jnc.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kmjevic K. GABAergic inhibition in neocortex. J Mind Behav. 1987;81:537–547. [Google Scholar]
- 7.Huntsman MM, Isackson PJ, Jones EG. Lamina-specific expression and activity-dependent regulation of seven GABAA receptor subunit mRNAs in monkey visual cortex. J Neurosci. 1994;14:2236–2259. doi: 10.1523/JNEUROSCI.14-04-02236.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsen RW, Tobin AJ. Molecular biology of GABAA receptors. FASEB J. 1990;4:1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 10.Mohler H. GABAA receptor diversity and pharmacology. Cell Tissue Res. 2006;326:505–516. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- 11.Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- 12.Smith SS, Gong QH, Hsu FC, Markowitz RS, Mffrench-Mullen J, Li X. GABA(A) receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- 13.Parsian A, Zhang ZH. Human chromosomes 11p15 and 4p12 and alcohol dependence: possible association with the GABRB1 gene. Am J Med Genet. 1999;88:533–538. doi: 10.1002/(sici)1096-8628(19991015)88:5<533::aid-ajmg18>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 14.Dhar SS, Liang HL, Wong-Riley MT. Nuclear respiratory factor 1 co-regulates AMPA glutamate receptor subunit 2 and cytochrome c oxidase: tight coupling of glutamatergic transmission and energy metabolism in neurons. J Neurochem. 2009;108:1595–1606. doi: 10.1111/j.1471-4159.2009.05929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Priya A, Johar K, Wong-Riley MT. Nuclear respiratory factor 2 regulates the expression of the same NMDA receptor subunit genes as NRF-1: both factors act by a concurrent and parallel mechanism to couple energy metabolism and synaptic transmission. Biochim Biophys Acta. 2013a;1833:48–58. doi: 10.1016/j.bbamcr.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Priya A, Johar K, Nair B, Wong-Riley MT. Nuclear respiratory factor 2 regulates the transcription of AMPA receptor subunit GluA2 (Gria2) Biochim Biophys Acta. 2014b;1843:3018–3028. doi: 10.1016/j.bbamcr.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ongwijitwat S, Wong-Riley MT. Is nuclear respiratory factor 2 a master transcriptional coordinator for all ten nuclear-encoded cytochrome c oxidase subunits in neurons? Gene. 2005;360:65–77. doi: 10.1016/j.gene.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Lahiri DK, Ge Y. Electrophoretic mobility shift assay for the detection of specific DNA-protein complex in nuclear extracts from the cultured cells and frozen autopsy human brain tissue. Brain Res Brain Res Protoc. 2000;5:257–265. doi: 10.1016/s1385-299x(00)00021-0. [DOI] [PubMed] [Google Scholar]
- 19.Ishimaru N, Tabuchi A, Hara D, Hayashi H, Sugimoto T, Yasuhara M, Shiota J, Tsuda M. Regulation of neurotrophin-3 gene transcription by Sp3 and Sp4 in neurons. J Neurochem. 2007;100:520–531. doi: 10.1111/j.1471-4159.2006.04216.x. [DOI] [PubMed] [Google Scholar]
- 20.Dhar SS, Wong-Riley MT. Coupling of energy metabolism and synaptic transmission at the transcriptional level: role of nuclear respiratory factor 1 in regulating both cytochrome c oxidase and NMDA glutamate receptor subunit genes. J Neurosci. 2009;29:483–492. doi: 10.1523/JNEUROSCI.3704-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhar SS, Johar K, Wong-Riley MT. Biogenomic transcriptional regulation of all thirteen cytochrome c oxidase subunit genes by specificity protein 1. Open Biol. 2013;3:120176. doi: 10.1098/rsob.120176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Whiting PJ. GABA-A receptor subtypes in the brain: a paradigm for CNS drug discovery? Drug Discov Today. 2003;8:445–450. doi: 10.1016/s1359-6446(03)02703-x. [DOI] [PubMed] [Google Scholar]
- 24.Xia T, Zeng G, Gao L, Yu RK. Sp1 and AP2 enhance promoter activity of the mouse GM3-synthase gene. Gene. 2005;351:109–118. doi: 10.1016/j.gene.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Suske G. The Sp-family of transcription factors. Gene. 1999;238:291–300. doi: 10.1016/s0378-1119(99)00357-1. [DOI] [PubMed] [Google Scholar]
- 26.Letovsky J, Dynan WS. Measurement of the binding of transcription factor Sp1 to a single GC box recognition sequence. Nucleic Acids Res. 1989;17:2639–2653. doi: 10.1093/nar/17.7.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao X, Yang SH, Simpkins JW, Barger SW. Glutamate receptor activation evokes calpain-mediated degradation of Sp3 and Sp4, the prominent Sp-family transcription factors in neurons. J Neurochem. 2007;100:1300–1314. doi: 10.1111/j.1471-4159.2006.04297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinacho R, Valdizán EM, Pilar-Cuellar F, Prades R, Tarragó T, Haro JM, Ferrer I, Ramos B. Increased Sp4 and Sp1 transcription factor expression in the postmortem hippocampus chronic schizophrenia. J Psychiatr Res. 2014;58:189–196. doi: 10.1016/j.jpsychires.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Ramos B, Gaudilliere B, Bonni A, Gill G. Transcription factor Sp4 regulates dendritic patterning during cerebellar maturation. Proc Natl Acad Sci USA. 2007;104:9882–9887. doi: 10.1073/pnas.0701946104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou X, Long JM, Geyer MA, Masliah E, Kelsoe JR, Wynshaw-Boris A, Chien KR. Reduced expression of the Sp4 gene in mice causes deficits in sensorimotor gating and memory associated with hippocampal vacuolization. Mol Psychiatry. 2005;10:393–406. doi: 10.1038/sj.mp.4001621. [DOI] [PubMed] [Google Scholar]
- 31.Zhou X, Nie ZG, Roberts A, Zhang DX, Sebat J, Malhotra D, Kelsoe JR, Geyer MA. Reduced NMDAR1 expression in the Sp4 hypomorphic mouse may contribute to endophenotypes of human psychiatric disorders. Hum Mol Genet. 2010;19:3797–3805. doi: 10.1093/hmg/ddq298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joyce CJ. In silico comparative genomic analysis of GABAA receptor transcriptional regulation. BMC Genomics. 2007;8:203. doi: 10.1186/1471-2164-8-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Connors BW, Malenka RC, Silva LR. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. J Physiol. 1988;406:443–468. doi: 10.1113/jphysiol.1988.sp017390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whiting PJ. GABA-A receptors: a viable target for novel anxiolytics? Curr Opin Pharmacol. 2006;6:24–29. doi: 10.1016/j.coph.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 36.Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res Brain Res Rev. 1999;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- 37.Baumann SW, Baur R, Siegel E. Subunit arrangement of gamma-aminobutyric acid type A receptors. J Biol Chem. 2001;276:36275–36280. doi: 10.1074/jbc.M105240200. [DOI] [PubMed] [Google Scholar]
- 38.Klausberger T, Sarto I, Ehya N, Fuchs K, Furtmuller R, Mayer B, Huck S, Sieghart W. Alternate use of distinct intersubunit contacts controls GABAA receptor assembly and stoichiometry. J Neurosci. 2001;21:9124–9133. doi: 10.1523/JNEUROSCI.21-23-09124.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tia S, Wang JF, Kotchabhakdi N, Vicini S. Developmental changes of inhibitory synaptic currents in cerebellar granule neurons: role of GABAA receptor α6 subunit. J Neurosci. 1996;16:3630–3640. doi: 10.1523/JNEUROSCI.16-11-03630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L, Yang C, Mower GD. Developmental changes in the expression of GABA(A) receptor subunits (alpha(1), alpha(2), alpha(3)) in the cat visual cortex and the effects of dark rearing. Brain Res Mol Brain Res. 2001;88:135–143. doi: 10.1016/s0169-328x(01)00042-0. [DOI] [PubMed] [Google Scholar]
- 41.Bosman LW, Rosahl TW, Brussaard AB. Neonatal development of the rat visual cortex: synaptic function of GABAA receptor alpha subunits. J Physiol. 2002;545:169–181. doi: 10.1113/jphysiol.2002.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Q, Wong-Riley MT. Developmental changes in the expression of GABAA receptor subunits alpha1, alpha2, and alpha3 in the rat pre-Botzinger complex. J Appl Physiol. 2004;96:1825–1831. doi: 10.1152/japplphysiol.01264.2003. [DOI] [PubMed] [Google Scholar]
- 43.Liu Q, Wong-Riley MT. Developmental changes in the expression of GABAA receptor subunits alpha1, alpha2, and alpha3 in the brain stem nuclei of rats. Brain Res. 2006;1098:129–138. doi: 10.1016/j.brainres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the alpha-subunit isoform: implications for structure-function relations and synaptic transmission. J Physiol. 1995;489:529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith GB, Olsen RW. Functional domains of GABAA receptors. Trends Pharmacol Sci. 1995;16:162–168. doi: 10.1016/s0165-6147(00)89009-4. [DOI] [PubMed] [Google Scholar]
- 47.Sigel E. Mapping of the benzodiazepine recognition site on GABA(A) receptors. Curr Top Med Chem. 2002;2:833–839. doi: 10.2174/1568026023393444. [DOI] [PubMed] [Google Scholar]
- 48.Golshani P, Truong H, Jones EG. Developmental expression of GABAA receptor subunit and GAD genes in mouse somatosensory barrel cortex. J Comp Neurol. 1997;383:199–219. doi: 10.1002/(sici)1096-9861(19970630)383:2<199::aid-cne7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 49.Yu ZY, Wang W, Fritschy JM, Witte OW, Redecker C. Changes in neocortical and hippocampal GABAA receptor subunit distribution during brain maturation and aging. Brain Res. 2006;1099:73–81. doi: 10.1016/j.brainres.2006.04.118. [DOI] [PubMed] [Google Scholar]
- 50.Murphy KM, Beston BR, Boley PM, Jones DG. Development of human visual cortex: A balance between excitatory and inhibitory plasticity mechanisms. Dev Psychobiol. 2005;46:209–221. doi: 10.1002/dev.20053. [DOI] [PubMed] [Google Scholar]
- 51.Rudolph U, Crestani F, Benke D, BrÜNIG I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, MöHLER H. Benzodiazepine actions mediated by specific γ-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 52.Fagiolini M, Fritschy JM, Löw K, Mohler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- 53.Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Scofield PR, Seeburg PH. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- 54.Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, Fritschy JM, Rülicke T, Bluethmann H, Möhler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 55.Hendrickson A, March D, Richards G, Erickson A, Shaw C. Coincidental appearance of the alpha1 subunit of the GABA-A receptor and the type I benzodiazepine receptor near birth in macaque monkey visual cortex. Int J Dev Neurosci. 1994;12:299–314. doi: 10.1016/0736-5748(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 56.Simeone TA, Donevan SD, Rho JM. Molecular biology and ontogeny of gamma-aminobutyric acid (GABA) receptors in the mammalian central nervous system. J Child Neurol. 2003;18:39–48. doi: 10.1177/08830738030180012101. [DOI] [PubMed] [Google Scholar]
- 57.Brooks-Kayal AR, Shumate MD, Jin H, Lin DD, Rikhter TY, Holloway KL, Coulter DA. Human neuronal gamma-aminobutyric acid(A) receptors: coordinated subunit mRNA expression and functional correlates in individual dentate granule cells. J Neurosci. 1999;19:8312–8318. doi: 10.1523/JNEUROSCI.19-19-08312.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brooks-Kayal AR, Shumate MD, Jin H, Lin DD, Rikhter TY, Kelly ME, Coulter DA. Gamma-aminobutyric acid(A) receptor subunit expression predicts functional changes in hippocampal dentate granule cells during postnatal development. J Neurochem. 2001;77:1266–1278. doi: 10.1046/j.1471-4159.2001.00329.x. [DOI] [PubMed] [Google Scholar]
- 59.Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect Biol. 2012;4:a005736. doi: 10.1101/cshperspect.a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skou JC. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta. 1957;23:394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]