Abstract
The present experiment monitored bilateral eyelid responses during eyeblink conditioning in rats trained with a unilateral unconditioned stimulus (US). Three groups of rats were used to determine if cross-modal savings occurs when the location of the US is switched from one eye to the other. Rats in each group first received paired or unpaired eyeblink conditioning with a conditioned stimulus (tone or light; CS) and a unilateral periorbital electrical stimulation US. All rats were subsequently given paired training, but with the US location (Group 1), CS modality (Group 2), or US location and CS modality (Group 3) changed. Changing the location of the US alone resulted in an immediate transfer of responding in both eyelids (Group 1) in rats that received paired training prior to the transfer session. Rats in groups 2 and 3 that initially received paired training showed facilitated learning to the new CS modality during the transfer sessions, indicating that cross-modal savings occurs whether or not the location of the US is changed. All rats that were initially given unpaired training acquired conditioned eyeblink responses similar to de novo acquisition rate during the transfer sessions. Savings of CR incidence was more robust than savings of CR amplitude when the US switched sides, a finding that has implications for elucidating the neural mechanisms of cross-modal savings.
Keywords: Learning, Transfer, Associative, Classical Conditioning
Mechanisms of associative learning have been investigated for decades using eyeblink conditioning (e.g., Gormezano, Kehoe, & Marshall, 1983; Gormezano, Schneiderman, Deaux, & Fuentes, 1962; Hilgard & Marquis, 1936; Spence, 1953). This form of learning is typically established by pairing a conditioned stimulus (CS), such as a tone or light, with an unconditioned stimulus (US), such as periorbital stimulation or an airpuff directed toward only one eye. After repeated CS-US pairings are given, an eyelid closure response (i.e., the conditioned response; CR) emerges during the CS period prior to the onset of the US.
Eyeblink conditioning is often considered to be lateralized to the eyelid where the US is directed, even though eyeblink CRs are often observed in both eyelids, which is especially evident in rats (Campolattaro & Freeman, 2009a) and humans (Hilgard & Campbell, 1936; Hilgard & Marquis, 1935; 1936). Typically, CR percentage is greater, CR amplitude is larger and CR onset is shorter in the eyelid responses ipsilateral to US presentation compared to the eyelid responses contralateral to US presentation (Campolattaro & Freeman, 2009a; Disterhoft, Kwan, & Lo, 1977; Hilgard & Campbell, 1936; Hilgard & Marquis, 1935; 1936). However, when the US is redirected toward the other eye during paired training, CR expression in its eyelid rapidly reaches an asymptotic percentage, maximum peak amplitude and well-timed onset latency. The facilitation of CR performance likely results from savings (i.e., persistence of memory) acquired during the previous paired training, when the US was directed toward the other eye (Lavond, Kanawa, Ivkovich, & Clark, 1994; Lavond & Steinmetz, 1989; Lavond, Wikgren & Nokia, 2011; Lee, Kim, & Wagner, 2008; Lincoln, McCormick, & Thompson, 1982; McCormick, Clark, Kettner, Rising, & Thompson, 1981).
Most eyeblink conditioning experiments that have used inter-eye transfer training procedures present the same CS (e.g., a tone CS) during both the acquisition and transfer phases (e.g., Clark, Zhang, & Lavond, 1997; Lee, Kim, & Wagner, 2008; Lavond et al., 2011; Lincoln et al., 1982). One exception is Pearce, Montgomery and Dickinson (1981) who trained rabbits for twelve sessions with a light or an auditory clicker CS paired with a unilateral airpuff US. All rabbits then received five additional paired training sessions with the clicker CS paired with the US redirected to the other eye. Pearce et al (1981) found that rabbits initially trained with the light CS acquired relatively high percentages of eyeblink CRs in both eyelids during training, whereas rabbits initially conditioned with the clicker CS only acquired CRs in the conditioned eye. Interestingly, during the subsequent sessions, when the US location was changed and all rabbits received training with the clicker CS, savings was only seen in the newly conditioned eyelid of rabbits that had been initially trained with the clicker CS. Rabbits that were initially trained with the light CS subsequently acquired eyeblink CRs to the clicker CS at a de novo conditioning rate, revealing that cross-modal savings (i.e., light CS to clicker CS transfer training) did not occur (Pearce et al., 1981). These findings may suggest that the same CS, or perhaps just the same CS sensory modality, used during initial training is needed to support savings during transfer-training.
In rabbits, cross-modal savings in the contralateral eyelid has not been seen when the CS modality and US location are changed together (Pearce et al., 1981). However, previous experiments have shown that changing the modality of the CS alone (e.g., from an auditory CS to visual CS) in well-trained rabbits, while keeping the location of the US the same, typically facilitates learning to the new modality CS (Kehoe & Holt, 1984; Holt & Kehoe, 1985; Schreurs & Kehoe, 1987; Kehoe, 1988; Kehoe, Horne, & Macrae, 1995). The same training procedures also produce cross-modal savings of eyeblink conditioning in rats (Brown & Stanton, 2008; Campolattaro & Freeman, 2009b; Campolattaro, Kashef, Lee, & Freeman, 2011). It is possible that rats show robust cross-modal savings in the contralateral eyelid during transfer training because, unlike rabbits, they tend to express eyeblink CRs bilaterally (Campolattaro & Freeman, 2009a). Therefore, the goal of the current experiment was to determine whether cross-modal savings occurs in rats when both the US location and CS modality are changed at the same time.
Bilateral eyelid responses were monitored during eyeblink conditioning in rats trained with unilateral US presentations. Three groups of rats were used to determine if cross-modal savings (e.g., tone to light transfer) occurs when the location of the US is switched from one eye to the other. Half of the rats in each group first received eyeblink conditioning with paired presentations of a CS (tone or light) and a periorbital shock US. The other half of rats in each group received unpaired presentations of the CS and US. All rats were then given paired training with the US location (Group 1), CS modality (Group 2) or US location and CS modality (Group 3) switched.
Method
Subjects
Subjects were 48 male Long Evans rats (200-250g), approximately 150 days old at the beginning of the experiment. The rats were housed in Spence Laboratories of Psychology at the University of Iowa with a 12-hr light-dark cycle, with light onset at 07:00 a.m. All procedures were approved by the University of Iowa Institutional Animal Care and Use Committee.
Surgery
One week prior to training, rats were removed from their home cage and anesthetized with isoflurane gas. The rats were fitted with differential electromyograph (EMG) electrodes that were implanted in the upper left and right eyelid muscles (orbicularis oculi) and ground electrodes were attached to a stainless steel skull screw. The EMG electrode leads terminated in gold pins held in a plastic connector, which were secured to the skull with bone cement. Bipolar stimulating electrodes (for delivering the shock US) were implanted subdermally, immediately caudal to each eye. The bipolar electrodes terminated in a plastic connector and were secured to the skull with Osteobond Copolymer Bone Cement (Zimmer, Warsaw, IN).
Conditioning Apparatus
The conditioning apparatus and eyelid EMG recording procedures used in the present study have been previously described in other reports (e.g., Campolattaro & Freeman, 2009a; Campolattaro, Schnitker, & Freeman, 2008; Freeman, Halverson & Poremba, 2005).
Conditioning Procedure
Rats were divided into three groups that were trained with one session per day. Each group (n = 16) received ten 100-trial sessions of either paired (n = 8, experimental rats) or unpaired (n = 8, control rats) conditioning with a 400 msec CS (2 kHz, 85d tone or 6 W light; modality counterbalanced) and a unilateral 25 msec periorbital shock US (1-2 mA; counterbalanced left vs right eye). All groups were then given five sessions of paired training with a changed US location (e.g., right eye to left eye), CS modality (e.g., light to tone) or both US location and CS modality. Every 10th trial in the paired training was a CS-alone probe trial (i.e., 10 probe trails per a 100-trial session). These trials were necessary to obtain eyelid EMG responses without contamination by the presence of the US. The offset of the CS coincided with the onset of the US for paired trials, yielding a non-overlapping 400 msec interstimulus interval. Paired trials were separated by a variable intertrial interval (ITI) that averaged 30 sec. The CS and US for unpaired training were separated by a variable ITI that averaged 15 sec.
Responses
CRs were defined as electromyography (EMG) activity that exceeded a threshold of 0.4 units (amplified and integrated units in volts) above the baseline mean during the CS period after 80 msec. CRs during CS-US trials were defined as responses obtained after the baseline period, but before the onset of the US. CR amplitude was the maximum EMG activity for responses that exceeded the threshold after CS-onset, during CS-alone probe trials. Sub-threshold and zero amplitude responses were not included in the calculation of CR amplitude.
Results
The graphs in Figures 1-3 are shown with “left”, “right”, “tone” and “light” labels to simplify the presentation of the results, although CS modality order and the location of the US were counterbalanced in this experiment. Data from one rat in Group 1 (paired-to-paired training condition) was excluded from the transfer session analysis because it did not complete transfer training. One rat from Group 2 (unpaired-to paired-training condition) did not complete any training and was therefore excluded from analysis.
Figure 1.
US location changed. Mean (±SEM) conditioned response (CR) percentage (A-C) and amplitude (D) recorded in the left (white plots) and right (black plots) eyelid during the different phases of training. The dashed line separates the sessions when the location of the US was changed from one eyelid (Left US) to the other (Right US). 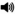 = tone CS. (A) Percentages of CRs were greater in the left eyelid than right eyelid for rats given paired→paired training during initial training. CR percentages maintained high percentages in both eyelids after the location of the US was changed. (B) Rats given unpaired-paired training showed low percentages of CRs during initial training in both eyelids and acquired CRs at a de novo rate after they received paired training. (C) CR percentage for rats given paired→paired training obtained from the first session (session 11) when the location of the US was changed was divided into ten 10-trial blocks. Percentage of CRs in the right eyelid increased to asymptote by the third block of training. (D) CR amplitude for rats given paired→paired training increased in the left eyelid before the location of the US was changed and then decreased after changing the location of the US. CR amplitude in the right eyelid steadily increased from baseline after the location of the US was changed to that side.
= tone CS. (A) Percentages of CRs were greater in the left eyelid than right eyelid for rats given paired→paired training during initial training. CR percentages maintained high percentages in both eyelids after the location of the US was changed. (B) Rats given unpaired-paired training showed low percentages of CRs during initial training in both eyelids and acquired CRs at a de novo rate after they received paired training. (C) CR percentage for rats given paired→paired training obtained from the first session (session 11) when the location of the US was changed was divided into ten 10-trial blocks. Percentage of CRs in the right eyelid increased to asymptote by the third block of training. (D) CR amplitude for rats given paired→paired training increased in the left eyelid before the location of the US was changed and then decreased after changing the location of the US. CR amplitude in the right eyelid steadily increased from baseline after the location of the US was changed to that side.
Figure 2.
CS modality changed. Mean (±SEM) conditioned response (CR) percentage (A-C) and amplitude (D) recorded in the left (white plots) and right (black plots) eyelids during the different phases of training. The dashed line separates the sessions when the modality of the CS was changed.  = tone CS;
= tone CS;  = light CS. (A) Percentages of CRs during initial paired training with the tone CS were greater in the left eyelid during paired→paired training. Cross-modal savings was observed in both eyelids after the CS modality was changed to the light. (B) Rats given unpaired-paired training showed low percentages of CRs during initial training in both eyelids and showed a higher percentage of CRs in the left eyelid than right eyelid after they received paired training with the light CS. (C). CR percentages for rats given paired→paired found during the first session of cross-modal training (session 11) were divided into ten 10-trial blocks. Percentage of CRs during session 11 started at ~10-20% and increase to 70-80% by the tenth block, and CR percentages were consistently greater in the left eyelid (ipsilateral to the US) than the right eyelid (contralateral to the US). CR amplitude for rats given paired→paired training was consistently larger in the left eyelid than the right eyelid.
= light CS. (A) Percentages of CRs during initial paired training with the tone CS were greater in the left eyelid during paired→paired training. Cross-modal savings was observed in both eyelids after the CS modality was changed to the light. (B) Rats given unpaired-paired training showed low percentages of CRs during initial training in both eyelids and showed a higher percentage of CRs in the left eyelid than right eyelid after they received paired training with the light CS. (C). CR percentages for rats given paired→paired found during the first session of cross-modal training (session 11) were divided into ten 10-trial blocks. Percentage of CRs during session 11 started at ~10-20% and increase to 70-80% by the tenth block, and CR percentages were consistently greater in the left eyelid (ipsilateral to the US) than the right eyelid (contralateral to the US). CR amplitude for rats given paired→paired training was consistently larger in the left eyelid than the right eyelid.
Figure 3.
US location and CS modality changed. Mean (±SEM) conditioned response (CR) percentage (A-C) and amplitude (D) recorded in the left (white plots) and right (black plots) eyelid during the different phases of training. The dashed line separates the sessions when the location of the US and modality of the CS was changed.  = tone CS;
= tone CS;  = light CS. (A) Rats given paired→paired showed greater percentages of CRs in the left eyelid than the right eyelid before the US location and CS modality were changed. Cross-modal savings was observed in both eyelids after the CS modality was changed to the light. (B) Low percentages of CRs in both eyelids were found during initial training for rats given unpaired-paired training, which increased at a de novo rate during paired training after the US location and modality of the CS changed. (C) CR percentage, in ten-trial blocks, for rats given paired→paired training obtained from the first session (session 11) of cross-modal training with the location of the US changed. Initial CR Percentage during session 11 started at ~10-20%, which increased to 70-80% by the sixth block. (D) CR amplitude for rats given paired→paired training increased in the left eyelid before the location of the US and CS modality were changed and then decreased after the transfer session. CR amplitude in the right eyelid steadily increased from baseline after the cross-modal training with location of the US was changed.
= light CS. (A) Rats given paired→paired showed greater percentages of CRs in the left eyelid than the right eyelid before the US location and CS modality were changed. Cross-modal savings was observed in both eyelids after the CS modality was changed to the light. (B) Low percentages of CRs in both eyelids were found during initial training for rats given unpaired-paired training, which increased at a de novo rate during paired training after the US location and modality of the CS changed. (C) CR percentage, in ten-trial blocks, for rats given paired→paired training obtained from the first session (session 11) of cross-modal training with the location of the US changed. Initial CR Percentage during session 11 started at ~10-20%, which increased to 70-80% by the sixth block. (D) CR amplitude for rats given paired→paired training increased in the left eyelid before the location of the US and CS modality were changed and then decreased after the transfer session. CR amplitude in the right eyelid steadily increased from baseline after the cross-modal training with location of the US was changed.
The rats that were given paired CS-US training during initial training acquired high percentages of CRs in both eyelids, but CR percentage was consistently higher in the eyelid that was ipsilateral to the US compared to the contralateral eyelid (Figures 1A, 2A & 3A). These findings were confirmed with analyses of variance (ANOVA) which each revealed a significant main effect of eyelid (ipsilateral vs contralateral) for Group 1 F(1,6) = 13.30, p < 0.02, Group 2, F(1,7) = 12.24, p < .01, and Group 3, F(1,7) = 22.72, p < 0.01. Rats given unpaired training showed significantly lower CR percentages (~10 %) than rats given paired training throughout initial training (Figures 1B, 2B, 3B). ANOVAs revealed this difference was significant for Group 1 [F(1,7) = 410.78, p < 0.001], Group 2 [F(1,7) = 96.75, p < .001], and Group 3 [F(1,7) = 192.19, p < 0.001].
Group 1: US location Changed
Changing the location of the US-alone (Group 1) after initial training resulted in an almost immediate transfer of CR percentage in both eyelids for the rats given paired-to-paired training (Figure 1A). Rats in Group 1 that were initially given unpaired training did not show any transfer when they were subsequently switched to paired training, rather they acquired CRs similar to a de novo rate (Figure 1B, sessions 11-15 vs Figure 1A, session 1-5). No significant differences in CR percentage were found between the eyelids during sessions 11-15 in either the paired-to-paired (Figure 1A, session 11-15) or unpaired-to-paired group (Figure 1B, sessions 11-15). Figure 1C shows the development of conditioning during session 11 (i.e., the transfer-training session when the location of the US was changed) for rats in the paired-to-paired group. CR percentage was lower in the eyelid ipsilateral to the US (~ 60%) on the first 10-trial block of session 11 compared to the eyelid contralateral to the US (~80%). An ANOVA revealed a significant interaction of the eyelid and session variables for session 11 [F(9,108) = 2.35, p < 0.02]. Follow-up tests (Tukey's honestly significant difference, HSD) confirmed that CR percentage obtained during the first block of session 11 differed significantly between the eyelids, p < 0.01. No significant differences in CR percentage between the eyelids were found for the remaining blocks of session 11.
Figure 1D shows CR amplitude for Group 1 rats in the paired-to-paired conditioning during the initial (1-10) and transfer (11-15) sessions. CR amplitude was greater in the ipsilateral eyelid compared to the contralateral eyelid during session 1-10 and CR amplitude increased in the other eyelid after the location of the US changed (sessions 11-15). Separate ANOVAs revealed significant interactions of the eyelid (ipsilateral vs contralateral) and session factors, before (i.e., session 1-10) [F(9,54) = 7.85, p < 0.01] and after (i.e., session 11-15), [F(4,24) = 13.55, p < 0.01] the location of the US was changed. CR amplitude was significantly larger in the eyelid ipsilateral to the US, relative to the other eyelid, during sessions 3-10, p < 0.05, but CR amplitude in that eyelid decreased during the transfer session and subsequently became significantly larger in the newly conditioned eyelid during sessions 12-15, p < 0.05. Overall, the amount of savings for CR amplitude was less robust compared to the amount of savings for CR percentage.
Group 2: CS Modality Changed
Changing the CS modality-alone (Group 2) after initial training resulted in robust cross-modal transfer in both eyelids. That is, conditioning with the new CS modality in the paired group developed more rapidly than with the first CS (Figure 2A, session 1-5 vs Figure 2B, sessions 11-15). Acquisition to the new CS modality in the paired-to-paired group was also faster than acquisition in the unpaired-to-paired (Figure 2A vs 2B, sessions 11-15). Rats in the paired-to-paired group had an overall CR percentage of ~60% on session 11 (i.e., the transfer session) whereas rats in the unpaired-to-paired group had CR percentage of ~15% on session 11. CR percentage in Group 2 rats was higher in the eyelid that was ipsilateral to the US during sessions 11-15 (Figure 2A, sessions 11-15 & Figure 2B, session 11-15). ANOVAs of the eyelid and session factors confirmed that CR percentages were significantly greater in the ipsilateral eyelid than contralateral eyelid for paired-to-paired rats [F(1,7) = 9.89, p < 0.02] and unpaired-to-paired rats [F(1,5) = 8.45, p < 0.05]. Figure 2C shows the development of cross-modal transfer during session 11 for rats in the paired-to-paired group. CRs were not immediately elicited to the new CS modality, but were acquired rapidly throughout the session. That is, CR percentage was initially low (~10%) on the first block of the transfer session, but steadily increased to ~75% by the tenth block, which indicates savings.
Figure 2D shows CR amplitude for the rats in the paired group during the initial (1-10) and transfer (11-15) sessions. Differences in CR amplitude were confirmed with using separate ANOVAs which revealed a significant interaction of the eyelid and session factors for initial training, [F(9,36) = 3.66, p < 0.01] and a main effect of eyelid during transfer training [F(1,7) = 24.4, p < 0.01]. Follow-up tests (HSD) showed that CR amplitude in the eyelid ipsilateral to the US was greater than the contralateral eyelid during sessions 4-10 and also throughout the transfer training sessions (i.e., sessions 11-15), ps < 0.01. The maintenance of CR amplitude from session 10 to session 11 indicates robust cross-modal transfer of CR amplitude.
Group 3: US Location and CS Modality Changed
Changing the US location and CS modality-together (Group 3) after initial training produced substantial cross-modal transfer in both eyelids (sessions 11-15; Figure 3A). Conditioning in paired rats was faster with the new CS modality compared to initial training with the other CS modality (Figure 3A, session 1-5 vs sessions 11-15) and the rate of conditioning during the transfer sessions was faster for rats in the paired-paired condition than rats in the unpaired-to-paired condition (Figure 3A, sessions 11-15 vs Figure 3B, sessions 11-15). During the transfer session, CR percentage became greater in the eyelid that was previously contralateral to the US in both groups of rats (Figure 3A, sessions 11-15; Figure 3B, session 11-15). These findings were confirmed with separate ANOVAs which revealed significant interactions of the eyelid and session variables for rats given paired-to-paired, [F(4,24) = 3.20, p < 0.04] and unpaired-to-paired, [F(4,28) = 2.81, p < 0.05]. Follow-up tests (HSD) showed that CR percentage in the eyelid ipsilateral to the US was greater than the eyelid contralateral to the US during session 13-15 for both training groups, ps < 0.01. Figure 3C shows the development of cross-modal transfer during session 11 for rats in the paired group. Similar to the findings in Group 2, CR percentage was initially low (~10%) on the first block of session 11 (i.e., the transfer session), but increased rapidly (up to ~80%) by the tenth block. No significant differences were observed between the eyelids during this session. Figure 3D shows CR amplitude for the rats in the paired group during the two phases of training. Similar to the findings in Group 1, CR amplitude became larger in the eyelid that was previously contralateral to the US after the location of the US was changed (sessions 11-15).
CR amplitude in the eyelid ipsilateral to the US increased with transfer training, whereas CR amplitude in the eyelid contralateral to the US decreased. An ANOVA revealed a significant interaction of the eyelid (ipsilateral vs contralateral) and session factors after the location of the US was changed, [F(4,24) = 23.57, p < 0.01]. Follow-up tests (HSD) confirmed that CR amplitude was significantly larger in the eyelid ipsilateral to the US during sessions 12-15, and CR amplitude was significantly greater in the eyelid contralateral to the US on session 11, ps < 0.05. Similar to Group 1, savings for CR percentage was more robust relative to savings for CR amplitude.
Discussion
The results of the present experiment show that cross-modal transfer occurs robustly in eyeblink conditioning with rats when both the US location (e.g., from left to right periorbital region) and CS modality (e.g., from a tone to light CS) are changed together during the transfer session. Cross-modal savings was also observed when the CS modality (e.g., from a tone to light CS) was switched and the US location was held constant. Changing the location of the US and keeping the CS modality constant produced immediate savings of CR percentage in both eyelids. One general finding in the present experiment was that CR percentages and amplitudes in the eyelid ipsilateral to the location of the US were usually greater, or showing a trend to become greater, than the eyelid contralateral to the location of the US. When the US location was changed during the transfer session, CR percentage and amplitude gradually became more dominant in the newly reinforced eyelid (Figures 1A&D and 3A&D). When the modality of the CS was changed, but not the location of the US, CR percentage and amplitude during cross-modal training remained greater in the reinforced eyelid compared to the non-reinforced eyelid (Figure 2A&D).
The present findings support the hypothesis that cross-modal savings results from a general transfer of the CS-US association, rather than immediate CS generalization (Kehoe, 1988; Campolattaro & Freeman, 2009b). The general transfer of the CS-US association, as previously found in other cross-modal savings experiments, has been characterized as a “learning-to-learn” phenomenon (e.g., Kehoe, 1988; Kehoe et al., 1995). Rats that were given paired training with first modality CS showed facilitated learning to the new modality CS, and this enhanced rate of learning occurred whether or not the location of the US was changed during the transfer session. Importantly, CR percentages started at baseline levels during the first block of cross-modal training, which gradually increased during the session (Figure 2C and 3C, session 11). Rats in Groups 2 and 3 showed general transfer of learning, whereas rats in Group 1, which were given transfer training with only the location of the US switched, showed immediate generalization of responding (Figure 1A, session 11 vs Figures 2A and 3A, session 11). Rats given unpaired training acquired eyeblink CRs at a de novo rate after they received paired training (Figures 1B, 2B and 3B, sessions 11-15). Moreover, a previous study did not find impaired acquisition following unpaired training (Campolattaro & Freeman, 2009b). Therefore, the enhanced rate of learning observed during the transfer session for those rats initially given paired training was likely associative in nature and not due to a stimulus pre-exposure effect.
Changing the location of the US alone (Group 1) resulted in immediate savings in CR percentage (Figure 1A) with a steady increase in CR amplitude (Figure 1D) on the newly reinforced side. This result is consistent with previous findings showing that CRs produced on the same side as the US presentation tend to be larger (i.e., have greater amplitude) than those produced on the contralateral side (Campolattaro & Freeman, 2009a; Disterhoft et al., 1977; Hilgard & Campbell, 1936; Hilgard & Marquis, 1935; 1936). Additionally, experiments using eyeblink conditioning procedures with rabbits have previously observed facilitation of learning, evident by a rapid acquisition of eyeblink CRs in the newly reinforced eye, when training was continued with the same exact CS (Pearce et al., 1981) or same sensory modality CS (e.g., a tone CS; Lee et al., 2008).
Changing the modality of the CS alone (Group 2) resulted in cross modal savings at a rate similar to that found in rabbits (Kehoe & Holt, 1984; Holt & Kehoe, 1985; Schreurs & Kehoe, 1987; Kehoe, 1988; Kehoe et al., 1995). Unlike the rabbits in the Pearce et al (1981) study, however, the present study with rats found that changing the sensory modality of the CS and the location of the US at the same time produced savings in the newly reinforced eye (Group 3). Inter-eye cross modal savings may occur more robustly in rats because memory formation of the eyeblink CR in rabbits is more highly lateralized to the conditioned side. The enhanced rate of responding to the new modality CS in the newly reinforced eyelid may also be quicker in rats because they tend to express more bilateral CRs than rabbits (Brandon, Betts, & Wagner, 1994; Campolattaro & Freeman, 2008).
The memory underlying the eyeblink CR is thought to result from plasticity in the cerebellar interpositus nucleus (IPN) and cerebellar cortex induced during paired training (Freeman, 2014). Prior studies have found that permanent or reversible lesions of the IPN ipsilateral to the US location prevent acquisition of eyeblink CRs (Freeman et al., 2005; Krupa, Thompson, & Thompson, 1993; Krupa & Thompson, 1997; McCormick, Clark, Lavond, & Thompson, 1982), whereas lesions to the contralateral IPN do not disrupt eyeblink conditioning on the side where the US is presented (Lavond et al., 1994). If an IPN lesion is made prior to initial paired training, eyeblink CRs are not observed in either eye, but CRs develop rapidly if conditioning is switched to the other side (Lavond et al., 1994). Savings observed on the contralateral eyelid may be due to the formation of a memory trace that is established when the non-lesion side is trained. However, if an ipsilateral IPN lesion is made after initial training, then no savings is seen when conditioning is switched to the other side (Lavond et al., 1994).
Savings observed for CR percentage in the present experiment was more robust than CR amplitude when the US location was changed during transfer training. This finding was observed whether or not the modality of the CS was also changed. It is possible that greater bilateral plasticity develops in the cerebellar cortex than the IPN during initial training (Figure 4A). When transfer training is giving, plasticity in the cerebellar cortex may mediate small amplitude CRs in the contralateral eye, but plasticity in the IPN for the “naïve” side needs to develop to generate higher amplitude CRs (Figure 4B).
Figure 4.
This proposed schematic represents how the cerebellar interpositus nucleus (IPN) and cerebellar cortex (CCTX) are important for acquisition and transfer training. (A) During acquisition, high amplitude conditioned responses (CR) are supported by the IPN and CCTX ipsilateral to the US, and low amplitude conditioned responses (cr) observed in the contralateral eyelid are mediated by learning-related changes that develop in the CCTX on that side, possibly through a contralateral CCTX projection (= dotted line). (B) Rapid conditioning occurs during transfer-training whether or not the CS modality is changed because a weak contralateral memory trace had formed during acquisition. High amplitude conditioned responses (CR) develop on the side where the US is now located and lower amplitude conditioned responses (cr) form on the side that was previously conditioned. The increase in CR amplitude is likely driven by learning-related changes in the IPN and CCTX ipsilateral to the US.  = tone CS;
= tone CS;  = light CS;
= light CS;  = periorbital stimulation US.
= periorbital stimulation US.
Learning-related modifications that support cross-modal savings in the IPN ipsilateral to the US may play an important role in establishing savings in the contralateral IPN (as suggested by Lavond et al., 2011). This effect may be driven by multimodal IPN neurons that support cross-modal transfer when only one side is trained (Campolattaro et al., 2011). It remains possible that some learning-related changes in neural activity occur in the contralateral IPN while the ipsilateral IPN side is conditioned (Lee et al., 2008), although Lavond et al (1994) argue against this hypothesis. Nevertheless, learning-related changes in the contralateral cerebellar hemisphere (e.g., cerebellar cortex), or even changes elsewhere in the brain (e.g., prefrontal cortex, hippocampus and/or amygdala), might contribute to the enhanced rate of learning during inter-eye transfer training sessions. Additional experiments will be necessary to test these possibilities.
Acknowledgments
This research was supported by National Institute for Mental Health grant 080005 to JHF.
References
- Brandon SE, Betts SL, Wagner AR. Discriminated lateralized eyeblink conditioning in the rabbit: An experimental context for separating specific and general associative influences. Journal of Experimental Psychology. Animal Behavior Processes. 1994;20:292–307. doi: 10.1037//0097-7403.20.3.292. [DOI] [PubMed] [Google Scholar]
- Brown KL, Stanton ME. Cross-modal transfer of the conditioned eyeblink response during interstimulus interval discrimination training in young rats. Developmental Psychobiology. 2008;50:647–664. doi: 10.1002/dev.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JH. Examination of bilateral eyeblink conditioning in rats. Behavioral Neuroscience. 2009a;123:1346–1352. doi: 10.1037/a0017314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JH. Cerebellar inactivation impairs cross modal savings of eyeblink conditioning. Behavioral Neuroscience. 2009b;123:292–302. doi: 10.1037/a0014483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolattaro MM, Kashef A, Lee I, Freeman JH. Neuronal correlates of cross-modal transfer in the cerebellum and pontine nuclei. Journal of Neuroscience. 2011;31:4051–4062. doi: 10.1523/JNEUROSCI.4142-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolattaro MM, Schnitker KM, Freeman JH. Changes in inhibition during differential eyeblink conditioning with increased training. Learning & Behavior. 2008;36:159–165. doi: 10.3758/lb.36.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Zhang AA, Lavond DG. The importance of cerebellar cortex and facial nucleus in acquisition and retention of eyeblink/NM conditioning: Evidence for critical unilateral regulation of the conditioned response. Neurobiology of Learning and Memory. 1997;67:96–111. doi: 10.1006/nlme.1996.3740. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Kwan HH, Lo WD. Nictitating membrane conditioning to tone in the immobilized albino rabbit. Brain Research. 1977;137:127–143. doi: 10.1016/0006-8993(77)91016-2. [DOI] [PubMed] [Google Scholar]
- Freeman JH. Cerebellar learning mechanisms. Brain Research. 2014 doi: 10.1016/j.brainres.2014.09.062. Advance online publication. doi:10.1016/j.brainres.2014.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr, Halverson HE, Poremba A. Differential effects of cerebellar inactivation on eyeblink conditioned excitation and inhibition. Journal of Neuroscience. 2005;25:889–895. doi: 10.1523/JNEUROSCI.4534-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormezano I, Kehoe EJ, Marshall BS. 20 years of classical-conditioning research with the rabbit. Progress in Psychobiology and Physiological Psychology. 1983;10:197–275. [Google Scholar]
- Gormezano I, Schneiderman N, Deaux E, Fuentes I. Nictitating membrane: Classical conditioning and extinction in the albino rabbit. Science. 1962;138:33–34. doi: 10.1126/science.138.3536.33. [DOI] [PubMed] [Google Scholar]
- Hilgard ER, Marquis DG. Acquisition, extinction, and retention of conditioned lid responses to light in dogs. Journal of Comparative Psychology. 1935;19:29–58. [Google Scholar]
- Hilgard ER, Campbell AA. The course of acquisition and retention of conditioned eyelid responses in man. Journal of Experimental Psychology. 1936;19:227–247. [Google Scholar]
- Hilgard ER, Marquis DG. Conditioned eyelid responses in monkeys, with a comparison of dog, monkey, and man. Psychological Monographs. 1936;47:186–198. [Google Scholar]
- Holt PE, Kehoe EJ. Cross-modal transfer as a function of similarities between training task in classical conditioning of the rabbit. Animal Learning & Behavior. 1985;13:51–59. [Google Scholar]
- Kehoe EJ. A layered network model of associative learning: learning to learn and configuration. Psychological Review. 1988;95:411–433. doi: 10.1037/0033-295x.95.4.411. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ, Holt PE. Transfer across CS-US intervals and sensory modalities in classical conditioning of the rabbit. Animal Learning & Behavior. 1984;12:122–128. [Google Scholar]
- Kehoe EJ, Horne AJ, Macrae M. Learning to learn: real-time features and a connectionist model. Adaptive Behavior. 1995;3:235–271. [Google Scholar]
- Krupa DJ, Thompson JK, Thompson RF. Localization of a memory trace in the mammalian brain. Science. 1993;260:989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Thompson RF. Reversible inactivation of the cerebellar interpositus nucleus completely prevents acquisition of the classically conditioned eye-blink response. Learning & Memory. 1997;3:545–556. doi: 10.1101/lm.3.6.545. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Kanzawa SA, Ivkovich D, Clark RE. Transfer of learning but not memory after unilateral cerebellar lesion in rabbits. Behavioral Neuroscience. 1994;108:284–293. doi: 10.1037//0735-7044.108.2.284. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Steinmetz JE. Acquisition of classical conditioning without cerebellar cortex. Behavioural Brain Research. 1989;33:113–164. doi: 10.1016/s0166-4328(89)80047-6. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Wikgren J, Nokia MS. Inside the Thompson laboratory during the “cerebellar years” and the continuing cerebellar story. Neurobiology of Learning & Memory. 2011;95:114–117. doi: 10.1016/j.nlm.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Lee T, Kim JJ, Wagner AR. Bilateral nature of the conditioned eyeblink response in the rabbit: Behavioral characteristics and potential mechanisms. Behavioral Neuroscience. 2008;122:1306–1317. doi: 10.1037/a0013591. [DOI] [PubMed] [Google Scholar]
- Lincoln JS, McCormick DA, Thompson RF. Ipsilateral cerebellar lesions prevent learning of the classically conditioned nictitating membrane/eyelid response. Brain Research. 1982;242:190–193. doi: 10.1016/0006-8993(82)90510-8. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Clark GA, Lavond DG, Thompson RF. Initial localization of the memory trace for a basic form of learning. Proceedings of the National Academy of Sciences. 1982;79:2731–2735. doi: 10.1073/pnas.79.8.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Lavond DG, Clark GA, Kettner RE, Rising CE, Thompson RF. The engram found? role of the cerebellum in classical conditioning of nictitating membrane and eyelid responses. Bulletin of the Psychonomic Society. 1981;18:103–105. [Google Scholar]
- Pearce JM, Montgomery A, Dickinson A. Contralateral transfer of inhibitory and excitatory eyelid conditioning in the rabbit. Quarterly Journal of Experimental Psychology Section B-Comparative and Physiological Psychology. 1981;33:45–61. [Google Scholar]
- Schreurs BG, Kehoe EJ. Cross-modal transfer as a function of initial training levels in classical conditioning with the rabbit. Learning & Behavior. 1987;15:47–54. [Google Scholar]
- Spence KW. Learning and performance in eyelid conditioning as a function of intensity of the UCS. Journal of Experimental Psychology. 1953;45:57–63. doi: 10.1037/h0058815. [DOI] [PubMed] [Google Scholar]






