Abstract
Background
In the general population, low serum magnesium (Mg) levels are associated with poor outcomes and death. While limited data suggest that low baseline Mg levels may be associated with higher mortality in hemodialysis (HD) patients, the impact of changes in Mg over time is unknown.
Study Design
We examined the association of time-varying serum Mg levels with all-cause mortality using multivariable time-dependent survival models adjusted for clinical characteristics and other time-varying laboratory measures.
Setting & Participants
9,359 maintenance HD patients treated in a large dialysis organization between 2007 and 2011.
Predictor
Time-varying serum Mg levels across 5 Mg increments (<1.8, 1.8–<2.0, 2.0–<2.2, 2.2–<2.4, ≥2.4 mg/dl).
Outcomes
All-Cause Mortality
Results
2,636 individuals died over 5 years. Time-varying serum Mg <2.0 mg/dl was associated with higher mortality after adjustment for demographics and co-morbidities including hypertension, diabetes, and malignancies (reference: Mg 2.2–<2.4 mg/dL): adjusted HRs for serum Mg <1.8 and 1.8–<2.0 mg/dl were 1.39 (95% CI, 1.23–1.58; p<0.001) and 1.20 (95% CI, 1.06–1.36; p=0.004), respectively. Some associations were attenuated to the null after incremental adjustment for laboratory tests, particularly serum albumin. However among patients with serum albumin measurements, low albumin levels (<3.5 g/dl) and Mg <2.0 mg/dl was associated with an additional death risk (adjusted HR, 1.17; 95% CI, 1.05–1.31; p=0.004), while patients with high serum albumin levels (≥3.5 g/dl) exhibited low death risk (adjusted HRs of 0.53 and 0.53 [p≤0.001] for Mg <2.0 mg/dl and ≥2.0 mg/dl, respectively; reference: albumin <3.5 g/dl and Mg ≥2.0 mg/dl).
Limitations
Causality cannot be determined, and residual confounding cannot be excluded given the observational study design.
Conclusions
Lower serum Mg levels are associated with higher mortality in HD patients including in those with hypoalbuminemia. Interventional studies are warranted to examine whether correction of hypomagnesemia ameliorates adverse outcomes in this population.
Keywords: Magnesium, hypomagnesemia, time-varying serum magnesium, dialysis, serum albumin, death risk, all-cause mortality, cohort, end-stage renal disease (ESRD), incident hemodialysis patients
Magnesium (Mg) is the second most abundant intracellular cation in the body, and it is essential for the regulation of various enzymatic and cellular functions. Mg homeostasis is tightly regulated in the body, and in patients with preserved kidney function, normal Mg levels are maintained by renal reabsorption and excretion 1,2. In contrast, in dialysis patients, serum Mg levels are largely dependent upon dietary intake and dialysate Mg concentrations.
In the general population, low serum Mg levels have been associated with higher risk of type 2 diabetes mellitus 3,4, hypertension 5–7, cardiac arrhythmia8 cardiovascular disease (CVD), and mortality9–13. Recent data show that hypomagnesemia may be a risk factor for the development of chronic kidney disease (CKD), including end-stage renal disease (ESRD) 14–17. In ESRD patients receiving hemodialysis (HD), limited data suggest that hypomagnesemia is associated with increased all-cause and cardiovascular mortality 18,19. However, these studies were limited by short follow-up time and failure to account for changes in Mg levels over time. Moreover, these studies were conducted outside the United States and thus may not be generalizable to the US dialysis population, given the varying dialysate Mg concentrations used in different countries. Therefore, we conducted what is to our knowledge the first observational study investigating the association between serum Mg and all-cause mortality among a large US cohort of maintenance HD (MHD) patients. We hypothesized that lower serum Mg levels are associated with higher death risk.
Methods
Study Cohort
This was a retrospective study of ESRD patients who were initiated on MHD treatment in one of the outpatient dialysis facilities of a large US dialysis organization, and were followed up over a period of 5 years (January 2007–December 2011)20. Patients were included provided that they were age 18 years or older, underwent in-center HD for at least 60 days, and had serum Mg levels measured at least once during the first 91-day period. The study was approved by the University of California Irvine and DaVita Clinical Research.
Demographic and Clinical Measures
Age was estimated by using date of birth and the date of study entry (date of dialysis initiation). Body mass index (BMI) was calculated at the baseline from post-HD dry body weight in kilograms divided by height in meters squared. Race and ethnicity determinations were based on self-selection and include the following: Caucasian, African-American, Hispanic, Asian, and Other. The following 9 comorbidities (ascertained from ICD-9 codes) were considered: diabetes mellitus, hypertension, atherosclerotic disease, congestive heart failure, cerebrovascular disease, dyslipidemia, chronic obstructive pulmonary disease, liver disease, and malignancy.
Magnesium and Other Laboratory Values
Blood samples were drawn using standardized techniques in all dialysis clinics and were transported within 24 hours to a single laboratory center (DaVita Laboratory, Deland, FL), where the laboratory values were measured by automated and standardized methods. All blood samples were collected pre-dialysis except the post-dialysis serum urea nitrogen for calculation of urea kinetics. Most laboratory values were measured monthly, including serum potassium, bicarbonate, blood urea nitrogen (BUN), calcium, phosphorus, albumin, alkaline phosphatase (ALP), normalized protein catabolic rate (nPCR) and white blood cell count (WBC). Serum intact parathyroid hormone (iPTH) and ferritin were measured at least quarterly. Hemoglobin was measured weekly to bi-weekly in most patients. Delivered dialysis dose was estimated by single-pool Kt/V (spKt/V) using the urea kinetic model.
All patients included in the study had serum Mg measurements within the first 91 days of study entry (baseline quarter). The subsequent serum Mg level was checked frequently but not routinely. To minimize measurement variability, all repeated measures for each 91-day interval from date of dialysis initiation were averaged, and used in all models. For iPTH, ALP, and ferritin, the distributions were skewed, thus they were logarithmically transformed in the adjusted models. The exposure of interest was time-varying serum Mg. Serum Mg per each patient quarter (91 day interval) was divided into 5 groups (<1.8, 1.8–<2.0, 2.0–<2.2, 2.2–<2.4, and ≥2.4 mg/dl). Serum Mg category cutoffs were chosen according to a normal reference range of 1.8–2.4 mg/dl, and a 0.2 mg/dl incremental change within this reference range. Time-varying serum Mg measurements would account for changes in the exposure of over time, and allow for estimation of short term exposure-mortality associations.
Outcome Ascertainment
The study outcome of interest was all-cause mortality from initiation of HD, and, again, patients were followed up over a 5-year follow-up period (January 2007–December 2011). Patients were censored for loss to follow-up, discontinuation of dialysis, kidney transplantation, or transfer to a non-affiliated dialysis clinic.
Statistical Methods
Patients’ baseline demographics, clinical characteristics, and laboratory values across serum Mg categories were summarized as proportions, means ± standard deviation, or medians (interquartile ranges [IQRs]) as dictated by data type, and were compared using analysis of variance for parametric variables (or Kruskal-Wallis test for non-parametric variables) or chi-square test. Correlations between baseline continuous Mg levels and other covariates were examined by Pearson correlation.
The association between 5 levels of time-varying serum Mg and mortality risk was determined using time-dependent Cox proportional hazards regression models, which included repeated and time-updated measures of covariates that were averaged over each 91 day interval from patients’ date of dialysis initiation. Time-varying models allow for the change in exposure and covariates and their association with the outcome over time in order to ascertain short term exposure-mortality associations21. Time varying serum Mg-mortality associations were examined with unadjusted models, and with two levels of multivariable adjustment: (1) Case-mix models, which adjusted for baseline characteristics: age, sex, race/ethnicity (Caucasian, African American, Hispanic, Asian, or other), comorbidities including diabetes mellitus, hypertension and history of cancer, and dialysis dose as indicated by spKt/V ; and (2) case-mix plus malnutrition-inflammation-cachexia syndrome (MICS) models, which included all the covariates in the case-mix model, plus baseline BMI and 11 time-updated laboratory variables that bear associations with clinical outcomes in HD patients: serum albumin, potassium, ALP, pre-dialysis BUN, nPCR, albumin-adjusted calcium, phosphorous, iPTH, hemoglobin, WBC, and ferritin. For sensitivity analyses, the association between time-varying serum Mg as a continuous variable and mortality was examined using non-parametric restricted cubic splines with best estimated knots defined at the 25th, 50th, and 75th percentiles of observed values (1.8, 2.05, and 2.3 mg/dl).
We also conducted Mg-mortality association analyses across a priori defined subgroups to investigate potential effect modification by socio-demographics, comorbid conditions, and laboratory levels. In addition, we examined the relationship between serum Mg and albumin over time using linear mixed regression models with unstructured variance to account for intra-subject correlations in repeated measurements. Associations of combined time-varying Mg and albumin levels and mortality were then examined, where patients were divided into 4 (2 × 2) groups according to their time-updated serum Mg (<2.0 vs. ≥2.0 mg/dl) and albumin levels (<3.5 vs. ≥3.5 g/dl).
For patients with data on serum Mg at baseline but missing for subsequent follow-up periods, the last available Mg level was assumed unchanged until the next measurement or occurrence of the event (death or censor). Missing quarterly laboratory values (< 0.5% for all the tests except nPCR, where 3.8% were missing) were otherwise imputed by population means or medians of the existing values in the same patient quarter in the multivariable models. All analyses were implemented using SAS version 9.3 (SAS Institute Inc) and Stata version 10.1 (Stata Corp LP).
Results
Study Cohort Description
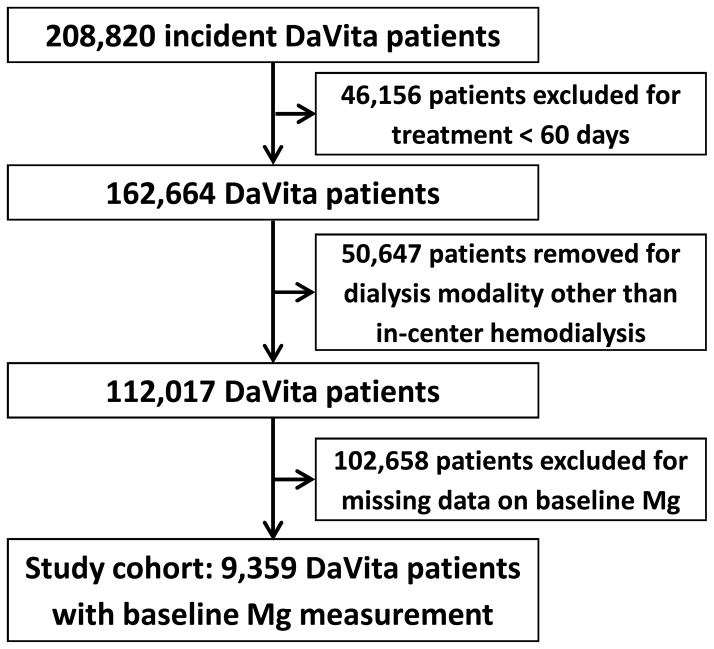
A total of 208,820 ESRD patients who initiated dialysis during January 2007–December 2011 within one of the outpatient facilities of a large dialysis organization were identified. After excluding patients who received treatment for < 60 days or those who underwent a dialysis modality other than thrice-weekly in-center hemodialysis at study entry, there were 112,017 remaining patients (Figure 1). Among these patients, 9,359 patients who had serum Mg levels measured during the first 91-day period following initiation of dialysis formed the final study cohort (Figure 1). The mean serum Mg of the cohort was 2.1 ± 0.4 (standard deviation) mg/dl; and median concentration was 2.1 [IQR, 1.8–2.3] mg/dl). The baseline clinical characteristics of these patients stratified according to five baseline serum Mg categories are presented in Table 1. Patients with lower serum Mg levels tended to be older and Caucasian; were more likely to have had prior malignancy but less likely to have had diabetes; had lower hemoglobin, serum albumin, nPCR, BUN, potassium, adjusted calcium, and phosphorus levels; and had higher ferritin and ALP levels. Serum Mg level positively correlated with nutritional markers including albumin, BUN, and nPCR (Pearson’s correlation coefficient of 0.21, 0.27 and 0.21, respectively; p<0.001 for all). However, the correlations of serum Mg with dialysis dose (i.e. spKt/V) and iPTH were weak and nonsignificant (Pearson’s correlation coefficients of −0.01 [p=0.2] and 0.01 [p=0.4], respectively).
Figure 1.
Algorithm (flow chart) of patient selection for the cohort.
Table 1.
Baseline characteristics of 9,359 hemodialysis patients according to baseline serum magnesium level.
| Characteristics | Total (N=9359) | <1.8 (n=1809) | 1.8 – <2.0 (n=1870) | 2.0 – <2.2 (n=2278) | 2.2 – <2.4 (n=1712) | ≥ 2.4 (n=1690) |
|---|---|---|---|---|---|---|
| Age (years) | 63.3 (14.9) | 64.5 (14.4) | 64.4 (14.6) | 63.2 (14.9) | 62.8 (14.8) | 61.3 (15.8) |
| Female Sex | 43.8 | 46.1 | 44.2 | 43.5 | 42.2 | 42.9 |
| Race* | ||||||
| Caucasian | 53.4 | 56.3 | 53.0 | 54.8 | 52.2 | 50.1 |
| African American | 29.2 | 31.5 | 31.5 | 28.7 | 28.0 | 26.1 |
| Hispanic | 13.0 | 9.1 | 11.4 | 12.8 | 15.4 | 17.0 |
| Asian | 2.0 | 1.3 | 1.6 | 1.6 | 1.9 | 3.4 |
| Other | 2.4 | 1.7 | 2.5 | 2.1 | 3.3 | 4.0 |
| Primary Insurance | ||||||
| Medicare | 56.4 | 55.8 | 57.6 | 57.4 | 55.7 | 55.2 |
| Medicaid | 5.5 | 4.2 | 5.3 | 5.1 | 5.7 | 7.2 |
| Other* | 38.1 | 40.0 | 37.1 | 37.5 | 38.7 | 37.6 |
| BMI kg/m2 | 28.4 (7.6) | 28.5 (7.8) | 28.8 (7.7) | 28.7 (7.7) | 28.3 (7.5) | 27.8 (7.3) |
| Co-morbidities | ||||||
| Diabetes | 59.1 | 54.2 | 60.5 | 58.9 | 61.9 | 60.2 |
| Hypertension* | 46.6 | 45.8 | 44.9 | 47.0 | 47.3 | 48.1 |
| CVD | 16.4 | 14.3 | 16.3 | 17.7 | 16.7 | 16.6 |
| CHF | 37.8 | 36.6 | 37.9 | 37.4 | 38.5 | 39.0 |
| CVA | 1.2 | 0.8 | 1.3 | 1.2 | 1.0 | 1.4 |
| Dyslipidemia | 26.1 | 25.5 | 26.6 | 24.0 | 26.5 | 28.4 |
| COPD* | 5.1 | 4.8 | 5.2 | 5.5 | 4.4 | 5.3 |
| Liver disease* | 1.5 | 2.1 | 1.7 | 1.5 | 1.3 | 1.2 |
| Cancer | 2.5 | 3.6 | 2.2 | 2.5 | 2.0 | 1.9 |
| Baseline Laboratory values | ||||||
| Hb (g/dl) | 11.1 (1.2) | 10.8 (1.2) | 11.0 (1.2) | 11.1 (1.1) | 11.3 (1.2) | 11.4 (1.2) |
| Hb < 10 g/dl, % | 17.9 | 25.3 | 19.7 | 16.9 | 14.6 | 12.8 |
| WBC (×103/dl)* | 7.87 (2.90) | 7.91 (2.70) | 7.91 (2.87) | 7.989 (3.17) | 7.78 (3.05) | 7.84 (2.61) |
| Ferritin (ng/ml)* | 302 [174–521] | 382 [217–634] | 316 [187–564] | 306 [177–514] | 271 [154–461] | 256 [144–427] |
| Albumin (g/dl) | 3.50 (0.48) | 3.35 (0.52) | 3.42 (0.48) | 3.52 (0.45) | 3.59 (0.45) | 3.64 (0.44) |
| Albumin <3.5 g/dl, % | 43.5 | 56.6 | 49.9 | 42.4 | 36.4 | 31.2 |
| nPCR (g/kg/d) | 0.79 (0.22) | 0.72 (0.21) | 0.76 (0.21) | 0.79 (0.21) | 0.82 (0.22) | 0.86 (0.22) |
| ALP (IU/l)* | 87 [69–116] | 91 [70–120] | 88 [71–118] | 86 [68–115] | 86 [68–115] | 86 [67–111] |
| Cholesterol | ||||||
| Total (mg/dl) | 150.8 (45.9) | 145.7 (45.3) | 150.5 (44.2) | 151.3 (47.2) | 151.7 (44.7) | 155.6 (47.1) |
| HDL (mg/dl) | 40.2 (14.1) | 38.8 (13.7) | 40.0 (14.5) | 39.5 (13.6) | 40.9 (14.4) | 42.3 (14.5) |
| LDL (mg/dl) | 78.6 (35.0) | 74.9 (34.3) | 78.5 (33.0) | 79.5 (35.8) | 79.2 (34.8) | 80.8 (36.5) |
| TG (mg/dl)* | 159.0 (91.7) | 160.2 (92.5) | 161.6 (91.9) | 156.1 (86.7) | 155.8 (86.1) | 162.0 (102.6) |
| Potassium (mEq/l) | 4.4 (0.5) | 4.3 (0.5) | 4.4 (0.5) | 4.4 (0.5) | 4.5 (0.5) | 4.6 (0.5) |
| Bicarbonate (mEq/l) | 23.7 (2.8) | 23.6 (2.9) | 23.8 (2.7) | 23.7 (2.6) | 23.7 (2.8) | 23.6 (2.9) |
| SUN (mg/dl) | 48.1 (14.5) | 42.9 (13.9) | 45.5 (13.7) | 47.8 (13.5) | 50.5 (14.3) | 54.2 (14.8) |
| Adj Ca (mg/dl) | 9.1 (0.6) | 8.9 (0.6) | 9.1 (0.5) | 9.1 (0.5) | 9.2 (0.6) | 9.2 (0.6) |
| Phosphorus (mg/dl) | 4.9 (1.2) | 4.5 (1.1) | 4.7 (1.1) | 4.8 (1.1) | 5.0 (1.2) | 5.3 (1.2) |
| iPTH (pg/ml)** | 303 [189, 476] | 306 [190, 484] | 313 [193, 483] | 302 [191, 471] | 299 [185, 468] | 296 [183, 468] |
| iPTH <150 pg/ml, % | 16.7 | 16.8 | 15.3 | 16.5 | 16.1 | 18.7 |
| Dialysis adequacy: spKt/V* | 1.58 (0.33) | 1.48 (0.33) | 1.48 (0.34) | 1.49 (0.34) | 1.47 (0.32) | 1.48 (0.34) |
Note: Magnesium expressed in mg/dL. Unless otherwise indicated, values for categorical variables are given as percentages; values for continuous variables, as mean ± standard deviation or median [interquartile range]. Conversion factors for units: calcium in mg/dL to mmol/L, ×0.2495; cholesterol in mg/dL to mmol/L, ×0.02586; phosphorus in mg/dL to mmol/L, ×0.3229; TGs in mg/dL to mmol/L, ×0.01129; SUN in mg/dL to mmol/L, ×0.357.
p-value not significant.
skewed distribution, median [1st quartile, 3rd quartile], and Kruskal-Wallis test.
Comparison across serum Mg levels by ANOVA or Kruskal-Wallis test for continuous variables, or chi-square test for categorical variables.
Abbreviations: BMI, body mass index; CHF, congested heart failure; CVD, cardiovascular disease; CVA, cerebrovascular accident; COPD, chronic obstructive pulmonary disease; Hb, hemoglobin; WBC, white blood cell; nPCR, normalized protein catabolic rate; ALP, alkaline phosphatase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglycerides; SUN, serum urea nitrogen; Adj Ca, albumin-adjusted calcium; iPTH, intact parathyroid hormone; spKt/V, single-pool Kt/V.
Serum Magnesium Levels and All-Cause Mortality
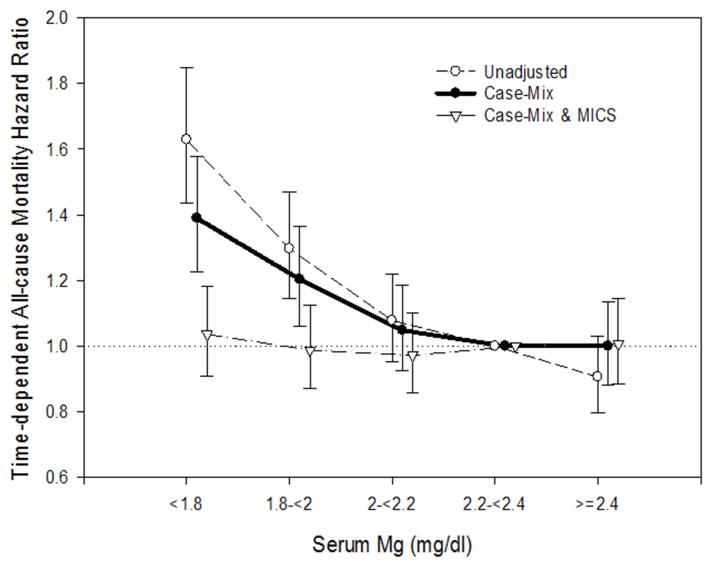
During the follow-up period, a total of 2,636 deaths occurred over a mean follow-up period of 19 ± 15 (range, 2.0–59.9) months. To account for changes in serum Mg levels over time and examine short term serum Mg-mortality associations, serum Mg and all-cause mortality associations were examined using time-varying Cox survival models as shown in Figure 2. In unadjusted time-varying models, with the reference group as Mg 2.2–<2.4 mg/dl, serum Mg levels below 2.0 mg/dl were significantly associated with increased risk for death: HRs of 1.63 (95% CI, 1.44–1.85; p<0.001) and 1.30 (95% CI, 1.15–1.47; p<0.001), for Mg levels of <1.8 and 1.8–<2.0 mg/dl, respectively. These associations were somewhat attenuated after case-mix adjustment but remained statistically significant: adjusted HRs were 1.39 (95% CI, 1.23–1.58; p<0.001) and 1.20 (95% CI, 1.06–1.36; p=0.004) for Mg <1.8 and 1.8–<2.2 mg/dl, respectively. However, these associations were further attenuated to the null after additional adjustment for case-mix and MICS covariates, particularly serum albumin.
Figure 2.
Time-dependent all-cause mortality hazard ratios (and 95% confidence interval error bars) by quarterly serum Mg levels. Cox regression with 3 levels of adjustments: (1) Unadjusted; (2) Case-mix adjusted for age, sex, race/ethnicity (Caucasian, African American, Hispanic, Asian, or other), comorbidities including diabetes mellitus, hypertension and history of cancer, and spKt/V; (3) Case-mix & MICS adjusted for all the covariates in the case-mix model, plus BMI and MICS surrogates markers (11 laboratory variables are described in text).
Within these case-mix- and MICS-adjusted models examining the association of serum Mg and mortality risk, higher serum albumin level had a strong association with improved survival; for every 1-g/dl increase in time-varying serum albumin level, there was a 62% decreased risk of mortality (adjusted HR, 0.38; 95% CI, 0.35–0.42; p<0.001). In associations examining time-varying continuous levels of serum Mg with all-cause mortality using non-parametric restricted cubic splines, we observed that both lower and higher serum Mg levels exhibited a trend towards increased mortality risk, although high Mg levels > 3 mg/dl were not statistically significant due to a small sample size (Figure S1, available as online supplementary material).
In case-mix adjusted subgroup analyses (Table S1 and Figure S2), we observed a consistent association between lower serum Mg level and higher mortality across most subgroups, except in patients with high serum albumin levels.
Relationship Between Serum Magnesium and Albumin
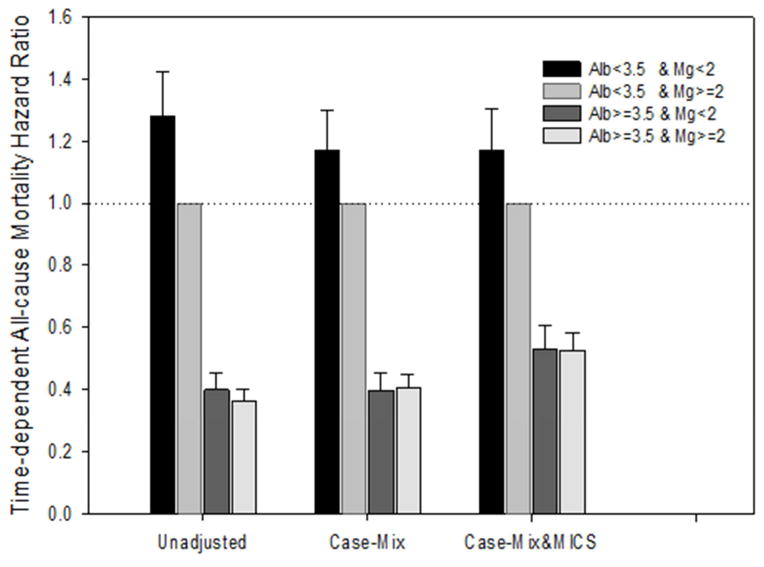
Baseline serum Mg and albumin levels were positively correlated in unadjusted Pearson correlation analysis. In addition, for every 1-g/dl higher albumin level, there was about a 0.2-mg/dl higher serum Mg (p<0.001) level after accounting for intra-subject correlations in repeated measurements over time. The association between serum Mg and mortality differed by serum albumin levels as evidenced by the subgroup analyses. The interaction between time-varying serum Mg and serum albumin was close to statistical significance (p=0.07) in the case-mix- and MICS-adjusted model. When examining associations of time-varying combined levels of serum Mg and serum albumin with mortality, compared with patients with low albumin (<3.5 g/dl) and high Mg (≥2.0 mg/dl) as reference, patients with high albumin (≥3.5 g/dl) had lower mortality irrespective of Mg levels in case-mix- and MICS-adjusted models: adjusted HRs of 0.53 (95% CI, 0.46–0.60; p<0.001) and 0.53 (95% CI, 0.47–0.58; p<0.001) for patients with high albumin/low Mg and high albumin/high Mg, respectively (Table 2 and Figure 3). However, compared to the reference group comprising patients with low albumin and high Mg, membership in the low albumin and low Mg group was associated with an additional 17% higher risk of mortality in case-mix- and MICS-adjusted models: adjusted HR, 1.17 (95% CI, 1.05–1.30; p=0.004).
Table 2.
Time-dependent all-cause mortality hazard ratios, by serum Mg and albumin concentration categories.
| Alb* (g/dl) | Mg* (mg/dl) | Unadjusted | Case-Mix | Case-Mix+MICS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | ||||||||
| <3.5 | <2.0 | 1.28 (1.15–1.42) | <0.001 | 1.17 | 1.05 | 1.30 | 0.004 | 1.17 | 1.05 | 1.31 | 0.004 | ||
| ≥2.0 | 1.00 (reference) | -- | 1.00 (reference) | -- | 1.00 (reference) | -- | |||||||
| ≥3.5 | <2.0 | 0.40 | 0.35 | 0.45 | <0.001 | 0.40 | 0.35 | 0.45 | <0.001 | 0.53 | 0.46 | 0.60 | <0.001 |
| ≥2.0 | 0.36 | 0.33 | 0.40 | <0.001 | 0.41 | 0.37 | 0.45 | <0.001 | 0.53 | 0.47 | 0.58 | <0.001 | |
Values are quarterly measurements. Reference group: serum Mg ≥2.0 mg/dl and Albumin <3.5 g/dl.
HR: all-cause mortality hazard ratio; CI: confidence interval; Mg, magnesium; MICS, malnutrition-inflammation-cachexia syndrome
Case-Mix analyses adjusted for age (years), sex (male, female), race (Caucasian, African American, Hispanic, Asian, or other), diabetes (yes, no), hypertension (yes, no), cancer (yes, no), and single-pool Kt/V.
Case-Mix + MICS: Case-Mix+ body mass index (kg/m2), hemoglobin (g/dl), white blood cell count (×103/dl), ferritin (log ng/ml), alkaline phosphatase (log IU/l), potassium (mEq/l), albumin-adjusted calcium (mg/dl), phosphorous (mg/dl), intact parathyroid hormone (log pg/ml), albumin (g/dl), urea nitrogen (mg/dl), nPCR (g/kg/d).
Figure 3.
Time-dependent all-cause mortality hazard ratios (and 95% confidence interval error bars) across 4 groups of serum Mg (<2 or ≥2 mg/dl) and albumin (<3.5 or ≥3.5 g/dl) combination, with Mg ≥2 mg/dl and albumin <3.5 g/dl as reference. Cox regression with 3 levels of adjustments: (1) Unadjusted; (2) Case-mix adjusted for age, sex, race/ethnicity (Caucasian, African American, Hispanic, Asian, or other), comorbidities including diabetes mellitus, hypertension and history of cancer, and spKt/V; (3) Case-mix & MICS adjusted for all the covariates in the case-mix model, plus BMI and MICS surrogates markers (11 laboratory variables are described in text)
Discussion
In this study, we found that lower serum Mg levels were significantly associated with increased all-cause mortality in MHD patients independent of sociodemographics and comorbidities using a time-varying model. However, we observed that the association was attenuated to the null when incrementally adjusted for inflammatory markers, especially with serum albumin. The association between time-varying serum Mg and mortality was modified by time-varying serum albumin level. Among hypoalbuminemic HD patients, hypomagnesemia contributed to an additional higher mortality risk.
To our knowledge, our study is the first to examine the relationship between time-varying serum Mg and mortality in a large MHD cohort in the United States over an extended follow up period. Our findings are consistent with previous studies of Japanese HD patients by Ishimura et al18 which used an institutional registry and by Sakaguchi et al19 which used national-registry HD data. However, both groups focused on baseline serum Mg alone, and Sakaguchi et al19 limited their follow up to 1-year.
Magnesium is an essential cation for vital cellular functions in the body. Under normal conditions, approximately 1% of total body Mg is found in extracellular fluid. In the general population, magnesium homeostasis is dependent upon the balance between dietary intake, kidney reabsorption and excretion by the renal tubules, particularly in the thick ascending limb of the loop of Henle and distal nephrons 1,2,22. Serum Mg levels are tightly regulated with a narrow normal range of 1.8 to 2.4 mg/dl. In anuric dialysis patients, there is loss of renal regulation of Mg homeostasis, and Mg levels are largely dependent upon dietary intake and dialysate Mg concentrations. In Japan and the United States, dialysate Mg concentrations of 1.0 mEq/l and 0.5 mEq/l, respectively, are typically used. Consequently, the mean serum Mg levels in the aforementioned Japanese HD studies were much higher than in our cohort (2.77 ± 0.33 mg/dl 18 and 2.61 ± 0.52 mg/dl 19, versus 2.07 ± 0.36 mg/dl).
Despite their impaired capacity for Mg renal excretion, low serum Mg levels have commonly been reported in patients receiving HD or peritoneal dialysis (PD) 23–25. Hypomagnesemia in this context has been attributed to decreased dietary intake 26, protein-energy wasting 18,19,27, and increased use of proton pump inhibitors21. In the present study, patients with lower serum Mg at baseline had a much higher prevalence of malnutrition, as assessed by protein-energy wasting markers including lower serum albumin, SUN, and nPCR. Malnutrition is common among dialysis patients 28. A low serum albumin level, which was attributed to low protein intake and a high state of inflammation 29, is one of the strongest predictors of all-cause and CVD mortality in dialysis patients 30–32. In our study, serum albumin was a dominant independent predictor of mortality after fully adjusting for all available potential comorbid and sociodemographic confounders (p<0.001). Indeed, after adjusting for serum albumin, associations between Mg and mortality were attenuated to the null in case-mix- and MICS-djusted survival models. However, among patients with low albumin levels, we found that low Mg was associated with an additional 17% higher death risk compared to those with high serum Mg levels. These findings suggest that serum albumin is not only a confounder but also a modifier of the association of serum Mg with all-cause mortality, and that additional pathogenic factors beyond protein-energy wasting may account for the link between lower serum Mg and death.
Hypomagnesemia may be associated with adverse outcomes via several mechanistic pathways. First, Mg deficiency has been shown to induce endothelial dysfunction and promote atherosclerosis in both in vitro 33 and in vivo studies 34. Second, low Mg level promotes vascular calcification and vascular stiffness in both animal 35,36 and human studies 37,38, including patients undergoing maintenance dialysis 39,40. Third, Mg possesses anti-inflammatory and antioxidant properties. Lower serum Mg is associated with increased inflammation in both non-dialyzed 41,42 and dialyzed subjects 18,19. In our study, patients with lower Mg had higher ferritin and lower albumin levels, an indication of increased inflammation. Fourth, Mg deficiency is associated with insulin resistance and metabolic syndrome 43,44, including higher incidence of hypertension and dyslipidemia 45,46. Furthermore, data from the general population suggest that Mg supplementation is associated with a lower incidence of diabetes 47, better control of diabetes 48 and hypertension 6, and less inflammation and endothelial dysfunction 49,50. In dialysis patients, long-term Mg supplementation has been reported to reduce carotid intima-medial thickness 40. However, further studies are needed to examine whether correction of hypomagnesemia by either oral supplement or higher Mg concentration in the dialysate would reduce risk for death in patients undergoing MHD, especially in those with hypoalbuminemia.
In this study, we observed an L-shaped association between serum Mg levels and mortality, such that mortality risk nadired at a serum Mg level of 2.2 mg/dl. When examined as a continuous variable, we found that there was a trend towards higher death risk with Mg levels > 3 mg/dl, although not statistically significant. Prior data suggest that hypermagnesemia may inhibit PTH secretion, leading to low bone turnover and vascular calcification 51,52 as potential risk factors for CVD and death. However, our study did not show a significant correlation between baseline serum Mg and iPTH. Further studies are needed to confirm the association between moderate to severe hypermagnesemia and mortality risk, and to explore underlying mechanisms.
Strengths of our study include a large sample size of more than 9000 MHD patients, follow-up for up to 5 years, and serial Mg measurements that enabled the time-varying survival analysis to account for short term effects of serum Mg levels. There are several limitations in this present study. First, a large proportion of patients were excluded due to lack of serum Mg measurements, increasing risk of selection bias. However, a comparison of included versus excluded patients in the cohort showed similarity in baseline characteristics (Table S2). A comparison of patients receiving one versus more than one Mg measurements showed that patients who only had a baseline serum Mg measurement had a shorter duration of follow up and were more likely to have diabetes, CHF, CVD and dyslipidemia at baseline (Table S3). Second, as there was no information on specific cause of death in this cohort, we could not investigate the association between serum Mg and cardiovascular mortality. Third, ionized serum Mg was not measured in this cohort. Approximately 30% of serum Mg is bound to protein, primarily albumin, and therefore total measured concentrations of Mg may be affected by hypoalbuminemia53. A currently accepted equation correcting Mg measurements for hypoalbuminemia has not been established, as it has for calcium. Additional studies that include analysis for ionized Mg are warranted. Fourth, although we rigorously adjusted for various plausible confounders, given the observational study design, we are unable to determine if associations were causal.
In conclusion, to our knowledge, this is the first study to examine the association between time-varying serum Mg and mortality risk among a large national MHD cohort. We observed that lower serum Mg was significantly associated with increased all-cause mortality when adjusted for comorbid and sociodemographic variables. We also found that there was a differential association between serum Mg and mortality across serum albumin levels such that hypomagnesemia had a particularly stronger association with death among patients with low albumin levels. Future studies are needed to determine the mechanisms underlying the association of hypomagnesemia with mortality, as well as the impact of correcting low Mg levels with Mg supplementation upon survival among hypomagnesemic dialysis patients.
Supplementary Material
* Reference group: serum Mg 2.2–<2.4 mg/dl
HR: all-cause mortality hazard ratio; CI: confidence interval
Case-Mix analyses adjusted for age (years), sex (male, female), race (Caucasian, African American, Hispanic, Asian, or other), diabetes (yes, no), hypertension (yes, no), cancer (yes, no), and spKt/V
Abbreviations: CHF, congested heart failure; BMI, body mass index; Hb, hemoglobin; ALP, alkaline phosphatase; Alb, albumin; iPTH, intact parathyroid hormone; spKt/V, single-pool Kt/V.
Data presented as mean (SD), median [interquartile range], or proportions.
Comparison between groups by t-test and Wilcoxon-Rank Sum for continuous variables, or chi-square test for categorical variables.
Data presented as mean (SD), median [interquartile range], or proportions.
Comparison between groups by t-test and Wilcoxon-Rank Sum for continuous variables, or chi-square test for categorical variables.
Figure S1. Association of time-varying continuous levels of serum Mg with all-cause mortality hazard ratios
Supplemental Figure 2A. Time-dependent all-cause mortality hazard ratios (and 95% confidence interval error bars) across pre-specified subgroups of Age (<65, ≥65 years). Sex (Male, Female), Race (Caucasian, Other), and Dialysis Dose (spKt/V: <1.2, ≥1.2) using the case-mix model
Supplemental Figure 2B. Time-dependent all-cause mortality hazard ratios (and 95% confidence interval error bars) across pre-specified subgroups of Comorbidties (Yes, No): Hypertension (HTN), Diabetes, and Congestive Heart Failure (CHF), and body mass index (BMI: <30, ≥30 kg/m2) using the case-mix model.
Supplemental Figure 2C. Time-dependent all-cause mortality hazard ratios (and 95% confidence interval error bars) across pre-specified subgroups of Hemoglobin (Hb: <10, ≥10 g/dl). Ferritin (<300, ≥300 ng/ml). Alkaline Phosphatase (ALP: <90, ≥90 IU/1), and Albumin (Alb: <3.5, ≥3.5 g/dl) using the case-mix model.
Supplemental Figure 2D. Time-dependent all-cause mortality hazard ratios (and 95% confidence interval error bars) across pre-specified subgroups of intact Parathyroid Hormone (iPTH: <300, ≥300 pg/ml) using the case-mix model.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Acknowledgments
We thank DaVita Clinical Research for providing the clinical data for this study.
Support: The work is supported by National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases grants K24-DK091419 (KKZ), R01-DK095668 (KKZ and RM), and a philanthropic grant from Mr. Harold Simmons and Mr. Louis Chang. CMR is supposed by the National Institute of Diabetes and Digestive and Kidney Diseases grant K23-DK102903.
Footnotes
Financial Disclosure: KKZ has received honoraria from Genzyme/Sanofi and Shire, and was the medical director of DaVita Harbor-UCLA/Medical Foundation Inc in Long Beach, CA during 2007–2012. The other authors declare that they have no other relevant financial interests.
Contributions: Study concept and design: LL, ES, CMR, KK-Z; data analyses: LL, ES, MS, CMR. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. KK-Z takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that discrepancies from the study as planned have been explained.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Konrad M, Schlingmann KP. Inherited disorders of renal hypomagnesaemia. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014;29(Suppl 4):iv63–71. doi: 10.1093/ndt/gfu198. [DOI] [PubMed] [Google Scholar]
- 2.Schlingmann KP, Konrad M, Seyberth HW. Genetics of hereditary disorders of magnesium homeostasis. Pediatric nephrology. 2004;19(1):13–25. doi: 10.1007/s00467-003-1293-z. [DOI] [PubMed] [Google Scholar]
- 3.Pham PC, Pham PM, Pham SV, Miller JM, Pham PT. Hypomagnesemia in patients with type 2 diabetes. Clinical journal of the American Society of Nephrology : CJASN. 2007;2(2):366–373. doi: 10.2215/CJN.02960906. [DOI] [PubMed] [Google Scholar]
- 4.Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H. Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysis. Archives of internal medicine. 2007;167(9):956–965. doi: 10.1001/archinte.167.9.956. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson HO, Nicolson DJ, Campbell F, et al. Magnesium supplementation for the management of essential hypertension in adults. The Cochrane database of systematic reviews. 2006;(3):CD004640. doi: 10.1002/14651858.CD004640.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Jee SH, Miller ER, 3rd, Guallar E, Singh VK, Appel LJ, Klag MJ. The effect of magnesium supplementation on blood pressure: a meta-analysis of randomized clinical trials. American journal of hypertension. 2002;15(8):691–696. doi: 10.1016/s0895-7061(02)02964-3. [DOI] [PubMed] [Google Scholar]
- 7.Peacock JM, Folsom AR, Arnett DK, Eckfeldt JH, Szklo M. Relationship of serum and dietary magnesium to incident hypertension: the Atherosclerosis Risk in Communities (ARIC) Study. Annals of epidemiology. 1999;9(3):159–165. doi: 10.1016/s1047-2797(98)00040-4. [DOI] [PubMed] [Google Scholar]
- 8.Altura BM, Altura BT. New perspectives on the role of magnesium in the pathophysiology of the cardiovascular system. I. Clinical aspects. Magnesium. 1985;4(5–6):226–244. [PubMed] [Google Scholar]
- 9.Del Gobbo LC, Imamura F, Wu JH, de Oliveira Otto MC, Chiuve SE, Mozaffarian D. Circulating and dietary magnesium and risk of cardiovascular disease: a systematic review and meta-analysis of prospective studies. The American journal of clinical nutrition. 2013;98(1):160–173. doi: 10.3945/ajcn.112.053132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsson SC, Orsini N, Wolk A. Dietary magnesium intake and risk of stroke: a meta-analysis of prospective studies. The American journal of clinical nutrition. 2012;95(2):362–366. doi: 10.3945/ajcn.111.022376. [DOI] [PubMed] [Google Scholar]
- 11.Liao F, Folsom AR, Brancati FL. Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study. American heart journal. 1998;136(3):480–490. doi: 10.1016/s0002-8703(98)70224-8. [DOI] [PubMed] [Google Scholar]
- 12.Reffelmann T, Ittermann T, Dorr M, et al. Low serum magnesium concentrations predict cardiovascular and all-cause mortality. Atherosclerosis. 2011;219(1):280–284. doi: 10.1016/j.atherosclerosis.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 13.Xu T, Sun Y, Xu T, Zhang Y. Magnesium intake and cardiovascular disease mortality: a meta-analysis of prospective cohort studies. International journal of cardiology. 2013;167(6):3044–3047. doi: 10.1016/j.ijcard.2012.11.090. [DOI] [PubMed] [Google Scholar]
- 14.Pham PC, Pham PM, Pham PT, Pham SV, Pham PA, Pham PT. The link between lower serum magnesium and kidney function in patients with diabetes mellitus Type 2 deserves a closer look. Clinical nephrology. 2009;71(4):375–379. doi: 10.5414/cnp71375. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi Y, Shoji T, Hayashi T, et al. Hypomagnesemia in type 2 diabetic nephropathy: a novel predictor of end-stage renal disease. Diabetes care. 2012;35(7):1591–1597. doi: 10.2337/dc12-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tin A, Grams ME, Maruthur NM, et al. Results from the Atherosclerosis Risk in Communities study suggest that low serum magnesium is associated with incident kidney disease. Kidney international. 2015 Apr;87(4):820–7. doi: 10.1038/ki.2014.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Laecke S, Nagler EV, Verbeke F, Van Biesen W, Vanholder R. Hypomagnesemia and the risk of death and GFR decline in chronic kidney disease. The American journal of medicine. 2013;126(9):825–831. doi: 10.1016/j.amjmed.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 18.Ishimura E, Okuno S, Yamakawa T, Inaba M, Nishizawa Y. Serum magnesium concentration is a significant predictor of mortality in maintenance hemodialysis patients. Magnesium research : official organ of the International Society for the Development of Research on Magnesium. 2007;20(4):237–244. [PubMed] [Google Scholar]
- 19.Sakaguchi Y, Fujii N, Shoji T, Hayashi T, Rakugi H, Isaka Y. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney international. 2014;85(1):174–181. doi: 10.1038/ki.2013.327. [DOI] [PubMed] [Google Scholar]
- 20.Kuttykrishnan S, Kalantar-Zadeh K, Arah OA, et al. Predictors of treatment with dialysis modalities in observational studies for comparative effectiveness research. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2015 doi: 10.1093/ndt/gfv097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dekker FW, de Mutsert R, van Dijk PC, Zoccali C, Jager KJ. Survival analysis: time-dependent effects and time-varying risk factors. Kidney international. 2008;74(8):994–997. doi: 10.1038/ki.2008.328. [DOI] [PubMed] [Google Scholar]
- 22.Musso CG. Magnesium metabolism in health and disease. International urology and nephrology. 2009;41(2):357–362. doi: 10.1007/s11255-009-9548-7. [DOI] [PubMed] [Google Scholar]
- 23.Eisenman K, Holley JL. A higher magnesium dialysate concentration treats hypomagnesemia. Peritoneal dialysis international : journal of the International Society for Peritoneal Dialysis. 2005;25(6):604–605. [PubMed] [Google Scholar]
- 24.Ejaz AA, McShane AP, Gandhi VC, Leehey DJ, Ing TS. Hypomagnesemia in continuous ambulatory peritoneal dialysis patients dialyzed with a low-magnesium peritoneal dialysis solution. Peritoneal dialysis international : journal of the International Society for Peritoneal Dialysis. 1995;15(1):61–64. [PubMed] [Google Scholar]
- 25.Markell MS, Altura BT, Sarn Y, et al. Deficiency of serum ionized magnesium in patients receiving hemodialysis or peritoneal dialysis. ASAIO journal. 1993;39(3):M801–804. [PubMed] [Google Scholar]
- 26.Wyskida K, Witkowicz J, Chudek J, Wiecek A. Daily magnesium intake and hypermagnesemia in hemodialysis patients with chronic kidney disease. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2012;22(1):19–26. doi: 10.1053/j.jrn.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Fein P, Suda V, Borawsky C, et al. Relationship of serum magnesium to body composition and inflammation in peritoneal dialysis patients. Advances in peritoneal dialysis Conference on Peritoneal Dialysis. 2010;26:112–115. [PubMed] [Google Scholar]
- 28.Mehrotra R, Kopple JD. Nutritional management of maintenance dialysis patients: why aren’t we doing better? Annual review of nutrition. 2001;21:343–379. doi: 10.1146/annurev.nutr.21.1.343. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y, Molnar MZ, Rattanasompattikul M, et al. Relative contributions of inflammation and inadequate protein intake to hypoalbuminemia in patients on maintenance hemodialysis. International urology and nephrology. 2013;45(1):215–227. doi: 10.1007/s11255-012-0170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, et al. Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2005;20(9):1880–1888. doi: 10.1093/ndt/gfh941. [DOI] [PubMed] [Google Scholar]
- 31.Mehrotra R, Duong U, Jiwakanon S, et al. Serum albumin as a predictor of mortality in peritoneal dialysis: comparisons with hemodialysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;58(3):418–428. doi: 10.1053/j.ajkd.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rambod M, Bross R, Zitterkoph J, et al. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: a 5-year prospective cohort study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009;53(2):298–309. doi: 10.1053/j.ajkd.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maier JA. Endothelial cells and magnesium: implications in atherosclerosis. Clinical science. 2012;122(9):397–407. doi: 10.1042/CS20110506. [DOI] [PubMed] [Google Scholar]
- 34.Adrian M, Chanut E, Laurant P, Gaume V, Berthelot A. A long-term moderate magnesium-deficient diet aggravates cardiovascular risks associated with aging and increases mortality in rats. Journal of hypertension. 2008;26(1):44–52. doi: 10.1097/HJH.0b013e3282f09f68. [DOI] [PubMed] [Google Scholar]
- 35.Inagaki O, Syono T, Nakagawa K, Nishian Y, Takenaka Y, Takamitsu Y. Influence of magnesium deficiency on concentration of calcium in soft tissue of uremic rats. Renal failure. 1996;18(6):847–854. doi: 10.3109/08860229609047711. [DOI] [PubMed] [Google Scholar]
- 36.Planells E, Llopis J, Peran F, Aranda P. Changes in tissue calcium and phosphorus content and plasma concentrations of parathyroid hormone and calcitonin after long-term magnesium deficiency in rats. Journal of the American College of Nutrition. 1995;14(3):292–298. doi: 10.1080/07315724.1995.10718510. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto T, Hara A, Ohkubo T, et al. Serum magnesium, ambulatory blood pressure, and carotid artery alteration: the Ohasama study. American journal of hypertension. 2010;23(12):1292–1298. doi: 10.1038/ajh.2010.168. [DOI] [PubMed] [Google Scholar]
- 38.Van Laecke S, Marechal C, Verbeke F, et al. The relation between hypomagnesaemia and vascular stiffness in renal transplant recipients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26(7):2362–2369. doi: 10.1093/ndt/gfq728. [DOI] [PubMed] [Google Scholar]
- 39.Meema HE, Oreopoulos DG, Rapoport A. Serum magnesium level and arterial calcification in end-stage renal disease. Kidney international. 1987;32(3):388–394. doi: 10.1038/ki.1987.222. [DOI] [PubMed] [Google Scholar]
- 40.Turgut F, Kanbay M, Metin MR, Uz E, Akcay A, Covic A. Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. International urology and nephrology. 2008;40(4):1075–1082. doi: 10.1007/s11255-008-9410-3. [DOI] [PubMed] [Google Scholar]
- 41.Guerrero-Romero F, Rodriguez-Moran M. Hypomagnesemia, oxidative stress, inflammation, and metabolic syndrome. Diabetes/metabolism research and reviews. 2006;22(6):471–476. doi: 10.1002/dmrr.644. [DOI] [PubMed] [Google Scholar]
- 42.King DE. Inflammation and elevation of C-reactive protein: does magnesium play a key role? Magnesium research : official organ of the International Society for the Development of Research on Magnesium. 2009;22(2):57–59. [PubMed] [Google Scholar]
- 43.Barbagallo M, Dominguez LJ. Magnesium metabolism in type 2 diabetes mellitus, metabolic syndrome and insulin resistance. Archives of biochemistry and biophysics. 2007;458(1):40–47. doi: 10.1016/j.abb.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Belin RJ, He K. Magnesium physiology and pathogenic mechanisms that contribute to the development of the metabolic syndrome. Magnesium research : official organ of the International Society for the Development of Research on Magnesium. 2007;20(2):107–129. [PubMed] [Google Scholar]
- 45.Corica F, Corsonello A, Ientile R, et al. Serum ionized magnesium levels in relation to metabolic syndrome in type 2 diabetic patients. Journal of the American College of Nutrition. 2006;25(3):210–215. doi: 10.1080/07315724.2006.10719534. [DOI] [PubMed] [Google Scholar]
- 46.Ma J, Folsom AR, Melnick SL, et al. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: the ARIC study. Atherosclerosis Risk in Communities Study. Journal of clinical epidemiology. 1995;48(7):927–940. doi: 10.1016/0895-4356(94)00200-a. [DOI] [PubMed] [Google Scholar]
- 47.Larsson SC, Wolk A. Magnesium intake and risk of type 2 diabetes: a meta-analysis. Journal of internal medicine. 2007;262(2):208–214. doi: 10.1111/j.1365-2796.2007.01840.x. [DOI] [PubMed] [Google Scholar]
- 48.Song Y, He K, Levitan EB, Manson JE, Liu S. Effects of oral magnesium supplementation on glycaemic control in Type 2 diabetes: a meta-analysis of randomized double-blind controlled trials. Diabetic medicine : a journal of the British Diabetic Association. 2006;23(10):1050–1056. doi: 10.1111/j.1464-5491.2006.01852.x. [DOI] [PubMed] [Google Scholar]
- 49.King DE, Mainous AG, 3rd, Geesey ME, Woolson RF. Dietary magnesium and Creactive protein levels. Journal of the American College of Nutrition. 2005;24(3):166–171. doi: 10.1080/07315724.2005.10719461. [DOI] [PubMed] [Google Scholar]
- 50.Song Y, Li TY, van Dam RM, Manson JE, Hu FB. Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. The American journal of clinical nutrition. 2007;85(4):1068–1074. doi: 10.1093/ajcn/85.4.1068. [DOI] [PubMed] [Google Scholar]
- 51.Courivaud C, Davenport A. Magnesium and the risk of all-cause and cardiac mortality in hemodialysis patients: agent provocateur or innocent bystander? Kidney international. 2014;85(1):17–20. doi: 10.1038/ki.2013.301. [DOI] [PubMed] [Google Scholar]
- 52.Naves-Diaz M, Passlick-Deetjen J, Guinsburg A, et al. Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26(6):1938–1947. doi: 10.1093/ndt/gfq304. [DOI] [PubMed] [Google Scholar]
- 53.Kroll MH, Elin RJ. Relationships between magnesium and protein concentrations in serum. Clinical chemistry. 1985;31(2):244–246. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
* Reference group: serum Mg 2.2–<2.4 mg/dl
HR: all-cause mortality hazard ratio; CI: confidence interval
Case-Mix analyses adjusted for age (years), sex (male, female), race (Caucasian, African American, Hispanic, Asian, or other), diabetes (yes, no), hypertension (yes, no), cancer (yes, no), and spKt/V
Abbreviations: CHF, congested heart failure; BMI, body mass index; Hb, hemoglobin; ALP, alkaline phosphatase; Alb, albumin; iPTH, intact parathyroid hormone; spKt/V, single-pool Kt/V.
Data presented as mean (SD), median [interquartile range], or proportions.
Comparison between groups by t-test and Wilcoxon-Rank Sum for continuous variables, or chi-square test for categorical variables.
Data presented as mean (SD), median [interquartile range], or proportions.
Comparison between groups by t-test and Wilcoxon-Rank Sum for continuous variables, or chi-square test for categorical variables.
Figure S1. Association of time-varying continuous levels of serum Mg with all-cause mortality hazard ratios
Supplemental Figure 2A. Time-dependent all-cause mortality hazard ratios (and 95% confidence interval error bars) across pre-specified subgroups of Age (<65, ≥65 years). Sex (Male, Female), Race (Caucasian, Other), and Dialysis Dose (spKt/V: <1.2, ≥1.2) using the case-mix model
Supplemental Figure 2B. Time-dependent all-cause mortality hazard ratios (and 95% confidence interval error bars) across pre-specified subgroups of Comorbidties (Yes, No): Hypertension (HTN), Diabetes, and Congestive Heart Failure (CHF), and body mass index (BMI: <30, ≥30 kg/m2) using the case-mix model.
Supplemental Figure 2C. Time-dependent all-cause mortality hazard ratios (and 95% confidence interval error bars) across pre-specified subgroups of Hemoglobin (Hb: <10, ≥10 g/dl). Ferritin (<300, ≥300 ng/ml). Alkaline Phosphatase (ALP: <90, ≥90 IU/1), and Albumin (Alb: <3.5, ≥3.5 g/dl) using the case-mix model.
Supplemental Figure 2D. Time-dependent all-cause mortality hazard ratios (and 95% confidence interval error bars) across pre-specified subgroups of intact Parathyroid Hormone (iPTH: <300, ≥300 pg/ml) using the case-mix model.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org