Abstract
Awareness and use of electronic cigarettes (e-cigarettes) is increasing. Questions regarding positive (e.g., smoking reduction/cessation) and negative (e.g., delay of cessation) potential public health consequences of e-cigarettes may be informed by studying dual users of e-cigarettes and tobacco cigarettes. A cross-sectional online survey assessed demographics, product use patterns, and beliefs about relative product benefits and harms among dual users (n = 350) in the United States using the website Amazon Mechanical Turk (MTurk). Compared to tobacco cigarettes, e-cigarettes were used less often and were associated with lower dependence. Participants reported a 30% reduction in self-reported tobacco cigarette smoking since beginning to use e-cigarettes. Reported primary reasons for e-cigarette use were harm reduction and smoking cessation. E-cigarette use was reported as more likely in settings with smoking restrictions and when others’ health could be adversely affected. Conversely, participants reported having used tobacco cigarettes more often than e-cigarettes in hedonic situations (e.g., after eating, drinking coffee or alcohol, or having sex), outdoors, or when stressed. Participants were twice as likely to report wanting to quit tobacco cigarettes compared to e-cigarettes in the next year and intended to quit tobacco cigarettes sooner. Tobacco cigarettes were described as more harmful and addictive, but also more enjoyable than e-cigarettes. Participants provided evidence consistent with both positive and negative public health consequences of e-cigarettes, highlighting the need for experimental research, including laboratory studies and clinical trials. Policies should consider potential public health benefits of e-cigarettes, in addition to potential harms.
Keywords: e-cigarette, smoking, cigarette, use patterns, dual use
Traditional (combustible) tobacco cigarette use is responsible for staggering morbidity and mortality in the United States and is a significant economic burden (U.S. Department of Health and Human Services, 2014). Electronic cigarettes or “e-cigarettes” are an evolving and increasingly prevalent technology with the potential to substitute for tobacco cigarettes. E-cigarettes work by heating a solution that contains nicotine to produce an aerosol or “vapor” to be inhaled or “vaped” by the user. E-cigarette use is accelerating rapidly: National tobacco use surveys report more than a two-fold increase in e-cigarette use and/or experimentation among youth and adult smokers over a 1-year period, as well as a three-fold increase among youth never-smokers of tobacco cigarettes (Bunnell et al., 2015; King, Alam, Promoff, Arrazola, & Dube, 2013; Pearson, Richardson, Niaura, Vallone, & Abrams, 2012; Zhu et al., 2013). Financial analysts estimate that sales of e-cigarette and related products have reached $2.5 billion per year and predict that sales will surpass tobacco cigarette sales by 2024 (Herzog, Gerberi, & Scott, 2014).
The increasing popularity of e-cigarettes has sparked debate among public health and tobacco control communities regarding the potential public health impact of these products. Critics are concerned about potential health risks, for example, the production of toxins during vaporization (Kosmider et al., 2014). Questions also remain as to whether e-cigarettes might prevent smoking cessation or cause relapse by presenting a purportedly healthier option, as with “light” cigarettes. “Light” cigarettes, which delivered less tar and nicotine than regular cigarettes when tested on machine inhalation systems, were purported to be healthier than regular cigarettes. However, research eventually showed that these “light” cigarettes did not afford reduced harm, and likely caused substantial public health harm by maintaining smoking behavior in many health-concerned individuals who would have otherwise quit smoking (Grana, Benowitz, & Glantz, 2014; Warner, 2005). E-cigarettes may also pose a risk for relapse in former smokers and for smoking initiation in nonsmokers (e.g., for novelty or cognitive-enhancing properties of nicotine without risk from tobacco smoking), although there is no current evidence to support these claims. Additional concerns include a potential return to the social acceptability of smoking-like behavior, which could increase nonsmoking youth initiation of smoking or vaping, renormalization of tobacco consumption in indoor workplaces and public spaces, and the use of e-cigarettes to administer other drugs (e.g., cannabis, cocaine) (Grana et al., 2014; Kinnunen et al., 2014; Pepper et al., 2013).
In contrast, supporters of e-cigarettes assert that e-cigarettes provide less toxin exposure than tobacco cigarettes (Burstyn, 2014; Cahn & Siegel, 2011; Farsalinos & Palosa, 2014), and suggest that these products will aid in smoking reduction and cessation and inspire new quit attempts in tobacco cigarette smokers by serving as nicotine replacement products. Increased acceptability of e-cigarettes over currently FDA-approved forms of nicotine replacement (e.g., gum, patch) may render e-cigarettes substantially more effective at reducing smoking at the population level than traditional nicotine replacement medications (Barbeau, Burda, & Siegel, 2013; Steinberg et al., 2014). Even if e-cigarettes cause some level of health harm and deter some users from quitting nicotine-containing products altogether, e-cigarettes could still result in net public health benefit considering that the overwhelming rate of death and disease from tobacco cigarettes may be decreased (Nitzkin, 2014).
Current tobacco cigarette smokers who also use e-cigarettes (i.e., “dual users”) are informative to addressing both the positive (e.g., smoking reduction/cessation) and negative (e.g., delay of cessation) public health claims regarding e-cigarettes (Walton et al., 2015). Moreover, although dual users constitute the majority of e-cigarette users (e.g., Giovenco, Lewis, & Delnevo, 2014; Sutfin, McCoy, Morrell, Hoeppner, & Wolfson, 2013), surveys of e-cigarette use have only recently focused on dual users (Farsalinos, Romagna, & Voudris, 2015; Rutten et al., 2015). A potential limitation of one of these studies (Farsalinos et al.) was that two-thirds of participants were recruited from e-cigarette forums, which may have resulted in an oversampling of individuals with a positive bias toward e-cigarettes. Moreover, Farsalinos et al.’s sample was largely European, making results potentially less representative of dual use in the United States, where increased tobacco control activity may influence user attitudes about smoking and related behaviors (Shiffman, 2009). By contrast,Rutten et al. (2015) used a panel-based approach to survey U.S. dual users. Unfortunately, e-cigarette use duration and nicotine content were not measured, resulting in a sample that potentially included e-cigarette users who were relatively inexperienced or used e-cigarettes containing zero nicotine. The present online survey was conducted concurrently with the Rutten et al. survey, and like their study recruited dual users in the United States to describe use patterns and harm perceptions of e-cigarettes and tobacco cigarettes. However, unlike Rutten et al., our sample included only dual users who had substantial experience with both products (i.e., at least 3 months use and past-week use) and used only nicotine-containing e-cigarettes, characteristics that we believe are critical for understanding public health consequences of dual use of e-cigarettes and tobacco cigarettes.
Methods
Participants
Participants were dual users of e-cigarettes and tobacco cigarettes who were registered on Amazon Mechanical Turk (MTurk), a crowdsourcing Internet marketplace that has been used extensively for research (e.g., Adkison, O’Connor, Chaiton, & Schwartz, 2015; Carter, DiFeo, Bogie, Zhang, & Sun, 2014; Johnson et al., 2015). Dual use of e-cigarettes and tobacco cigarettes was defined as use of e-cigarettes and tobacco cigarettes for ≥ 3 months, each; use of e-cigarettes and smoking of tobacco cigarettes in the past week; and use of a nicotine-containing e-cigarette. Participants were required to have a 95% or higher approval rating on MTurk, be ≥18 years of age, and reside in the United States (confirmed during initial registration on MTurk). Participation was voluntary and anonymous (no name or IP address were recorded). The Institutional Review Board at Johns Hopkins University approved this study.
Materials
Surveys were hosted by Qualtrics (Provo, UT). The screening questionnaire included the survey description (e.g., purpose of the study, confidentiality and anonymity of responses, compensation), and questions determining demographics and use status of e-cigarettes and tobacco cigarettes. Demographic questions were included to obscure inclusion criteria based on dual use. If a participant met inclusion criteria, he or she was given a code to access the password-protected survey.
Participants were asked about e-cigarette and tobacco cigarette use patterns as well as perceived harm and legality of these products (survey available in Supplemental Materials). Dependence was assessed using the Fagerström Test for Cigarette Dependence (FTCD; Fagerström, 2012; Heatherton, Kozlowski, Frecker, & Fagerström, 1991) to examine tobacco cigarettes, and a modified version to examine dependence to e-cigarettes; the FTCD was modified by substituting the word “e-cigarette” for any instance of “cigarette” and substituting “using” for any instance of “smoking.”
Procedure
The survey was advertised on MTurk with the title, “Survey about e-cigarettes and decision making.” Prior to completing the survey, participants were instructed to complete the brief screening questionnaire to determine qualification status. If a participant qualified, he/she completed the survey in a new tab of the active Internet browser. Participants were instructed to complete the survey in one sitting. Once finished with the survey, participants were instructed to enter their unique MTurk identification number. Upon returning to MTurk, participants were prompted to enter their identification number into a text box to verify that they had completed the survey. Participants whose identification number matched the identification number submitted at the end of the survey were paid $1. The survey was active from May 7–20, 2014.
Two tactics were employed with the intention of improving the quality of participant responses. First, in the MTurk advertisement and survey descriptions, participants were instructed that paying attention during the survey and answering questions carefully could potentially result in a $0.25 bonus payment. Second, distractor questions and attention check questions were included at multiple stages. For example, in one question, participants were asked to select adjectives consistent with their current mood state, but embedded in the instructions for the question was a prompt to select “none of the above” as the response. Participants who indicated inattention (e.g., failed a distractor question; provided internally inconsistent answers) were excluded from all analyses.
Data Analysis
Descriptive analyses (e.g., percentage endorsing a given item) were used to characterize demographic and categorical items. In most cases, data pertaining to e-cigarette and tobacco cigarette use patterns were non-normally distributed; summary data for these variables are therefore reported as medians with interquartile ranges (IQR). Non-normally distributed data also necessitated the use of Spearman rank-order correlations, which measured the relationship between product use duration or frequency and product use behavior for e-cigarettes and tobacco cigarettes. Differences in daily tobacco cigarette use before and after e-cigarette initiation were examined using a Wilcoxon signed-rank test.
In total, 400 participants completed the survey. Data from 50 participants were excluded based on inattention (n = 12) or failure to meet dual-use inclusion criteria despite having qualified via the screening questionnaire (n = 38). All analyses were conducted using data from the remaining 350 participants.
Results
Demographic Characteristics
Table 1 shows demographic characteristics of the sample. The mean participant age was 32 years (SD = 10; Range = 18–70), and 53% were male, 83% were Caucasian, and 85% were non-Hispanic. The most commonly endorsed range of annual income was ≤ $35,000 (48%) and most participants were employed full time (53%). Most had never been married (53%) and 83% had completed at least some college. Although most participants (57%) reported never having received a diagnosis of a psychiatric disorder, 20% and 23% reported a diagnosis of one or multiple disorders in their lifetime, respectively; these rates are consistent with past research on psychiatric diagnosis rates in smokers (Lawrence, Mitrou, & Zubrick, 2009; Minichino et al., 2013).
Table 1.
Demographic Characteristics
| n | % | |
|---|---|---|
| Age | ||
| 18–24 | 78 | 22% |
| 25–39 | 203 | 58% |
| 40–54 | 55 | 16% |
| 55 + | 14 | 4% |
| Gender | ||
| Male | 184 | 53% |
| Female | 166 | 47% |
| Race | ||
| Caucasian/White | 292 | 83% |
| African American/Black | 18 | 5% |
| Mixed Race | 13 | 4% |
| Asian | 13 | 4% |
| Native American | 4 | 1% |
| Other | 3 | 1% |
| I prefer not to answer | 7 | 2% |
| Ethnicity | ||
| Hispanic | 39 | 11% |
| Non-Hispanic | 296 | 85% |
| I prefer not to answer | 15 | 4% |
| Income | ||
| < $25,000 | 89 | 25% |
| $25,000 – $34,999 | 81 | 23% |
| $35,000 – $49,999 | 56 | 16% |
| $50,000 – $74,999 | 70 | 20% |
| ≥ $75,000 | 47 | 13% |
| I prefer not to answer | 7 | 2% |
| Marital Status | ||
| Married | 114 | 33% |
| Divorced/Separated/Widowed | 52 | 15% |
| Never been married | 184 | 53% |
| Education | ||
| No high school diploma | 2 | 1% |
| High school or GED | 44 | 13% |
| Post-high school trade/vocational training | 15 | 4% |
| Some college credit | 118 | 34% |
| College degree (Associate, Bachelor’s) | 153 | 44% |
| Graduate or Professional degree | 17 | 5% |
| I prefer not to answer | 1 | 0% |
| Employment | ||
| Full Time | 184 | 53% |
| Part Time | 76 | 22% |
| Unemployed | 50 | 14% |
| Student | 40 | 11% |
| History of Psychiatric Diagnosis | ||
| Never | 199 | 57% |
| One disorder | 70 | 20% |
| More than one disorder | 81 | 23% |
| Anxiety | 94 | 27% |
| Depression | 105 | 30% |
| Bipolar Disorder | 28 | 8% |
| Schizophrenia or Schizoaffective Disorder | 2 | 1% |
| Attention Deficit Hyperactivity Disorder (ADHD) | 22 | 6% |
| Post Traumatic Stress Disorder (PTSD) | 29 | 8% |
| Other | 4 | 1% |
Note. Percentages may not sum to 100% due to rounding error.
Use Patterns and Dependence
Tables 2 and 3 describe tobacco cigarette and e-cigarette use patterns and dependence, respectively. Participants reported smoking tobacco cigarettes more times per day and more days per week than using e-cigarettes. Compared with e-cigarette use, tobacco cigarette use was associated with higher dependence scores, less time to first use of the day, greater reluctance to give up the first use of the day, greater likelihood of daily use, and stronger cravings. The percentage of participants reporting daily use was lower for e-cigarettes (47%) than tobacco cigarettes (86%).
Table 2.
E-cigarette and tobacco cigarette use behavior
| E-cigarettes |
Tobacco cigarettes |
|||||
|---|---|---|---|---|---|---|
| Median | IQR | Range | Median | IQR | Range | |
| Use duration (years) a | 1 | 1–2 | 0.25–6 | 10 | 5–16 | 0.25–53 |
| Days used per week, prior to e-cigarettes b | – | – | – | 7 | 6–7 | 1–7 |
| Days used per week, past 30 days c | 4 | 2–7 | 1–7 | 7 | 4–7 | 1–7 |
| Times used per day d, prior to e-cigarettes bde | – | – | – | 10 | 5–20 | 1–50 |
| Times used per day d, past 30 days | 5 | 2–10 | 1–200 | 7 | 3–13 | 1–40 |
| Estimated puffs per bout | 6 | 4–10 | 1–100 | 14 | 10–20 | 3–50 |
| Nicotine Dependence (FTCD score) | 2 | 1–4 | 1–9 | 3 | 1–5 | 0–9 |
| Quit intent (months) f | 6 | 3–10 | 0–12 | 5 | 3–8 | 0–12 |
Note. IQR = interquartile range, FTCD = Fagerström Test for Cigarette Dependence.
E-cigarette use duration data are missing for 5 participants (all reported smoking for at least 3 months to qualify for the survey).
Data on tobacco cigarette smoking rates (cigarettes per day, days smoked per week prior to e-cigarettes) are missing from two participants.
Data on days vaped per week are missing from one participant.
Times used per day = cigarettes per day for tobacco cigarettes and ‘bouts’ per day (defined as an instance of at least one puff or vape) for e-cigarettes.
Data on e-cigarettes used per day prior to initiation of tobacco cigarette smoking are not included for the single participant who reported this pattern of use.
A subsample of participants intended to quit e-cigarettes (n = 127) and tobacco cigarettes (n = 254) in the next 12 months.
Table 3.
E-cigarette and tobacco dependence and craving
| E-cigarettes | Tobacco cigarettes |
|
|---|---|---|
| FTCD Level | ||
| 0–2 (Very low) | 62% | 42% |
| 3–4 (Low) | 21% | 25% |
| 5 (Moderate) | 8% | 13% |
| 6–7 (High) | 6% | 17% |
| 8–10 (Very high) | 3% | 4% |
| FTCD Q1: Time to first cigarette/use | ||
| Within 5 minutes | 7% | 13% |
| 6–30 minutes | 13% | 38% |
| 31–60 minutes | 12% | 19% |
| After 60 minutes | 68% | 30% |
| FTCD Q2: Difficult to abstain when use is restricted (Yes) | 16% | 27% |
| FTCD Q3: Use time hate the most to give up | ||
| First one in the morning | 19% | 50% |
| All others | 81% | 50% |
| FTCD Q4: Times used/cigarettes per day | ||
| 10 or less | 76% | 68% |
| 20 to 30 | 14% | 25% |
| 21 to 30 | 6% | 5% |
| 31 or more | 4% | 1% |
| FTCD Q5: Use most frequently right after waking? (Yes) | 19% | 38% |
| FTCD Q6: Smoke/Use when ill? (Yes) | 100% | 76% |
| Strong cravings to smoke/vape? (Yes) | 21% | 76% |
| Ever smoked/vaped daily? (Yes) | 47% | 86% |
Note. FTCD = Fagerström Test for Cigarette Dependence. Percentages may not sum to 100% due to rounding error.
E-cigarette use was associated with a significant reduction in self-reported tobacco cigarette use. Since initiation of e-cigarette use, the median number of tobacco cigarettes smoked per day (CPD) decreased significantly from 10 to 7 CPD (30% reduction), n = 346, Z = −10.41, p < .001. For participants whose CPD changed after e-cigarette use initiation (n = 189), most (83%) agreed that e-cigarette use played a role in this change. Half of the sample reported reductions in CPD, with a median reduction of 5 CPD (IQR = 3 to 10) from a median of 15 to 6 CPD. An almost equal percentage (45%) of participants reported no change in CPD, remaining at median of 10 CPD. Only 5% of participants reported an increase in CPD, with a median increase of 4 CPD (IQR = 1 to 7) from a median of 3 to 10 CPD. An exploratory comparison of daily compared to nondaily e-cigarette users found minimal differences in tobacco use behavior, with the exception of change in median tobacco CPD: following e-cigarette initiation, daily users (n=91) decreased from 15 to 8 CPD (47% reduction) compared to non-daily users (n=256), who decreased from 10 to 7 CPD. An independent samples t-test confirmed that daily users had significantly greater reduction in CPD than non-daily users, t(343)=7.607, p<.001.
Table 4 shows results of correlational analyses between aspects of e-cigarette and tobacco cigarette use. Duration of e-cigarette use was positively and significantly correlated with duration of tobacco use. More frequent use of e-cigarettes was significantly associated with lower current CPD and greater decreases in CPD and days smoked per week following initiation of e-cigarette use. Longer duration of tobacco cigarette use was significantly correlated with a smaller change in CPD. Frequency of tobacco cigarette use was significantly correlated with the lower dependence (modified FTCD score) for e-cigarettes.
Table 4.
Spearman correlations between duration or frequency of use and product use behavior for e-cigarettes and tobacco cigarettes.
| E-cigarettes |
Tobacco cigarettes |
||||
|---|---|---|---|---|---|
| Use duration (years) |
Days vaped/week (past 30d) |
Use duration (years) |
Days smoked/week (past 30d) |
||
| E-cigarettes | Use duration (years) | - | 0.02 | 0.19*** | 0.02 |
| Days vaped/week (past 30d) | 0.02 | - | −0.03 | −0.03 | |
| Times used/day | 0.03 | 0.48*** | 0.01 | −0.10 | |
| Estimated puffs/bout | 0.02 | 0.05 | −0.06 | 0.01 | |
| Modified FTCD score | 0.04* | 0.23*** | 0.01 | −0.11* | |
| Tobacco cigarettes | Use duration (years) | 0.19*** | −0.03 | - | 0.34*** |
| Days smoked/week (past 30d) | 0.02 | −0.03 | 0.34*** | - | |
| Cigarettes/day | 0.01 | −0.12* | 0.52*** | 0.64*** | |
| Cigarettes/day prior to e-cigarettes | 0.01 | 0.11* | 0.53*** | 0.41*** | |
| Cigarettes/day change score | −0.01 | −0.37*** | −0.13* | 0.11* | |
| Days smoked/week prior to e-cigarettes | 0.05* | 0.19*** | 0.36*** | 0.62*** | |
| Days smoked/week change score | 0.02 | −0.22*** | 0.03 | 0.52*** | |
| Estimated puffs/bout | −0.02 | −0.01 | 0.20*** | 0.20*** | |
| FTCD score | 0.02 | 0.05* | 0.43*** | 0.43*** | |
Note FTCD = Fagerström Test for Cigarette Dependence. Change score = current use minus use prior to e-cigarette initiation.
p < .05;
p < .01;
p < .001
Past Quit Attempts and Intention to Quit
Sixty-eight percent of participants reported a past serious quit attempt for tobacco cigarettes that lasted at least 24 hours and 41% reported a serious quit attempt in the past year. Sixty-one percent of participants reported personally knowing someone who had quit using tobacco cigarettes for at least one month with the help of e-cigarettes. Twice as many participants were planning to quit using tobacco cigarettes (73%) compared to planning to quit e-cigarettes (36%) in the next year.
Sixty-eight percent of participants reported having used nicotine replacement therapy (NRT), other medications (e.g., varenicline, bupropion), or other methods (e.g., counseling, quitline) to assist in quitting tobacco cigarettes. Notably, 20% of participants reported having used NRT, varenicline, or bupropion for unspecified reasons other than to quit smoking. Of participants reporting past use of NRT to quit smoking, 27% reported having used only one method, whereas 33% reported having used more than one method. The most commonly used NRT products were nicotine gum (39%) and nicotine patch (38%).
Product Experience
Regarding experience with tobacco products, most participants reported most commonly using commercially available, machine-rolled tobacco cigarettes (91%) and cigarettes without menthol flavor (59%). In the past 30 days, 40% of participants reported using one or more other tobacco products; hookah (52%) and cigars (40%) were most likely to be used, whereas chew was used most frequently (Mdn = 6 days) (Table 5). Most participants either did not know if their e-cigarette model was modified (43%) or reported not using a modified product (37%). Many participants reported not using menthol or other flavored e-liquid (53%); however, those who reported having tried flavored e-liquid (59%) continued to use it (79%).
Table 5.
Other tobacco products used in the past 30 days.
| %n |
Median days used |
IQR | |
|---|---|---|---|
| Hookah | 52% | 2 | 1–4 |
| Cigars | 40% | 2 | 1–4 |
| Bidis/Cloves | 24% | 2 | 1–4 |
| Cigarillos | 22% | 2 | 2–5 |
| Pipe Tobacco | 14% | 5 | 2–24 |
| Little Cigars | 13% | 2 | 1–3 |
| Snus | 9% | 2.5 | 1–4 |
| Chew | 8% | 6 | 1–10 |
| Snuff | 6% | 3 | 1–16 |
Note. Data represents distribution and frequency of other tobacco product use in dual users reporting any other product use (n=141, 40%).
Twenty-seven participants (8%) reported using products other than e-liquid or nicotine in an e-cigarette. Cannabis was the most commonly used substance (n = 22), followed by “synthetic cannabis (e.g., K2, Spice)” (n = 3), methamphetamine (n = 2), “bath salt compounds (e.g., MDPV, mephedrone)” (n = 2), and “prescription pain pills (e.g., Vicodin, Oxycontin)” (n = 1). The 22 participants reporting cannabis use in e-cigarettes reported using it this way a median of 10 times in their lifetime (IQR = 2.75–20). Twenty of these participants reported being “able to get high,” and 14 reported continued use of this method. Other drugs were associated with success in achieving a “high” (“bath salt” compounds: n = 2; methamphetamine: n = 1, synthetic cannabis: n = 1), but minimal continuation of use.
Use Reasons and Settings
Participants were asked to endorse all applicable reasons for their use of e-cigarettes (Table 6). Among the most popular reasons for e-cigarette use was the belief that they are less harmful to their health than tobacco cigarettes (64%), that e-cigarettes were used to deal with cravings (57%), and the belief that e-cigarettes are less harmful to others than tobacco cigarettes (52%). When asked to identify the single-most important reason for e-cigarette use, participants most often endorsed that they believed that e-cigarettes were less harmful to their health than tobacco cigarettes (25%). The second most commonly endorsed important reason for use was to cut down smoking in preparation for a quit attempt (21%).
Table 6.
Reported reasons for use of e-cigarettes
| Select all that apply |
Select most important reason |
|
|---|---|---|
| I believe it is less harmful to my health than regular cigarettes | 64% | 25% |
| To cut down smoking tobacco in preparation for a quit attempt | 40% | 21% |
| To be able to deal with situations where I can't smoke (e.g., at work, in a restaurant) | 45% | 13% |
| To quit smoking or avoid relapsing | 34% | 7% |
| To deal with cravings for tobacco | 57% | 5% |
| I believe it is less harmful to others | 52% | 5% |
| With an e-cigarette, it is easier to just smoke one or a few puffs at a time rather than a whole cigarette | 45% | 5% |
| I can breathe easier for feel more fit | 30% | 5% |
| To avoid having to go outside to smoke | 43% | 4% |
| E-cigarettes are cheaper than regular cigarettes | 27% | 3% |
| I prefer the taste of an e-cigarette | 22% | 2% |
| To cut down smoking tobacco with no intention to quit | 12% | 2% |
| Other (e.g., prefer the smell, reduce stress) | 3% | 2% |
| To avoid withdrawal from tobacco | 35% | 1% |
| To increase my ability to concentrate | 8% | 1% |
| I can't stop using it | 1% | 0% |
Note. Categories are sorted by “Select most important reason.”
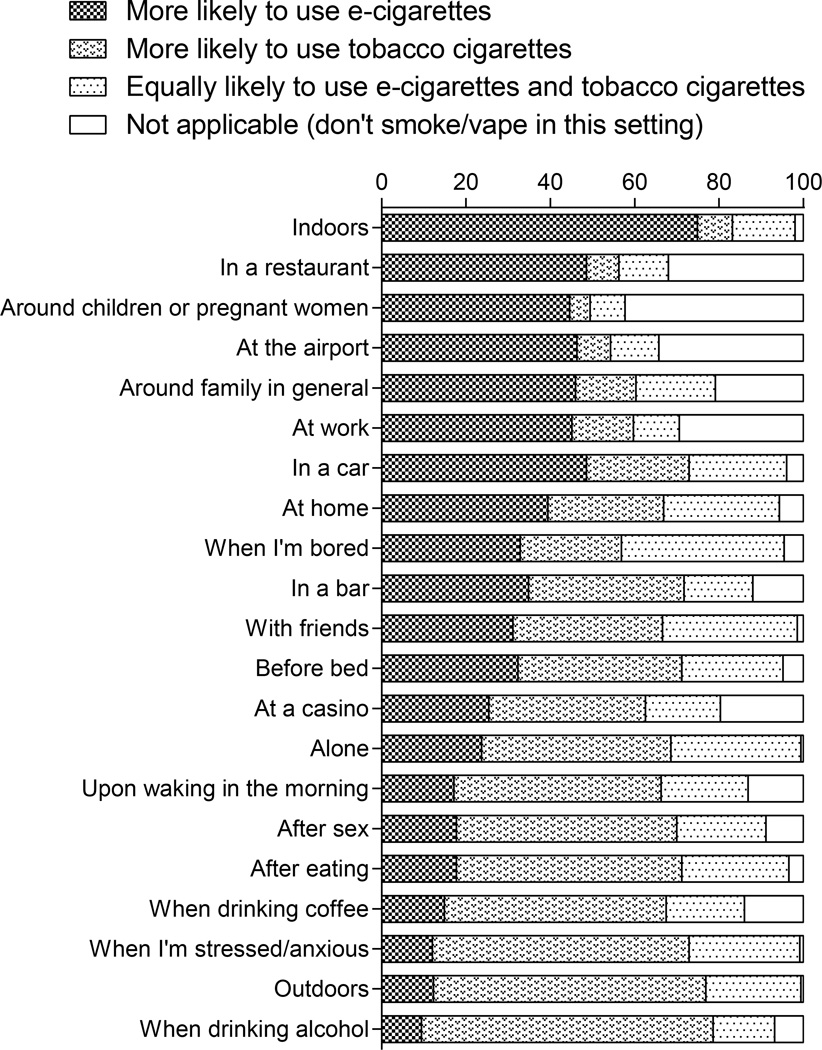
As shown in Figure 1, participants reported a greater likelihood of using e-cigarettes than tobacco cigarettes indoors (75%), in a restaurant (49%), in a car (49%), at the airport (46%), around family in general (46%), and at work (45%). By comparison, tobacco cigarettes were more likely than e-cigarettes to be used in settings with alcohol (69%), outdoors (65%), when stressed or anxious (61%), after eating (53%), when drinking coffee (53%), and after having sex (52%). E-cigarette use was reported to be more likely than tobacco cigarette use around children or pregnant women, with 45% of participants reporting being more likely to use e-cigarettes than tobacco cigarettes in those settings and 42% reporting using neither product.
Figure 1.
Likelihood ratings of e-cigarette and tobacco cigarette use in different settings.
Harm Perceptions
Among participants who agreed with the statement that e-cigarettes and tobacco cigarettes are enjoyable (n = 281), 63% reported that e-cigarettes were less enjoyable than tobacco cigarettes. Among participants who agreed with the statement that e-cigarettes and tobacco cigarettes are addictive (n = 238), 57% reported that e-cigarettes were less addictive than tobacco cigarettes. Of the whole sample, 30% reported that e-cigarettes were not at all addictive. On average, participants rated e-cigarettes as having a lower harm on an 11-point Likert-type scale than tobacco cigarettes for harming self and others (Table 7). When asked if one product was more harmful than the other, more participants reported being unsure about the dangers of e-cigarettes (22%) than tobacco cigarettes (2%). Eighty-seven percent endorsed the belief that tobacco cigarettes are much more (57%) or somewhat more (30%) harmful than e-cigarettes. Over half (59%) of participants agreed with the statement that NRT was equally as harmful as e-cigarettes (the most commonly endorsed response). The analogous statement regarding e-cigarettes being equally as harmful as non-NRT medications was also the most commonly endorsed response (37%).
Table 7.
Reported perceived harmfulness of e-cigarettes, tobacco cigarettes, and other products.
| E-Cigarettes |
Tobacco Cigarettes |
|||||
|---|---|---|---|---|---|---|
| Beliefs | Median | IQR | Range | Median | IQR | Range |
| Risk harming self | 5 | 3–6 | 0–10 | 9 | 8–10 | 0–10 |
| Risk harming other | 2 | 0–4 | 0–10 | 7 | 6–9 | 0–10 |
| Accuracy of media representation | 5 | 4–7 | 0–10 | 5 | 5–7 | 0–10 |
| True | False |
Don't Know |
True | False |
Don't Know |
|
| Safe for human use | 49% | 30% | 20% | 11% | 85% | 3% |
| Potentially dangerous or harmful | 52% | 26% | 22% | 93% | 5% | 2% |
| This product would not be sold commercially if it were not safe to use |
21% | 67% | 12% | 7% | 86% | 7% |
| Product is regulated by the Food and Drug Administration |
16% | 36% | 47% | 41% | 23% | 36% |
| Comparison Product |
E-cigarettes much more harmful |
E-cigarettes somewhat more harmful |
Both products about the same |
Product somewhat more harmful |
Product much more harmful |
|---|---|---|---|---|---|
| Tobacco cigarettes | 0% | 0% | 12% | 30% | 57% |
| NRT | 3% | 12% | 59% | 15% | 10% |
| Other medications | 3% | 13% | 37% | 27% | 21% |
Note. IQR = interquartile range. NRT = nicotine replacement therapy, e.g. nicotine gum, patch, lozenge, inhaler, or nasal spray. Other medications refer to smoking cessation medications, e.g., Varenicline/Chantix or Buproprion/Zyban/Wellbutrin. Risk of harm was as based on an 11-point Likert-type scale from 0 (no risk) to 10 (extreme risk). Accuracy of media representation was based on an 11-point Likert-type scale from 0 (not at all accurate) to 10 (extremely accurate). Percentages may not sum to 100% due to rounding error.
Gender Effects
Gender analyses showed minor effects on use patterns and perceptions of relative harm. Females reported a longer duration of tobacco cigarette use than males, t(348) = −2.1, p = .03, and reported using tobacco cigarettes on more days per week than males both before, t(348) = −0.1, p = .04, and after e-cigarette initiation, t(346) = −3.3, p = .001. No gender differences were detected for quit intent regarding e-cigarettes or tobacco cigarettes. Males and females also did not differ on reported harmfulness of e-cigarettes. Compared to males, females reported greater perceived harm from tobacco cigarettes to self, t(348) = −4.2, p < 001, and others, t(348) = −4.3, p < .001. Females were more likely than males to endorse using e-cigarettes to deal with tobacco cravings, Χ2(1, N = 350) = 14.6, p < .001, to quit smoking or avoid relapsing, Χ2(1, N = 350) = 4.2, p = .04, and because e-cigarettes are cheaper than tobacco cigarettes, Χ2(1, N = 350) = 5.7, p = .02. Gender differences in reported use likelihood were found for several variables. Specifically, a greater percentage of males than females reported being more likely to use e-cigarettes over tobacco cigarettes at home, and a greater percentage of females than males reported equal likelihood of using either type of product at home, Χ2(3, N = 350) = 8.7, p = .03. A greater percentage of males than females reported being more likely to use e-cigarettes over tobacco cigarettes upon waking in the morning, and a greater percentage of females than males reported using tobacco cigarettes over e-cigarettes upon waking in the morning, Χ2(3, N = 350) = 11.4, p = .01. A greater percentage of males than females reported being more likely to use e-cigarettes over tobacco cigarettes outdoors, and a greater percentage of females than males reported equal likelihood of using either product outdoors, Χ2(3, N = 350) = 10.0, p = .01. Despite these differences, both male and female participants reported the greatest likelihood of using e-cigarettes at home and using tobacco cigarettes upon waking in the morning and outdoors.
Discussion
The present research sought to characterize e-cigarette and tobacco cigarette use patterns and relative harm perceptions in current dual users of both products. We observed several noteworthy findings regarding dual use with potential implications for public health. Each will be discussed in turn.
The first major finding was that more frequent e-cigarette use (i.e., more days per week) was associated with fewer CPD and greater decrease in tobacco cigarette use (CPD and days per week), a result suggestive of a systematic effect of e-cigarettes substituting for smoking and one that is consistent with previous findings (Farsalinos et al., 2015). Cross-sectional studies of smokers outside of a quit context found a reduction in CPD, suggesting that e-cigarettes may aid in reduction or cessation efforts (Adkison et al., 2013; Etter & Bullen, 2011a; Lechner et al., 2015; Rutten et al., 2015). Dual users in the Rutten et al. (2015) sample reported smoking “fewer cigarettes” (54.1%), “about the same number of cigarettes” (40.6%), or reported smoking “more cigarettes” (1.6%) following e-cigarette initiation; these findings were very similar to our survey sample, which reported a decrease (50%), no change (45%), or increase (5%) in CPD. Smoking reduction may be associated with an intermediate stage between regular use and total cessation, which has been shown to increase the motivation of daily heavy smokers to quit (Cheong, Yong, & Borland, 2007; Schane, Ling, & Glantz, 2010). Therefore, e-cigarette-related reductions in smoking may present an opportunity for re-engaging smokers in cessation efforts (e.g., counseling, medication), which can double or triple the chances for quitting (Centers for Disease Control and Prevention, 2011; Hughes, 2003; Rutten et al., 2015). Given the fact that reduction of cigarette smoking is associated with some reduction in morbidity (Gerber, Myers, & Goldbourt, 2012; Joseph et al., 2008; Lee, 2013; Tverdal & Bjartveit, 2006), a promising outcome from our study is that we observed a group-level decrease in the number of cigarettes smoked per day in our sample of dual users, despite a minority endorsing smoking cessation as a reason for e-cigarette use.
In contrast to concerns that e-cigarette use could promote initiation to tobacco cigarette smoking, we observed initiation of tobacco use after e-cigarette use in only one of 350 participants, suggesting a low rate of transitioning from e-cigarettes to tobacco cigarettes. This finding is consistent with previous results showing higher rates of current and ever use of e-cigarettes among current cigarette smokers than non-smokers (Czoli, Hammond, & White, 2014; McMillen, Maduka, & Winickoff, 2012; Nitzkin, 2014; Pearson et al., 2012; Pepper et al., 2013; Regan, Promoff, Dube, & Arrazola, 2013; Sutfin et al., 2013). Finally, our data also suggest that only a small percentage of the sample modified their e-cigarette or used non-nicotine drugs in their e-cigarette device. Therefore, it appears that use of other drugs in these devices is a relatively minor issue at this time.
A second major finding was that a large percentage of dual users reported intending to quit smoking tobacco cigarettes within the next year, and many had previous experience with one or more cessation products (e.g., NRTs, cessation pharmacotherapy). We found that 68% of dual users reported a serious quit attempt (≥24 hours) and 41% planned to quit in the next 6 months, consistent with Rutten et al., who found 65% of dual users were considering quitting smoking cigarettes or cigars in the next 6 months. Although limited, there is some evidence to support e-cigarettes as helping to reduce smoking rates (Bullen et al., 2013; Caponnetto et al., 2013; McRobbie, Bullen, Hartmann-Boyce, & Hajek, 2014) and motivate new cessation attempts. For instance, one randomized controlled trial found that e-cigarettes performed similarly to nicotine patch with respect to six-month quit rates, and were well tolerated (Bullen et al., 2013). Additionally, e-cigarette appeal and client motivation may provide better treatment compliance compared to pharmacotherapies and NRT products due to sensorimotor characteristics, socioeconomic factors, convenience, and mild side effects (Caponnetto et al., 2013; Farsalinos, Romagna, Tsiaparas, Kyrzopoulos, & Voudris, 2014). The modest success rates of NRT products may be improved by adjunctive use of low-nicotine or nicotine-free e-cigarettes as a cessation tool (Moore et al., 2009; Walker et al., 2012). It is worth noting that not all e-cigarette users are interested in smoking cessation or reduction. In these individuals, e-cigarettes may potentially facilitate smoking and reduce motivation for eventual cessation, although there is currently no evidence to support this.
Third, similar to previous research, participants reported their primary reasons for using e-cigarettes as being less harmful to one’s health (relative to tobacco cigarettes) and to cut down smoking tobacco cigarettes in preparation for a quit attempt (Adkison et al., 2013; Farsalinos et al., 2014; Goniewicz, Lingas, & Hajek, 2013; Rutten et al., 2015). Despite offering fewer and different options as use reasons,Rutten et al. (2015), found that dual users endorsed reasons for e-cigarette use related to smoking reduction, smoking cessation, and reduction of health risks; they did not ask about one’s most important reason for use. Participants reported being more likely to use e-cigarettes in settings where smoking may be restricted (e.g., in a restaurant, indoors), providing some confirmation that e-cigarettes are being used to comply with smoke-free laws. Participants were also more likely to use e-cigarettes when concerned about adverse effects of smoking on others’ health. The sample largely endorsed either not using any product, or using e-cigarettes only, around children or pregnant women. Further research is needed to evaluate the effects of acute and chronic exposure to nicotine and other low-level toxins from second- or third-hand vapor in vulnerable populations (e.g., infants and children, pregnant women, individuals with chronic illness) to develop evidence-based health policies and recommendations (Ballbe et al., 2014; Collaco, Drummond, & McGrath-Morrow, 2015; Durmowicz, 2014). Finally, participants reported being more likely to use tobacco cigarettes in hedonic situations, when there were no use restrictions, or when stressed/anxious, suggesting that situations such as these might be the most difficult circumstances for dissuading cigarette smoking. Longitudinal studies (e.g., Population Assessment of Tobacco and Health; https://pathstudyinfo.nih.gov) and studies with long retrospective report would be better suited to measure changes in use motives (e.g., desire to quit or reduce smoking) and public health outcomes, such as delayed cessation.
Lastly, with respect to perceptions of relative harm, participants reported that they perceived e-cigarettes to be safer and less addictive than tobacco cigarettes, a finding consistent with previous reports (e.g., Amrock, Zakhar, Zhou, & Weitzman, 2015; Farsalinos et al., 2015; Goniewicz, Lingas, et al., 2013; Pearson et al., 2012). Dual users in our sample also reported that e-cigarettes were less harmful than pharmacotherapy for smoking cessation, and were no more harmful than NRT (e.g., patch, gum). Although no studies of long-term safety of e-cigarettes are available, recently proposed federal regulations may reduce some of the heterogeneity in e-cigarettes that contributes to significant health concerns with regard to poor quality control (e.g., inaccurate labeling of nicotine content, variable nicotine delivery) and health risks (e.g., nicotine toxicity, low-level toxic contaminants and carcinogens, and particulate matter) (Chatham-Stephens et al., 2014; Cheng, 2014; Grana et al., 2014; Lippi et al., 2014; Nitzkin, 2014; Pearson et al., 2012; Riker, Lee, Darville, & Hahn, 2012). Education and outreach regarding the science and regulatory status may be potentially effective in improving public knowledge regarding e-cigarettes and tobacco products.
Limitations to the study should be considered when interpreting our major findings. The survey relied on self-report, although biochemical verification of smoking behavior would have improved validity. The cross-sectional design and convenience sampling allowed for efficient data collection, but generalizability of the results may be limited due to participant self-selection and representativeness of individuals registered on MTurk relative to the population at large (e.g., education, race). Additionally, we were not able to accurately measure e-cigarette use history (e.g., progression from nicotine containing liquid to nicotine-free liquid) or e-cigarette nicotine dose. However, one benefit of recruitment via MTurk without advertising on e-cigarette forums is greater representativeness of e-cigarette users in general, reducing a potential sampling bias from e-cigarette enthusiasts. Given the length of the survey, we did not obtain information on the rate of using one or both products within a day. Future studies could explore such details as well as study more experienced e-cigarette users (e.g., Spindle, Breland, Karaoghlanian, Shihadeh, & Eissenberg, 2014) and include single-product user comparison groups. The FTCD has not been validated to assess e-cigarette nicotine dependence in dual users, and dependence on the two nicotine products was measured separately, within-subjects. The latter point can be emphasized by considering dependence measures of other drugs: for example, opioid dependence is typically not measured separately for heroin and prescription opioids. It is also important to keep in mind that the features and addictive qualities of e-cigarettes are likely to continue to change with modifications to their technology. Finally, self-report responses are susceptible to social desirability biases or errors in retrospective memory, a limitation common to survey research.
Based on our survey results, dual users of e-cigarettes and tobacco cigarettes tend to be light smokers with low-to-moderate tobacco dependence who use e-cigarettes for harm reduction and/or supplementing nicotine use in settings with smoking restrictions. To provide useful recommendations for regulatory decisions, further research is needed on this dual user population, which is central to understanding potential positive and negative health consequences. Longitudinal studies are needed to determine evolving beliefs and use behavior, while laboratory research can provide data on abuse liability and nicotine discrimination.
Supplementary Material
References
- Adkison SE, O'Connor RJ, Bansal-Travers M, Hyland A, Borland R, Yong HH, Fong GT. Electronic nicotine delivery systems: International tobacco control four-country survey. American Journal of Preventive Medicine. 2013;44:207–215. doi: 10.1016/j.amepre.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkison SE, O'Connor RJ, Chaiton M, Schwartz R. Development of measures assessing attitudes toward contraband tobacco among a web-based sample of smokers. Tobacco Induced Diseases. 2015;13:7. doi: 10.1186/s12971-015-0032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrock SM, Zakhar J, Zhou S, Weitzman M. Perception of e-cigarette harm and its correlation with use among U.S. adolescents. Nicotine & Tobacco Research. 2015;17:330–336. doi: 10.1093/ntr/ntu156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballbè M, Martínez-Sánchez JM, Sureda X, Fu M, Pérez-Ortuño R, Pascual JA, Fernández E. Cigarettes vs. e-cigarettes: Passive exposure at home measured by means of airborne marker and biomarkers. Environmental Research. 2014;135:76–80. doi: 10.1016/j.envres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Barbeau AM, Burda J, Siegel M. Perceived efficacy of e-cigarettes versus nicotine replacement therapy among successful e-cigarette users: A qualitative approach. Addiction Science & Clinical Practice. 2013;8:5. doi: 10.1186/1940-0640-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, Walker N. Electronic cigarettes for smoking cessation: A randomised controlled trial. Lancet. 2013;382:1629–1637. doi: 10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- Bunnell RE, Agaku IT, Arrazola RA, Apelberg BJ, Caraballo RS, Corey CG, King BA. Intentions to smoke cigarettes among never-smoking US middle and high school electronic cigarette users: National Youth Tobacco Survey, 2011–2013. Nicotine & Tobacco Research. 2015;17:228–235. doi: 10.1093/ntr/ntu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstyn I. Peering through the mist: Systematic review of what the chemistry of contaminants in electronic cigarettes tells us about health risks. BMC Public Health. 2014;14:18. doi: 10.1186/1471-2458-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn Z, Siegel M. Electronic cigarettes as a harm reduction strategy for tobacco control: A step forward or a repeat of past mistakes? Journal of Public Health Policy. 2011;32:16–31. doi: 10.1057/jphp.2010.41. [DOI] [PubMed] [Google Scholar]
- Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, Polosa R. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: A prospective 12-month randomized control design study. PLoS One. 2013;8:e66317. doi: 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RR, DiFeo A, Bogie K, Zhang GQ, Sun J. Crowdsourcing awareness: Exploration of the ovarian cancer knowledge gap through Amazon Mechanical Turk. PLoS One. 2014;9:e85508. doi: 10.1371/journal.pone.0085508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Quitting smoking among adults–United States, 2001–2010. MMWR: Morbidity and Mortality Weekly Report. 2011;60:1513–1519. [PubMed] [Google Scholar]
- Chatham-Stephens K, Law R, Taylor E, Melstrom P, Bunnell R, Wang B, Schier JG. Notes from the field: Calls to poison centers for exposures to electronic cigarettes–United States, September 2010-February 2014. MMWR: Morbidity and Mortality Weekly Report. 2014;63:292–293. [PMC free article] [PubMed] [Google Scholar]
- Cheng T. Chemical evaluation of electronic cigarettes. Tobacco Control. 2014;23:ii11–ii17. doi: 10.1136/tobaccocontrol-2013-051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong Y, Yong HH, Borland R. Does how you quit affect success? A comparison between abrupt and gradual methods using data from the International Tobacco Control Policy Evaluation Study. Nicotine & Tobacco Research. 2007;9:801–810. doi: 10.1080/14622200701484961. [DOI] [PubMed] [Google Scholar]
- Collaco JM, Drummond MB, McGrath-Morrow SA. Electronic cigarette use and exposure in the pediatric population. JAMA Pediatrics. 2015;169:177–182. doi: 10.1001/jamapediatrics.2014.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoli CD, Hammond D, White CM. Electronic cigarettes in Canada: Prevalence of use and perceptions among youth and young adults. Canadian Journal of Public Health. 2014;105:e97–e102. doi: 10.17269/cjph.105.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmowicz EL. The impact of electronic cigarettes on the paediatric population. Tobacco Control. 2014;23:ii41–ii46. doi: 10.1136/tobaccocontrol-2013-051468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF, Bullen C. Electronic cigarette: Users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011a;106:2017–2028. doi: 10.1111/j.1360-0443.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine & Tobacco Research. 2012;14:75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Characteristics, perceived side effects and benefits of electronic cigarette use: A worldwide survey of more than 19,000 consumers. International Journal of Environmental Research and Public Health. 2014;11:4356–4373. doi: 10.3390/ijerph110404356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, Voudris V. Factors associated with dual use of tobacco and electronic cigarettes: A case control study. The International Journal on Drug Policy. 2015 doi: 10.1016/j.drugpo.2015.01.006. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: A systematic review. Therapeutic Advances in Drug Safety. 2014;5:67–86. doi: 10.1177/2042098614524430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber Y, Myers V, Goldbourt U. Smoking reduction at midlife and lifetime mortality risk in men: A prospective cohort study. American Journal of Epidemiology. 2012;175:1006–1012. doi: 10.1093/aje/kwr466. [DOI] [PubMed] [Google Scholar]
- Giovenco DP, Lewis MJ, Delnevo CD. Factors associated with e-cigarette use: A national population survey of current and former smokers. American Journal of Preventative Medicine. 2014;47:476–480. doi: 10.1016/j.amepre.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: An internet survey. Drug and Alcohol Review. 2013;32:133–140. doi: 10.1111/j.1465-3362.2012.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grana R, Benowitz N, Glantz SA. E-cigarettes: A scientific review. Circulation. 2014;129:1972–1986. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Herzog B, Gerberi J, Scott A. Wells Fargo Securities: Equity Research. 2014. Tobacco-Nielsen C-Store Data-E-Cig $ Sales Decline Moderates; pp. 1–17. [Google Scholar]
- Hughes JR. Motivating and helping smokers to stop smoking. Journal of General Internal Medicine. 2003;18:1053–1057. doi: 10.1111/j.1525-1497.2003.20640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PS, Herrmann ES, Johnson MW. Opportunity costs of reward delays and the discounting of hypothetical money and cigarettes. Journal of the Experimental Analysis of Behavior. 2015;103:87–107. doi: 10.1002/jeab.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph AM, Hecht SS, Murphy SE, Lando H, Carmella SG, Gross M, Hatsukami DK. Smoking reduction fails to improve clinical and biological markers of cardiac disease: A randomized controlled trial. Nicotine & Tobacco Research. 2008;10:471–481. doi: 10.1080/14622200801901948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BA, Alam S, Promoff G, Arrazola R, Dube SR. Awareness and ever-use of electronic cigarettes among U.S. adults 2010–2011. Nicotine & Tobacco Research. 2013;15:1623–1627. doi: 10.1093/ntr/ntt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen JM, Ollila H, El-Amin SE, Pere LA, Lindfors PL, Rimpelä AH. Awareness and determinants of electronic cigarette use among Finnish adolescents in 2013: A population-based study. Tobacco Control. 2014 doi: 10.1136/tobaccocontrol-2013-051512. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, Goniewicz ML. Carbonyl compounds in electronic cigarette vapors: Effects of nicotine solvent and battery output voltage. Nicotine & Tobacco Research. 2014;16:1319–1326. doi: 10.1093/ntr/ntu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D, Mitrou F, Zubrick SR. Smoking and mental illness: Results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285. doi: 10.1186/1471-2458-9-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner WV, Tackett AP, Grant DM, Tahirkheli NN, Driskill LM, Wagener TL. Effects of duration of electronic cigarette use. Nicotine & Tobacco Research. 2015;17:180–185. doi: 10.1093/ntr/ntu061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PN. The effect of reducing the number of cigarettes smoked on risk of lung cancer, COPD, cardiovascular disease and FEV(1)--a review. Regulatory Toxicology and Pharmacology. 2013;67:372–381. doi: 10.1016/j.yrtph.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Lippi G, Favaloro EJ, Meschi T, Mattiuzzi C, Borghi L, Cervellin G. E-cigarettes and cardiovascular risk: Beyond science and mysticism. Seminars in Thrombosis and Hemostasis. 2014;40:60–65. doi: 10.1055/s-0033-1363468. [DOI] [PubMed] [Google Scholar]
- McMillen R, Maduka J, Winickoff J. Use of emerging tobacco products in the United States. Journal of Environmental and Public Health. 2012;2012:989474. doi: 10.1155/2012/989474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database of Systematic Reviews. 2014;12:CD010216. doi: 10.1002/14651858.CD010216.pub2. [DOI] [PubMed] [Google Scholar]
- Minichino A, Bersani FS, Calò WK, Spagnoli F, Francesconi M, Biondi M. Smoking behaviour and mental health disorders--mutual influences and implications for therapy. International Journal of Environmental Research and Public Health. 2013;10:4790–4811. doi: 10.3390/ijerph10104790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D, Aveyard P, Connock M, Wang D, Fry-Smith A, Barton P. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: Systematic review and meta-analysis. BMJ. 2009;338:b1024. doi: 10.1136/bmj.b1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitzkin JL. The case in favor of E-cigarettes for tobacco harm reduction. International Journal of Environmental Research and Public Health. 2014;11:6459–6471. doi: 10.3390/ijerph110606459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. e-Cigarette awareness, use, harm perceptions in US adults. American Journal of Public Health. 2012;102:1758–1766. doi: 10.2105/AJPH.2011.300526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper JK, Reiter PL, McRee AL, Cameron LD, Gilkey MB, Brewer NT. Adolescent males' awareness of and willingness to try electronic cigarettes. The Journal of Adolescent Health. 2013;52:144–150. doi: 10.1016/j.jadohealth.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: Adult use and awareness of the 'e-cigarette' in the USA. Tobacco Control. 2013;22:19–23. doi: 10.1136/tobaccocontrol-2011-050044. [DOI] [PubMed] [Google Scholar]
- Riker CA, Lee K, Darville A, Hahn EJ. E-cigarettes: Promise or peril? The Nursing Clinics of North America. 2012;47:159–171. doi: 10.1016/j.cnur.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Rutten LJ, Blake KD, Agunwamba AA, Grana RA, Wilson PM, Ebbert JO, Leischow SJ. Use of e-cigarettes among current smokers: Associations among reasons for use, quit intentions, and current tobacco use. Nicotine & Tobacco Research. 2015 doi: 10.1093/ntr/ntv003. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schane RE, Ling PM, Glantz SA. Health effects of light and intermittent smoking: A review. Circulation. 2010;121:1518–1522. doi: 10.1161/CIRCULATIONAHA.109.904235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Light and intermittent smokers: Background and perspective. Nicotine & Tobacco Research. 2009;11:122–125. doi: 10.1093/ntr/ntn020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Breland AB, Karaoghlanian NV, Shihadeh AL, Eissenberg T. Preliminary results of an examination of electronic cigarette user puff topography: The effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine & Tobacco Research. 2015;17:142–149. doi: 10.1093/ntr/ntu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MB, Zimmermann MH, Delnevo CD, Lewis MJ, Shukla P, Coups EJ, Foulds J. E-cigarette versus nicotine inhaler: Comparing the perceptions and experiences of inhaled nicotine devices. Journal of General Internal Medicine. 2014;29:1444–1450. doi: 10.1007/s11606-014-2889-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutfin EL, McCoy TP, Morrell HE, Hoeppner BB, Wolfson M. Electronic cigarette use by college students. Drug and Alcohol Dependence. 2013;131:214–221. doi: 10.1016/j.drugalcdep.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tverdal A, Bjartveit K. Health consequences of reduced daily cigarette consumption. Tobacco Control. 2006;15:472–480. doi: 10.1136/tc.2006.016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- Walker N, Howe C, Bullen C, Grigg M, Glover M, McRobbie H, Whittaker R. The combined effect of very low nicotine content cigarettes, used as an adjunct to usual Quitline care (nicotine replacement therapy and behavioural support), on smoking cessation: A randomized controlled trial. Addiction. 2012;107:1857–1867. doi: 10.1111/j.1360-0443.2012.03906.x. [DOI] [PubMed] [Google Scholar]
- Walton KM, Abrams DB, Bailey WC, Clark D, Connolly GN, Djordjevic MV, Hatsukami DK. NIH electronic cigarette workshop: Developing a research agenda. Nicotine & Tobacco Research. 2015;17:259–269. doi: 10.1093/ntr/ntu214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner KE. Will the next generation of "safer" cigarettes be safer? Journal of Pediatric Hematology/Oncology. 2005;27:543–550. doi: 10.1097/01.mph.0000184574.00717.6c. [DOI] [PubMed] [Google Scholar]
- Zhu SH, Gamst A, Lee M, Cummins S, Yin L, Zoref L. The use and perception of electronic cigarettes and snus among the U.S. population. PLoS One. 2013;8:e79332. doi: 10.1371/journal.pone.0079332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.