Abstract
Background
Intestinal colonization during infancy is important to short and long term health outcomes. Bacteroides, an early member of the intestinal microbiome, are necessary for breaking down complex molecules within the intestine and function to assist the body’s immune system in fighting against potentially harmful pathogens. Little is known about the colonization pattern of Bacteroides in preterm infants during the early neonatal period.
Purpose
This study measured Bacteroides colonization during the early neonatal period in a population of preterm infants based on clinical factors including mode of birth, antibiotics, and nutrition.
Methods
Bacterial DNA was isolated from 144 fecal samples from 29 preterm infants and analyzed using quantitative real time polymerase chain reaction (PCR). Analyses included liner mixed models to determine which clinical factors affect Bacteroides colonization of the infant gut.
Results
We found that infants born via vaginal canal had a higher rate of increase in Bacteroides than infants born via Cesarean section (p<.001). We did not find significant associations between antibiotic administration and differences in nutritional exposures with Bacteroides colonization.
Implications for Practice
These findings highlight the significant influence of mode of birth on Bacteroides colonization. While mode of birth is not always modifiable, these study findings may help develop interventions for preterm infants born via Cesarean section aimed at overcoming delayed Bacteroides colonization.
Implications for Research
Greater study of the intestinal microbiome and the clinical factors relevant to the preterm infant is needed so that interventions may be developed and tested, resulting in optimal microbial and immune health.
Keywords: Preterm Infant, microbiome, bacteroides, mode of birth, newborn intensive care, cesarean, bacterial colonization, neonate
The Human Microbiome has emerged as an important contributor to human health and disease, as well as a potential target for therapeutic manipulation. The microbiome refers to the collective genetic make-up of the bacteria present in any particular human habitat such as the mouth, gastrointestinal tract, respiratory tract, vaginal canal, or skin.1 From initial colonization during infancy and into adulthood, the microbiome is influenced by many factors including genetics, perinatal and neonatal issues such as mode of delivery, use of antibiotics, nutrition, environment, health and disease status. Understanding how the microbiome evolves early in life and eventually matures to reflect an adult like composition is important when considering the impact that the microbiome has in shaping the immune system, influencing the inflammatory response and ultimately, the predisposition for health or disease.
The molecular based tools and bioinformatic analyses now used to study the microbiome have allowed us to describe the entire community structure of bacteria present in a given habitat. Measures of richness and diversity allow us to see not only how many different types of bacteria are present, but also their proportions or abundance to one another. Most of the microbiome studies published to date have been observational and descriptive in nature. However, as more of these studies are conducted and findings are generated, we will eventually have the ability to capitalize on the collective nature of this work such that we can begin to manipulate the microbiome for therapeutic purposes.
Microbiome studies typically characterize the entire microbial community of a specific habitat rather than focus on specific bacteria of interest. While there is great value in studying the microbiome as a whole, there is also a place for the study of specific bacteria that contribute to the microbiome. In the study reported here, we focused our work on Bacteroides present in the preterm infant intestine as measured in fecal material, and showed how they are influenced by various clinical factors over time.
Background and Significance
Early intestinal colonization is important to establishing a functional immune response, developing immunological tolerance, and ultimately to prevention of infectious disease. 2 The infant gut is no longer thought to be sterile at birth and there appears to be a pattern by which the intestinal microbiome is acquired and assembled within the infant gut.3,4 In a recent study published by La Rosa and colleagues, this pattern was described in the preterm infant as beginning with Bacilli, followed by Gammaproteobacteria, and then Clostridia.3 This study showed that by 33 to 36 weeks postconceptual age, the gut was well colonized by anaerobes. Some of the dominant anaerobic bacteria (ie. Firmicutes and Bacteroidetes) do not appear to grow outside the gut and as a result, need to be transmitted from one human host to another.5 The extent to which this transmission occurs during birth is not fully understood, but studies have shown differences in the intestinal microbiome and specifically the presence of anerobes such as Bacteroides based on mode of birth in infants born at term.6,7 Thus, we focused our study on Bacteroides in the early neonatal period, and specifically sought to explore how our findings in preterm infants would compare to those in term infants.
The majority of the bacteria in the gut are anaerobes, and of these, approximately 25% are Bacteroides.8 Bacteroides are gram-negative rods that play an important role in breaking down complex molecules within the intestine and function to assist the body’s immune system in fighting against potentially harmful pathogens.8 As stated, they are transmitted from mother to child during vaginal birth and as a result, are present in the human microbiome early in life, typically measurable by around 10 days following birth. Bacteroides are generally commensal in nature, benefiting the health of the host when they are located in the intestine. However, these bacteria can quickly become pathogenic if they are translocated outside the gastrointestinal tract, and have been associated with abscess formation in multiple body sites such as the abdomen, brain, liver, pelvis, and lungs, as well as serious blood stream infections.8
Like the majority of microbial species, colonization with Bacteroides is influenced by clinical factors, most notably those that occur during the perinatal and neonatal period. One of the first clinical factors influencing intestinal colonization occurs at birth. Mode of birth has been associated with differences in Bacteroides colonization, with infants born via Cesarean section having overall lower levels and a delayed pattern of colonization when compared to those born vaginally.7,9 In addition to delayed colonization with Bacteroides, Cesarean section has also been shown to negatively influence colonization with Lactobacillus and Bifidobacteria6 when compared with infants who are born via vaginal canal.
Following birth, the majority of preterm infants are administered antibiotics upon admission to the newborn intensive care unit. By definition, antibiotics kill bacteria. In term infants and adults, antibiotic administration has been shown to influence the intestinal microbiome.9,10 Interestingly, it is not only the prophylactic antibiotics that are administered to preterm infants soon after birth that influence intestinal colonization3 but also the antibiotics that are commonly administered to mothers during the perinatal period that have been shown to influence the intestinal microbiome of preterm infants.11 While the majority of studies on antibiotics have not focused specifically on Bacteroides colonization, there is evidence to suggest that this type of bacteria is decreased as a result of antibiotic administration early in the neonatal period.9
Nutrition is a major contributor to the microbiome over the lifespan, but is likely to play the most significant role during infancy when the intestinal microbiome is first acquired. Specifically, feeding with human milk from the mother of the infant influences the development of the microbiome as a result of the many bioactive factors present in human milk. Human milk oligosaccharides, bacteria, fatty acids, and immune cells all play a role in colonizing the gut with commensal bacteria and result in optimal immune programming. In studies evaluating the microbiome based on different diet regimens during the early neonatal period, infants fed human milk typically have a greater abundance of Lactobacillus and Bifidobacteria.12 These differences are important, because evidence suggests that not only is there a difference in the structure of the microbiome associated with differences in diet, these differences also result in distinct immune programming. In an animal study involving newborn monkeys (rhesus macaques) who were fed mother’s milk or nursery formula, the milk fed group developed greater T cell function that appeared to persist for as long as one year following birth.13 In addition to differences in immune programming, the fatty acid profile associated with human milk or infant formula feeding is different. A study involving preterm infants has shown that fecal short-chain fatty acids, which are important to the inflammatory response, are strongly influenced by nutrition. Specifically, preterm infants fed human milk had greater levels of total short chain fatty acids, and specific fatty acids including acetate, propionate, and chloride.14
We have provided a brief summary of what is known about Bacteroides and some the clinical factors that influence intestinal colonization with these bacteria during infancy. While more research is needed, especially under conditions of prematurity, mode of delivery, antibiotic exposure, and nutrition were all found to be important factors influencing intestinal colonization and thus, were included in this study. Our research objective was as follows: To study the pattern of Bacteroides colonization during the early neonatal period in a population of preterm infants based on clinical factors including mode of delivery, antibiotic administration, and nutritional exposures (i.e. proportion of feeding with human milk).
Methods
Subjects and Samples
Stool samples were collected from the diapers of preterm infants born prior to 32 weeks of gestation and frozen to −80°C within 24 hours of collection as reported elsewhere.15 Subjects who were unaffected by necrotizing enterocolitis or any other gastrointestinal or infectious disease were selected for the study based on sample availability every week for at least the first four weeks following birth, and ideally, out to six weeks following birth. A total of 29 infants were identified as having adequate samples available for study with the following sampling frames: 12 infants had samples available each week for four weeks, 6 infants has samples available each week for five weeks, and 11 infants had samples available each week for 6 weeks. This resulted in 144 total samples for analysis.
Laboratory Methods
Samples were systematically stored at −80°C until the time of analysis. At the time of analysis, DNA was isolated from approximately 200 mg of fecal material using a bead beating step and a commercially available kit designed to isolate DNA from fecal material (Qiagen, Valencia, CA). Quality control of DNA extraction was assured by following the Qiagen guidelines and optimizing our specimen for this protocol using the bead beating step. In addition, a 260/280 DNA/protein ratio was assured and DNA extraction yields were measured using a NanoDrop 2000 (Thermo Scientific, Waltham, MA), with DEPC water as a control. Isolated DNA from these samples (n=144) was then prepared for polymerase chain reaction (PCR) analysis as outlined below.
Quantitative real-time PCR (qPCR) was run in duplicate on a total volume of 25 μl. Final reaction mixtures contained 800 nM of each primer (forward and reverse) and 50 ng of template DNA, and 25 μl of SYBR@Green (Promega). Primers were designed based on previously published work16 that used a group of species-specific 16s rDNA-targeted primers. To measure the Bacteroides as a group, species-specific forward and reverse primers for Bacteroides fragilis were used to represent the Bacteroides-Prevotella-Porphyromonas group.16 The primer sequences were as follows:
Forward: 5′-GGTGTCGGCTTAAGTGCCAT-3′ (Tm: 62.5° C)
Reverse: 5′-CGGA(C/T)GTAAGGGCCGTGC-3′ (Tm: 65.5° C)
Using the molecular weights specified for each primer, a 100μl primer stock containing 20 pmol of each primer was prepared in DEPC water. 1 μl from this stock made up to 25 μl resulted in a final concentration of 800 nM for each primer (forward and reverse) in the final PCR mixture. The DNA concentration of bacteria standard was 262 ng/μl. Bacterial DNA standards were prepared in the following concentration ranges: 500ng, 50ng, 5ng, 0.5ng, .05ng, and 0.005ng.
The PCR cycle parameters for Bacteroides were as follows: 95°C for 3 minutes, 95°C for 30 seconds, 61°C for 30 seconds, 72°C for 1 minute, incubate 86°C for 1 second, 95°C step 2, 40 times, melting curve from 60°C to 95°C every 1°C. Hold 10 sec, END. The colony forming units (CFU) calculation used was 100 uL per plate. The clone number calculation for Bacteroides fragilis was as follows: following overnight incubation in brain heart infusion (BHI) broth at 37°C in an anaerobic incubator, CFU/mL was determined by serial dilution technique. 1 mL from the overnight culture was used for DNA extraction. The concentration of DNA was measured, and the value was incorporated into the CFU/mL values for determining the clone numbers. Serial dilutions were performed in quadruplicates and the average CFU/mL value for the final clone number calculation was used in the analysis. A concentration of 246 ng/uL of DNA was purified from 5 * 106 CFU/uL of Bacteroides fragilis. The CFU calculations were then based on the following:
500ng of DNA: 1.0 * 107CFU
50 ng of DNA: 1.0 * 106CFU
5 ng of DNA: 1.0* 105CFU
5 ng of DNA: 1.0* 104CFU
1 ng of DNA: 2.0 *103 CFU
Statistical Analysis
We performed statistical data analysis to compare nanograms of bacteria divided by the wet weight of stool, which we then analyzed based on our clinical variables of interest: birth mode, antibiotics, and nutrition. We applied linear mixed models to account for repeated measures over the first six weeks following birth. Subject specific intercepts were modeled as random effects to account for the repeated sampling within individual subjects over the first six weeks following birth. The candidate predictors were modeled as fixed effects. All two-way interactions between the candidate predictors and time (i.e week after birth) were considered. Terms were dropped from the model based on likelihood ratio testing. The response exhibited a heavily skewed distribution and was log-transformed. IBM SPSS 21.0 (IBM, Inc.) was used for all statistical analyses.
Results
Overall, we found that postnatal time was a significant driver to Bacteroides colonization, and that infants born via the vaginal canal were colonized with a greater amount of Bacteroides and had a significantly greater increase over time in the Bacteroides present when compared to those born via cesarean birth (p<.001). Interestingly, antibiotic administration and nutritional exposures did not influence the amount of Bacteroides measured in this study.
On average, the infants included in this study were born at 29 weeks of gestation and at a birth weight of 1280 grams. The majority of the infants were born via Cesarean section (85%), received antibiotics at birth (84%), and were fed human milk (83%). We carefully accounted for antibiotic administration and nutritional exposures in proximity to sample collection. We found that while nearly all of the infants received antibiotics at birth, more than half of the samples (55%) had no antibiotic exposures within seven days of sample collection, 26% of the samples had exposure to antibiotics within seven days of sample collection, and 16% of the samples had no antibiotic exposures at all. When accounting for nutritional exposures, we found that for the majority of samples (83%) the infant was fed >50% human milk within 7 days of the sample collection. Data describing the subjects and the samples analyzed are presented in Tables 1a and 1b.
Table 1.
Subject Demographics (n=29)
| n (%) | |
|---|---|
|
| |
| Sex | |
| Male | 17 (58.6%) |
| Female | 12 (41.4%) |
|
| |
| Mean (SD) | |
|
| |
| Gestational age (weeks) | 29.4 (1.29) |
|
| |
| Birth Weight (grams) | 1279 (263) |
|
| |
| Birth Mode | |
| Cesarean section | 25 (84%) |
| Vaginal | 4 (15%) |
| Table 1b. Specimen characteristics (n=144)
| |
|---|---|
| n (%) | |
|
| |
| Percent of total feeding comprised by breast milk within seven days of sample collection | |
| <50% | 24 (16.6%) |
| >50% | 120 (83.3%) |
|
| |
| Any antibiotic exposure prior to sample collection | |
| Yes | 121 (84.0%) |
| No | 23 (16.0%) |
|
| |
| Any antibiotic exposure within 7 days of sample collection | |
| Yes | 79 (54.9%) |
| No | 38 (26.4%) |
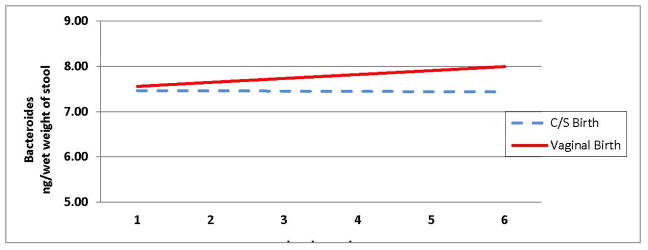
Based on the results of the likelihood ratio tests, we excluded the variables “antibiotics within seven days” and “feeding with human milk” from the statistical model as they provided no additional information about the measure of Bacteroides. Significant terms that remained in the model are the time of measurement of Bacteroides over six weeks, birth mode, and the two-way interaction between these variables. The change in Bacteroides over time for vaginal births was .0883 ng/week. In contrast, the rate of change in Bacteroides over time for caesarian births was 0.006 ng/week. These findings result in a difference in slopes of 0.0823 ng/week. This difference in slopes was significant with p-value < 0.001 (see Table 2). This implies that the rate of change in levels of Bacteroides over time differs for infants who were born via cesarean section when compared with those who were born vaginally. Thus, two colonization trajectories characterized by different slopes were calculated and shown in Figure 1, Colonization of Bacteroides in Preterm Infants Born via Cesarean Section and Vaginal Canal. Table 3 displays the average measurement of Bacteroides at each postnatal week for the infants based on mode of birth. In sum, we found that infants born vaginally had significantly more Bacteroides present in their stool over time when compared to infants born via Cesarean section (p<0.000).
Table 2.
Estimates of Change in Amount of Bacteroides Over Time
| Slope of Line | Standard Error | |
|---|---|---|
| Cesarean | 0.0060 | 0.0153 |
| Vaginal | 0.0883 | 0.0185 |
| Difference | 0.0823* | 0.0285 |
Significant Difference between slopes (Difference of slopes greater than zero). p-value < 0.001, Likelihood Ratio Test.
Figure 1.
Colonization of Bacteroides in preterm infants born via cesarean section and vaginal canal during the first six weeks following birth
The slope for vaginal birth is 7.47 + 0.088 at each incremental time point
The slope for cesarean birth is 7.47 + (0.006) at each incremental time point
The differences in these slopes is .082 (p<.000)
Table 3.
Bacteroides over the first six weeks following birth based on mode of birth
| Week1 | Week2 | Week3 | Week4 | Week5 | Week6 | |
|---|---|---|---|---|---|---|
| Cesarean Birth | 7.464 | 7.458 | 7.452 | 7.446 | 7.44 | 7.434 |
| Vaginal Birth | 7.557 | 7.644 | 7.731 | 7.818 | 7.905 | 7.992 |
Data reported as (ng/wet weight of stool)
Discussion
The findings of this study highlight that mode of birth in the preterm infant has a significant influence on Bacteroides colonization. These findings are similar to those identified by studies involving term infants 6,7,9 and require us to ask why in particular Bacteroides colonization appears to be delayed in the infant born via Cesarean section. It may be that passage through the vaginal canal either provides an important inoculation of Bacteroides at the time of birth, or more likely, the Lactobacillus that is present in the vaginal canal seeds the infant microbiome such that Bacteroides can begin to colonize over the early neonatal period. 6,17 Alternatively, the intestinal colonization of infants, especially preterm infants, who are born via Cesarean section may be overcome by the microbes that live on the mother’s skin at the time of birth (i.e. Staphlococcus and Streptococcus). It is known that these infants have a lower overall diversity of bacteria present in their intestinal microbiome,6,7 which may in large part be explained by the lack of Bacteroides that typically contributes a large proportion of the bacteria to the intestinal microbiome.
Another explanation for the lower proportion of Bacteroides found in infants born via Cesarean section is the lack of exposure to the mother’s intestinal bacteria. Often, regardless of gestational age, infants born vaginally interact with the intestinal bacteria present in the mother’s gut when fecal material is passed by the mother at the time of birth. The bacterial transmission of Bacteroides between the maternal intestinal microbiome and the infant born vaginally has not been confirmed. However, it is likely that the maternal intestinal microbiome, which is rich in Bacteroides is shared with the infant during vaginal birth.18 Since infants born via Cesarean section have no exposure to the perineal or anal area during birth, they do not have the advantage of directly interacting with maternal microbes such as Bacteroides.
It was somewhat surprising that the other two clinical factors of interest, antibiotic exposures and feeding with human milk, did not influence the Bacteroides colonization. However, this may be explained by the somewhat small and homogenous sample of preterm infants available for this observational study. Due to the overwhelmingly routine practice of administering antibiotics to preterm infants immediately following birth, it is difficult for studies to evaluate the influence that these exposures have on early intestinal colonization. Until there is new evidence that provides more precise guidance relative to which preterm infants are at greatest risk of sepsis and as a result, should receive antibiotics following birth, it will be challenging to understand the short and long term effects of antibiotics on the intestinal microbiome. Unlike administration of antibiotics, nutritional practices are typically more variable within a NICU setting. The largely mother’s milk fed preterm infant population included in this study reflects several years of effort focused on increasing the number of NICU mother’s who pump human milk and aim to breastfeed their infant upon discharge to home. While this is an outstanding achievement, it prevents our ability to more fully evaluate differences in the intestinal microbiome based on diet. Future studies that involve a more targeted enrollment of preterm infants based on specific clinical factors such diet will enable us to better answer these questions.
One of the limitations of this study is the sample size and distribution of the descriptive variables, namely the number of infants who were born vaginally (n=4) versus via Cesarean section (n=25). The significantly greater number of infants who were born via Cesarean section reflects current clinical practice relative to preterm birth, where infants are more frequently born via Cesarean section. Given that the aim of this observational study was to describe patterns in Bacteroides colonization without manipulating any clinical variables, we did not purposefully select infants based on specific clinical characteristics. Rather, we selected on availability of samples at specific time points for analysis. Our longitudinal study design helped mitigate the difference in the group sizes, but future work in this area will require larger and balanced group sizes, in addition to longitudinal data collection and analysis.
The findings generated by this study are specific to the preterm infant. This is unique from the majority of studies investigating the influence of clinical factors such as mode of birth on acquisition of the microbiome in that these studies have primarily involved infants born at term. While more study is needed in both the setting of preterm and term birth, the findings relative to mode of birth and colonization with Bacteroides appear similar: colonization with Bacteroides is delayed following Cesarean section when compared with vaginal birth. These findings highlight a potential opportunity for future intervention using a specific probiotic based on knowledge of the microbiome during the perinatal and neonatal period.
Implications for Practice
While mode of birth is not typically modifiable, we may use these study findings to develop interventions for preterm infants born via Cesarean section aimed at overcoming delayed Bacteroides colonization. For example, we may learn that in infants who are at an otherwise low risk of sepsis, a more judicious use of antibiotics is warranted to allow optimal colonization with Bacteroides to occur following Cesarean birth. In addition to more careful consideration of antibiotic administration, we may also determine that there are other aspects of nursing practice that put the preterm infant born via Cesarean section at a microbial disadvantage. For example, these infants are often separated from their mothers for a longer period of time while the mother recovers from surgical birth. While the influence of this separation on intestinal colonization requires further study, it is likely that skin-to-skin contact between mother and infant following birth, even during a NICU hospitalization, has a positive influence on acquisition of a diverse microbiome.
Another area of practice that we may consider analyzing as a result of these study findings is how we feed preterm infants in the NICU. While this study did not find differences in Bacteroides colonization over time as a result feeding, we know from other studies that feeding, specifically feeding with human milk, provides many advantages to the preterm infant’s early intestinal colonization.14 For preterm infants born via Cesarean section, we may consider introducing human milk feedings as early in the neonatal period as possible as a strategy to promote an optimal intestinal microbiome. Along these lines, future consideration may be given to the administration of a specific probiotic that is targeted at influencing the pattern of Bacteroides colonization following Cesarean birth. The administration of probiotics to preterm infants requires a great deal more research.19,20,21 However, targeting these efforts towards specific clinical scenarios where there is a body of evidence to suggest that an aspect of practice (i.e. mode of birth) influences intestinal colonization may be a promising path towards manipulating the microbiome for therapeutic purposes through the use of a specific prebiotic or probiotic.
Implications for Research
Greater study of the intestinal microbiome and the influence of specific clinical factors relevant to the preterm infant is needed. Findings from these studies will be important to the development and testing of interventions aimed at promoting health and preventing disease. These studies must be carefully constructed and include large sample sizes so that we can truly compare the microbiome between groups and evaluate the influence of clinical interventions on intestinal colonization. In these studies, special attention must also be paid to the influence of the intrauterine environment and the maternal microbiome on the preterm infant’s intestinal colonization. Identifying factors associated with a pattern of intestinal colonization that might be modifiable during pregnancy and birth has the potential to result in novel interventions that may protect against diseases associated with aberrant inflammation.
Finally, it is important to note that the majority of research conducted on the human microbiome to date has been observational in nature. Future studies, including studies of the preterm infant intestinal microbiome, will be well served by a longitudinal design and the inclusion of measures (i.e. inflammatory markers) representing the host response to microbial colonization. Without these data points, we will continue to provide observational reports that do not provide insights about how the infant is responding to a specific pattern of intestinal colonization. Future studies that lead the field forward will be more robust in that they include information about both the microbiome, or a specific aspect of the microbiome, and also how these microbes influence the patient’s inflammatory response and long-term health. Studies of this type that focus on infants, specifically preterm infants, will be especially important because they will have the potential to improve health and prevent disease across the entire lifespan.
What This Study Adds.
Similar to the term infant, intestinal colonization of the preterm infant is influenced by mode of birth.
Preterm infants born via Cesarean section have a delayed pattern of intestinal colonization with Bacteroides, and important bacterial species within the intestinal microbiome, when compared to infants born via the vaginal canal.
More research is needed to better understand how clinical factors relevant to the perinatal and neonatal period influence early intestinal colonization and the degree to which these influences persist throughout infancy and into childhood.
Summary of Recommendations for Practice and Research.
What we know
Early intestinal colonization that occurs during infancy plays a role in both short and long term health outcomes.
The patterned acquisition of the intestinal microbiome is important to the development of the immune response in the preterm infant.
Several factors relevant to the neonatal and perinatal period influence early intestinal colonization, perhaps most notably mode of birth and diet.
The colonization of specific bacterial species within the intestinal microbiome such as Bacteroides changes over time and is influenced by mode of birth in the setting of both term and preterm birth.
What needs to be studied
Greater numbers of preterm infants over longer periods of time, thereby increasing the sample sizes available for longitudinal analyses assessing the influence of clinical factors on the intestinal microbiome.
In addition to studies that aim to describe the community structure of the microbiome, more research is needed that will highlight the functional nature of the bacterial species present within the microbiome. This will be accomplished using new technologies that not only identify which bacterial species are present in a given human habitat, but also how they interact with the human host.
The preterm infant’s response to intestinal colonization with specific bacterial species that goes beyond the presence or absence of a specific disease process.
What we can do today
Raise awareness of the importance of early intestinal colonization during infancy, and why preterm infants are at especially high-risk for disturbances in the acquisition of their intestinal microbiome.
When possible for mother and infant, promote vaginal birth for both the term and preterm infant as an important factor in establishing a diverse intestinal microbiome during infancy.
When possible, provide clinical interventions (i.e. early feeding with human milk and avoidance of unnecessary antibiotics) that have been shown to help facilitate optimal microbial and immune health during infancy and early childhood.
References
- 1.Turnbaugh P, Ley R, Hamady M, Fraser-Liggett C, Knight R, Gordon J. The human microbiome project. Nature. 2007;449:804–10. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLoughlin RM, Mills KH. Influence of gastrointestinal commensal bacteria on the immune responses that mediate allergy and asthma. J of Allergy Clin Immunology. 2011;127:1097–107. doi: 10.1016/j.jaci.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 3.La Rosa PS, Warner BB, Zhou Y, et al. Patterned progression of bacterial populations in the premature infant gut. Proc Nat Aca of Sci. 2014;111:12522–7. doi: 10.1073/pnas.1409497111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koenig JE, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Nat Aca of Sci. 2011;108 (Suppl 1):4578–85. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ley R, Peterson D, Gordon J. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–48. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Nat Aca of Sci. 2010;107:11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jakobsson HE, Abrahamsson TR, Jenmalm MC, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2013;63:559–66. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 8.Wexler HM. Bacteroides: the good, the bad, and the nitty-gritty. Clin Micro Reviews. 2007;20:593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–21. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Cobas AE, Artacho A, Knecht H, et al. Differential effects of antibiotic therapy on the structure and function of human gut microbiota. PloS One. 2013;8:e80201. doi: 10.1371/journal.pone.0080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arboleya S, Sanchez B, Milani C, et al. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J of Peds. 2015;166:538–44. doi: 10.1016/j.jpeds.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 12.Marques TM, Wall R, Ross RP, Fitzgerald GF, Ryan CA, Stanton C. Programming infant gut microbiota: influence of dietary and environmental factors. Curr Opin in Biotec. 2010;21:149–56. doi: 10.1016/j.copbio.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Ardeshir A, Narayan NR, Méndez-Lagares G, et al. Breast-fed and bottle-fed infant rhesus macaques develop distinct gut microbiotas and immune systems. Science Trans Med. 2014;6:252ra120. doi: 10.1126/scitranslmed.3008791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pourcyrous M, Nolan VG, Goodwin A, Davis SL, Buddington RK. Fecal short-chain fatty acids of very-low-birth-weight preterm infants fed expressed breast milk or formula. J Ped Gastro Nut. 2014;59:725–31. doi: 10.1097/MPG.0000000000000515. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y, Shan G, Sodergren E, Weinstock G, Walker WA, Gregory KE. Longitudinal analysis of the premature infant intestinal microbiome prior to necrotizing enterocolitis: a case-control study. PloS One. 2015;10:e0118632. doi: 10.1371/journal.pone.0118632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rinttila T, Kassinen A, Malinen E, Krogius L, Palva A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Micro. 2004;97:1166–77. doi: 10.1111/j.1365-2672.2004.02409.x. [DOI] [PubMed] [Google Scholar]
- 17.Petrova MI, Lievens E, Malik S, Imholz N, Lebeer S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front Physio. 2015;6:81. doi: 10.3389/fphys.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaishampayan PA, Kuehl JV, Froula JL, Morgan JL, Ochman H, Francino MP. Comparative metagenomics and population dynamics of the gut microbiota in mother and infant. Genome Bio Evol. 2010;2:53–66. doi: 10.1093/gbe/evp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau C, Ambalavanan N, Chakraborty H, Wingate MS, Carlo WA. Extremely low birth weight and infant mortality rates in the United States. Pediatrics. 2013;131:855–60. doi: 10.1542/peds.2012-2471. [DOI] [PubMed] [Google Scholar]
- 20.Asmerom M, Crowe L, Marin T. Understanding the Biologic Therapies of Probiotics, Prebiotics, and Synbiotics: Exploring Current Evidence for Use in Premature Infants for the Prevention of Necrotizing Enterocolitis. J Peri Neo Nsg. 2015;29:240–7. doi: 10.1097/JPN.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 21.Robinson J. Cochrane in context: probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid-Based Child Health. 2014;9:672–4. doi: 10.1002/ebch.1977. [DOI] [PubMed] [Google Scholar]