Abstract
Aims
We assessed gender differences in pre-event health status (symptoms, functioning, quality of life) in young patients with acute myocardial infarction (AMI), and whether or not this association persists following sequential adjustment for important covariates. We also evaluated the interaction between gender and prior coronary artery disease (CAD), given that aggressive symptom control is a cornerstone of care in those with known coronary disease.
Methods and Results
A total of 3,501 AMI patients (2,349 women) aged 18–55 years were enrolled from 103 United States/24 Spanish hospitals (2008–2012). Clinical/health status information was obtained by medical record abstraction and patient interviews. Pre-event health status was measured by generic [Short Form-12 (SF-12), EuroQoL [EQ-5D)] and disease-specific [Seattle angina questionnaire (SAQ)] measures. T-test/chi-square and multivariable linear/logistic regression analysis was utilized, sequentially adjusting for covariates. Women had more co-morbidities and significantly lower generic mean health scores than men [SF-12 physical health =43±12 vs. 46±11 and mental health= 44±13 vs. 48±11]; EQ-5D utility index=0.7±0.2 vs. 0.8±0.2, and visual analog scale=63±22 vs. 67±20, P<0.0001 for all. Their disease-specific health status was also worse, with more angina [SAQ angina frequency=83±22 vs. 87±18], worse physical function [physical limitation=78±27 vs. 87±21] and poorer quality of life [55±25 vs. 60±22, P<0.0001 for all]. In multivariable analysis, the association between female gender and worse generic physical/mental health persisted, as well as worse disease-specific physical limitation and quality of life. The interaction between gender and prior CAD was not significant in any of the health status outcomes.
Conclusion
Young women have worse pre-event health status as compared with men, regardless of their CAD history. While future studies of gender differences should adjust for baseline health status, an opportunity may exist to better address the pre-event health status of women at risk for AMI.
Keywords: Health Status, women, acute myocardial infarction, quality of life
Introduction
Young women (≤55 years) have a 2–3 fold greater in-hospital mortality following acute myocardial infarction (AMI) as compared with young men.1, 2 Because health status has been shown to predict short and long-term adverse cardiovascular events,3, 4 it is possible that earlier recognition of patients’ compromised health status could create opportunities to organize more aggressive treatment and intensify preventive strategies. Beyond its prognostic importance, patient-reported health status measures are increasingly used to quantify patients’ perceptions of how their disease affects them in terms of their symptoms, function and quality of life5 and have been advocated as a marker of healthcare quality.4, 6 Patients’ health status is not only an important outcome,7 but can be used clinically to optimize treatment in stable coronary artery disease (CAD). Importantly, while an AMI represents a critical clinical event for CAD patients, it is often preceded by worsening symptoms and health status. Despite the high rates of morbidity and mortality outcomes for young women with an AMI,1, 2 no prior study has explored whether or not their pre-admission health status is worse than that of men. Understanding the profiles of young women with an AMI is an important first step in tailoring therapy to these patients and improving their outcomes. To address this gap in knowledge, we sought to (a) assess gender differences in pre-event patient-reported health status (including angina symptoms, quality of life, general physical/mental functioning), (b) investigate whether any gender differences in pre-event health status persist after sequential adjustment for important covariates, and (c) evaluate the interaction between gender and prior CAD given that among those with known CAD, aggressive treatment of symptoms is a standard of care. Based on previous research8–10 we hypothesized that young women with AMI would have increased angina, more physical limitations, poorer disease-specific quality of life and worse physical/mental functioning, measured at the time of their AMI, as compared with men.
Methods
Study sample
Between August 21st 2008 and May 1st 2012, 3,501 AMI patients from 103 United States (US) hospitals and 24 Spanish hospitals were enrolled into the VIRGO study (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients – called Infarto de miocardio en la Mujer Joven (IMJOVEN) in Spain) (VIRGO Grant: #5 R01 HL081153-05). The study was designed to investigate factors associated with higher mortality in young women with AMI.11 Patients were prospectively recruited and enrolled in the VIRGO study, which used a 2:1 female to male enrollment design to enrich the study’s inclusion of young women Every attempt was made to enroll consecutive young women with an AMI at each center. After 2 women were recruited the next young man with an AMI was recruited at each site. A total of 5,585 patients with AMI were screened at participating sites, of which 3,572 were eligible and enrolled. The final cohort used in this study consisted of 2,985 from the US (2,009 women, 976 men), and 516 patients from Spain (340 women, 176 men), with a total of 3,501 patients (N=2,349 women; N=1,152 men).
Participants
The VIRGO methodology and design have been previously described.11 In brief, participants were 18–55 years old and AMI was confirmed by increased cardiac biomarkers within 24 hours of admission, and at least either ischemic symptoms or electrocardiographic changes. Participants must have presented directly to the enrolling site, or been transferred within 24 hours of presentation, thus insuring that primary clinical decision-making occurred at the enrolling site. Exclusion criteria included (a) non-English/non-Spanish speaking patients’ (b) inability to provide informed consent, (c) incarceration and (d) those patients who developed elevated cardiac markers as a result of elective coronary revascularization. Institutional Review Board approval was obtained at each participating institution, and patients provided informed consent for their study participation including baseline and follow-up interviews.
Socio-demographics and clinical variables
Baseline data (i.e. index AMI admission) were collected by medical chart abstraction and standardized in-person interviews during the index AMI admission by trained personnel.11 The patient domains collected include detailed information on demographics, socio-economic status (SES), cardiovascular risk factors, prior co-morbidities and chronic risk factors (Table 1, Appendix 1).
Table 1.
Baseline clinical characteristics for the overall population stratified by gender.
| Variable | Men (N=1,152); % |
Women (N=2,349); % |
P-value |
|---|---|---|---|
| Socio-demographics (%) | |||
| Age, range (years) | 23 – 55 | 18 – 55 | |
| Age, median (IQR), years | 48 (43, 52) | 48 (44, 52) | 0.2663a |
| Race | |||
| White | 84 | 76 | |
| Black | 10 | 19 | <0.0001 |
| Other | 6 | 5 | |
| Married | 58 | 48 | <0.0001 |
| Education | |||
| < High school | 4 | 6 | |
| High school | 42 | 41 | |
| > High school | 54 | 53 | |
| Work full- or part-time | 73 | 56 | <0.0001 |
| Health insurance | 79 | 81 | 0.0628 |
| Avoid getting healthcare due to cost | 29 | 31 | 0.1393 |
| Cardiac risk factors (%) | |||
| Current Smoker (last 30 days) | 59 | 60 | 0.8106 |
| Diabetes Mellitus | 27 | 39 | <0.0001 |
| Hypertension | 62 | 64 | 0.3907 |
| Dyslipidemia | 92 | 83 | <0.0001 |
| Sleep apnea | 5 | 4 | 0.3046 |
| Illicit drug use | 8 | 8 | 0.8163 |
| Co-morbidities (%) | |||
| Congestive heart failure | 2 | 5 | <0.0001 |
| Renal dysfunction | 8 | 12 | 0.0006 |
| Chronic lung disease | 5 | 13 | <0.0001 |
| Depression | 24 | 48 | <0.0001 |
| Cancer | 2 | 4 | 0.0008 |
| Obesity (BMI ≥30kg/m2) | 45 | 51 | 0.0004 |
| Medical History (%) | |||
| Prior AMI or PCI or CABG | 20 | 19 | 0.1742 |
| Angina | 26 | 28 | 0.3726 |
| Prior cardiac catheterization | 21 | 20 | 0.6470 |
| Prior stroke/TIA | 2 | 5 | 0.0001 |
| Prior PAD | 2 | 2 | 0.4706 |
| Autoimmune Disorders | 1 | 4 | <0.0001 |
| Alcohol abuse | 11 | 4 | <0.0001 |
P-value from Wilcoxon Rank Sum Test
BMI: body mass index; AMI: acute myocardial infarction; PCI: percutaneous coronary intervention; CABG: coronary artery bypass grafting; TIA: transient ischemic attack; PAD: peripheral artery disease.
Health status measures
Both generic [Short Form-12 (SF-12),12 the EuroQoL Quality of Life Scale (EQ-5D)13 and disease-specific health status instruments [Seattle angina questionnaire (SAQ14)] were administered at the baseline interview to capture pre-event health status. The SF-12 and SAQ have 4-week recall periods, while the EQ-5D inquires about current health.
Short Form-12 (SF-12)
The SF-12 has been demonstrated to be a valid and reliable instrument and is widely used to quantify patients’ overall mental/physical functional status12. The SF-12 physical (PCS) and mental (MCS) component summary scores were calculated and range from 0–100, with higher scores indicating higher functioning. A score of 50 represents the US population average, with a standard deviation of 10 points. Additionally, a mean PCS score of 48±10.6 and a MCS score of 53±10.7 represent the population averages for Spain.15 A mean score of ≥5–10 for a 0.5–1.0 standard deviation change is considered clinically significant.16, 17
EuroQoL scale (EQ-5D)
The EQ-5D is a standardized measure of generic health status13 and has previously been validated in AMI patients.18 This questionnaire has two parts; a descriptive section that classifies patients into one of 243 health states consisting of the following dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression), and a 20cm visual analog scale (EQ-VAS), which ranges from 0–100, with higher scores indicating better health states.19 A mean VAS score ranging from 79–86 and 73–82 represent the US and Spanish population average for patients between 18–55 years. Similarly, a utility index score ranging from 0.80–0.89 and 0.91–0.96 represent the US and Spanish population average for patient’s 18–55 years.20 The mean (standard deviation) minimally important difference is 0.040 (0.026), based on the US algorithm.21, 22
Seattle angina questionnaire (SAQ)
The SAQ is a 19-item disease-specific health status measure for patients with CAD, that has demonstrated validity, reliability and clinical responsiveness14, 23 and is predictive of mortality and re-hospitalization4. The five clinically relevant domains of the SAQ include physical limitation, angina stability, angina frequency, treatment satisfaction, and quality of life. For the purpose of this study the physical limitation, angina frequency, treatment satisfaction and quality of life domains were used. Each domain has a range of 0 to 100 points with higher scores indicating higher levels of functioning. The mean scores for the US population average with CAD include the following: physical limitation (50), angina frequency (67), treatment satisfaction (78) and quality of life (56).14 A mean difference of greater than 5 points between groups for all scales is considered clinically significant14.
Statistical analysis
Baseline characteristics were examined for the total sample and compared between men and women using Chi-squared tests for categorical variables and t-tests for continuous variables. Baseline health status results (SF-12, EQ-5D, SAQ) are reported as mean and standard deviation (SD). To examine the relationship between gender and health status, as indicated by SAQ physical limitation and angina frequency scores, we used a sequential logistic modeling approach by dichotomizing the physical limitation score into no physical limitations due to angina (SAQ PL=100) vs. any (SAQ PL<100) and the angina frequency score into no angina over the past 4 weeks (SAQ AF=100) vs. any (SAQ AF<100). The SAQ quality of life scores, SF-12 PCS and MCS were modeled using a sequential linear regression modeling approach. For all models, we sequentially adjusted for potential confounders and, at each step, assessed the association of gender with the outcome. The first model included only gender (Model 1). The second model included Model 1 and other demographics (age, race, and marital status) (Model 2). The third model included Model 2 and SES (education level, employment status, and insurance status) (Model 3). The fourth model included Model 3 and cardiovascular risk factors (hypertension, diabetes mellitus, dyslipidemia, current smoking status, and obesity [body mass index (BMI≥30 kg/m2)] (Model 4). The fifth model included Model 4 and prior comorbidities, including prior angina, prior heart failure, prior AMI/percutaneous coronary intervention/coronary artery bypass grafting, prior cardiac catheterization, prior peripheral artery disease (PAD) and prior stroke/transient ischemic attack (TIA) (Model 5). The sixth model included Model 5 and other important risk factors (renal dysfunction, chronic lung disease, and depression) (Model 6). In these sequential models, interaction terms between gender and country (i.e. US vs. Spain) were also evaluated (Appendix 1).
In addition, we conducted a subgroup analysis of the baseline health status results for men and women with and without prior CAD (defined as prior AMI/PCI/CABG) to explore whether the pre-event health status differed by gender among those with known CAD as compared with a new diagnosis. This analysis permitted an evaluation of patients’ symptoms and health status prior to an AMI among those with known CAD in whom optimizing their symptom control is a guideline-endorsed goal. Interaction terms between gender and prior CAD were evaluated for the SAQ physical limitation, angina frequency and quality of life scores as well as the SF-12 PCS and MCS scores. For all analyses, the complete available dataset was used with no imputation for missing variables (for all three-health status measures only <5% were missing data on both genders). A p-value < 0.05 was considered statistically significant. All analyses were computed using SAS 9.3 (SAS Institute Inc., Cary, NC).
Results
Baseline characteristics
Baseline characteristics of the total sample (N=3,501), stratified by gender (N=2,349 67% women) are shown in Table 1. Both genders were of similar age, but women were more likely to be black, to be single, have health insurance and to be unemployed as compared with men. In addition, women were more likely to have diabetes mellitus, obesity, congestive heart failure, renal dysfunction, chronic lung disease, prior stroke/TIA, autoimmune disorders and depression, but less likely to have dyslipidemia or alcohol abuse.
Health status analyses
The generic and disease-specific health status of women was significantly lower on admission than men (Table 2). On presentation, women had significantly poorer physical [PCS (Mean±SD)=43±12 vs. 46±11, P<0.0001] and mental (MCS= 44±13 vs. 48±11, P<0.0001) generic health status than men. In addition, women scored significantly poorer on the EQ-5D index score (0.7±0.2 vs. 0.8±0.2, P<0.0001) and the EQ-5D VAS (VAS= 63±22 vs. 67±20, P<0.0001). Women reported more disease-specific physical limitations (78±27 vs. 87±21, P<0.0001), more angina frequency (83±22 vs. 87±18, P<0.0001), less treatment satisfaction (91±14 vs. 93±11, P<0.0001) and worse quality of life (55±25 vs. 60±22, P<0.0001) than men. When dichotomizing physical function and symptoms, women were more likely to report having physical limitations due to angina (57% vs. 47% of men; P<0.0001) and angina (55% of women vs. 50% of men, P=0.0061) than men prior to their AMI.
Table 2.
Pre-event Generic and Disease Specific Health Status Measures Stratified by gender.
| Men (N=1,152) |
Women (N=2,349) |
P-value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| General Health (SF-12)b | |||
| PCS score | 46 (11) | 43 (12) | <0.0001 |
| MCS score | 48 (11) | 44 (13) | <0.0001 |
| CVD Functional Status (SAQ)c | |||
| Physical Limitation Sub-score | 87 (21) | 78 (27) | <0.0001 |
| Angina Frequency Sub-score | 87 (18) | 83 (22) | <0.0001 |
| Patients with no prior angina (%) | 74% | 72% | 0.3726 |
| Treatment Satisfaction Sub-score | 93 (11) | 91 (14) | <0.0001 |
| Quality of Life Sub-score | 60 (22) | 55 (25) | <0.0001 |
| Health Related Quality of Life (EQ-5D)d | |||
| Index Score | 0.8 (0.2) | 0.7 (0.2) | <0.0001 |
| VAS Score | 67 (20) | 63 (22) | <0.0001 |
Of those who completed the instrument.
A mean score of ≥5–10 for a 0.5–1.0 standard deviation change is considered clinically significant.
A mean difference of greater than 5 points between groups for all scales is considered clinically significant.
The mean (standard deviation) minimally important difference is 0.040 (0.026) based on the US algorithm.
PCS: SF-12 physical component summary score; MCS: SF-12 mental component summary score; VAS: EQ-5D visual analogue score; SAQ: seattle angina questionnaire.
Multivariable analyses of gender differences in pre-event health status
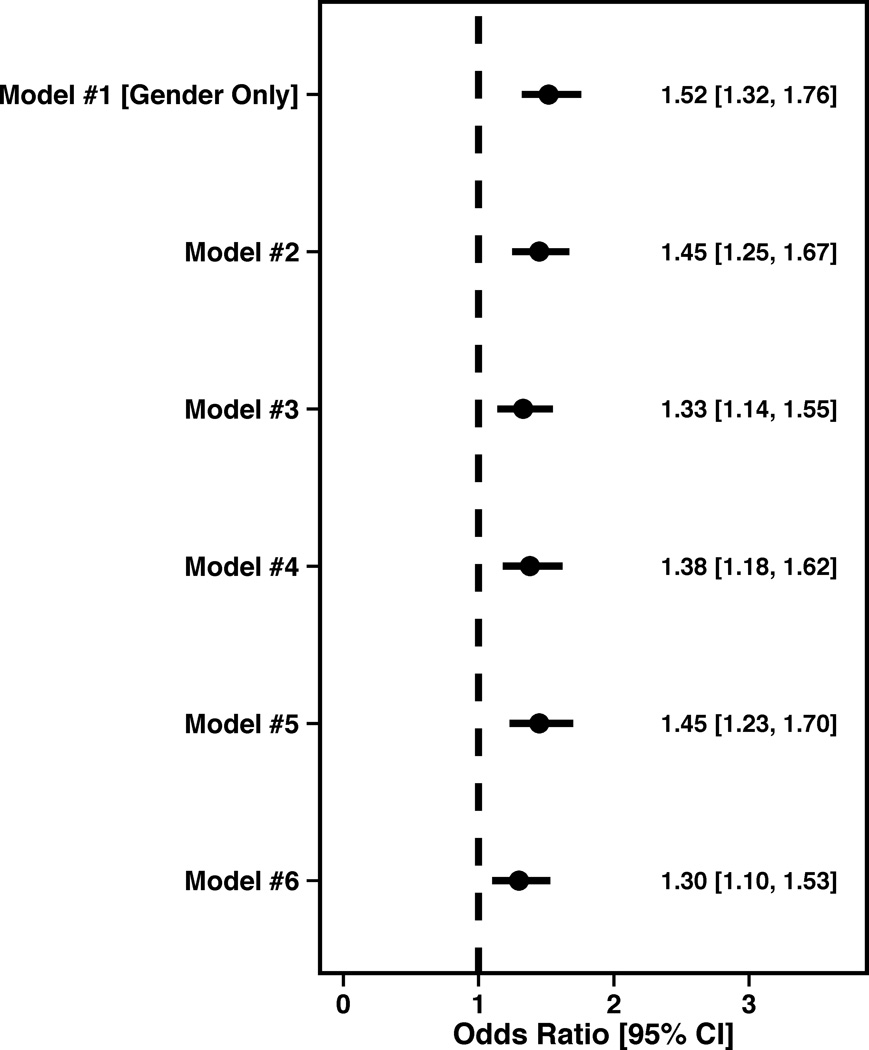
In the model only including gender, women were more likely to report physical limitations due to angina, with the odds of having any limitations being 1.52 (95% CI 1.32,1.76). After sequentially adjusting for the numerous differences between women and men (Figure 1A), women were significantly more likely to report physical limitations due to angina prior to their AMI [OR= 1.29 (95% CI 1.09, 1.53)].
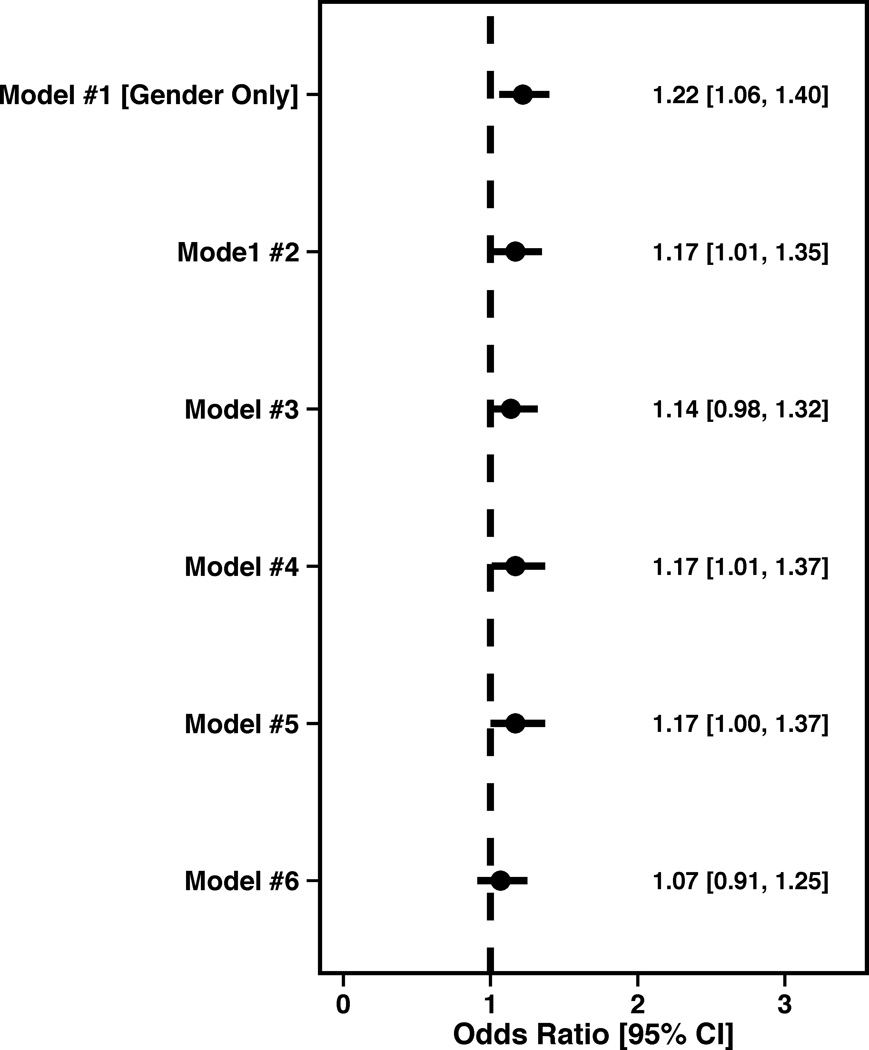
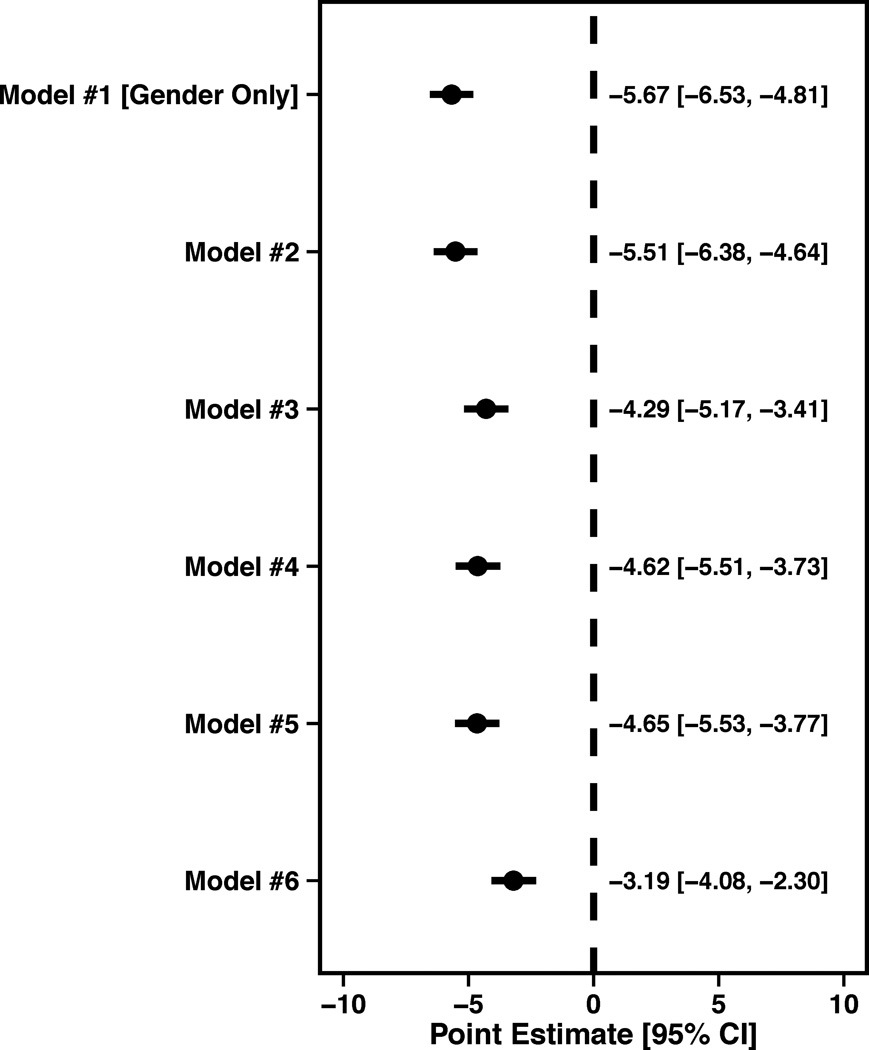
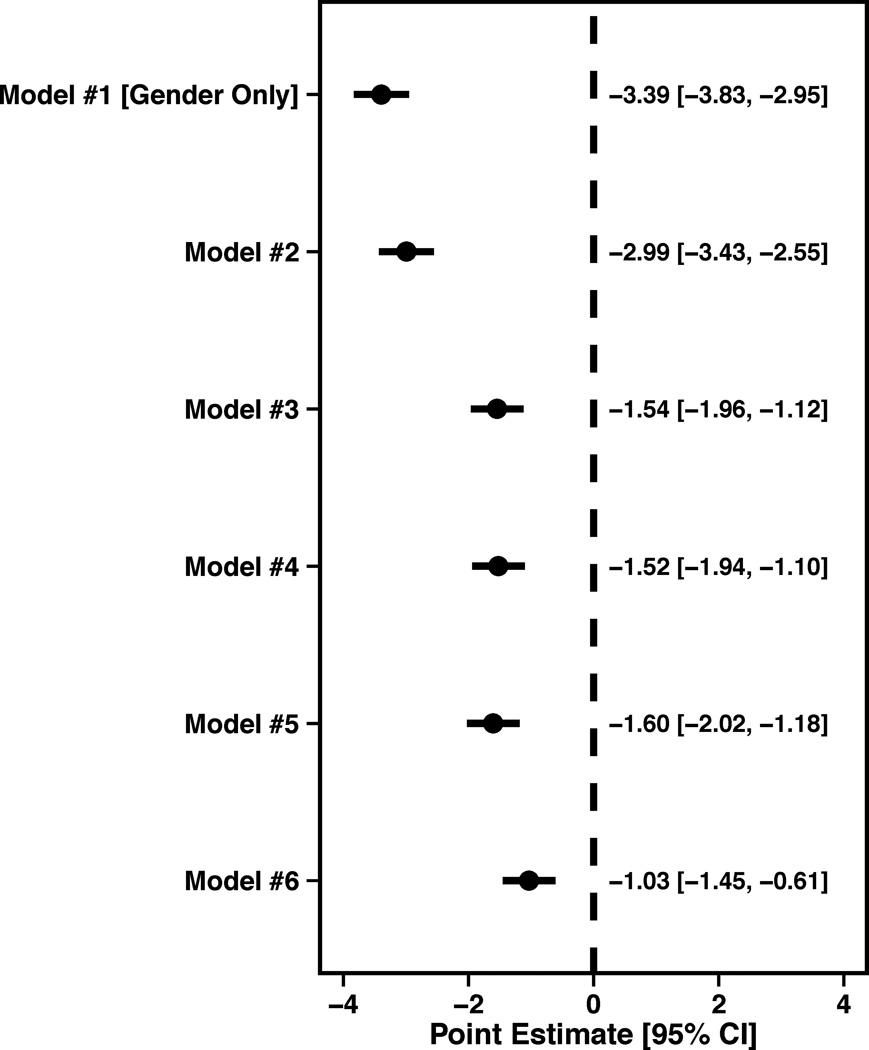
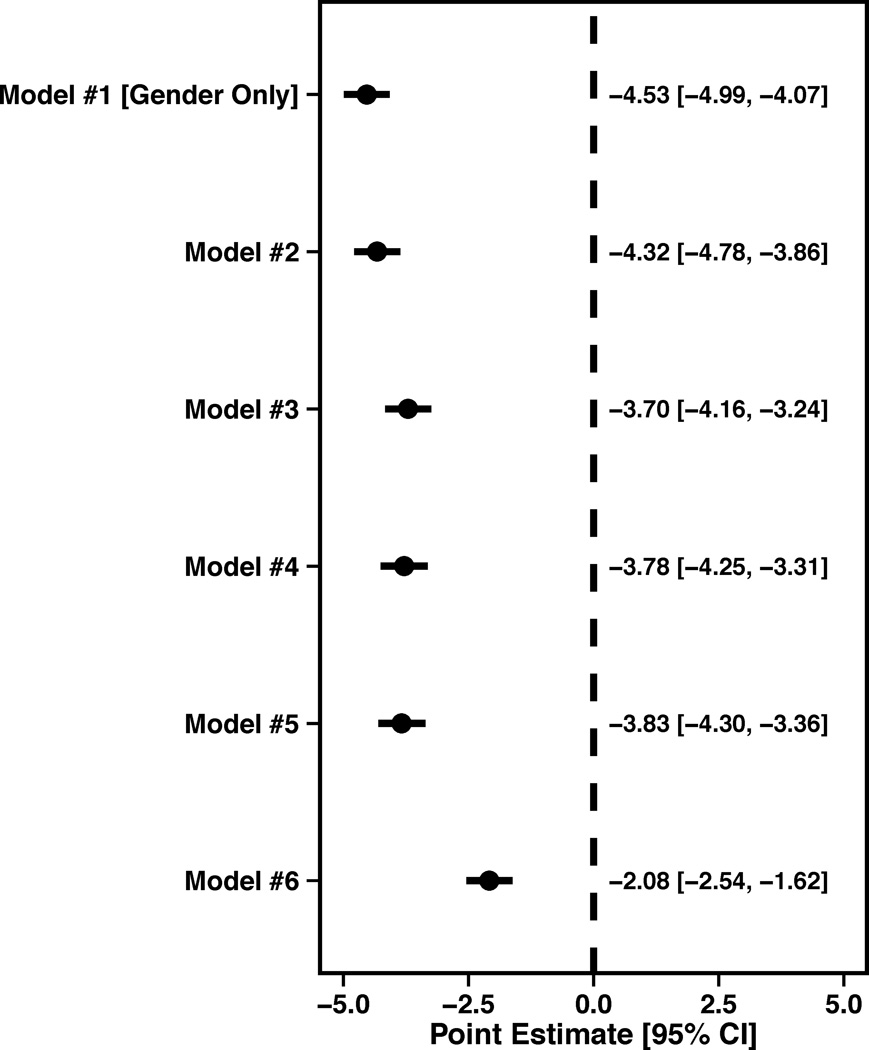
Figure 1.
Forest plots illustrating the effect of sequential adjustment on the relationship between gender and SAQ physical limitation, angina frequency and quality of life domains and SF-12 PCS and MCS domains. All models were sequentially adjusted for the following: Model 1: Female gender; Model 2: Model 1 + demographics; Model 3: Model 2 + SES; Model 4: Model 3 + cardiovascular risk factors; Model 5: Model 4 + prior comorbidities; Model 6: Model 5 + other important risk factors.
(A) Physical limitation forest plot showing the OR and 95% CI for female gender.
(B) Angina frequency forest plot showing the female OR and 95% CI.
(C) Quality of life forest plot showing the female Beta (95% CI).
(D) SF-12 PCS forest plot showing the female Beta (95% CI).
(E) SF-12 MCS forest plot showing the female Beta (95% CI).
Women were also more likely to report having angina prior to their AMI (Female OR=1.22, 95% CI 1.06,1.40). However, after adjusting for socio-demographics, SES, cardiovascular risk factors, prior co-morbidities and chronic risk factors, gender was no longer independently associated with having angina (Female OR=1.07, 95% CI 0.91,1.25) prior to an AMI (Figure 1B).
Women were also more likely to report a poorer quality of life than men, as per the SAQ quality of life score in unadjusted analyses (β= −5.67±0.86, P<0.0001). After full adjustment, the effect of gender was attenuated, but still statistically significant (β= -3.19, SE=0.89, P=0.0004; Figure 1C).
In regards to generic health status, women reported having a poorer PCS in unadjusted analyses (β=−3.39, SE=0.44, P<0.0001). Following adjustment for socio-demographics, SES, cardiovascular risk factors, prior co-morbidities and chronic risk factors, the effect of gender was attenuated (β=−1.04, SE=0.42, P=0.0136) (Figure 1D). Lastly, women also reported a poorer MCS in unadjusted analyses (β=−4.53, SE=0.46, P<0.0001), which was also attenuated with full adjustment (β=−2.07, SE=0.46, P<0.0001) (Figure 1E).
We tested the gender vs. country interaction (US VS. Spain), which was not significant in the SF-12 (PCS, MCS) or the SAQ health status outcomes (physical limitation, angina frequency, quality of life), suggesting that the relationship between health status and gender did not vary by country (Appendix 1).
Pre-event health status of patients with prior CAD/No prior CAD
In patients with known prior CAD before their AMI, women (n=436) had poorer scores than men on the SF-12 PCS and MCS indicating poorer physical and mental functioning. In regards to the SAQ domains, women had more physical limitations, more angina, less treatment satisfaction, and a poorer quality of life than men (n=236). Women with prior CAD also reported a poorer EQ-5D index utility score and VAS score (Appendix 2).
Similarly, in those patients with no previous CAD, women (n=1,913) again had poorer scores on the SF-12 PCS and MCS indicating poorer physical and mental functioning. In regards to the SAQ domains, women had more physical limitations, more angina, less treatment satisfaction, and a poorer quality of life (Appendix 3). Women with no prior CAD also reported a poorer EQ-5D index utility score and VAS score (Appendix 3). The gender versus prior CAD interaction was not significant in any of the SF-12 generic health status outcomes or the SAQ health status outcomes, suggesting that the worse health status prior to AMI was present in women, regardless of their CAD history.
Discussion
This is the first study, to our knowledge, to compare the pre-event health status of young women (≤55 years), with that of young men. We found that young women not only present with more co-morbidities at initial hospitalization, but also report significantly poorer physical and mental functioning, more symptoms of angina, and poorer quality of life in the weeks leading up to their AMI. The poorer health status outcomes persisted even after we considered the <45 and ≥45 cutoff as a surrogate for hormonal status. After adjusting for a myriad of potential confounders, women still had poorer pre-event physical limitations and quality of life, as well as poorer physical/mental functioning compared with men. These associations existed, regardless of country and whether or not patients had known CAD prior to their infarct. The latter findings suggest that the management of patients’ health status prior to their AMI differs for men and women and/or that women have the tendency to report lower health status due to other potential mechanisms (i.e. disposition to report worse health states, societal roles/burden, hormonal/genetic explanations).24, 25 Importantly, health status scores for women and men reached the threshold for what is defined as a clinically relevant difference (i.e. mean difference of >5 points on the SAQ)14 for the physical limitation, angina frequency and quality of life domains, but not for the treatment satisfaction domain. Similarly, health status scores for both genders reached the threshold for a clinically relevant difference on the SF-12 MCS score but not the PCS.16, 17, 26 We observed small to medium effect sizes, in all of our multivariable models, which suggest that more effective therapies in those with new or known CAD may alleviate the health status burden of these young patients. In terms of population norms, young women and men with AMI had decreased SF-12 PCS and MCS scores when compared with US and Spanish general population averages.15–17 In addition, both genders rated their current health strikingly lower on the EQ-5D VAS score, in comparison to US/Spanish population norms, however their index scores where similar.20, 22 These findings extend the existing literature by providing novel insights into gender differences in health status prior to an AMI.8–10, 27–30 Gender differences in patient-centered outcomes, such as angina, disease-specific quality of life and general health status, have rarely been studied in AMI, particularly in a younger cohort of patients (i.e. ≤55 years). Preliminary data have suggested that manifestations of CAD, depression, co-morbidities, infarct severity and baseline health related quality of life are associated with quality of life after AMI.31–33 Although studies have recognized that women have a poorer quality of life as compared with men,24–31, 34 particularly in the early recovery period (i.e. 1–12 months post AMI),9, 29, 34 our results demonstrate that young women report poorer health even before their infarct. From a methodological standpoint, future gender-based comparisons of health status outcomes should adjust for patients’ pre-event health status, given its strong association with subsequent health status (i.e. 1-month or 12-month endpoints).10, 35
The underlying factors contributing to the observed gender disparity in health status at the time of an AMI may include differences in clinical characteristics (i.e. disease severity)32, psychosocial factors or the quality of outpatient care.25 In regards to psychosocial factors, younger women may have less social supports,9 more anxiety and a higher inclination to stress compared with men, due to an increased work/home burden,36, 37 thereby impacting their daily functioning and quality of life. Furthermore, an important observation in this study is the greater burden of pre-existing risk factors and co-morbidities in women, including diabetes, chronic heart failure, renal dysfunction, lung disease, cancer, obesity prior stroke and autoimmune disorders. These findings confirm prior research indicating that young women, but not older women, have more risk factors and co-morbidities at the time of their AMI.1, 2, 38 Collectively, these observations highlight a need to better understand the outpatient care of young women at risk for an AMI.
In the present study we found a significant effect of gender in terms of physical limitations, quality of life and physical/mental functioning, even following adjustment for a range of covariates, including depressive symptoms. This finding is an important observation given prior research9, 39 demonstrating more depressive symptoms in younger women (<60 years) with AMI. Our work not only confirms these prior studies, but raises the possibility that even after adjusting for patients’ depressive symptoms that there are associated characteristics, such as greater delays in seeking treatment for angina symptoms (i.e. in outpatient care) or perhaps less engagement with health care systems and/or access to prevention strategies, that warrant further evaluation in future studies.
Our findings should be interpreted within the context of several potential limitations. Firstly, this was an observational study and consequently the observed differences between genders in health status outcomes may be due to residual confounding. However, our detailed data collection enabled us to adjust for a broad range of patient-level factors that are typically not included in gender-based research. Secondly, although we inquire about prior health, patients were interviewed during their index hospitalization for AMI (i.e. health status measures have a 4 week recall period). As such, there is a possibility for poor recall and report bias. Thirdly, it is possible that only healthier patients agreed to participate in this study, which may have impacted on the results (i.e. patients had to survive to consent), however the long-term objectives of VIRGO are to better illuminate the recovery after AMI and this is not a relevant question in those who die early in their hospitalization. Lastly, we only focused on health status outcomes for all three measures at 1 time point (i.e. baseline) and future examination of the long-term health status of these young AMI patients is needed. Importantly, these findings may underscore the importance of measuring pre-event health status at the time of admission to be able to adjust for these differences when looking at longer-term outcomes.
Conclusion
In conclusion, this study has demonstrated that even after adjusting for important clinical confounders, young women have poorer pre-event physical limitations and quality of life as well as a poorer physical and mental functioning, in comparison to similarly aged men. These associations existed, regardless of whether or not patients had known CAD prior to their AMI. These results indicate the potential in targeting the pre-event setting in order to identify high-risk women early on. This information may necessitate public health policies, which specifically address risk factors and education in this young female group.
Supplementary Material
Acknowledgements
None.
Funding: The VIRGO study was supported by a 4-year National Heart, Lung, and Blood Institute grant [number 5R01HL081153). IMJOVEN was supported in Spain by grant PI 081614 from the Fondo de Investigaciones Sanitarias del Instituto Carlos III, Ministry of Science and Technology, and additional funds from the Centro Nacional de Investigaciones Cardiovasculares (CNIC). Dr. Krumholz is supported by grant U01 HL105270-04 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute.
Footnotes
Conflict of interest: The senior author, John Spertus owns the copyright to the SAQ.
References
- 1.Vaccarino V, Parsons L, Every NR, et al. Sex-based differences in early mortality after myocardial infarction. National registry of myocardial infarction 2 participants. N Engl J Med. 1999;341(4):217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 2.Vaccarino V, Horwitz RI, Meehan TP, et al. Sex differences in mortality after myocardial infarction: Evidence for a sex-age interaction. Arch Intern Med. 1998;158(18):2054–2062. doi: 10.1001/archinte.158.18.2054. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen SS, Martens EJ, Denollet J, et al. Poor health-related quality of life is a predictor of early, but not late, cardiac events after percutaneous coronary intervention. Psychosomatics. 2007;48(4):331–337. doi: 10.1176/appi.psy.48.4.331. [DOI] [PubMed] [Google Scholar]
- 4.Spertus JA, Jones P, McDonell M, et al. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106(1):43–49. doi: 10.1161/01.cir.0000020688.24874.90. [DOI] [PubMed] [Google Scholar]
- 5.Spertus JA. Evolving applications for patient-centered health status measures. Circulation. 2008;118(20):2103–2110. doi: 10.1161/CIRCULATIONAHA.107.747568. [DOI] [PubMed] [Google Scholar]
- 6.Spertus JA, Radford MJ, Every NR, et al. Challenges and opportunities in quantifying the quality of care for acute myocardial infarction: Summary from the acute myocardial infarction working group of the american heart association/american college of cardiology first scientific forum on quality of care and outcomes research in cardiovascular disease and stroke. J Am Coll Cardiol. 2003;41(9):1653–1663. doi: 10.1016/s0735-1097(03)00415-7. [DOI] [PubMed] [Google Scholar]
- 7.Spertus J. Selecting end points in clinical trials: What evidence do we really need to evaluate a new treatment? Am Heart J. 2001;142(5):745–747. doi: 10.1067/mhj.2001.119135. [DOI] [PubMed] [Google Scholar]
- 8.Brink E, Grankvist G, Karlson BW, et al. Health-related quality of life in women and men one year after acute myocardial infarction. Qual Life Res. 2005;14(3):749–757. doi: 10.1007/s11136-004-0785-z. [DOI] [PubMed] [Google Scholar]
- 9.Emery CF, Frid DJ, Engebretson TO, et al. Gender differences in quality of life among cardiac patients. Psychosom Med. 2004;66(2):190–197. doi: 10.1097/01.psy.0000116775.98593.f4. [DOI] [PubMed] [Google Scholar]
- 10.Garavalia LS, Decker C, Reid KJ, et al. Does health status differ between men and women in early recovery after myocardial infarction? J Womens Health (Larchmt) 2007;16(1):93–101. doi: 10.1089/jwh.2006.M073. [DOI] [PubMed] [Google Scholar]
- 11.Lichtman JH, Lorenze NP, D'Onofrio G, et al. Variation in recovery: Role of gender on outcomes of young ami patients (virgo) study design. Circ Cardiovasc Qual Outcomes. 2010;3(6):684–693. doi: 10.1161/CIRCOUTCOMES.109.928713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 13.The EuroQol Group. Euroqol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 14.Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the seattle angina questionnaire: A new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 15.Monteagudo Piqueras O, Hernando Arizaleta L, Palomar Rodriguez JA. population based norms of the spanish version of the sf-12v2 for murcia (spain) Gac Sanit. 2011;25(1):50–61. doi: 10.1016/j.gaceta.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Wyrwich KW, Bullinger M, Aaronson N, et al. Estimating clinically significant differences in quality of life outcomes. Qual Life Res. 2005;14(2):285–295. doi: 10.1007/s11136-004-0705-2. [DOI] [PubMed] [Google Scholar]
- 17.Ware JE, Kosinski M, Keller SD, Lincoln RI. Sf-12: How to score the sf-12 physical and mental health summary scales. 3rd ed. Boston MA: Quality Metric incorporated; 1998. [Google Scholar]
- 18.Ellis JJ, Eagle KA, Kline-Rogers EM, et al. Validation of the eq-5d in patients with a history of acute coronary syndrome. Curr Med Res Opin. 2005;21(8):1209–1216. doi: 10.1185/030079905X56349. [DOI] [PubMed] [Google Scholar]
- 19.Shaw JW, Johnson JA, Coons SJ. Us valuation of the eq-5d health states: Development and testing of the d1 valuation model. Med Care. 2005;43(3):203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Janssen BSA. Self-reported population health: An international perspective based on eq-5d. Springer, Link; 2014. Population norms for the eq-5d; pp. 19–30. [PubMed] [Google Scholar]
- 21.Luo N, Johnson J, Coons SJ. Using instrument-defined health state transitions to estimate minimally important differences for four preference-based health-related quality of life instruments. Med Care. 2010;48(4):365–371. doi: 10.1097/mlr.0b013e3181c162a2. [DOI] [PubMed] [Google Scholar]
- 22.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: Eq-5d and sf-6d. Qual Life Res. 2005;14(6):1523–1532. doi: 10.1007/s11136-004-7713-0. [DOI] [PubMed] [Google Scholar]
- 23.Spertus JA, Winder JA, Dewhurst TA, et al. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74(12):1240–1244. doi: 10.1016/0002-9149(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 24.Levit RD, Reynolds HR, Hochman JS. Cardiovascular disease in young women: A population at risk. Cardiol Rev. 2011;19(2):60–65. doi: 10.1097/CRD.0b013e31820987b5. [DOI] [PubMed] [Google Scholar]
- 25.Beckie T. Biopsychosocial determinants of health and quality of life among young women with coronary heart disease. Current Cardiovascular Risk Reports. 2014;8(366):1–10. [Google Scholar]
- 26.Sloan JST, Vargas-Chanes D and Fridley B. Practical guidelines for assessing the clinical significance of health-related quality of life changes within clinical trials. Drug information Journal. 2003:3723–3731. [Google Scholar]
- 27.Agewall S, Berglund M, Henareh L. Reduced quality of life after myocardial infarction in women compared with men. Clin Cardiol. 2004;27(5):271–274. doi: 10.1002/clc.4960270506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pettersen KI, Reikvam A, Rollag A, et al. Understanding sex differences in health-related quality of life following myocardial infarction. Int J Cardiol. 2008;130(3):449–456. doi: 10.1016/j.ijcard.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 29.Wiklund I, Herlitz J, Johansson S, et al. Subjective symptoms and well-being differ in women and men after myocardial infarction. Eur Heart J. 1993;14(10):1315–1319. doi: 10.1093/eurheartj/14.10.1315. [DOI] [PubMed] [Google Scholar]
- 30.Kristofferzon ML, Lofmark R, Carlsson M. Perceived coping, social support, and quality of life 1 month after myocardial infarction: A comparison between swedish women and men. Heart Lung. 2005;34(1):39–50. doi: 10.1016/j.hrtlng.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Beck CA, Joseph L, Belisle P, et al. Predictors of quality of life 6 months and 1 year after acute myocardial infarction. Am Heart J. 2001;142(2):271–279. doi: 10.1067/mhj.2001.116758. [DOI] [PubMed] [Google Scholar]
- 32.Lane D, Carroll D, Ring C, et al. Mortality and quality of life 12 months after myocardial infarction: Effects of depression and anxiety. Psychosom Med. 2001;63(2):221–230. doi: 10.1097/00006842-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 33.McBurney CR, Eagle KA, Kline-Rogers EM, et al. Health-related quality of life in patients 7 months after a myocardial infarction: Factors affecting the short form-12. Pharmacotherapy. 2002;22(12):1616–1622. doi: 10.1592/phco.22.17.1616.34121. [DOI] [PubMed] [Google Scholar]
- 34.Duenas M, Ramirez C, Arana R, et al. Gender differences and determinants of health related quality of life in coronary patients: A follow-up study. BMC Cardiovasc Disord. 2011;1124 doi: 10.1186/1471-2261-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maddox TM, Reid KJ, Spertus JA, et al. Angina at 1 year after myocardial infarction: Prevalence and associated findings. Arch Intern Med. 2008;168(12):1310–1316. doi: 10.1001/archinte.168.12.1310. [DOI] [PubMed] [Google Scholar]
- 36.Kristofferzon ML, Lofmark R, Carlsson M. Myocardial infarction: Gender differences in coping and social support. J Adv Nurs. 2003;44(4):360–374. doi: 10.1046/j.0309-2402.2003.02815.x. [DOI] [PubMed] [Google Scholar]
- 37.Brezinka V, Kittel F. Psychosocial factors of coronary heart disease in women: A review. Soc Sci Med. 1996;42(10):1351–1365. doi: 10.1016/0277-9536(95)00284-7. [DOI] [PubMed] [Google Scholar]
- 38.Canto JG, Rogers WJ, Goldberg RJ, et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA. 2012;307(8):813–822. doi: 10.1001/jama.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mallik S, Spertus JA, Reid KJ, et al. Depressive symptoms after acute myocardial infarction: Evidence for highest rates in younger women. Arch Intern Med. 2006;166(8):876–883. doi: 10.1001/archinte.166.8.876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.