Abstract
Objective
To develop an adaptive behavioral treatment for African American adolescents with obesity.
Method
In a sequential multiple assignment randomized trial, 181 youth ages 12 to 16 years with primary obesity and their caregiver were first randomized to 3 months of home-based versus office-based delivery of motivational interviewing plus skills building. After 3 months, non-responders to first phase treatment were re-randomized to continued home-based skills or contingency management. Primary outcome was percent overweight and hypothesized moderators were adolescent executive functioning and depression
Results
There were no significant differences in primary outcome between home-based or office-based delivery or between continued home-based skills or contingency management for non-responders to first-phase treatment. However, families receiving home-based treatment initially attended significantly more sessions in both phases of the trial, and families receiving contingency management attended more sessions in the second phase. Overall, participants demonstrated decreases in percent overweight over the course of the trial (3%), and adolescent executive functioning moderated this effect such that those with higher functioning lost more weight.
Conclusions
More potent behavioral treatments to address the obesity epidemic are necessary, targeting new areas such as executive functioning. Delivering treatment in the home with contingency management may increase session attendance for this population.
Keywords: adolescents, obesity, SMART
Obesity has become a world-wide epidemic. Rates of pediatric obesity have steadily risen over the past 30 years, particularly among minority children (Ogden, Carroll, Kit, & Flegal, 2014). Current estimates suggest this disparity has persisted in school- and high school-aged youth despite significant attention and funding efforts (Khan et al., 2009; Let’s Move, 2011; National Collaborative on Childhood Obesity Research [NCCOR]). According to 2011–2012 data from the National Health and Nutrition Examination Survey, almost one out of every three US children is overweight or obese (Ogden et al., 2014). The prevalence of obesity, or body mass index (BMI) greater than or equal to the 95th percentile on the CDC sex-specific BMI-forage growth charts, was one in five for African American adolescents; significantly higher than non-Hispanic white adolescents (20.2% vs. 14.1%) (Ogden et al., 2014). Significant weight-related health complications occur as a result of obesity, even during childhood and adolescence (Friend, Craig, & Turner, 2013; Liese et al., 2006). Therefore, excessive body weight is one of the most significant medical problems facing children and adolescents today.
The American Academy of Pediatrics (AAP) strongly recommends the use of behavioral interventions to increase healthy eating and activity level (Barlow & Dietz, 1998) among overweight and obese adolescents. Despite the clear need to develop effective treatments for African American adolescents with obesity (AAAO), most clinical trials have included predominantly White adolescents. When minorities have participated, they have been at high risk for drop-out (Jelalian et al., 2008). Reports of the effectiveness of community-based weight loss programs have demonstrated similar lack of success in retaining minority adolescents (Zeller, Kirk, et al., 2004). In general, ethnic minority youth underutilize services (Atkinson & Gim, 1989; Garland et al., 2005), terminate treatment prematurely (Armbruster & Fallon, 1994; Sue, 1977), attend fewer sessions (Bui & Takeuchi, 1992; Miller, Southham-Gerow, & Allin, 2008), and realize fewer clinical benefits (Weersing & Weisz, 2002). Most of the studies testing behavioral treatments for minority youth with obesity have either not shown significant weight loss or have shown rapid regain of weight over time (Barr-Anderson, Adams-Wynn, DiSantis, & Kumanyika, 2013; Resnicow, Taylor, Baskin, & McCarthy, 2005; Savoye et al., 2007; Sung-Chan, Sung, Zhao, & Brownson, 2013; Wadden et al., 1990; Williamson, Walden, & White, 2006).
There are several possible explanations for the lack of intervention success with AAAO. First, as noted above, minority families have low rates of intervention retention. Successful interventions for this population may need to increase access to services by providing interventions in the home or other accessible community settings. Second, motivation to engage in the behaviors necessary for weight loss may also be a factor. These skills include controlling portion sizes, monitoring food intake and activity level, and exerting environmental control so that cues to eat unhealthy foods and being inactive are reduced, and using techniques (e.g., distraction) to manage hunger and cravings (Dietz & Robinson, 2005). Although empirically shown to be related to weight loss, such behaviors are time consuming at best and can be aversive at worst. Not surprisingly, then, several studies have indicated low motivation on the part of overweight participants to engage in these behaviors. For example, one study focusing on African American youth (Resnicow et al., 2005) reported that structured diets with reduced caloric intake were not included as part of the program because early focus groups with the target population indicated opposition to their use. In a large clinical trial (Savoye et al., 2007), retention in one study arm was so poor that it had to be discontinued; noteworthy is the report by the authors that drop-out in this arm occurred because families did not want to adhere to recommendations for using structured family meal planning.
In the only home-based skills-building behavioral intervention tested with AAAO, a pilot study demonstrated that the home-based approach increased intervention retention compared to earlier trials (Naar-King et. al., 2009). In the same study, youth motivation for weight loss predicted dose of treatment, which predicted response to intervention (MacDonell, Ellis, Naar-King, & Cunningham, 2010). Qualitative analyses of the intervention condition’s treatment session recordings suggested that caregivers and youth who attended a low number of treatment sessions did not make implementation of treatment recommendations a priority due to low motivation for change which reflected a combination of beliefs about weight and weight loss that were discrepant from those of interventionists (e.g., weight status is genetic and is not affected by one’s behavior) and their own personal problems (e.g., poor health status, multiple job demands, housing problems) that made weight loss appear less urgent (Idalski Carcone et al., 2011). Clearly, addressing these family motivational factors may be critical in intervention studies with AAAOs in order to increase intervention efficacy.
Motivation for weight loss behaviors can be addressed either through increasing intrinsic motivation for change or extrinsic motivation for change. Motivational Interviewing (MI) is a method of communication designed to increase intrinsic motivation for change in adults (Miller & Rollnick, 2013) and adolescents (Naar-King & Suarez, 2011). It has been tested alone and in combination with other weight loss skills building interventions, primarily in adult populations (Armstrong et al., 2011; Brennan, Walkley, Fraser, Greenway, & Wilks, 2008). However, one pilot study of a 4-session MI intervention for AAAO resulted in small changes in weight loss behaviors (MacDonell, Brogan, Naar-King, Ellis, & Marshall, 2012). Contingency management (CM) is a primary evidence-based strategy for increasing extrinsic motivation for behavior change that has been tested extensively in the adolescent substance abuse literature (Petry, 2012). CM interventions offer competing behavioral incentives (e.g., money, prizes) to counteract the reinforcement inherent in unhealthy foods and sedentary activities such as TV watching or electronic games. The use of some type of reinforcer to encourage weight loss for obese youth has frequently been recommended (Barlow & the Expert Committee, 2007) though studies with AAAO have not explicitly employed this strategy.
In summary, it is clear that behavioral weight loss interventions to date have had limited success for African American youth. While the behavioral strategies necessary for weight loss are well known, intervention retention and motivation for treatment is often poor and may account for variability in treatment response. Integrating MI with family-based weight loss skills training is one possible approach to improving treatment response (Irby, Kaplan, Garner-Edwards, Kolbash, & Skelton, 2010). Delivering services in the home to increase access and incorporating contingency management may also improve treatment response, but these interventions are costly and may not be necessary for families with greater resources and more motivation for weight loss. Optimal interventions would be those that are 1) initially tailored and individualized based on participants’ presenting characteristics and 2) can change based upon the participants’ success or failure (Almirall, Compton, Gunlicks-Stoessel, Duan, & Murphy, 2012; Nahum-Shani et al., 2012). The use of several, sequential treatments (e.g., an educational intervention followed by a motivation building intervention) is often necessary in clinical practice because treatment outcomes are heterogeneous across patients, treatment goals change over time, and in the long-term it is necessary to balance benefits of a given treatment with its risks and costs. However, in clinical practice, decisions to select an intervention that has a particular content or dose or to change interventions are typically made based on clinical judgments and impressions rather than evidence-based decision rules.
Adaptive treatment interventions are also known as clinical pathways or treatment algorithms and provide a framework for operationalizing key clinical decisions about the nature and timing of treatment (Collins, Murphy, Nair, & Stretcher, 2005). Key questions in developing an adaptive treatment include the determination of the best sequencing of treatments and when to transition from more intensive treatments to less intensive treatments or vice versa. Sequential multiple assignment randomization trials (SMART) are part of the newest generation of improvements in clinical trial design and methodology which allow the development of such adaptive treatments. The advantages of a SMART design over separate experiments testing different treatment strategies at different critical decision points includes the involvement of the same participants in all phases of intervention development, being able to understand how initial and subsequent stage treatments work with (synergistically) or against (antagonistically) each other, and the ability to generate hypotheses about baseline and post-baseline moderators of sequenced treatments. The end goal of the SMART approach is the development of an evidence-based adaptive intervention strategy that can be implemented in real-world settings.
The Current Study
Primary aims and hypotheses
The goal of the present SMART was to develop a seven-month adaptive treatment for weight loss in AAAO. Consistent with previous research with obese adolescents, our primary outcome was percent overweight (Ellis et al., 2010; Epstein, Valoski, Wing, & McCurley, 1994; MacDonell et al., 2010; Naar-King et al, 2009; Paluch, Epstein, & Roemmich, 2007). The first primary aim was to compare adolescents’ percent overweight after completing three months of home-based cognitive-behavioral weight loss skills treatment integrated with MI (Home-based MI Skills; HB-MIS) versus office-based delivery of the same intervention (OB-MIS). We hypothesized first that HB-MIS would result in greater weight loss at Post-Test 1 compared to OB-MIS. Adolescents who did not respond to HB-MIS or OB-MIS (i.e., those who lost less than 3% of their original weight), were re-randomized to three months of contingency management (CM) versus continued skills training (CS). Both of these conditions were integrated with MI and delivered in the home. Thus, our second and related primary aim was to compare non-responding adolescents’ percent overweight after completing the CM versus CS. Our second hypothesis was that CM would result in greater weight loss at Post-Test 2 than CS. Our third primary aim was to examine the overall effect of time on changes in percent overweight, because it is important to know whether all participants lost weight regardless of treatment condition. Our third hypothesis was that participants would significantly decrease their percent overweight at Post-Test 1 and Post-Test 2.
Secondary aims and hypotheses
An additional goal of a SMART design is to determine how to vary intervention components in response to characteristics of the individual or environment. These characteristics are called tailoring variables. Identifying moderator variables that predict for whom a particular intervention is most likely to succeed may best determine how to most effectively tailor treatments and how to develop the best decision rules for delivering different treatment alternatives. Based on our own prior research and clinical experience and the limited available literature, our first secondary aim was to explore whether the pretreatment tailoring variables of adolescent executive functioning and adolescent depression were differentially related to changes in percent overweight at Post-Test 1 and Post-Test 2. Executive functioning refers to cognitive processes involved in self-regulation including inhibitory control, mental flexibility and attention, reward sensitivity, and working memory. Studies have shown that adolescents with obesity have more impairment in executive functioning (Reinert, Po’e, & Barkin, 2013), and youth with poorer executive functioning have reduced likelihood of success in health behavior interventions (Ellis, Naar-King, Jen, & Brogan, 2013; Joseph et al., 2010). Depression has long been linked to obesity (Luppino et al., 2010), with studies showing that depression significantly predicts attrition in pediatric obesity treatment (Jensen, Aylward, & Steele, 2012; Zeller, Saelens, Roehrig, Kirk, & Daniels, 2004). Thus, our fourth set of exploratory hypotheses was that adolescents with lower executive functioning and higher depression would be more likely to benefit from the more intensive and potentially costly treatments. In other words, adolescents with a) more deficits in executive functioning or b) more depressive symptoms, would have greater decreases in percent overweight at 1) Post-Test 1 if they completed HB-MIS versus OB-MIS, and 2) Post-Test 2 if they completed CM versus CS
Our secondary outcome was treatment engagement. Engagement was examined by comparing proportions of participants who received the allocated treatment (i.e., completing at least three treatment sessions in each phase), and by comparing the number of sessions attended in each phase. We expected that because of reduced participant burden, treatment engagement would be higher in HB-MIS than OB-MIS. Because attendance was rewarded in CM, we expected greater engagement in CM than CS.
Method
Participants
Inclusion criteria were 1) self-identifying as African American, 2) being between the ages of 12 years 0 months and 16 years 11 months, 3) having BMI≥95th percentile for age and gender, 4) residing primarily with primary caregiver, 5) living within 30 miles of the urban children’s hospital affiliated with the university, 6) having a primary caregiver willing to participate in treatment, and 7) speaking English. Exclusion criteria were 1) obesity secondary to medication use for another medical condition (e.g., steroids, antipsychotics) or secondary to a chronic condition (e.g., Down syndrome, Prader-Willi syndrome, Cushing’s syndrome), 2) conditions causing potential daily fluid fluctuations (e.g., diabetes insipidus, congestive heart failure, dialysis), 3) medical conditions that prevent participation in normal exercise, 4) pregnancy, 5) medical condition where weight loss is contraindicated, 6) thought disorder (e.g., schizophrenia or other psychosis), suicidal, or homicidal, or 7) serious cognitive impairment (e.g., inability to complete the questionnaires). Potential participants were required to have a medical provider give clearance prior to enrollment if the participant had 1) a diagnosis of asthma, diabetes, or hypertension; 2) an initial blood pressure readings averaging above 140/90; or 3) problems after physical activity reported the Physical Activity Readiness Questionnaire (Arraiz, Wigle, & Mao, 1992; Mottola & Wolfe, 1994; Thomas, Reading, & Shepard, 1992).
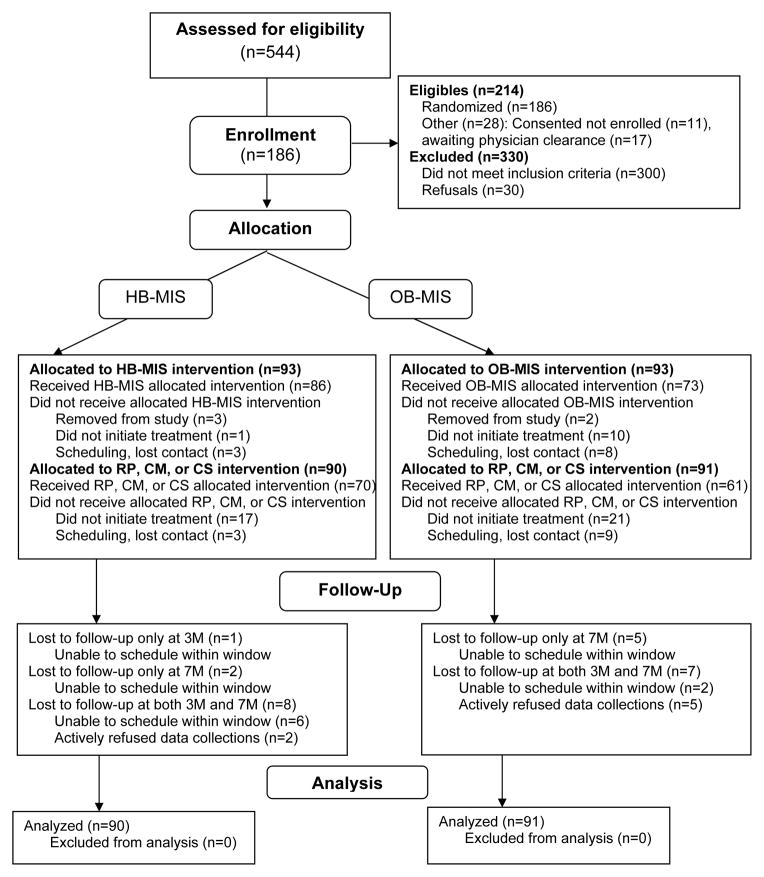
Figure 1 shows the Consolidated Standards of Reporting Trials (CONSORT) diagram of participant flow throughout the study. Review of electronic medical records from the local children’s hospital, direct provider referral, and community inquiry identified 544 potentially eligible adolescents who were screened for eligibility and interest. Thirty families refused to participate, 300 youth were ineligible (241 after more extensive electronic chart review: 39 did not meet age criteria, 36 had BMI below inclusion criteria, 140 did not identify as African American, 20 had an exclusionary diagnosis, 3 lived further than 30 miles away, and 3 were ineligible for other reasons; and 59 after phone or in-home screening: 12 did not meet age criteria, 1 participated in a pilot study, 10 had BMI below inclusion criterion, 4 did not identify as African American, 19 had an exclusionary diagnosis, and 13 were ineligible for other reasons), and 28 interested families did not enroll because research staff lost contact before a baseline data collection could be scheduled or because the family failed to obtain a physical activity clearance from their health care provider.
Figure 1.
Participant flow chart following Consolidated Standards of Reporting Trials (CONSORT) guidelines. HB-MIS = Home-based motivational interviewing and skills; OB-MIS = Office-based motivational interviewing and skills; RP = Relapse prevention; CM = Contingency management; CS = Continued skills.
In total, 186 adolescents and their primary caregivers were enrolled in the study (86.9% of eligible families). Five families were removed from the study by the research team (2 because of interventionist error; 3 because of ineligibility discovered after initiation of the study), leaving 181 youth-caregiver pairs who were included in the current analyses. Among the youth, 67% (n=122) were girls; mean age was 13.75 years (SD=1.35) at baseline. Baseline weight ranged from 133.00 to 451.00 pounds (M=229.97, SD=51.13), BMI ranged from 25.70 to 60.50 (M=38.15, SD=7.45), and percent overweight ranged from 35.38% to 218.47% (M=96.81, SD=37.59). Among caregivers, 87.3% (n=158) were the youth’ biological mothers; other caregivers included fathers, adoptive mothers, guardians, and others. Ninety-five percent (n=172) of caregivers indicated they were of African American race. Caregivers’ past year personal income ranged from “less than $5,000” (n=33) to “$100,000 or greater” (n=2) (median= “$12,000-$15,999”). Caregivers’ educational attainment varied from finished some high school (n=19) to Masters or Doctoral degree (n=11); median and modal education level was some college or associate degree. Just fewer than half of caregivers were employed outside the home (n=88, 48.6%). Caregivers’ weight ranged from 133.00 to 625.00 pounds (M=245.29, SD=67.29), BMI ranged from 22.40 to 92.67 (M=40.90, SD=10.16), and 88.4% (n=160) were considered obese (i.e., BMI≥30.0).
Procedure
All aspects of the study were approved by the university’s Institutional Review Board, and the trial was registered with ClinicalTrials.gov (identifier: NCT01350531). Potential participants recruited from pediatric clinics were first approached by medical staff. Interested caregivers completed a release of contact information form; research assistants then contacted the family to complete additional phone screening and schedule a consent and baseline data collection. A HIPAA waiver was obtained for conducting review of electronic medical records to identify additional potential families. For this recruitment method, a letter was sent to caregivers from the medical staff and a two-week opt-out window was offered. After the two-week window, research staff began calling the caregivers. Families recruited from community settings (e.g., health fairs) also completed a release of contact information form and study staff followed up with the caregivers.
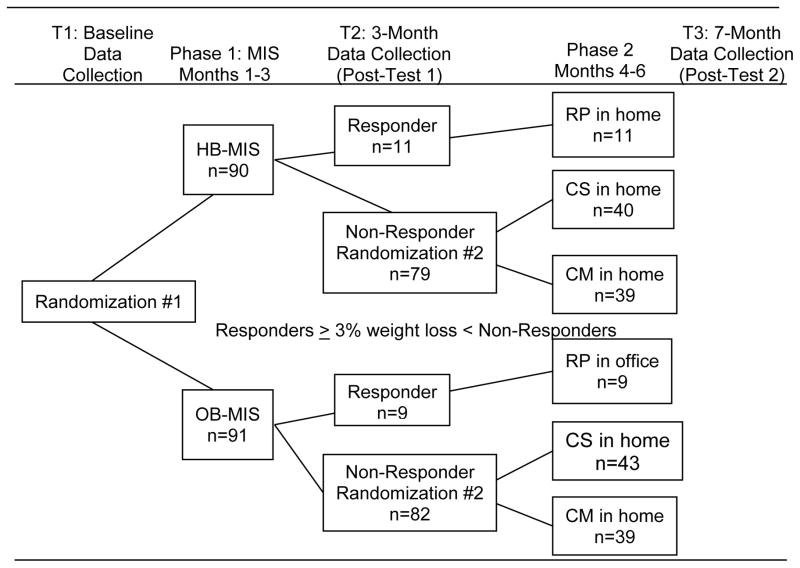
See Figure 2 for a diagram of the data collection time-points and study flow. Parental informed consent and adolescent assent were obtained during the first home visit, after the caregiver completed the phone screening. Given our experience working with the study population and their notoriously high attrition rates, we collected all data in home at baseline, 3, and 7 months. After baseline, participants were first randomized to either HB-MIS (n=90) or OB-MIS (n=91). Randomization was stratified based on the presence (yes/no) of adolescent comorbidities (e.g., asthma, hypertension) and adolescent percent overweight (with high percent overweight defined as ≥88.0% above the CDC’s median age and gender normed BMI and low percent overweight as <88.0% above). Data collectors were kept blind to participants’ group assignments.
Figure 2.
Sequential Multiple Assignment Randomization Trial (SMART) design and participant flow. 5 families were removed from the study by the research team and are not included in the numbers shown. MIS = Motivational interviewing and skills; HB-MIS = Home-based motivational interviewing and skills; OB-MIS = Office-based motivational interviewing and skills; RP = Relapse Prevention; CS = Continued Skills; CM = Contingency Management.
At the 3-month data collection, the research assistant collected two weights within the same week. After the 3-month data collection, families were re-randomized based on response and non-response to Phase 1 treatment. Response was defined as weight loss of ≥3% of original body weight. Nonresponse was defined as weight loss of <3%. This point was chosen because 1% weight loss per month would be a reasonable rate of weight loss as recommended by the National Heart Lung and Blood Institute (National Institutes of Health, 2000). Non-responding families were re-randomized into CM or CS for the second phase of treatment. Responding families were reassigned to a relapse prevention (RP) intervention. The 7-month data collection was completed approximately one month after the second phase ended.
In order to reduce attrition for follow-up data collections, caregivers and youth were contacted via mail, telephone, text message, email, and in person. Quarterly newsletters were also sent to participants, with information about upcoming local events, recipes, and healthy eating tips. There were no differences in attrition at 3 (i.e., T2) or 7 (i.e., T3) months; 90.0% of families randomized to HB-MIS and 92.3% of families in OB-MIS completed their T2 data collection, and 88.9% and 86.8% completed their T3 data collection. Families were compensated for their time with $50 each for the baseline (i.e., T1) and T3 data collections, and $10 for the T2 data collection.
Intervention
Phase 1: HB-MIS and OB-MIS
The interventions delivered in first phase of the SMART were designed to be very similar except for delivery setting. In both cases, a community health worker (a paraprofessional counselor) delivered weekly 1-hour face-to-face sessions, with the first session focusing engaging the family in treatment. The second two sessions were conjointly delivered with a registered dietitian to provide education in nutrition and physical activity and to develop a plan to either reduce their food intake by 500kcal or to consume a maximum of 1600–2000kcal per day. The remaining sessions focused on behavioral skills training integrated with MI. Content included self-monitoring food and physical activity/inactivity levels, stimulus control of food and activity/inactivity triggers both in and out of the home, managing hunger and food cravings, and parenting. Youth were also weighed weekly with a discussion of the factors that might have promoted weight loss or gain. A second session (15-45 minutes) was delivered each week in both conditions. Counselors used this session to check-in with families (both groups), and to practice skills and trouble-shoot implementation barriers (HB-MIS). For HB-MIS this check-in was done in the home, and for OB-MIS it was done by phone. Phase 1 intervention lasted for three months. Families in OB-MIS received a $10 gift card per session for attendance. A parking voucher (valued at $2.50) or transportation via taxi was also provided.
Phase 2: CS, CM and RP
The second phase of treatment was also three months. Adolescents who did not respond to treatment (i.e., did not lose ≥3% body weight) were re-randomized into CS or CM in the second phase of the SMART. Both CS and CM were implemented in participants’ homes. In CS, the counselor first conducted a functional assessment with the family and then together with the family selected which additional skills to focus on during the three months of treatment (possible modules included managing distorted thinking regarding weight loss, reducing emotional eating, increasing planning and organizational skills, and strengthening refusal skills). Families also had the option to repeat any session from the first phase HB-MIS, or to complete mini-modules (covering, for example, going out to eat or holiday planning) for 10–15 minutes based on assessed need. Consistent with Phase 1, planned dose was a weekly home-based 1-hour session with a second 15-minute home-based weekly refresher. All modules were integrated with MI strategies. No incentives for weight loss were provided.
In CM, a voucher-based system was used to provide incentives to the teen for weight loss and to the caregiver for adherence to CM. Caregivers and youth signed a CM contract with their counselor describing the CM program. Each point was equivalent to $1. Youth and parents could each earn up to $624 in vouchers for products available from amazon.com (except dietary supplements, food, weapons, alcoholic beverages, and cigarettes). Youth earned 20 points for losing at least one pound each week (calculated as an average between weight in session and a mid-week weigh-in). Youth earned 4 additional points each successive week they met their goal and 40 bonus points if they lost at least four pounds in one 4-week period. If youth missed more than one weekly goal in a 4-week period, the points available to earn was reset to 20. When caregivers missed any of their goals (delivering adolescent incentives, attending sessions, and ensuring that the youth recorded daily weights), their points were reset to 20, but they could still earn 40 bonus points every four weeks if they met their goals each week of that window. No points were deducted once earned.
Rather than training in new skills during this part of treatment, the counselor’s role in CM was to guide the caregiver in administering the CM (one 40-minute session per week) and to lead discussions about barriers and facilitators of weight loss (second 40-minute session per week). Youth goals were discussed with the counselor and the caregiver; whereas caregivers’ goals were discussed without the youth present. If the caregiver did not administer the CM, the counselor did so that the youth would still receive points for success.
Youth who responded to the first phase interventions (i.e., lost at least 3% of their original weight) were all assigned to relapse prevention (RP) for the second phase. For RP, the location of treatment remained the same as it was for phase 1 (office-based or home-based), and session frequency was reduced to one face-to-face session per week. Treatment consisted of modules designed to explore values and commitment to treatment, managing slips, and maintenance of dietary and physical activity changes based on functional analysis. If families had not initiated changes in both nutrition and physical activity domains, they had the option of engaging in new skills training for this domain.
Quality Assurance Procedures
In order to promote counselors’ fidelity to the treatment protocols, state-of-the-art quality assurance procedures were used. First, counselors were hired based a structured behavioral interview targeting knowledge, skills, and past performance and experience and performance in a video assessment of simulated encounters-revised (Rosengren, Hartzler, Baer, Wells, & Dunn, 2008) that assesses potential for MI competence. Once hired, counselors were initially trained for two months. Specifically, counselors completed a total of 80 hours of didactic training with a psychologist and dietitian who were both MI trainers. They also spent 50 hours role-playing and 170 hours in individual or interactive training activities (e.g. rating audio and video training clips).
Counselors received one hour per week of individual supervision and two hours per week of group supervision with clinical supervisor and dietitian. The team (counselors, supervisor, and dietitian) met weekly by phone with the expert consultant to address difficult cases. Monthly, each counselor had two-hour case review sessions with the clinical supervisor and dietitian. The supervisor and dietitian also provided quarterly three-hour booster trainings. Finally, all sessions were recorded and rated for adherence to the treatment modules (audio for home-based interventions and video for office-based). During the initial training period, sessions were coded based on the Motivational Interviewing Treatment Integrity (MITI 3.1) (Moyers, Martin, Manuel, Hendrickson, & Miller, 2005) to assess MI fidelity.
Measures
Primary Outcome: Percent overweight
Percent overweight was calculated as the percentage their BMI was above the CDC’s median BMI for age and gender. This is the recommended primary outcome measure for pediatric obesity, especially with samples of obese adolescents for whom zBMI and BMI are not considered optimal measures (Epstein et al., 1994; Paluch et al., 2007), and the measure has been shown to respond to intervention in other studies with this population (Ellis et al., 2010; MacDonell et al., 2010; Naar-King et al, 2009). BMI was computed using Epi Info software version 3.5.1 (CDC, Atlanta, GA). Weight was assessed with the Seca 869 scale (Seca, Hanover, MD) at T1, T2, and T3. Youth were weighed twice for each data collection point, between 1 and 9 days apart (M=4.42, SD=2.10) with the average weight used to calculate BMI, and height was assessed using the Seca 213 Stadiometer.
Moderator: Adolescent executive functioning
The Behavior Rating Inventory of Executive Function – Parent Report (BRIEF) (Gioia, Isquith, Guy, & Kenworthy, 2000) is an 86-item scale assessing caregiver report of youths’ abilities regarding inhibition, shifting situations, modulating emotions, working memory, planning/organizing, organizing materials, and monitoring work. At baseline, caregivers rated their youth’s level of difficulty with each task in the past 6 months on a 1, never, to 3, often, scale; thus, higher scores reflect more problems with executive functioning. In a standardization sample with over 1400 participants matched to US population for gender and ethnicity (13.5% African American, 38.0% lower-middle or lower class socioeconomic status), overall α=.93 (Gioia et al., 2000). The total score (Global Executive Composite) was used for analyses.
Moderator: Adolescent depressive symptoms
The 8-item Patient-Reported Outcome Measurement Information System (PROMIS) – Pediatric Short Form v1.0 – Depressive Symptoms (Irwin et al., 2010) assessed youths’ level of depressive symptoms in the past week at baseline. The PROMIS is a National Institutes of Health-funded system designed to develop a variety of highly reliable and valid tools to measure patient-reported physical, mental, and social well-being. The PROMIS depressive symptoms assesses negative mood, decreases in positive affect, and negative views of the self. At baseline, participants indicated how often they felt each symptom on a 1, never, to 5, almost always, scale, which was rescaled to a 0–4 metric for analyses. The PROMIS depressive symptoms scale was normed with a sample of over 1,500 youth recruited from public schools, hospital-based outpatient clinics, and specialty pediatrics clinics and 21% of the item calibration sample were African American youth. In the current study, α=.90.
Data Analysis Plan
The linear mixed effects (LME) model with a random intercept and fixed slopes was used to test the study hypotheses. A primary advantage of this approach is, under the assumption that the data are missing at random, all model parameters are estimated in the presence of missing data, thus, precluding the need for data estimation and avoiding casewise deletion. Different models for the within subject error covariance matrix were compared. The simple compound symmetry model, which assumes equal error variance at each data collection point had the best parsimony adjusted fit. We used SPSS, version 22, for the analyses. The one-tailed alpha was set at p<.05 for all analyses using the intent-to-treat sample. With a predicted sample size of 180 and a two-tailed alpha of .05, this study was powered (1−β=.80) to detect a change in percent overweight of 2.46%.
Coding the time metric for LME models
The primary outcome was collected at each study visit: Baseline (T1/0 months), Post-Test 1 (T2/3 months), and Post-Test 2 (T3/7 months). Study visit (T1, T2, and T3) was used to represent time in the LME models.
Phase 1 treatment effects
There were two primary treatment effects of interest. Each treatment effect was represented by a single degree of freedom (df) interaction contrast defined as follows: C1 = Group x (T2-T1) and C2 = Group x (T3-T1). The group factor was the randomized treatment group (HB-MIS vs. OB-MIS). A significant C1 contrast indicates differential weight loss over the course of the 3-month intervention. A significant C2 contrast indicates differential weight loss over the duration of the study (3-month intervention plus 4-month follow-up). The C2 contrast is complicated by the fact that most of the participants (161 of 181) were randomly assigned to the Phase 2 intervention at T2. Thus the C2 contrast effect includes the net effect of the Phase 2 intervention on weight change from T2 to T3. Because the net effects are randomized across treatment groups, the test of the primary contrasts is not confounded. Plots of the simple effect means following a significant interaction were used to confirm or disconfirm the study hypothesis. However, only the C1 contrast is free of the Phase 2 net effect.
Phase 2 treatment effects
In Phase 2, there was one treatment effect of interest, weight loss during the course of the Phase 2 treatment. This treatment effect was defined by one single df interaction contrast as follows: Group x (T3-T2). A significant interaction indicates differential weight loss between CM and CS groups. Plots of the simple effects means following a significant interaction were used to confirm or disconfirm study hypotheses.
Covariates
Bivariate statistics were used to examine baseline differences in adolescent and caregiver demographic characteristics across treatment groups. Significance was determined using a Bonferroni corrected α=.05/7=.007. Significant demographic variables were considered possible covariates in analysis of treatment effects.
Treatment attendance
Because participants attended a variable number of treatment sessions (differential treatment dose), we compared dose received across treatment groups for each study phase and overall. We also examined demographic predictors of dose. This analysis included the randomization variable (Phase 1 and Phase 2 randomization in separate analyses) and an interaction term, demographic predictor x intervention group, in order to identify demographic variables that would explain differential treatment attendance (Rochon, 1999).
Moderator variables
Adolescent executive functioning and adolescent depression, measured at baseline (T1), were used as time invariant moderator variables. Factors were created from these variables using a median split to define categories. Each moderator was tested in a separate analysis for each phase of treatment. For Phase 1, the complete LME model was a 2 (OB-MIS vs. HB-MIS) x 3 (T1/baseline, T2/3 month, T3/7 month) x 2 (moderator variable) model with a random intercept. For Phase 2, the complete LME model was a 2 (CM vs. CS) x 2 (T2/3 month, T3/7 month) x 2 (moderator variable) mixed factor model with random intercept. Two kinds of moderator effects were of interest: the Group X Time X Moderator interaction and the Time X Moderator interaction. This second interaction was of interest because, regardless of treatment differences, it is important to know if the moderator was related to change in weight status.
Results
Descriptive Analyses
Table 1 compares participants’ characteristics at baseline by Phase 1 and 2 treatment group assignment. There were no significant differences in baseline characteristics after using the Bonferroni correction for multiple comparisons.
Table 1.
Demographic and Baseline Characteristics of Participants by Treatment Condition
| N | M (SD) or % | N | M (SD) or % | Test of Group Differences at Baseline | |
|---|---|---|---|---|---|
| Phase 1 Condition | HB-MIS | OB-MIS | |||
|
| |||||
| Adolescent Age | 90 | 14.1 (1.36) | 91 | 14.4 (1.52) | t(179)=−1.06, p=.289 |
| Adolescent Gender | |||||
| Female | 67 | 74.4 | 55 | 60.4 | X2(1)=4.04, p=.044 |
| Male | 23 | 25.6 | 36 | 39.6 | |
| Caregiver Type | |||||
| Biological Parent | 83 | 92.2 | 84 | 92.3 | X2 (1)=0.00, p=.983 |
| Other | 7 | 7.8 | 7 | 7.7 | |
| Caregiver Age | 90 | 43.7 (8.36) | 90 | 42.3 (7.49) | t(179)=1.29, p=.228 |
| Caregiver Gender | |||||
| Female | 87 | 96.7 | 85 | 93.4 | X2(1)=4.04, p=.044 |
| Male | 3 | 3.3 | 6 | 6.6 | |
| Caregiver Marital Status | |||||
| Single Parent | 51 | 56.7 | 64 | 70.3 | X2(1)=3.65, p=.056 |
| Two-Parents | 39 | 43.3 | 27 | 29.7 | |
| Caregiver Income* | 88 | $12k-15,999 ($5k-11,999, $16k-24,999) | 90 | $12k-15,999 ($5k-11,999, $25k-34,999) | X2(8)=10.67, p=.221 |
| Percentage Overweight | 90 | 93.8 (36.10) | 91 | 99.8 (38.97) | t(179)=−1.09, p=.278 |
| Executive Functioning** | 89 | 123.4 (26.10) | 91 | 124.4 (29.30) | t(178)=−0.25, p=.805 |
| Depressive Symptoms | 90 | 6.32 (6.00) | 91 | 6.62 (6.99) | t(179)=−0.30, p=.763 |
|
| |||||
| Phase 2 Condition | CM | CS | |||
|
| |||||
| Adolescent Age | 72 | 14.3 (1.42) | 72 | 14.7 (1.39) | t(142)=−1.57, p=.118 |
| Adolescent Gender | |||||
| Female | 52 | 71.2 | 48 | 66.7 | X2(1)=0.35, p=.552 |
| Male | 21 | 28.8 | 24 | 33.3 | |
| Caregiver Type | |||||
| Biological Parent | 66 | 94.3 | 69 | 92.0 | X2(1)=0.29, p=.587 |
| Other | 4 | 5.7 | 6 | 8.0 | |
| Caregiver Age | 73 | 42.9 (9.05) | 72 | 42.5 (7.10) | t(143)=0.30, p=.765 |
| Caregiver Gender | |||||
| Female | 70 | 95.9 | 69 | 95.8 | X2(1)=0.00, p=.986 |
| Male | 3 | 4.1 | 3 | 4.2 | |
| Caregiver Marital Status | |||||
| Single Parent | 45 | 61.6 | 52 | 72.2 | X2(1)=1.83, p=.176 |
| Two-Parents | 28 | 38.4 | 20 | 27.8 | |
| Caregiver Income* | 73 | $16k-24,999 ($5k-11,999, $25k-34,999) | 72 | $12k-15,999 ($5k-11,999, $16k-24,999) | |
| Percentage Overweight | 72 | 99.50 (42.51) | 72 | 95.71 (38.28) | t(142)=0.57, p=.570 |
| Executive Functioning** | 73 | 127.58 (32.32) | 72 | 122.78 (22.94) | t(143)=1.03, p=.305 |
| Depressive Symptoms | 73 | 7.51 (7.10) | 72 | 5.64 (6.20) | t(143)=1.69, p=.094 |
Notes.
Median caregiver income and the interquartile range are reported.
Higher scores reflect more executive functioning problems.
HB-MIS=Home-based Motivational Interviewing and Skills; OB-MIS=Office-based Motivational Interviewing and Skills; CM=Contingency management; CS= Continued skills.
Treatment Attendance
Among families randomized to HB-MIS, 95.6% received the allocated treatment (consistent with other intensive interventions with a similar population, defined as completing at least three treatment sessions; Ellis et al., 2005; Naar-King et al., 2014), and 80.2% of families in the OB-MIS received the allocated treatment. Furthermore, 77.8% of families who were in HB-MIS in Phase 1 received the allocated Phase 2 treatment (again defined as completing at least three sessions, regardless of new treatment condition) compared to 67.0% of families first assigned to OB-MIS. Families assigned to HB-MIS completed significantly more sessions in Phase 1 (M=13.17, SD=5.68) than families in OB-MIS (M=8.62, SD=5.73), F(1, 179)=28.79, p<.001. There was also an effect of Phase 1 group on Phase 2 session attendance. Families who were in HB-MIS also completed more Phase 2 sessions across conditions (M=11.49, SD=8.19) than families initially assigned to OB-MIS (M=8.87, SD=7.87), F(1, 179)=4.84, p<.05. There was also a Phase 2 group difference on Phase 2 session attendance. Among CM families (regardless of Phase 1 allocation), 80.8% received allocated CM treatment and 65.1% of CS families received the allocated CS treatment. Families in CM completed twice as many sessions (M=14.63, SD=8.56) as families in CS (M=7.06, SD=6.17), F(1, 157)=43.24, p<.001. Using the intent to treat principle, data were analyzed without attempting to take differences in treatment dose into account. No demographic variable significantly predicted differential treatment attendance, nor was treatment attendance related to change in percent overweight (Phase 1: r=.08, p=.34, Phase 2: r=−.05, p=.51).
Overall Reduction in Percent Overweight Across Treatment Conditions
There were significant main effects of time for Phase 1 treatments at Post-Test 1 [t(317.89)=2.11, p=.035] and at Post-Test 2 [t(317.95)=4.22, p<.001] suggesting that regardless of assignment to HB-MIS or OB-MIS adolescents demonstrated weight loss over time. From baseline to Post-Test 1 (end of Phase 1 treatment) participants reduced their percentage overweight by 1.39% (95% CI: 0.10%, 2.69%). For Phase 2, there was a marginally significant main effect of time (F(1,134.12)=3.76, p=.055) suggesting that regardless of CM or CS assignment, adolescents demonstrated weight loss from Post-Test 1 (beginning of Phase 2) to Post-Test 2 (end of Phase 2). Post hoc tests indicated that Phase 2 participants reduced their percentage overweight by 1.27% (95% CI: −.03%, 2.57%). Over the course of the trial, from baseline to the end of Phase 2, participants across conditions reduced percent overweight by 2.96% (95% CI: 1.64%, 4.27%). Further, 16 participants who were re-randomized into CM (n=9) or CS (n=7) achieved 3% weight loss in Phase 2, the criteria set for treatment response in Phase 1.
Effects of Group Assignment
The Phase 1 hypothesis was not supported (see Table 2). Participants assigned to HB-MIS did not experience greater weight loss than those assigned to OB-MIS at Post-Test 1 [t(313.89)=0.65, p=.515] or Post-Test 2 [t(313.95)=−0.21, p=.831]. The Phase 2 hypothesis was also not supported. Participants assigned to CM did not experience greater weight loss than those assigned to CS (F(1,134.12)=1.29, p=.257).
Table 2.
Comparison of Percentage Overweight and Hypothesized Moderators by Treatment Condition
| N | Median | M (SD) | N | Median | M (SD) | Cohen’s d*** | |
|---|---|---|---|---|---|---|---|
| Phase 1 Condition | HB-MIS | OB-MIS | |||||
|
| |||||||
| Percent Overweight | |||||||
| T1/Baseline | 90 | 84.82 | 93.75 (36.10) | 91 | 94.26 | 99.83 (38.97) | |
| T2/Post-test 1 | 80 | 79.37 | 91.25 (38.26) | 84 | 91.92 | 100.16 (39.95) | 0.23 |
| T3/Post-test 2 | 78 | 81.95 | 91.13 (38.89) | 79 | 91.07 | 98.32 (41.19) | 0.18 |
| Moderators* | |||||||
| Executive Functioning** | |||||||
| Low | 43 | 103.50 | 103.54 (14.43) | 47 | 101.00 | 99.45 (15.35) | |
| High | 46 | 141.00 | 144.67 (17.58) | 44 | 144.00 | 147.83 (17.40) | |
| Depressive Symptoms | |||||||
| Low | 43 | 1.00 | 1.47 (1.59) | 47 | 0.00 | 1.19 (1.53) | |
| High | 47 | 11.00 | 10.77 (5.01) | 44 | 11.00 | 12.41 (5.78) | |
|
| |||||||
| Phase 2 Condition | CM | CS | |||||
|
| |||||||
| Percent Overweight | |||||||
| T2/Post-test 1 | 72 | 89.60 | 99.50 (41.51) | 72 | 88.58 | 95.71 (38.28) | |
| T3/Post-test 2 | 71 | 90.14 | 98.55 (43.23) | 68 | 88.93 | 95.15 (37.73) | 0.08 |
| Moderators* | |||||||
| Executive Functioning** | |||||||
| Low | 35 | 97.00 | 99.89 (16.58) | 41 | 104.00 | 102.34 (14.50) | |
| High | 42 | 145.50 | 150.55 (21.19) | 42 | 140.50 | 141.60 (11.18) | |
| Depressive Symptoms | |||||||
| Low | 37 | 1.00 | 1.62 (1.59) | 44 | 0.00 | 1.09 (1.49) | |
| High | 41 | 14.00 | 12.59 (5.87) | 39 | 11.00 | 10.67 (5.02) | |
Notes.
Moderators were measured at T1/Baseline.
Higher scores reflect more executive functioning problems.
The effect size calculations represent between group differences at the specific time point.
HB-MIS= Home-based Motivational Interviewing and Skills; OB-MIS=Office-based Motivational Interviewing and Skills; CM=Contingency management; CS=Continued skills.
Moderator Variables
Executive Function
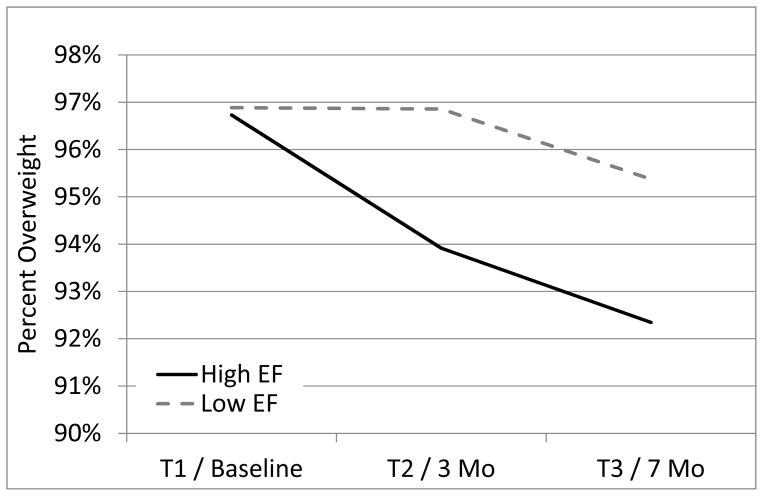
For Phase 1 treatment, the hypothesis that adolescents with lower executive functioning would experience a greater treatment effect in HB-MIS versus OB-MIS was not supported at Post-Test 1 [t(313.84)=−0.51, p=.614] or Post-Test 2 [t(313.90)=−0.18, p =.857]. For Phase 2, the hypothesis that adolescents with lower executive functioning would experience greater weight loss with CM versus CS was not supported [F(1,132.11)=1.38, p =.243]. Executive functioning did, however, moderate weight loss over time, from baseline to the end of Phase 1 treatment [t(313.84)=2.12, p=.034]. Figure 3 illustrates that, at the end of Phase 1 treatment, regardless of treatment condition, participants with greater executive functioning reduced their percent overweight by 2.82% (95% CI: 0.98%, 4.65%) as compared to those with lower executive functioning who reduced their percent overweight by 0.03% (95% CI: −1.79%, 1.85%). Executive functioning did not moderate weight loss during Phase 2, from 3-month to 7-month follow-up [F(1,132.11)=0.01, p=.912]. Overall, executive functioning moderated weight loss from baseline to Post-Test 2 [t(313.90)= 2.15, p=.032] with participants with greater executive functioning reducing their percent overweight by 4.39% (95% CI: 2.532%, 6.240%) as compared to those with lower executive functioning who reduced their percent overweight by 1.51% (95% CI: −0.35%, 3.38%).
Figure 3.
Percent overweight over time for participants with high and low executive functioning levels. EF = executive functioning.
Depression
The hypothesis that adolescents with higher levels of depression would experience a greater treatment effect in Phase 1 HB-MIS versus OB-MIS was not supported at either Post-Test 1 [t(313.89)=0.80, p=.937] or Post-Test 2 [t(313.96)=0.97, p=.923]. The Phase 2 hypothesis that adolescents with higher levels of depression would experience greater weight loss with CM versus CS was not supported [F(1,132.12)=0.10, p=.749]. At the end of Phase 1 treatment, there was marginally significant support for adolescent depression as a moderator of weight loss across conditions, [t(313.89)=1.73, p=.085] but no significance at Post-Test 2 [t(313.96)=0.65, p=.514]. At the end of Phase 1 treatment, participants with lower levels of depression reduced their percent overweight by 2.55% (95% CI: 0.70%, 4.40%) as compared to those with higher levels of depression who reduced their percent overweight by 0.27% (95% CI: −1.55%, 2.10%). There was marginally significant support for depression as a moderator of weight loss in Phase 2 [F(1,132.12)=3.57, p=.061]. From Post-Test 1 to the end of Phase 2 treatment, participants with higher levels of depression reduced their percent overweight by 2.51% (95% CI: 0.66%, 4.35%) as compared to those with lower levels of depression who reduced their percent overweight by 0.04% (95% CI: −1.77%, 1.85%).
Discussion
Weight loss skills training integrated with MI resulted in small but significant reductions in percent overweight in African American adolescents with obesity (AAAO), a population with high rates of obesity but typically poor response to behavioral treatments. The majority of such trials with this population have shown weight stabilization at best or at worst, weight gain. Thus, the delivered interventions resulted in more weight loss than typically seen in this population, though less than what has been seen in non-minority samples (Oude Luttikhuis, et al. 2009). In addition, the use of a SMART design allowed for testing of multiple treatment components (office versus home-based and contingency management versus continued skills). None of the various treatments had an advantage over another with regard to weight loss in a seven-month period, suggesting that any of these may have health benefits. Decisions regarding superiority could therefore be made based on other factors such as cost of delivery, participant preference/satisfaction, etc. The study may have been underpowered to detect the effect of delivery of the same intervention strategies when being delivered in different settings as the office-based versus home-based comparison had a small but non-significant effect size.
The strongest difference between treatment components was seen in intervention retention. Significantly more families in the home-based treatment received both first and second phase interventions. This suggests that not only did home-based treatment result in greater session attendance during the treatment itself, but this first phase intervention “primed the pump” such that those receiving home-based treatment in phase 1 also were more likely to be retained in phase 2 treatments compared to those who received office-based treatments in phase 1.
Although treatment retention dropped in the second three months of treatment across the sample, families who received contingency management in Phase 2 attended significantly more sessions than those receiving continued skills. Clearly, both home-based delivery and a voucher-based reinforcement program that includes vouchers for caregiver session attendance resulted in greater treatment retention. However, more potent treatments are still necessary for session attendance to result in greater weight loss for AAAO.
Results from the moderator analyses also suggest target areas for develop more potent weight loss interventions. While adolescent executive functioning did not moderate effects of the various treatments, overall those with higher executive functioning lost more weight. While the relationship between executive function and overweight is now well established in descriptive studies (Reinert et al., 2013), this is the first study to our knowledge to demonstrate that executive functioning moderates weight loss in an adolescent obesity treatment trial. Future directions based on such findings could include intervention development focusing on strategies that have been shown to have direct effects on improving executive function such as increasing physical activity (Davis et al., 2011), improving treatment strategies to inhibit cravings and working memory training (Verbeken, Braet, Goossens, & van der Oord, 2013).
Study limitations include the reliance on subjective measures of depression and executive functioning and being underpowered to detect small but potentially significant differences between different treatments. However, the present study was able to address multiple questions in a single trial due to the advantages of the SMART approach. Further analysis will focus on individual sequences that may be more or less effective in producing weight loss (e.g., HB-MIS plus CM versus OB-MIS plus CS) and analyzing additional moderators, in particular those related to characteristics in the caregiver that may affect response to a family-based treatment. Furthermore, further analyses of other important secondary outcomes such as changes in physical activity and nutrition behaviors, percent body fat, and biomarkers may reveal differences between treatment components to inform an adaptive treatment.
In summary, the current SMART suggested minimal difference between treatments in reducing percent overweight, but beginning with home-based MI and weight loss skills training and following up with CM for non-responders increased intervention retention for a population particularly at high risk for obesity and related complications. Future efforts to develop more potent behavioral treatments to address the obesity epidemic may target new areas such as executive functioning while delivering treatment in the home with CM for those with poor treatment retention.
Acknowledgments
This work was funded by the National Heart, Lung, and Blood Institute and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U01HL097889), PI’s: S. Naar-King and K.-L. C. Jen. K. Brogan is now at Florida International University.
Contributor Information
Sylvie Naar-King, Email: snaarkin@med.wayne.edu, Wayne State University, Detroit, MI.
Deborah A. Ellis, Email: dellis@med.wayne.edu, Wayne State University, Detroit, MI
April Idalski Carcone, Email: acarcone@med.wayne.edu, Wayne State University, Detroit, MI.
Thomas Templin, Email: t.templin@wayne.edu, Wayne State University, Detroit, MI.
Angela J. Jacques-Tiura, Email: atiura@med.wayne.edu, Wayne State University, Detroit, MI
Kathryn Brogan Hartlieb, Email: kabrogan@fiu.edu, Wayne State University, Detroit, MI.
Phillippe Cunningham, Email: cunninpb@musc.edu, Medical University of South Carolina, Charleston, SC.
Kai-Lin Catherine Jen, Email: cjen@wayne.edu, Wayne State Univesity, Detroit, MI.
References
- Almirall D, Compton SN, Gunlicks-Stoessel M, Duan N, Murphy SA. Designing a pilot sequential multiple assignment randomized trial for developing an adaptive treatment strategy. Statistics in Medicine. 2012;31:1887–1902. doi: 10.1002/sim.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster P, Fallon T. Clinical, sociodemographic, and systems risk factors for attrition in a children’s mental health clinic. American Journal of Orthopsychiatry. 1994;64:577–585. doi: 10.1037/h0079571. [DOI] [PubMed] [Google Scholar]
- Armstrong MJ, Mottershead TA, Ronksley PE, Sigal RJ, Campbell TS, Hemmelgarn BR. Motivational interviewing to improve weight loss in overweight and/or obese patients: A systematic review and meta-analysis of randomized controlled trials. Obesity Reviews. 2011;12:709–723. doi: 10.1111/j.1467-789X.2011.00892.x. [DOI] [PubMed] [Google Scholar]
- Arraiz GA, Wigle DT, Mao Y. Risk assessment of physical activity and physical fitness in the Canada health survey follow-up study. Journal of Clinical Epidemiology. 1992;45:419–428. doi: 10.1016/0895-4356(92)90043-M. [DOI] [PubMed] [Google Scholar]
- Atkinson DR, Gim RH. Asian-American cultural identity and attitudes toward mental health services. Journal of Counseling Psychology. 1989;36:209. doi: 10.1037//0022-0167.36.2.209. [DOI] [Google Scholar]
- Barlow SE, Dietz WH. Obesity evaluation and treatment: Expert committee recommendations. Pediatrics. 1998;102:e29–e29. doi: 10.1542/peds.102.3.e29. [DOI] [PubMed] [Google Scholar]
- Barlow SE the Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics. 2007;120:S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- Barr-Anderson DJ, Adams-Wynn AW, DiSantis KI, Kumanyika S. Family-focused physical activity, diet and obesity interventions in African–American girls: A systematic review. Obesity Reviews. 2013;14:29–51. doi: 10.1111/j.1467-789X.2012.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan L, Walkley J, Fraser SF, Greenway K, Wilks R. Motivational interviewing and cognitive behaviour therapy in the treatment of adolescent overweight and obesity: study design and methodology. Contemporary Clinical Trials. 2008;29:359–375. doi: 10.1016/j.cct.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Bui KVT, Takeuchi DT. Ethnic minority adolescents and the use of community mental health care services. American Journal of Community Psychology. 1992;20:403–417. doi: 10.1007/BF00937752. [DOI] [PubMed] [Google Scholar]
- Collins LM, Murphy SA, Nair VN, Strecher VJ. A strategy for optimizing and evaluating behavioral interventions. Annals of Behavioral Medicine. 2005;30:65–73. doi: 10.1207/s15324796abm3001_8. [DOI] [PubMed] [Google Scholar]
- Davis CL, Tomporowski PD, McDowell JE, Austin BP, Miller PH, Yanasak NE, Naglieri JA. Exercise improves executive function and achievement and alters brain activation in overweight children: A randomized, controlled trial. Health Psychology. 2011;30:91. doi: 10.1037/a0021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz WH, Robinson TN. Overweight children and adolescents. New England Journal of Medicine. 2005;352:2100–2109. doi: 10.1056/NEJMcp043052. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Frey MA, Naar-King S, Templin T, Cunningham P, Cakan N. Use of multisystemic therapy to improve regimen adherence among adolescents with type 1 diabetes in chronic poor metabolic control a randomized controlled trial. Diabetes Care. 2005;28:1604–1610. doi: 10.2337/diacare.28.7.1604. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Naar-King S, Jen KLC, Brogan K. Executive functioning affects weight loss among African American adolescents with obesity. 34th Annual Meeting and Scientific Session of the Society for Behavioral Medicine; San Francisco, CA. 2013. Mar, [Google Scholar]
- Ellis DA, Janisse H, Naar-King S, Kolmodin K, Jen KLC, Cunningham P, Marshall S. The effects of multisystemic therapy on family support for weight loss among obese African-American adolescents: findings from a randomized controlled trial. Journal of Developmental & Behavioral Pediatrics. 2010;31:461–468. doi: 10.1097/DBP.0b013e3181e35337. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychology. 1994;13:373–383. doi: 10.1037/0278-6133.13.5.373. [DOI] [PubMed] [Google Scholar]
- Friend A, Craig L, Turner S. The prevalence of metabolic syndrome in children: A systematic review of the literature. Metabolic Syndrome and Related Disorders. 2013;11:71–80. doi: 10.1089/met.2012.0122. [DOI] [PubMed] [Google Scholar]
- Garland AF, Lau AS, Yeh M, McCabe KM, Hough RL, Landsverk JA. Racial and ethnic differences in utilization of mental health services among high-risk youths. American Journal of Psychiatry. 2005;162:1336–1343. doi: 10.1176/appi.ajp.162.7.1336. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function professional manual. Lutz, FL: PAR; 2000. [Google Scholar]
- Idalski Carcone A, MacDonell KE, Naar-King S, Ellis DA, Cunningham PB, Kaljee L. Treatment engagement in a weight loss intervention for African American adolescents and their families. Children’s Health Care. 2011;40:232–252. doi: 10.1080/02739615.2011.590398. [DOI] [Google Scholar]
- Irby M, Kaplan S, Garner-Edwards D, Kolbash S, Skelton JA. Motivational interviewing in a family-based pediatric obesity program: A case study. Families, Systems, & Health. 2010;28(3):236. doi: 10.1037/a0020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DE, Stucky B, Langer MM, Thissen D, DeWitt EM, Lai JS, DeWalt DA. An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Quality of Life Research. 2010;19:595–607. doi: 10.1007/s11136-010-9619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelalian E, Hart CN, Mehlenbeck RS, Lloyd-Richardson EE, Kaplan JD, Flynn-O’Brien KT, Wing RR. Predictors of attrition and weight loss in an adolescent weight control program. Obesity. 2008;16:1318–1323. doi: 10.1038/oby.2008.51. [DOI] [PubMed] [Google Scholar]
- Jensen CD, Aylward BS, Steele RG. Predictors of attendance in a practical clinical trial of two pediatric weight management interventions. Obesity. 2012;20:2250–2256. doi: 10.1038/oby.2012.96. [DOI] [PubMed] [Google Scholar]
- Joseph CLM, Havstad SL, Johnson D, Saltzgaber J, Peterson EL, Resnicow K, Strecher VJ. Factors associated with nonresponse to a computer-tailored asthma management program for urban adolescents with asthma. Journal of Asthma. 2010;47:667–673. doi: 10.3109/02770900903518827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan LK, Sobush K, Keener D, Goodman K, Lowry A, Kakietek J, Zaro S. MMWR Recommendations Report. Vol. 58. Centers for Disease Control; 2009. Recommended community strategies and measurements to prevent obesity in the United States; pp. 1–26. [PubMed] [Google Scholar]
- Let’s move. [Accessed March 4, 2014];2011 http://www.letsmove.gov/
- Liese AD, D’Agostino RB, Jr, Hamman RF, Kilgo PD, Lawrence JM, Liu LL SEARCH for Diabetes in Youth Study Group. The burden of diabetes mellitus among US youth: Prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118:1510. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Archives of General Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury VA, O’Malley C, Stolk RP, Summerbell CD. Interventions for treating obesity in children. Cochrane Database of Systematic Reviews. 2009;3:1–57. doi: 10.1002/14651858.CD001872. [DOI] [PubMed] [Google Scholar]
- MacDonell K, Brogan K, Naar-King S, Ellis D, Marshall S. A pilot study of motivational interviewing targeting weight-related behaviors in overweight or obese African American adolescents. Journal of Adolescent Health. 2012;50:201–203. doi: 10.1016/j.jadohealth.2011.04.018. [DOI] [PubMed] [Google Scholar]
- MacDonell K, Ellis D, Naar-King S, Cunningham P. Predictors of home-based obesity treatment efficacy for African American youth. Children’s Health Care. 2010;39:1–14. doi: 10.1080/02739610903455087. [DOI] [Google Scholar]
- Miller LM, Southam-Gerow MA, Allin RB., Jr Who stays in treatment? Child and family predictors of youth client retention in a public mental health agency. Child & Youth Care Forum. 2008;37:153–170. doi: 10.1007/s10566-008-9058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. New York, NY: Guilford Press; 2013. [Google Scholar]
- Mottola M, Wolfe LA. Active living and pregnancy. In: Quinney A, Gauvin L, Wall T, editors. Toward active living: Proceedings of the international conference on physical activity, fitness and health. Champaign, IL: Human Kinetics; 1994. [Google Scholar]
- Moyers TB, Martin T, Manuel JK, Hendrickson SM, Miller WR. Assessing competence in the use of motivational interviewing. Journal of Substance Abuse Treatment. 2005;28:19–26. doi: 10.1016/j.jsat.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Naar-King S, Ellis D, King PS, Lam P, Cunningham P, Secord E, Templin T. Multisystemic therapy for high-risk African American adolescents with asthma: a randomized clinical trial. Journal of Consulting and Clinical Psychology. 2014;82:536–545. doi: 10.1037/a0036092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naar-King S, Ellis D, Kolmodin K, Cunningham P, Jen KLC, Saelens B, Brogan K. A randomized pilot study of multisystemic therapy targeting obesity in African-American adolescents. Journal of Adolescent Health. 2009;45:417–419. doi: 10.1016/j.jadohealth.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Naar-King S, Suarez M. Motivational interviewing with adolescents and young adults. New York, NY: Guilford Press; 2011. [Google Scholar]
- Nahum-Shani I, Qian M, Almirall D, Pelham WE, Gnagy B, Fabiano GA, Murphy SA. Experimental design and primary data analysis methods for comparing adaptive interventions. Psychological Methods. 2012;17:457. doi: 10.1037/a0029372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Collaborative on Childhood Obesity Research (NCCOR) [Accessed March 4, 2014]; http://www.nccor.org/
- National Institutes of Health. Clinical Guidelines on the Identification, Evaluation and Treatment of Overweight and Obesity in Adults. NHLBI Obesity Education Initiative 2000 [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. Journal of the American Medical Association. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluch RA, Epstein LH, Roemmich JN. Comparison of methods to evaluate changes in relative body mass index in pediatric weight control. American Journal of Human Biology. 2007;19:487–494. doi: 10.1002/ajhb.20608. [DOI] [PubMed] [Google Scholar]
- Petry NM. Contingency management for substance abuse treatment: A guide to implementing evidence-based practice. New York, NY: Routledge/Taylor & Francis Group; 2012. [Google Scholar]
- Reinert KRS, Po’e EK, Barkin SL. The relationship between executive function and obesity in children and adolescents: A systematic literature review. Journal of Obesity. 2013:1–10. doi: 10.1155/2013/820956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnicow K, Taylor R, Baskin M, McCarty F. Results of go girls: A weight control program for overweight African-American adolescent females. Obesity Research. 2005;13:1739–1748. doi: 10.1038/oby.2005.212. [DOI] [PubMed] [Google Scholar]
- Rosengren DB, Hartzler B, Baer JS, Wells EA, Dunn CW. The video assessment of simulated encounters-revised (VASE-R): Reliability and validity of a revised measure of motivational interviewing skills. Drug and Alcohol Dependence. 2008;97:130–138. doi: 10.1016/j.drugalcdep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochon J. Issues in adjusting for covariates arising postrandomization in clinical trials. Drug Information Journal. 1999;33:1219–1228. [Google Scholar]
- Savoye M, Shaw M, Dziura J, Tamborlane WV, Rose P, Guandalini C, Caprio S. Effects of a weight management program on body composition and metabolic parameters in overweight children: A randomized controlled trial. Journal of the American Medical Association. 2007;297:2697–2704. doi: 10.1001/jama.297.24.2697. [DOI] [PubMed] [Google Scholar]
- Sue DW. Counseling the culturally different: A conceptual analysis. The Personnel and Guidance Journal. 1977;55:422–425. doi: 10.1002/j.2164-4918.1977.tb05281.x. [DOI] [Google Scholar]
- Sung-Chan P, Sung YW, Zhao X, Brownson RC. Family-based models for childhood-obesity intervention: A systematic review of randomized controlled trials. Obesity Reviews. 2013;14:265–278. doi: 10.1111/obr.12000. [DOI] [PubMed] [Google Scholar]
- Thomas S, Reading J, Shephard RJ. Revision of the physical activity readiness questionnaire (PAR-Q) Canadian Journal of Sport Sciences. 1992;17:338–345. [PubMed] [Google Scholar]
- Verbeken S, Braet C, Goossens L, Van der Oord S. Executive function training with game elements for obese children: A novel treatment to enhance self-regulatory abilities for weight-control. Behaviour Research and Therapy. 2013;51(6):290–299. doi: 10.1016/j.brat.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Stunkard AJ, Rich L, Rubin CJ, Sweidel G, McKinney S. Obesity in black adolescent girls: A controlled clinical trial of treatment by diet, behavior modification, and parental support. Pediatrics. 1990;85:345–352. http://pediatrics.aappublications.org/content/85/3/345. [PubMed] [Google Scholar]
- Weersing VR, Weisz JR. Community clinic treatment of depressed youth: Benchmarking usual care against CBT clinical trials. Journal of Consulting and Clinical Psychology. 2002;70:299. doi: 10.1037//002-006X.70.2.299. [DOI] [PubMed] [Google Scholar]
- Williamson DA, Walden HM, White MA, York-Crowe E, Newton RL, Alfonso A, Ryan D. Two-year internet-based randomized controlled trial for weight loss in African-American girls. Obesity. 2006;14:1231–1243. doi: 10.1038/oby.2006.140. [DOI] [PubMed] [Google Scholar]
- Zeller M, Kirk S, Claytor R, Khoury P, Grieme J, Santangelo M, Daniels S. Predictors of attrition from a pediatric weight management program. The Journal of Pediatrics. 2004;144:466–470. doi: 10.1016/j.jpeds.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Zeller MH, Saelens BE, Roehrig H, Kirk S, Daniels SR. Psychological adjustment of obese youth presenting for weight management treatment. Obesity Research. 2004;12:1576–1586. doi: 10.1038/oby.2004.197. [DOI] [PubMed] [Google Scholar]