Abstract
AIM: To improve the preparation of adherent lymphokine-activated killer (A-LAK) cells and to study the effects of cryopreservation and phenylacetate (PA) on biological characters of A-LAK cells.
METHODS: A-LAK cells were obtained from peripheral blood mononuclear cells (PBMCs) of the patients with hepatocellular carcinoma (HCC) by using L-phenylalanine methyl ester (PME) to deplete immunosuppressive monocytes. Proliferative activity of SMMC7721 cell line after treatment with phenylacetate (PA) was observed. A-LAK cells were treated with the supernatant of SMMC7721 cells that had been pretreated with PA. The changes of proliferation, cytotoxicity and phenotype of A-LAK cells were investigated after cryopreservation.
RESULTS: The expansion of A-LAK cells (96.79 ± 69.10 folds on Day 14) was significantly higher than that of non-adherent LAK (NA-LAK) cells (22.77 ± 13.20) as well as conventional LAK cells (4.64 ± 0.91). PA significantly suppressed the growth of SMMC7721 cells, and the inhibitor ratio was 46%. The supernatant of cultured tumor cells intensively suppressed the proliferation and cytotoxicity of A-LAK cells, but the suppressive effect of the supernatant was previously decreased after treatment with PA. Impairments in proliferation and cytotoxicity of A-LAK cells immediately after thawing of cryopreservation and recovery after reincubation with IL-2 were observed. The cytotoxicity of thawed A-LAK cells on Day 5 was significantly higher than that of fresh A-LAK before freezing (54.8% ± 10.2% vs 40.5% ± 6.4%). No significant change in the percentage of lymphocyte subsets was identified in frozen A-LAK cells as compared with that in the fresh control cells.
CONCLUSION: A-LAK cells can be simply prepared by using PME, and showed a synergistic anti-tumor effect with the combination of PA. Cryopreservation can increase the immunoactivities of A-LAK cells from the patients with hepatocellular carcinoma.
INTRODUCTION
Biotherapy of cancer, as the forth modality of tumor treatment, has been accepted[1-4]. Adoptive transfer of LAK cells together with IL-2 has been found to be an effective immunotherapeutic modality for eradication of several tumors[5-8]. However, some reports indicated that clinical responses have been infrequent and transient, and that severe toxicity was associated with the administration of high doses of rIL-2[6,9,10]. Adherent-lymphokine activated killer (A-LAK) cells have significantly higher cytolytic activity on a per cell basis and greater proliferative capacity than LAK cells[9-14]. But the usage of A-LAK cells was limited by the complex traditional preparation by using nylon wool columns[15-17]. To harvest a large number of actively immunocompetent cells at the right time now becomes an important point of adoptive immunotherapy. The freezing of peripheral blood mononuclear cells (PBMCs)is a common practice, and does not significantly alter the functional activities of immunocompetent cells[18]. The recent reports showed that frozen PBMCs produced significantly larger quantities of IL-1, IL-2, IL-6, and IFN-γ than the fresh cells[19]. These results indicated that cryopreservation could increase the immunoactivities of immunocytes. We improved the preparation of A-LAK cells and studied the effects of cryopreservation and phenylacetate (PA) on biological characters of A-LAK cells from the patients with hepatocellular carcinoma.
MATERIALS AND METHODS
Cell lines and Reagents
A human hepatocellular carcinoma cell line, SMMC7721, was maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS, Gibco) at 37 °C in a 5% CO2 humidified atmosphere. Phenylacetate (PA, Sigma) and L-phenylalanine methyl ester (PME, Sigma) were dissolved in RPMI 1640 medium, brought to PH7.0 and 7.4, respectively and stored in aliquots at 4 °C.
Preparation of A-LAK cells
PBMCs were isolated from the patients with hepatocellular carcinoma (HCC) by using a cell separator (CS-3000 plus, Baxter), and then by centrifugation on Ficoll-Hypaque gradients. Cells collected from the gradient interface were washed twice in RPMI 1640 medium (GIBCO) and incubated in medium containing PME (5 mmol/L) for 40 min at room temperature to remove monocytes. After two washes with complete tissue culture medium (TCM) containing RPMI 1640 medium supplemented with 10% (v/v) heat-inactivated pooled AB human serum. The monocyte-depleted PBMCs were adjusted to the concentration of 2 × 106/mL and incubated in TCM containing rIL-2 (1000 U/mL) in a plastic culture flasks positioned on its flat side for 24 h at 37 °C in humidified atmosphere of 5% CO2. Following 24 h of activation in rIL-2, supernatants containing cells that did not adhere to plastic were decanted from the flasks, collected, and centrifuged. The recovered non-adherent cells (NA-LAK cells) were resuspended in fresh TCM plus rIL-2 (1000U/L) and cultured for 8-12 d. The cell-free supernatant of the non-adherent cell population was collected and used as autologous conditioned medium (AuCM). The cells adherent to plastic (A-LAK cells) were washed twice with prewarmed TCM to remove cells that were not firmly attached to plastic, and then were supplemented with TCM containing 50% (v/v) of AuCM and rIL-2 (1000 U/mL). These A-LAK cells were also cultured for 8-12 d and maintained at a concentration of 2 × 106 cells/mL by supplying fresh TCM containing rIL-2 (1000 U/mL) as needed. PBMCs were also established in cultures with rIL-2 (1000U/L) and cultured for 4 d as standard, regular LAK cells.
Procedures of freezing and thawing
After 10 d of incubation with rIL-2, A-LAK cells were washed and counted. Then the cells were diluted in the cold freezing medium in an ice bath to give a final concentration of 5 × 106 cells/mL. The freezing medium consisted of 10% (v/v) heat-inactivated pooled AB human serum, 10% dimethyl sulfoxide (DMSO), and 80% RPMI-1640 containing rIL-2 (1000 U/mL). The cells were distributed in precooled plastic vials. The vials were frozen according to the routine procedure: At 4 °C for 30 min → at 0 °C for 30 min → at -20 °C for 30 min → at -80 °C for 60 min → transferred to liquid nitrogen for storage.
The frozen cells were thawed in a 37 °C water bath, and washed at 1000 rpm in phosphate-buffered saline twice to remove DMSO. Cell recovery and viability were calculated by trypan blue exclusion. After thawing, the cells were cultured in complete media as described above with 1000 U of rIL-2/mL at the concentration of 2 × 106 cells/mL for 7-10 d.
Tumor cell culture and PA treatment
The SMMC7721 cells growing in logarithmic phase were seeded into flasks at a concentration of 2 × 105 cells/flask. The following day, the medium was changed, and PA was added to the medium at 0 and 5 mmol/L for 7 d. After cell counts, the mediums were changed. Twenty-four hours later, the cell-free supernatants of SMMC7721 cells with PA pre-treatment and non-treatment were collected.
Proliferative activity of A-LAK cells
Fold expansions of fresh A-LAK cells, NA-LAK cells, regular LAK and frozen A-LAK cells were determined by cell counts performed in the presence of trypan blue on day 1, 4, 7, 10, and 14, respectively.
On the other hand, A-LAK cells were incubated by supplying fresh TCM containing 10% (v/v) cell-free supernatant derived from SMMC7721 cells with and without PA administration previously for 14 d. Cell numbers were calculated by hemocytometer cell counts in trypan blue.
Cytotoxicity Assays[20]
The fresh A-LAK cells, frozen A-LAK cells and A-LAK cells treated with SMMC7721 cell supernatant administered with or without PA were tested as effector cells for cytotoxicity against cultured SMMC7721 cells in MTT assays. SMMC7721 cells as target cells were seeded 2 × 104 cells/well in wells of U-bottomed 96-well plates and incubated for 24 h. Effector cells were added to target cells in effector-to-target ratio of 10∶1. After 48-h incubation at 37 °C, stock MTT solution (50 μL/well) was added to all wells of an assay, and plates were incubated at 37 °C for 6 h. Acid-isopropanol was added to all wells and mixed thoroughly to dissolve the dark blue crystals. After a few minutes at room temperature to ensure that all crystals were dissolved, the plates were read on a Microplate Reader (3550-UV, Bio-Rad), using a test wavelength of 570 nm, a reference wavelength of 630 nm.
Analyses of phenotype
The surface phenotype of cells was analyzed before freezing, after thawing and after 3 d of restimulation with rIL-2 using monoclonal antibodies CD3, CD4, CD8, CD16, and CD25. Analyses were performed on a flow cytometer (EPI CV-V, Culter Corp.).
Statistics
Statistics analysis of the data was performed using the Student's t-test; P < 0.05 was considered statistically significant.
RESULTS
Proliferation of A-LAK cells
A-LAK cells expanded better and faster than autologous non-adherent cells or regular LAK cells under the same growth conditions (Table 1). For HCC patients, the expansions of A-LAK cells varied from 23- to 243- fold by 14 d in culture, which were much higher than those of NA-LAK cells and regular LAK cells.
Table 1.
Proliferative capacity of A-LAK cells
| Culture Days |
Cells expansion folds |
||
| A-LAK | NA-LAK | Regular LAK | |
| 1 | 1.35 ± 0.39 | 0.87 ± 0.21a | 0.83 ± 0.09a |
| 4 | 2.22 ± 0.85 | 1.09 ± 0.33a | 0.99 ± 0.03a |
| 7 | 4.42 ± 2.05 | 2.13 ± 0.83a | 1.55 ± 0.14b |
| 10 | 22.52 ± 10.47 | 8.49 ± 3.91a | 3.25 ± 0.64b |
| 14 | 96.79 ± 69.10 | 22.77 ± 13.20a | 4.64 ± 0.91a |
P < 0.05, vs compared with α-LAK cells of the same day;
P < 0.01, vs compared with α-LA cells of the same day
The recovery rate after thawing of the cryopreserved A-LAK cells was 84.3% ± 4.2%. The viability of frozen cells was 96.4% ± 3.8%. The length of storage in liquid nitrogen (ranged from 1 d to 6 mo) did not have any detectable effect. The frozen A-LAK cells after thawing maintained a high ability of proliferation. After 3 d of reculture with rIL-2, the expansion folds of thawed A-LAK cells was in the same range as A-LAK cells before freezing (Figure 1).
Figure 1.
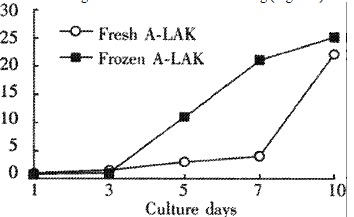
Proliferative capacity of A-LAK cells
Effect of PA on SMMC7721 cell proliferation
Having treated with PA at 5 mmol/L for 7 d, the SMMC7721 cells propagated from 2.0 × 105 cells/flask to 1.54 × 106 cells/flask. At the same time, the tumor cells without PA treatment proliferated to 2.86 × 106 cells/flask. The inhibitory ratio of PA affecting SMMC7721 proliferation was 46%.
Effect of PA and supernatant of Tumor cells on A-LAK propagation
On day 14 of cell culture, the expansion folds of A-LAK cells treated with the supernatant of SMMC7721 cells were only 55.22 ± 42.37, which were significantly lower than those of normal cultured A-LAK cells (96.79 ± 69.10, P < 0.01). However, the expansion folds of A-LAK cells treated with the supernatant of SMMC7721 cells with PA administration previously recovered to 84.72 ± 58.98, which had no significant statistical difference from those normal cultured A-LAK cells (P > 0.05).
Effect of PA and supernatant of tumor cells on A-LAK cell cytotoxicity
The cytotoxicity of A-LAK cells treated with the supernatant of SMMC7721 cells 31.2% ± 9.01% was significantly lower than that of normal cultured A-LAK cells 68.4% ± 8.82% (P < 0.001). However, the cytotoxicity of A-LAK cells treated with the supernatant of SMMC7721 cells with PA administration previously approached the normal level 57.1% ± 10.1% (P > 0.05).
Effect of cryopreservation on A-LAK cell cytotoxicity
Immediately after thawing, there was a significant decrease in cytotoxic activities. This impairment in activity was temporary, and the complete cytotoxicity could be restored by reincubation of the thawed A-LAK cells with rIL-2 for 1 d (Figure 2). After 5 d of postthaw culture with rIL-2, the cytotoxicity of the thawed A-LAK cells (54.8% ± 10.2%) was significantly higher than that of fresh A-LAK cells before freezing (40.5% ± 6.4%, P < 0.05).
Figure 2.
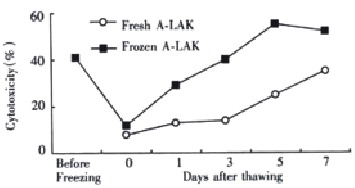
Effect of cryopreservation on A-LAK cells cytotoxicity
Change of phenotype of frozen A-LAK cells
As shown in Table 2, no significant change in the percentage of lymphocyte subpopulations of A-LAK cells before freezing, immediately after thawing, and 3 d after thawing was identified (P > 0.05).
Table 2.
Changes of phenotype of cryopteserved A-LAK cells
| Case No. | CD3 | CD4 | CD8 | CD4/CD8 | CD16 | CD25 | |
| Before freezing | 6 | 76.5 ± 10.3 | 41.4 ± 7.5 | 40.3 ± 5.3 | 1.03 ± 0.31 | 10.4 ± 3.1 | 52.3 ± 5.9 |
| Day 0 after thawing | 6 | 80.5 ± 11.4 | 35.9 ± 6.4 | 36.5 ± 4.9 | 1.07 ± 0.38 | 14.1 ± 4.3 | 49.7 ± 3.1 |
| Day 3 after thawing | 6 | 86.3 ± 8.9 | 33.1 ± 7.5 | 42.6 ± 6.0 | 0.78 ± 0.19 | 15.4 ± 2.7 | 46.5 ± 4.7 |
DISCUSSION
The application of LAK cells in immunotherapeutic treatment of cancer began in the past decade. The biotherapy may be the most effective method in delayed treatment of residual neoplastic diseases after classic reductive therapy (i.e. chemotherapy and bone marrow transplantation). A large number of immunocompetent cells are required for each adoptive immunotherapy. In some patients, because of the complexity of the preparation of immunocytes, such as LAK cells and A-LAK cells, the immunosuppressive status of the tumor host, and the procedures of the treatment of malignant tumor (i.e. the side effects of chemotherapy on the immune system of the host), it is difficult to obtain sufficient effector cells when they are needed. Therefore, we have carried out experiments to improve the preparation of A-LAK cells, and to determine the effects of cryopreservation and PA on the immunologic function of A-LAK cells obtained from the patients with HCC.
A major contributor to host immunity and immune surveillance against infection, tissue or cell damage and malignancy is the monocyte/macrophage system. The monocytes/macrophages play both positive and negative regulator roles in immune responses[21-23]. They can secret some kinds of cytokines (i.e. IL-1, IL-6), which may lead to an enhancement effect on both humoural and cellular immunity of human bodies[24,25]. Meanwhile, they can also produce some chemotactic factors (such as prostaglandin E2, lipocortin) to intensively suppress the immune responses[26,27]. In addition, some reports indicated that monocytes/macrophages could inhibit the proliferation and cytotoxicity of LAK cells[28]. Thus, the therapeutic effects of LAK cells would be improved by removing monocytes/macrophages from PBMC. The monocyte-depleted PBMC cells could be incubated with rIL-2 in a plastic flask and the plastic-adherent cells could be harvested as adherent LAK cells. There are several methods that can be used to get rid of monocytes/macrophages from peripheral blood. The routine one is to load PBMC onto prewarmed nylon wool column to remove monocytes/macrophages by adherence to nylon wool. But the complexity of this procedure limited the usage of A-LAK cells. Some reports demonstrated that PME could remove the monocytes/macrophages efficiently without changing the phenotype and cytotoxicity of lymphocytes. Triozzi and colleagues used this procedure to harvest A-LAK cells with efficient anti-tumor activities from the patients with renal cancer[15]. Our results using the same method to prepare A-LAK cells from patients with HCC are similar to those reports.
There are evidences that the immune system of tumor hosts has been destroyed, and that the immune function of hosts is suppressed[29-32]. Thus, LAK cells and A-LAK cells prepared from peripheral blood of tumor hosts were different from those of normal individuals. Melden et al[9] reported that for normal individuals, the expansion of A-LAK cells varied from 190- to 800- folds by 14 d in culture, whereas the numbers of A-LAK cells harvested from HCC patients in our study were only increased by 23- to 243- folds. We also indicated that the human HCC cell line (SMMC7721) intensively suppressed the proliferation and cytotoxic activity of A-LAK cells by secreting some immunosuppressive factors. The suppression of immune system of tumor host caused by secretion of immunosuppressive factors from tumor cells has become one reason for the limitation of the usage of the adoptive immunotherapy such as LAK/rIL-2.
The cryopreservation of human mononuclear cells is a common practice, but the effect of the cryopreservation of the effector cells on the functional activities is not well documented. Some reports indicated that high concentration of DMSO, rapid cooling rate, and rapid diluting after thawing could injure the hemopietic stem cells and lymphocytes[33]. Recent results showed that freezing not only did not significantly affect the functional activities of immunocompetent cells, but also increased the secreting abilities of some cytokines of these cells[19]. In our tests, optimal proliferation and cytotoxicity of A-LAK cells from the patients with HCC were generated after 10 d of culture with rIL-2. Cryopreservation was performed on cells after 10 d of culture. Our results demonstrated that impairments in proliferation and cytotoxicity of A-LAK cells immediately after thawing of cryopreservation and recovery after reincubation with IL-2 were observed. The cytotoxicity of thawed A-LAK cells on Day 5 was significantly higher than that of fresh A-LAK before freezing. The results indicated that cryopreservation could increase the immunoactivities of A-LAK cells from the patients with hepatocellular carcinoma. The difference of the immunologic activities of A-LAK cells during the distinct periods after thawing may explain the divergence of views from different investigators.
Phenylacetate (PA) is a member of a class of aromatic fatty acids and common metabolite of phenylalanine that is present in human plasma and cerebrospinal fluid in micromolar concentrations[34]. Experimental data indicated that PA was able to promote differentiation, growth inhibition, tumor maturation, and apoptosis in vitro and in vivo of various human leukemia cells, prostate carcinoma cells, breast cancer cells, brain tumor cells and renal cancer cells[35-38]. The phase I clinical trials at the National Cancer Institute with phenylacetate as a novel, nontoxic differentiation inducers have been finished[39]. Currently in clinical trials, PA was examined for its ability to modify tumor immunogenicity and revealed that it was able to reduce biosynthesis and secretion of TGF-β2, the tumor-derived T-cell suppressor factor, and reverse the tumor associated inhibition of IL-2 secretion by PHA-treated peripheral blood lymphocytes[40]. In our study, PA could significantly inhibit the growth of tumor cells, and the suppressive effect of the supernatant of tumor cells with pre-treatment of PA on the proliferation and cytotoxicity of A-LAK cells was decreased. Our data indicated that PA could decrease the immunosuppressive factors derived from tumor cells, antagonize the inhibition of tumor in biotherapy, and coordinate to attack tumor cells.
Footnotes
Edited by Zhang JZ
Supported by the National 9th Five-Year Program of China, No. 96-906-01-20
References
- 1.Ye SL. Biotherapy of tumor. In: Tang ZY, editor. Contemporary oncology. 2nd editor. Shanghai: Shanghai Med University Press; 2000. pp. 513–522. [Google Scholar]
- 2.Bremers AJ, Parmiani G. Immunology and immunotherapy of human cancer: present concepts and clinical developments. Crit Rev Oncol Hematol. 2000;34:1–25. doi: 10.1016/s1040-8428(99)00059-1. [DOI] [PubMed] [Google Scholar]
- 3.Cheng JD, Rieger PT, von Mehren M, Adams GP, Weiner LM. Recent advances in immunotherapy and monoclonal antibody treatment of cancer. Semin Oncol Nurs. 2000;16:2–12. doi: 10.1053/sonu.2000.19775. [DOI] [PubMed] [Google Scholar]
- 4.Kobari M, Egawa S, Shibuya K, Sunamura M, Saitoh K, Matsuno S. Effect of intraportal adoptive immunotherapy on liver metastases after resection of pancreatic cancer. Br J Surg. 2000;87:43–48. doi: 10.1046/j.1365-2168.2000.01336.x. [DOI] [PubMed] [Google Scholar]
- 5.Ye SL. Biotherapy of hepatocellular carcinoma. In: Tang ZY, Yu YQ, editors. Primary liver cancer, 2nd editor. Shanghai: Shanghai Sci Technical Press; 1999. pp. 382–296. [Google Scholar]
- 6.Hoffman DM, Gitlitz BJ, Belldegrun A, Figlin RA. Adoptive cellular therapy. Semin Oncol. 2000;27:221–233. [PubMed] [Google Scholar]
- 7.Pawelec G, Rees RC, Kiessling R, Madrigal A, Dodi A, Baxevanis C, Gambacorti-Passerini C, Masucci G, Zeuthen J. Cells and cytokines in immunotherapy and gene therapy of cancer. Crit Rev Oncog. 1999;10:83–127. [PubMed] [Google Scholar]
- 8.Semino C, Martini L, Queirolo P, Cangemi G, Costa R, Alloisio A, Ferlazzo G, Sertoli MR, Reali UM, Ratto GB, et al. Adoptive immunotherapy of advanced solid tumors: An eight year clinical experience. Anticancer Res. 1999;19:5645–5649. [PubMed] [Google Scholar]
- 9.Melder RJ, Whiteside TL, Vujanovic NL, Hiserodt JC, Herberman RB. A new approach to generating antitumor effectors for adoptive immunotherapy using human adherent lymphokine-activated killer cells. Cancer Res. 1988;48:3461–3469. [PubMed] [Google Scholar]
- 10.Schwarz RE, Vujanovic NL, Hiserodt JC. Enhanced antimetastatic activity of lymphokine-activated killer cells purified and expanded by their adherence to plastic. Cancer Res. 1989;49:1441–1446. [PubMed] [Google Scholar]
- 11.Kiremidjian-Schumacher L, Roy M, Wishe HI, Cohen MW, Stotzky G. Supplementation with selenium augments the functions of natural killer and lymphokine-activated killer cells. Biol Trace Elem Res. 1996;52:227–239. doi: 10.1007/BF02789164. [DOI] [PubMed] [Google Scholar]
- 12.Koberda J, Przepiorka D, Moser RP, Grimm EE. Sequential TNF and TGF-beta regulation of expansion and induction of cytotoxicity in long-term cultures of lymphokine-activated killer cells. Lymphokine Cytokine Res. 1994;13:139–145. [PubMed] [Google Scholar]
- 13.Boiardi A, Silvani A, Ruffini PA, Rivoltini L, Parmiani G, Broggi G, Salmaggi A. Loco-regional immunotherapy with recombinant interleukin-2 and adherent lymphokine-activated killer cells (A-LAK) in recurrent glioblastoma patients. Cancer immunol immunother. 1994;39:193–197. doi: 10.1007/BF01533386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basse PH, Herberman RB, Hokland ME, Goldfarb RH. Tissue distribution of adoptively transferred adherent LAK cells: role of the route of administration. Nat immune. 1992;11:193–202. [PubMed] [Google Scholar]
- 15.Triozzi PL, Aldrich W, Kim J, Kinney P, Sagone A, Rinehart J. Biologic effects of the adoptive transfer of cells depleted of monocytes with L-phenylalanine methyl ester. Immunopharmacology. 1994;28:39–45. doi: 10.1016/0162-3109(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 16.Koyama S, Fukao K. Phenotypic analysis of nylon-wool-adherent suppressor cells that inhibit the effector process of tumour cell lysis by lymphokine-activated killer cells in patients with advanced gastric carcinoma. J Cancer Res Clin Oncol. 1994;120:240–247. doi: 10.1007/BF01372563. [DOI] [PubMed] [Google Scholar]
- 17.Jaso-Friedmann L, Leary JH, Evans DL. Role of function-associated molecules in target cell lysis: Analysis of rat adherent lymphokine-activated killer cells. Nat immun. 1993;12:316–325. [PubMed] [Google Scholar]
- 18.Martí F, Miralles A, Peiró M, Amill B, de Dalmases C, Piñol G, Rueda F, García J. Differential effect of cryopreservation on natural killer cell and lymphokine-activated killer cell activities. Transfusion. 1993;33:651–655. doi: 10.1046/j.1537-2995.1993.33893342746.x. [DOI] [PubMed] [Google Scholar]
- 19.Venkataraman M. Effects of cryopreservation on immune responses. VIII. Enhanced secretion of interferon-gamma by frozen human peripheral blood mononuclear cells. Cryobiology. 1995;32:528–534. doi: 10.1006/cryo.1995.1055. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro-Dias F, Marzagão Barbuto JA, Tsujita M, Jancar S. Discrimination between NK and LAK cytotoxic activities of murine spleen cells by MTT assay: differential inhibition by PGE (2) and EDTA. J immunol Methods. 2000;241:121–129. doi: 10.1016/s0022-1759(00)00206-4. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh CL, Chen DS, Hwang LH. Tumor-induced immunosuppression: A barrier to immunotherapy of large tumors by cytokine-secreting tumor vaccine. Hum Gene Ther. 2000;11:681–692. doi: 10.1089/10430340050015581. [DOI] [PubMed] [Google Scholar]
- 22.Williams MA, Newland AC, Kelsey SM. The potential for monocyte-mediated immunotherapy during infection and malignancy. Part I: Apoptosis induction and cytotoxic mechanisms. Leuk Lymphoma. 1999;34:1–23. doi: 10.3109/10428199909083376. [DOI] [PubMed] [Google Scholar]
- 23.Rhoades CJ, Williams MA, Kelsey SM, Newland AC. Monocyte-macrophage system as targets for immunomodulation by intravenous immunoglobulin. Blood Rev. 2000;14:14–30. doi: 10.1054/blre.1999.0121. [DOI] [PubMed] [Google Scholar]
- 24.Schwacha MG, Schneider CP, Bland KI, Chaudry IH. Resistance of macrophages to the suppressive effect of interleukin-10 following thermal injury. Am J Physiol Cell Physiol. 2001;281:C1180–C1187. doi: 10.1152/ajpcell.2001.281.4.C1180. [DOI] [PubMed] [Google Scholar]
- 25.Marselli L, Marchetti P, Tellini C, Giannarelli R, Lencioni C, Del Guerra S, Lupi R, Carmellini M, Mosca F, Navalesi R. Lymphokine release from human lymphomononuclear cells after co-culture with isolated pancreatic islets: effects of islet species, long-term culture, and monocyte-macrophage cell removal. Cytokine. 2000;12:503–505. doi: 10.1006/cyto.1999.0583. [DOI] [PubMed] [Google Scholar]
- 26.Luo JS, Kammerer R, von Kleist S. Comparison of the effects of immunosuppressive factors from newly established colon carcinoma cell cultures on human lymphocyte proliferation and cytokine secretion. Tumour Biol. 2000;21:11–20. doi: 10.1159/000030106. [DOI] [PubMed] [Google Scholar]
- 27.Sakata T, Iwagami S, Tsuruta Y, Teraoka H, Hojo K, Suzuki S, Sato K, Suzuki R. The role of lipocortin I in macrophage-mediated immunosuppression in tumor-bearing mice. J immunol. 1990;145:387–396. [PubMed] [Google Scholar]
- 28.Bergmann L, Schui DK, Brieger J, Weidmann E, Mitrou PS, Hoelzer D. The inhibition of lymphokine-activated killer cells in acute myeloblastic leukemia is mediated by transforming growth factor-beta 1. Exp Hematol. 1995;23:1574–1580. [PubMed] [Google Scholar]
- 29.Pardoll D. T cells and tumours. Nature. 2001;411:1010–1012. doi: 10.1038/35082676. [DOI] [PubMed] [Google Scholar]
- 30.Dalgleish AG. Current problems in the development of specific immunotherapeutic approaches to cancer. J Clin Pathol. 2001;54:675–676. doi: 10.1136/jcp.54.9.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biggs MW, Eiselein JE. Suppression of immune surveillance in melanoma. Med Hypotheses. 2001;56:648–652. doi: 10.1054/mehy.2000.1211. [DOI] [PubMed] [Google Scholar]
- 32.Karimine N, Arinaga S, Inoue H, Nanbara S, Ueo H, Akiyoshi T. Lymphokine-activated killer cell function of peripheral blood mononuclear cells, spleen cells and regional lymph node cells in gastric cancer patients. Clin Exp immunol. 1994;96:484–490. doi: 10.1111/j.1365-2249.1994.tb06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai JM, Ding ZH, Mai GZ, Li XC. Different susceptibility of murine hemopoietic cells and lymphocytes to freezing-thawing process. Dier Junyi Daxue Xuebao. 1994;15:110–114. [Google Scholar]
- 34.Samid D, Hudgins WR, Shack S, Liu L, Prasanna P, Myers CE. Phenylacetate and phenylbutyrate as novel, nontoxic differentiation inducers. Adv Exp Med Biol. 1997;400A:501–505. doi: 10.1007/978-1-4615-5325-0_67. [DOI] [PubMed] [Google Scholar]
- 35.Han S, Wada RK, Sidell N. Differentiation of human neuroblastoma by phenylacetate is mediated by peroxisome proliferator-activated receptor gamma. Cancer Res. 2001;61:3998–4002. [PubMed] [Google Scholar]
- 36.Vasse M, Thibout D, Paysant J, Legrand E, Soria C, Crépin M. Decrease of breast cancer cell invasiveness by sodium phenylacetate (NaPa) is associated with an increased expression of adhesive molecules. Br J Cancer. 2001;84:802–807. doi: 10.1054/bjoc.2000.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe M, Sugano S, Imai J, Yoshida K, Onodera R, Amin MR, Uchida K, Yamaguchi R, Tateyama S. Suppression of tumourigenicity, and induction of differentiation of the canine mammary tumour cell line MCM-B2 by sodium phenylacetate. Res Vet Sci. 2001;70:27–32. doi: 10.1053/rvsc.2000.0437. [DOI] [PubMed] [Google Scholar]
- 38.Thibout D, Di Benedetto M, Kraemer M, Sainte-Catherine O, Derbin C, Crépin M. Sodium phenylacetate modulates the synthesis of autocrine and paracrine growth factors secreted by breast cancer cell lines. Anticancer Res. 1998;18:2657–2661. [PubMed] [Google Scholar]
- 39.Thibault A, Samid D, Cooper MR, Figg WD, Tompkins AC, Patronas N, Headlee DJ, Kohler DR, Venzon DJ, Myers CE. Phase I study of phenylacetate administered twice daily to patients with cancer. Cancer. 1995;75:2932–2938. doi: 10.1002/1097-0142(19950615)75:12<2932::aid-cncr2820751221>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 40.Liu L, Bar-ner M, Weber J, Danielpour D, Qian SW, Shearer GM, Bernutt RM. Enhancement of tumor immunogenicity by phenylacetate and derivatives: Changes in surface antigens and tumor-derived immunosuppressive factors. Proc Am Assoc Cancer Res. 1994;35:481. [Google Scholar]


