Abstract
AIM: To study the viscoelastic properties of human hepatocytes and hepatocellular carcinoma (HCC) cells under cytoskeletal perturbation, and to further to study the viscoelastic properties and the adhesive properties of mouse hepatoma cells (HTC) in different cell cycle.
METHODS: Micropipette aspiration technique was adopted to measure viscoelastic coefficients and adhesion force to collagen coated surface of the cells. Three kinds of cytoskeleton perturbing agents, colchicines (Col), cytochalasin D (CD) and vinblastine (VBL), were used to treat HCC cells and hepatocytes and the effects of these treatment on cell viscoelastic coefficients were investigated. The experimental results were analyzed with a three-element standard linear solid. Further, the viscoelastic properties of HTC cells and the adhesion force of different cycle HTC cells were also investigated. The synchronous G1 and S phase cells were achieved through thymine-2-desoryriboside and colchicines sequential blockage method and thymine-2-desoryriboside blockage method respectively.
RESULTS: The elastic coefficients, but not viscous coefficient of HCC cells (K1 = 103.6 ± 12.6 N·m-2, K2 = 42.5 ± 10.4 N·m-2, μ = 4.5 ± 1.9 Pa·s), were significantly higher than the corresponding value for hepatocytes (K1 = 87.5 ± 12.1 N·m-2, K2 = 33.3 ± 10.3 N·m-2, μ = 5.9 ± 3.0 Pa·s, P < 0.01). Upon treatment with CD, the viscoelastic coefficients of both hepatocytes and HCC cells decreased consistently, with magnitudes for the decrease in elastic coefficients of HCC cells (K1: 68.7 N·m-2 to 81.7 N·m-2, 66.3% to 78.9%; K2: 34.5 N·m-2 to 37.1 N·m-2, 81.2% to 87.3%, P < 0.001) larger than those for normal hepatocytes (K1: 42.6 N·m-2 to 49.8 N·m-2, 48.7% to 56.9%; K2: 17.2 N·m-2 to 20.4 N·m-2, 51.7% to 61.3%, P < 0.001). There was a little decrease in the viscous coefficient of HCC cells (2.0 to 3.4 Pa•s, 44.4 to 75.6%, P < 0.001) than that for hepatocytes (3.0 to 3.9 Pa•s, 50.8 to 66.1% P < 0.001). Upon treatment with Col and VBL, the elastic coefficients of hepatocytes generally increased or tended to increase while those of HCC cells decreased. HTC cells with 72.1% of G1 phase and 98.9% of S phase were achieved and high K1, K2 value and low μ value were the general characteristics of HTC cells. G1 phase cells had higher K1 value and lower μ value than S phase cells had, and G1 phase HTC cells had stronger adhesive forces [(275.9 ± 232.8) × 10-10 N] than S phase cells [(161.2 ± 120.4) × 10-10 N, P < 0.001).
CONCLUSION: The difference in both the pattern and the magnitude of the effect of cytoskeletal perturbing agent on the viscoelastic properties between HCC cells and hepatocytes may reflect differences in the state of the cytoskeleton structure and function and in the sensitivity to perturbing agent treatment between these two types of cells. Change in the viscoelastic properties of cancer cells may affect significantly tumor cell invasion and metastasis as well as interactions between tumor cells and their micro-mechanical environments.
INTRODUCTION
Current advances in oncology have shown that the continuous growth of malignancy, invasion and metastasis are a multi-step pathophysiological process, which consists of successive steps of tumor cell deformation and locomotion[1-3]. For an understanding of the mechanisms involved, advanced methodologies of cellular and molecular biology have been extensively used in the study of the related oncogenes and anti-oncogenes, as exemplified in hepatocellular carcinoma (HCC)[4-8], and in the elucidation of the interaction between tumor cells and vascular endothelial cells[9,10]. These studies have already led to considerable knowledge in the event involved in tumor metastasis.
The mechanical properties are very important for biologic behaviors of tumor cells in following reasons. Firstly, tumor cells are destined to experience shear-induced deformation in blood flow if they metastasize through the blood vasculature. The mechanical properties determine whether tumor cells can pass through the microvasculature to form metastases, and probably whether they can survive in the blood shear environment.Secondly, the mechanical properties are related to active pseudopod formation and motility, in which they probably have a similar structural basis and are the main cellular events of tumor cell invasion, and a relationship between active pseudopod formation and cytoskeletal structures has already been demonstrated[11-13]. To the aim of this study was to try to understand how the viscoelastic properties of HCC cells are altered compared to those of normal hepatocytes, how the viscoelastic properties of these two types of cells respond to treatment with cytoskeleton perturbing agents [cytochalasin D (CD), colchicines (Col) and vinblastine (VBL)] and what changes occur in viscoelastic properties and the adhesive properties of hepatoma cells in different phases.
MATERIALS AND METHODS
Cell sample preparation
HCC cells (SMMC 7721) were obtained from the 2nd Military Medical University (Shanghai, China); HTC cells were kindly given by department of clinical biochemistry of Chongqing Medical University. Normal hepatocytes were prepared from human fetal liver tissue by a combination of 0.5 g•L⁻¹ collagenases IV (Sigma) digestion and density gradient centrifugation[14-17]. Cells were maintained in an incubator at 37 °C in an atmosphere of 950 mL•L⁻¹ humidified air and 50 mL•L⁻¹ carbon dioxide. The final concentration of the cells for micropipette experiment was of the order of 109 cells•L⁻¹.
Preparation of synchronous G1 an d S phase HTC cells
The synchronous G1 phase cells were achieved through thymine-2-desoryriboside and colchicines sequential blockage method[18].
Micropipette system and analysis of the viscoelastic properties of cells
The structure of micropipette system and experimental procedures were described in literatures[19-21]. Micropipettes were pulled from capillary glass tubes in a micropipette puller (P87, Sutler Instrument Co, USA). The weighted average values of the internal radius of the pipette used in the present investigation were 2.47 ± 0.91 μm.
Experimental results were analyzed with a three-element standard linear solid model[22], in which an elastic element, K1, was in parallel with a Maxwell element composed of another elastic element, K2, in series with a viscous element, μ. Viscoelastic coefficients were expressed as ¯x ± s. Student's t-test was used for statistical analysis.
Analysis of the adhesive properties of HTC cells in different cycle
The adhesive model used was schematically shown in Figure 1, Fa was adhesive force of cell, Rp was the inner radius of micropipette, △P was negative pressure pulled the adhesive cell away from basement membrane coated by collagen IV, and theta; was the angle of micropipette between basement membrane, Fa can be calculated from the following equation: Fa = Rp2 × △P × costheta;
Figure 1.
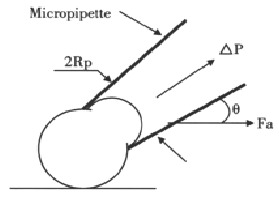
Geometry of adhesive model
RESULTS
Viscoelastic properties of HCC cells and hepatocytes and effects of cytoskeleton inhibitions
The values of the viscoelastic coefficients of hepatocytes and HCC and the effects of treatment with CD, Col and VBL were shown in Table 1, Table 2, Table 3, respectively. The results were summarized as follows: (1) Compared to those of normal hepatocytes, the values of the elastic coefficients K1 and K2 of HCC cells were significantly higher (P < 0.01). However, the viscous coefficient, μ, of the HCC cells was not significantly different from that of hepatocytes; (2) Treatment with 1-60 mg•L⁻¹ of Col resulted in a little but significant increase in K1 for hepatocytes with independent of [Col], whereas in the case of K2 there appeared to be no significant change. In the case of the viscous coefficient, there was a significant decrease with independent of [Col]. In contrast to hepatocytes, the HCC cells resulted in a significant decrease in all 3 coefficients with dependence on [Col] (Table 1); (3) Treatment of the hepatocytes with VBL in the concentration range of 0.05-2.00 mg•L⁻¹ resulted in a marked increase in the elastic coefficients at all concentrations, whereas the viscouscoefficient only increased significantly at 0.25 mg•L⁻¹ and 0.75 mg•L⁻¹of VBL. In the case of the HCC cells, K1 exhibited a little but significant increase at 0.05 mg•L⁻¹ of VBL, but then decreased continuously with increasing [VBL], whereas the values of K2 and μ decreased monotonously with increasing [VBL] (Table 2) and (4) Upon treatment with 0.25 to 5.00 mg•L⁻¹ of CD, the coefficients K1, K2 and μ decreased significantly from the control values, but the decrease exhibited no significant dependence on the perturbing agent concentration. In the case of the K1 and K2, the magnitude of the above decrease was significantly greater for the HCC cells. For μ, the magnitude of the decrease for HCC cells was less than that of the hepatocytes (Table 3).
Table 1.
Viscoelastic properties of hepatocytes and HCC cells under the action of colchicines (¯x ± s)
| [colchicine] (mg•L-1) |
Hepatocytes |
HCC cells |
||||
| K1 (N•m-2) | K2 (N•m-2) | μ (Pa•s) | K1 (N•m-2) | K2 (N•m-2) | μ (Pa•s) | |
| 0.0 | 87.5 ± 12.1 | 33.3 ± 10.3 | 5.9 ± 3.0 | 103.6 ± 12.6 | 42.5 ± 10.4 | 4.5 ± 1.9 |
| 1.0 | 95.4 ± 14.1a | 33.2 ± 7.7 | 3.9 ± 1.7b | 86.7 ± 10.0b | 20.6 ± 2.9b | 4.5 ± 1.5 |
| 15.0 | 107.1 ± 23.0b | 39.6 ± 12.2a | 5.3 ± 1.9 | 31.4 ± 8.0b | 7.0 ± 1.9b | 1.3 ± 0.6b |
| 30.0 | 99.5 ± 11.1b | 28.0 ± 7.3a | 4.0 ± 1.8b | 53.5 ± 12.9b | 12.3 ± 4.8b | 2.1 ± 1.0b |
| 60.0 | 104.4 ± 13.0b | 30.6 ± 6.5 | 3.5 ± 1.1b | 61.6 ± 16.0b | 16.5 ± 6.5b | 2.3 ± 1.2b |
P < 0.05,
P < 0.01 vs normal control
Table 2.
Viscoelastic properties of hepatocytes and HCC cells under the action of vinblastine (¯x ± s)
| [vinblastine] (mg•L-1) |
Hepatocytes |
HCC cells |
||||
| K1 (N•m-2) | K2 (N•m-2) | μ (Pa•s) | K1 (N•m-2) | K2 (N•m-2) | μ (Pa•s) | |
| 0.0 | 87.5 ± 12.1 | 33.3 ± 10.3 | 5.9 ± 3.0 | 103.6 ± 12.6 | 42.5 ± 10.4 | 4.5 ± 1.9 |
| 0.05 | 115.9 ± 15.9b | 42.4 ± 8.8b | 6.1 ± 2.3 | 118.4 ± 19.7b | 23.1 ± 6.0b | 5.6 ± 2.3 |
| 0.25 | 128.7 ± 2.4b | 54.0 ± 6.3b | 9.8 ± 1.6b | 93.2 ± 11.3b | 17.0 ± 3.2b | 2.9 ± 1.0b |
| 0.75 | 138.3 ± 23.2b | 51.4 ± 13.4b | 8.2 ± 3.3a | 84.5 ± 6.2b | 15.2 ± 3.1b | 2.6 ± 0.8b |
| 2.00 | 117.0 ± 9.1b | 43.9 ± 7.7b | 6.2 ± 2.3 | 53.4 ± 12.0b | 8.7 ± 2.8b | 1.0 ± 0.5b |
P < 0.05,
P < 0.01 vs normal control
Table 3.
Viscoelastic properties of hepatocytes and HCC cells under the action of cytochalasin D (¯x ± s)
| [cytochalasin D] (mg•L-1) |
Hepatocytes |
HCC cells |
||||
| K1 (N·m-2) | K2 (N•m-2) | μ (Pa•s) | K1 (N•m-2) | K2 (N•m-2) | μ (Pa•s) | |
| 0.00 | 87.5 ± 12.1 | 33.3 ± 10.3 | 5.9 ± 3.0 | 103.6 ± 12.6 | 42.5 ± 10.4 | 4.5 ± 1.9 |
| 0.25 | 37.7 ± 7.1b | 12.9 ± 3.3b | 2.0 ± 0.8b | 29.5 ± 11.4b | 6.5 ± 2.6b | 1.6 ± 0.8b |
| 0.50 | 39.5 ± 6.4b | 13.8 ± 3.4b | 2.6 ± 1.1b | 21.9 ± 5.2b | 5.4 ± 1.7b | 1.1 ± 0.5b |
| 2.50 | 42.4 ± 5.9b | 16.1 ± 3.3b | 2.9 ± 1.4b | 31.7 ± 3.8b | 7.1 ± 1.8b | 2.5 ± 1.0b |
| 5.00 | 44.9 ± 7.5b | 16.1 ± 3.0b | 2.6 ± 1.3b | 34.9 ± 9.4b | 8.0 ± 2.7b | 1.9 ± 0.7b |
aP < 0.05,
P < 0.01 vs normal control
Viscoelastic and adhesive properties of HTC in different cycle
The synchronization results detected with flow cytometer showed that it could meet the requirements of the experiments nicely. HTC with 72.1% of G1 phase and 98.9% of S phase were achieved. The values of the adhesive force of HTC on different concentration of artificial basement membrane (collagen IV coated) were shown in Table 4. The adhesive force of G1phase HTC on basement membrane coated by collagen IV 5 mg•L-1 was (275.9 ± 232.8) × 10-10 N, and the corresponding value of S phase HTC was (161.2 ± 120.4) × 10-10 N. Difference between them was considered significant (P < 0.001). The viscoelastic coefficients of HTC cells in different cycle were shown in (Table 5).
Table 4.
Adhesive forces of HTC on artificial basement membrane (¯x ± s)
| Concentration of collagen IV (mg•L-1) | Fa (10-10 N) |
| 1 | 107.8 ± 65.4 |
| 2 | 182.6 ± 107.9b |
| 5 | 298.9 ± 144.1 d |
P < 0.001 vs collagen IV 1 mg•L-1; dP < 0.001 vs collagen IV 2 mg•L-1
Table 5.
Viscoelastic coefficients of HTC in different cycle (¯x ± s)
| Viscoelastic coefficients | General | G1 phase | S phase |
| K1 (N•m-2) | 186.5 ± 35.6 | 215.3 ± 50.2 | 179.7 ± 33.0b |
| K2 (N•m-2) | 224.4 ± 114.5 | 181.9 ± 102.9 | 188.6 ± 87.1 |
| μ (Pa•s) | 3.1 ± 2.3 | 2.9 ± 1.3 | 4.7 ± 2.4b |
P < 0.001 vs G1 phase.
DISCUSSION
Viscoelasticity is an important mechanical property of a cell that is related to its motility and deformability[23-25]. For HCC cells, the viscoelasticity has probably significant bearing on tumor cell invasion and metastasis, in which it determines the flowing behavior of tumor cells in the circulation and whether such cells can be arrested to form metastasis. In addition, mechanical stiffness is closely related to cell adhesion behavior[26,27], which is the first step in tumor cell invasion. With the three-element standard linear solid viscoelastic model, we clearly showed that HCC cells have higher values for the elastic coefficients but not the viscous coefficient than hepatocytes. This result indicated that HCC cells were more rigid than normal hepatocytes under the experimental conditions. One possible explanation of this result is that, in HCC cells, interconnections between microfilaments and microtubules might have changed as compared to those in normal hepatocytes, and thus microfilaments are affected upon disorganization of microtubules.
As the primary force-bearing structure of a cell, the cytoskeleton may be very important in determining cell mechanical behaviors[28-30]. We used three microfilament- or microtubule- targeting perturbing agents (Col, VBL and CD) to treat hepatocytes and HCC cells and found the effects of agents on viscoelastic properties of HCC cells were different obviously in both pattern and extents from those on hepatocytes. Such differences might reflect differences in the state of the cytoskeleton structure and organization, and in the cell's sensitivity to agents. These results also suggested that cytoskeleton play a role in the maintenance of cell viscoelasticities.
Our results of the viscoelastic properties of HTC in different cycle indicated that high K1, K2 and low μ were the general characteristics of HTC, and these were coincided with the result of HCC cells. G1 phase cells had higher K1 value and lower μ value than S phase cells had, but there was no obviously difference in K2 between two phase cells, which reflected the discrepancies of cytoskeletal protein assemble and synthesis in different cell cycle. Those resultson relevance of cytoskeletal structure to viscoelastic coefficient of HCC cells suggested that microfilaments could play a major rule in the maintenance of cell viscoelasticity, especially in G1 phase cells. In contrary to these, synthesis of microtubules in S phase cells increased, and more microtubules took part in determination the cells viscoelasticity, which could endow G1 phase cells with higher elasticity and lower viscosity than S phase of cells. These characteristics evidently contributed to G1 phase cells survival from the blood shear environment and arrest to form metastases.
The adhesive properties of HTC in different cycle
Current study has shown that action of tumor cells on basement membrane is a multi-step pathophysiological process[31].Our results showed the adhesive force of HTC cells on basement membrane coated by collagen VI had the obvious correlation with the concentration of collagen. Wang et al[32] reported that the content of collagen IV and laminin in basement membrane increased along with the growth of tumor, but basement membrane became to decrease, even damaged when tumor transferred. So, the different thickness of basement membrane could reflected the different interaction between tumor cells and the membrane. Increased thickness of basement membrane might play the important rule in tumor cell invasion, which was conducive to the chemotactic motion of tumor cell, active orientation movement, and supplied strong adhesive force and adhesive site for tumor cell.The adhesive force of G1 phase of HTC was obviously greater than that of S phase cells. In general, expression of fibronectin receptor in transferred tumor cell would decrease and laminin receptor would increase. Fibronectin play a more important rule in improving tumor cell spreading and increasing the synthesis and proliferation of DNA in S/G2 phase. Laminin has many functions on the tumor cell adhesion and movement[33]. So, the difference of adhesive force between G1 phase cell and S phase cell could reflect the difference of expression of adhesive molecule receptor on the cell surface, especially the difference of periodic distribution of fibronectin receptor and laminin receptor. In addition, a strong affinity existed between laminin and thrombolysin, and both of them binded together to form thrombolysin by activating profibrinolysin and hydrolyzing laminin and fibronectin, and finally activation of procollagen and degradation of basement membrane occured. The phenomenon of high adhesive force value in G1 phase cell may be also relevant to these changes, which made G1 phase cells in active condition in adhesion and movement. In the course of tumor invasion and metastasis, G1phase cell were more capable of adhering to and getting through basement membranes than S phase cells.
Footnotes
Edited by Zhang JZ
Supported by the National Science Foundation of China, No.39370198
References
- 1.Okuda K. Hepatocellular carcinoma. J Hepatol. 2000;32:225–237. doi: 10.1016/s0168-8278(00)80428-6. [DOI] [PubMed] [Google Scholar]
- 2.Wu MC. Clinical research advances in primary liver cancer. World J Gastroenterol. 1998;4:471–474. doi: 10.3748/wjg.v4.i6.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang YF, Yang ZH, Hu JQ. Recurrence or metastasis of HCC: predictors, early detection and experimental antiangiogenic therapy. World J Gastroenterol. 2000;6:61–65. doi: 10.3748/wjg.v6.i1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445–454. doi: 10.3748/wjg.v7.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu WW. Etiological studies of hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:93–95. [Google Scholar]
- 6.Ji Y, Ling MY, Li Y, Xie H. Effect of cell fusion on metastatic ability of mouse hepatocarcinoma cell lines. World J Gastroenterol. 1999;5:22–24. doi: 10.3748/wjg.v5.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao XY, Liu J, Lian ZR, Clayton M, Hu JL, Zhu MH, Fan DM, Feitelson M. Cloning of differentially expressed genes in human hepatocellular carcinoma and nontumor liver. World J Gastroenterol. 2001;7:579–582. doi: 10.3748/wjg.v7.i4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou XP, Wang HY, Yang GS, Chen ZJ, Li BA, Wu MC. Cloning and expression of MXR7 gene in human HCC tissue. World J Gastroenterol. 2000;6:57–60. doi: 10.3748/wjg.v6.i1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun JJ, Zhou XD, Zhou G, Liu YK. Expression of intercellular adhesive molecule-1 in liver cancer tissues andliver cancer metastasis. World J Gastroenterol. 1998;4:202–205. doi: 10.3748/wjg.v4.i3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun JJ, Zhou XD, Liu YK, Zhou G. Phase tissue intercellular adhesion molecule-1 expression in nude mice human liver cancer metastasis model. World J Gastroenterol. 1998;4:314–316. doi: 10.3748/wjg.v4.i4.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong C, Aznavoorian S, Liotta LA. Two phases of pseudopod protrusion in tumor cells revealed by a micropipette. Microvasc Res. 1994;47:55–67. doi: 10.1006/mvre.1994.1005. [DOI] [PubMed] [Google Scholar]
- 12.You J, Mastro AM, Dong C. Application of the dual-micropipet technique to the measurement of tumor cell locomotion. Exp Cell Res. 1999;248:160–171. doi: 10.1006/excr.1999.4388. [DOI] [PubMed] [Google Scholar]
- 13.Sun WB, Han BL, Peng ZM, Li K, Ji Q, Chen J, Wang HZ, Ma RL. Effect of aging on cytoskeleton system of Kupffer cell and its phagocytic capacity. World J Gastroenterol. 1998;4:77–79. doi: 10.3748/wjg.v4.i1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang YJ, Li MD, Wang YM, Nie QH, Chen GZ. Experimental study of bioartificial liver with cultured human liver cells. World J Gastroenterol. 1999;5:135–137. doi: 10.3748/wjg.v5.i2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen K, Gao Y, Pan YX, Yang JZ. Separation and cultivation of porcine hepatocytes. Shijie Huaren Xiaohua Zazhi. 1999;7:200–202. [Google Scholar]
- 16.Gong JP, Han BL. Technique of isolation, culture and identification of liver cells. Shijie Huaren Xiaohua Zazhi. 1999;7:375–378. [Google Scholar]
- 17.Wang YJ, Lin GD, Lin J, Li MD. Large-scale isolation of sucking pig hepatocytes. Shijie Huaren Xiaohua Zazhi. 1999;7:661–662. [Google Scholar]
- 18.Yu QU, Song GB, Wu ZZ, Long M, Cai SX. Investigation of the viscoelasticity of synchronous HTC cells. Shengwu Wuli Xuebao. 1999;15:431–435. [Google Scholar]
- 19.Zhang G, Long M, Wu ZZ. Experiment study on viscoelastic properties of hepatocellular carcinoma cells. Jiefangjun Yixue Zazhi. 2001;26:204–207. [Google Scholar]
- 20.Zhang G, Wu ZZ, Wang HB, Long M, Song GB, Cai SX. Effect of vinblastine and cytochalasin D on viscoelastic properties of hepatocellular carcinoma cells. Zhongguo Shengwu Yixue Gongcheng Xuebao. 2000;19:213–217. [Google Scholar]
- 21.Zhang G, Long M, Wu ZZ. Comparative study on the viscoelastic properties of hepatocytes and hepatocellular carcinoma cells. Disan Junyi Daxue Xuebao. 2001;23:751. [Google Scholar]
- 22.Chien S, Sung KL. Effect of colchicine on viscoelastic properties of neutrophils. Biophys J. 1984;46:383–386. doi: 10.1016/S0006-3495(84)84034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riquelme BD, Foresto PG, Valverde JR, Rasia JR. Alterations to complex viscoelasticity of erythrocytes during storage. Clin Hemorheol Microcirc. 2000;22:181–188. [PubMed] [Google Scholar]
- 24.Chung TW, Ho CP. Changes in viscosity of low shear rates and viscoelastic properties of oxidative erythrocyte suspensions. Clin Hemorheol Microcirc. 1999;21:99–103. [PubMed] [Google Scholar]
- 25.Undar A, Henderson N, Thurston GB, Masai T, Beyer EA, Frazier OH, Fraser CD. The effects of pulsatile versus nonpulsatile perfusion on blood viscoelasticity before and after deep hypothermic circulatory arrest in a neonatal piglet model. Artif Organs. 1999;23:717–721. doi: 10.1046/j.1525-1594.1999.06408.x. [DOI] [PubMed] [Google Scholar]
- 26.Chang KC, Hammer DA. Adhesive dynamics simulations of sialyl-Lewis (x)/E-selectin-mediated rolling in a cell-free system. Biophys J. 2000;79:1891–1902. doi: 10.1016/S0006-3495(00)76439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heymann B, Grubmüller H. Dynamic force spectroscopy of molecular adhesion bonds. Phys Rev Lett. 2000;84:6126–6129. doi: 10.1103/PhysRevLett.84.6126. [DOI] [PubMed] [Google Scholar]
- 28.Svetina S, Bozic B, Derganc J, Zeks B. Mechanical and functional aspects of membrane skeletons. Cell Mol Biol Lett. 2001;6:677–690. [PubMed] [Google Scholar]
- 29.Wang N, Stamenović D. Contribution of intermediate filaments to cell stiffness, stiffening, and growth. Am J Physiol Cell Physiol. 2000;279:C188–C194. doi: 10.1152/ajpcell.2000.279.1.C188. [DOI] [PubMed] [Google Scholar]
- 30.Stamenović D, Coughlin MF. The role of prestress and architecture of the cytoskeleton and deformability of cytoskeletal filaments in mechanics of adherent cells: A quantitative analysis. J Theor Biol. 1999;201:63–74. doi: 10.1006/jtbi.1999.1014. [DOI] [PubMed] [Google Scholar]
- 31.Okada Y. Tumor cell-matrix interaction: pericellular matrix degradation and metastasis. Verh Dtsch Ges Pathol. 2000;84:33–42. [PubMed] [Google Scholar]
- 32.Wang F, Gao J. [Relationship between extracellular matrix and progressive growth of malignant tumor] Zhonghua Zhongliu Zazhi. 1998;20:112–115. [PubMed] [Google Scholar]
- 33.Kitayama J, Nagawa H, Tsuno N, Osada T, Hatano K, Sunami E, Saito H, Muto T. Laminin mediates tethering and spreading of colon cancer cells in physiological shear flow. Br J Cancer. 1999;80:1927–1934. doi: 10.1038/sj.bjc.6690622. [DOI] [PMC free article] [PubMed] [Google Scholar]


