Abstract
AIM: To generate soluble single chain variable fragments (ScFv) of monoclonal antibody MC3 recognizing colorectal and gastric carcinomas.
METHODS: mRNA was isolated from the hybridoma cell line producing MC3 and the DNAs encoding variable domains of heavy and light chains (VH and VL) of the antibody were amplified separately by RT-PCR and assembled into ScFv DNA with a linker DNA.The ScFv DNA was ligated into the phagemid vector pCANTAB5E and the ligated sample was transformed into E. coli TG1.The transformed cells were infected with M13KO7 helper phage to yield recombinant phages. After two rounds of panning with gastric carcinoma cell line AGS highly expressing MC3-binding antigen, the phage clones displaying ScFv fragments of the antibody were selected by ELISA. 4 phage clones showing strong signal in ELISA were used to infect E. coli HB2151 to express soluble ScFvs. The soluble ScFvs were identified by Dot blot and Western blot, and their antigen-binding activity was assayed by ELISA. The VH and VL DNAs of the ScFv DNA derived from phage clone 19 were sequenced.
RESULTS: The VH, VL and ScFv DNAs were about 340 bp, 320 bp and 750 bp respectively. After two rounds of panning to the recombinant phages, 18 antigen-positive phage clones were selected from 30 preselected phage clones by ELISA. All the soluble ScFvs derived from the 4 out of the 18 antigen-positive phage clones were about Mr32000 and concentrated in periplasmatic space under the given culture condition. The soluble ScFvs could bind the antigen, and they shared the same binding site with MC3. The sequences of the VH and VL DNAs of the MC3 ScFv showed that the variable antibody genes belonged to the IgG1 subgroup, κ-type.
CONCLUSION: The soluble ScFv of MC3 is successfully produced, which not only provides a possible novel targeting vehicle for in vivo and in vitro study on associated cancers, but also offers the anuibody a stable genetic source.
INTRODUCTION
Progress in the use of murine monoclonal antibodies (McAbs) for the in vivo study on diagnosis and treatment of human tumors is limited by a number of factors, including poor penetration of the intact antibody molecule into the tumors, their inability to reach the tumor in sufficient quantities without significant toxicity to normal tissue, and the development of a human anti-mouse antibody response to the injected McAb[1-5]. One possible way to alter the pharmacology of antibody is via the use of smaller molecular weight antibody fragment called ScFv. ScFv molecules offer several advantages as carriers for the selective delivery of radionuclides and toxins to tumors, including rapid blood clearance, low kidney uptake, small size suitable for rapid penetration through tumor tissue and less possibility of developing antimouse antibody response[6-18]. Colorectal and gastric carcinomas are frequent causes of death of the cancers of digestive system. MC3 is a specific monoclonal antibody directed against colorectal and gastric carcinomas[19], which has a potential use for in vivo diagnosis and therapy of the corresponding carcinomas. In order to overcome the disadvantages of the intact McAb applied in vivo and to offer the antibody a stable genetic source, soluble ScFv of MC3 was generated by advanced recombinant phage antibody technique, which may provide a novel tumor-targeting vehicle for in vivo study on the diagnosis and treatment of colorectal and gastric carcinomas.
MATERIALS AND METHODS
Materials
The hybridoma cell line producing McAb MC3 (isotype IgG1, κ) was generated by the Institute of Digestive Disease, Xijing Hospital, Fourth Military Medical University, Xi'an, China[19]. Mouse ScFv DNA construction kit, phage-displayed ScFv expression and detection kits, anti-E tag antibody and pCANTAB5 Sequencing Primer Set were purchased from Pharmacia, Sweden. mRNA isolation kit, Taq DNA polymerase, T4 DNA ligase, Sfi I and Not I restriction enzymes were bought from Promega, USA. The gastric carcinoma cell line AGS highly expressing MC3-binding antigen was from ATCC, USA.Horseradish peroxidase (HRP)-labeled goat anti-mouse IgG was from Sino-American Biotechnology Company, China.
Preparation of the phage-displayed ScFv
Total RNA was extracted from guanidine thiocyanate homogenates from 5 × 106 MC3-producing hybridoma cells[20], and the mRNA was isolated from the total RNA using mRNA isolation system according to the protocal supplied by the manufacturer. Subsequently the phage-displayed ScFvs were generated using the Mouse ScFv DNA construction kit and ScFv expression kit[21-27]. The purified mRNA was transcribed into cDNA using random primers, and the VH and VL DNAs were separately amplified through PCR program 1 (30 cycles: 94 °C × 1 min, 55 °C × 2 min, 72 °C × 2 min). Gel-purified VH and VL DNAs were mixed with linker primers at an equimolar ratio and assembled inuo ScFv DNA in fill-in reaction, designed program 2 (7 cycles: 94 °C × 1 min, 63 °C × 4 min). In a second PCR reaction (same as program 1), the ScFv DNA was amplified and provided with a SfiI site at the 5'end and a Not I site at the 3'end. After digestion with restriction enzymes SfiI and Not I, the ScFv DNA was ligated into the phagemid vector pCANTAB5E, and the ligated sample was transformed into competent E. coli TG1 cells to express phage-displayed ScFv. The transformants were grown in 2 × YT medium containing ampicillin and glucose (2 × YT-AG medium) up to an OD600 = 0.5. Bacteria were infected with M13KO7 helper phage for 1 h at 37 °C with shaking. The cells were sedimented by centrifugation, and the supernatant was carefully removed and discarded. The pellet was gently resuspended in 2 × YT medium containing ampicillin and kanamycin (2 × YT-AK medium) and incubated overnight at 37 °C with shaking.The supernatant containing the recombinant phages was harvested by centrifugation and the phages were precipitated with PEG8000 and NaCl and resuspended in 2 × YT medium, filtered through a 0.45 μm filter and store at 4 °C.
Panning to select for antigen-positive phage-displayed ScFvs
Recombinant phage expressing the MC3 ScFv were selected by panning[25-27]. The AGS cells highly expressing MC3-binding antigen were grown as an attached monolayer in 25-cm2 flasks until almost confluent, washed with PBS, and fixed with 0.25% glutaraldehyde for 8 min at room temperature (RT). The fixed cells were washed with PBS and blocked with 50 g/L nonfat dry milk in PBS for 2 h at RT. The cells were washed 3 times with PBS and the PEG-precipitated recombinant phages diluted 8:7 with 100 g/L nonfat dry milk in PBS containing 0.1 g/L sodium azide were added to the fixed cells. The culture flask was shaken gently for 2 h at 37 °C and the cells were washed 20 times with PBS and 20 times with PBS containing 0.1% Tween20 (PBST). Log phase E. coli TG1 cells were incubated with bound phages for 1 h at 37 °C with shaking. Ampicillin, glucose and M13KO7 were added to the bacterial suspension, and the culture was incubated for 1 h at 37 °C with shaking. Subsequently, preparation of PEG-precipitated recombinant phage was completed as above, and the second round of panning was performed as described for the first panning step. After the second round of panning, the reinfected TG1 bacteria were plated on SOBAG plates and grown overnight at 30 °C, and single colonies were grown in 2 × YT-AG medium overnight at 30 °Cwith shaking. 40 μL of the saturated culture of single clone was infected with 400 μL of M13KO7 helper phage in 2 × YT-AG medium for 2 h at 37 °C with shaking. Cells were pelleted by centrifugation, resuspended in 400 μL of 2 × YT-AK medium and grown overnight at 37 °C with shaking. After centrifugation, phage supernatants were harvested and screened by ELISA.
Detection of phage-displayed MC3 ScFv by ELISA
Phage supernatants obtained from single recombinant cell clones were screened for binding to the AGS cells by ELISA[25,26,28-33]. The AGS cells were grown in 96-well plates, fixed and blocked as described in the panning step. Phage supernatants obtained from single recombinant cell clones were diluted 4:1 with 20 g/L nonfat dry milk in PBS and incubated for 10 min at RT, then added to the cell-grown wells (100 μL/well) and shaken gently for 1.5 h at RT. The cells were washed 6 times with PBST, and the bound phages were detected by incubation for 1 h at RT with 1:5000 diluted HRP-labeled mouse McAb directed against major coat protein (gene 8 protein) of M13 (100 μL/well). Plates were washed 6 times, ABTS substrate was added and incubated for 40 min in the dark at RT with shaking, and the A value was measured at 405 nm (A405). M13KO7 helper phage served as a negative control, and the phage antibodies were regarded as antigen-positive when A405 was at least 2 × higher compared to the negative control.
Expression of soluble MC3 ScFv
Log-phase E. coli HB2151 cells were infected separately with phage clones12, 19, 23 and 30 (which previously showed strong signal in phage ELISA, clone 19 showed the strongest signal) for 30 min at 37 °C with shaking and the infected cells were plated on SOBAG plates containing 100 mg/L of nalidixic acid. Single colonies were grown in 5 mL of SB-AG medium overnight at 30 °C with shaking and diluted with 50 mL of fresh SB-AG medium for further incubation for 1 h at 30 °C with shaking. After centrifugation, the sedimented cells were resuspended in 50 mL freshly prepared SB-AI (I: IPTG, 1 mmol/L) and incubated for 5 h at 30 °C with shaking to induce the expression of soluble ScFv. Bacterial culture was divided into two separate centrifuge tubes and centrifuged and supernatants (extracellular fraction) were stored at -20 °C until use. One of the two cell pellets was resuspended in 0.5 mL of ice-cold 1 × TES (0.2 mol/L Tris-HCl, pH8.0, 0.5 mmol/L EDTA, 0.5 mol/L sucrose) followed by adding 0.75 mL of 1/5 × TES. After incubation for 30 min on ice, the supernatant containing the periplasmatic fraction was centrifugated and stored at -20 °C. Whole-cell extract was prepared by resuspending the second pellet in 0.5 mL PBS and boiling for 5 min following centrifugation.
Detection of soluble ScFv by Dot blot
Extracellular fraction and its concentrate precipitated by ice-cold 200 g/L trichloroacetic acid (TCA) (to 1/5 of the original volume), periplasmatic extract diluted 1:4 with PBS, and whole-cell extracts were spotted onto the nitrocellulose membrane separately (2 μL/spot). The membrane was blocked for 2 h at RT with PBS containing 100 g/L nonfat dry milk (blocking buffer, BB)followed by incubation for 1 h at RT with anti-E tag antibody (directed against the E tag-peptide fused to the VL region of the ScFv) diluted to a final concentration of 8 mg/L with an equal volume mixture of PBS and BB containing 0.05% Tween20 (PBS/BBT). After being washed once with PBS containing 0.05% Tween20 and 4 times with PBS and followed by a 5 min soak in PBS, the membrane was incubated for 1 h at RT with HRP-labeled goat anti-mouse IgG diluted 1:150 with PBS/BBT, and washed as above and developed using diaminobenzidine (DAB) substrate.
Detection of soluble ScFv by Western blot
Routine method was applied[34,35]. Periplasmatic extract diluted 1:4 with PBS and TCA-concentrated extracellular fraction were loaded into the wells (10 μL/well) of 120 g/L SDS polyacrylamide gels and followed by electrophoresis, and the fractionated proteins were then transferred onto a nitrocellulose membrane. The membrane was blocked and developed as discribed in Dot blot.
ELISA for assay of the reactivity with antigen of soluble ScFv
The AGS cells were grown in 96-well plates, fixed and blocked as described in phage ELISA followed by incubation with 50 μL of periplasmatic and extracellular fraction for 1 at RT. After washed 6 times with PBST, anti-E tag antibody diluted 1:1000 with PBS/BBT was added (50 μL/well) and incubated as above. The plate was washed and 50 μL of HRP-labeled goat anti-mouse IgG diluted 1:1000 with PBS/BBT was added and incubated as above. After being washed, ABTS substrate was added and A405 was measured. PBS was as a negative control, and the ScFv was considered as a binder when the ELISA response was at least 2 × higher compared to the negative control.
ELISA for assay of the competition of soluble ScFv with MC3 in antigen-binding
It was done as described in references[36-38].The AGS cells were grown in 96-well plates, fixed and blocked as described in phage ELISA followed by incubation with 50 μL of periplasmatic fraction for 1 h at RT, with PBS as a negative control. After washed 6 times with PBST, MC3 was added to the plate (50 μL/well) at a final concentration of 20 mg/L, and incubated for 1 h at RT. After washed as above, HRP-labeled goat anti-mouse IgG diluted 1:1000 was added (50 μL/well) and incubated and washed as above. Then TMB substrate was added and A450 was measured. Binding of MC3 with the cells was inhibited by the soluble ScFv, which was described as the inhibition rate: Inhibition rate (%) = 1- (A450 of the tested sample/A450 of the control) × 100%.
DNA Sequencing
The phagemid derived from phage antibody clone 19 was used for DNA sequencing of VH and VL DNAs of the ScFv DNA, based on the dideoxy method, with the primers taken from the pCANTAB5 Sequencing Primer Set.
RESULTS
Cloning of MC3 ScFv DNA and Screening of phage-dispalyed ScFv
The amplified VH, VL and ScFv DNAs were about 340 bp, 320 bp and 750 bp respectively. After two rounds of panning to the recombinant phages, 18 antigen-positive phage clones were selected from 30 preselected phage clones by ELISA. 4 out of the 18 clones (clones 12, 19, 23 and 30), which showed strong signal (clone 19 showed the strongest signal), were selected for expression of soluble MC3 ScFv in E. coli HB2151.
Identification of soluble MC3 ScFv
After expression of soluble ScFv in E. coli HB2151, extracellular, periplasmatic, and whole-cell fractions were checked for the presence and reactivity of ScFv by Dot blot, Western blot and ELISA. In a Dot blot, no visible signal was achieved for the whole-cell extracts, and only weak signal was shown for the extracellular extracts, while a strong signal for periplasmatic and TCA-precipitated extracellular extracts. Based on the results in the Dot blot, Western blot analysis was performed only with periplasmatic and TCA-precipitated extracellular extracts, and ELISA for reactivity of soluble ScFv with antigen was performed only with periplasmatic and extracellular fractions. Western blot showed that in both periplasmatic and TCA-precipitated extracellular extracts, a distinct band of Mr32000, lying within the expected molecular weight range of soluble ScFv, was visualized, and the periplasmatic fractions showed stronger reactivity in comparison to the TCA-precipitated extracellular extracts (Figure 1 and Figure 2). The results displayed in Dot blot and Western blot indicated that most of soluble ScFv was secreted into the periplasm under the given culture condition.
Figure 1.
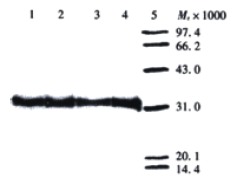
Detection of soluble MC3 ScFv in periplasmatic extract by Western blot. Lanes 1-4: periplasmatic extracts derived from phage clones 12, 19, 23 and 30 respectively, diluted 1:4 with PBS; Lane 5: low molecular weight protein marker.
Figure 2.
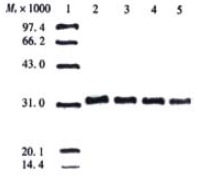
Detection of soluble MC3 ScFv in TCA-precipitated extracellular fraction by Western blot. Lane 1: low molecular weight protein marker; Lane 2-5: TCA-precipitated extracellular fractions (to 1/5 of the original volume) derived from the phage clones 12, 19, 23 and 30 respectively.
ELISA showed that MC3 ScFv secreted into the periplasm could react with AGS cells expressing MC3-binding antigen at a high level, whereas no significant binding signal was obtained in extracellular extracts. The periplasmatic fraction containing soluble ScFv derived from the phage clone 19 showed the strongest signal in ELISA.
Based on the above result in ELISA, the competition ELISA was performed only with periplasmatic fraction, and the result showed that all the soluble ScFv-containing periplasmatic extracts derived from the clones 12, 19, 23 and 30 could inhibit the binding of MC3 with its corresponding antigen, and the inhibition rates were 36.9%, 41.2%, 31.9% and 33.8% respectively, which indicated that the soluble ScFvs shared the same antigen binding site with McAb MC3.
DNA Sequence analysis
The phagemid derived from clone 19 was used for DNA sequencing, and the result showed that the VH and VL DNAs of MC3 ScFv DNA were 336 bp and 312 bp respectively, and they were identified as variable antibody genes belonging to the IgG1 subgroup, κ-type. The detail sequence of the VH and VL DNAs were listed as follows.
VH DNA of MC3 ScFv (336 bp)
5'-ATGGCCCAGGTGAAGCTGCAGCAG TCAGGACCTGAACTGAAGAAGCCT GGAGAGACAGTCAGGATCTCCTGC AAGGCTTCTGGATATACCTTCACA ACTGCTGGAATGCAGTGGGTGCAA AAGATGCCAGAAAGGGTTTGAAGT GGATTGGCTGGATAAACACCCACT CTGGAGTGCCAAAGTATGCAGAAG AGTTCAAGGGACGCTTTGCCTTCT CTTTGGAAACCTCTGCCAGCACTG CATATTTACAGATAAGCAACCTCA AAAATGAGGACACGGCTACGTATT TCTGTATGAGATGGGATTACGACG GGGGGTTTGCTTACTGGGGCCAA-3'
VL DNA of MC3 ScFv (312 bp)
5'-GCTTCTTTGGCTGTGTCTCTAGGG CAGAGGGCCACCATCTCCTGCAGA GCCAGCGAAAGTGTTGATAATATT GGCATTAGTTTTATGAACTGGTTC CAGCAGAAACCAGGACAGCCACCC AAACTCCTCATCTATGCTGCATCC AAGCAAGGATCCGGGGTCCCTGCC AGGTTTACTGGCAGTGGGTCTGGG ACAGATTTCAGCCTCAACATATAT CCTATGGAGGAGGATGATCCTGCA GTGTATTTCTGTCACCAAAGTAAG GAGGTTCCTTACACGTTCGGAGGG GGGACCAAGCTGGAAATAAAACGG-3'
DISCUSSION
Recent progress in antibody engineering have been directed toward the expression of antibody fragments in bacterial and phage display systems, leading to increase of various applications in biology, clinical diagnosis and therapy.The power of the phage display system to express antibody fragments offers several advantages over hybridoma technology, allowing quick, economical production and generation of antibodies with increasing affinity and specificity by mimicking affinity maturation in the normal immune system, and offering the antibody a stable genetic source which can be easily manipulated[39-51]. Antibody fragments showed less possibility of developing anti-antibody response in comparison to the intact antibodies when applied in vivo. In this study, the ScFv of murine McAb MC3 directed against colorectal and gastric carcinomas was successfully produced by advanced phage antibody technology, which offered it the above advantages of the engineering antibody. MC3 ScFv was displayed on the surface of M13 phage as a fusion protein with gene III protein (g3p) and additionally expressed in a soluble form secreted in the bacterial periplasm. Both expression forms retained their reactivity to the antigen. Because of the advantage of MC3 ScFv compared to the intact MC3 for in vivo diagnosis and therapy of the associated carcinomas, we have focused on the production of soluble ScFv.
A critical step in cloning of the ScFv DNA was the assembly of VH and VL DNAs with linker DNA. The linker primers consist of two 93-base oligonucleotides which are complementary to each other and have homology with the 3'end of the VH gene and the 5'end of the VL gene. 24 bases on either end of the linkers are complementary to the ends of the VH and the VL. The central 45 bases of the linkers encode the flexible (Gly-4Ser)a-3 linker which joins the VH and the VL to form a ScFv fragment. In the assembly and fill-in reactions, an exact quantification of purified VH and VL products and linker DNA was very essential. Even slight deviations of the equimolar ratio VH:VL:linker lead to either no visible ScFv product or to the formation of VH-linker or VL-linker dimers, apparently 450 bp in size.
McAb MC3 was prepared by immunizing mice with human colorectal carcinoma cells, but immunohistochemical detection indicated that the MC3-binding antigen was highly expressed in both colorectal and gastric carcinomas[19]. Because no purified antigen was available so far, the recombinant phages were selected in a panning reaction with AGS cells highly expressing MC3-binding antigen. After two rounds of panning, 18 antigen-positive phage clones were selected by ELISA, and 4 clones showing strong signal were used to express soluble ScFv. Clone 19, which showed the strongest signal in ELISA, was used for DNA sequencing and identified as variable antibody genes belonging to the IgG1 subgroup, κ-type.
The E. coli strain HB2151 was used to express soluble MC3 ScFv because in this strain the amber stop codon interposed between the E-tag sequence and g3p gene prevented the expression of a ScFv-g3p fusion protein. The soluble ScFv is secreted into the periplasm via the g3p leader sequence, where it is folded in its native form. Dot blot, Western blot, and ELISA verified that soluble ScFv was concentrated in periplasmatic fraction under the given culture condition. Location of functional ScFv in the periplasm is preferred for large-scale preparation because of smaller volume in comparison to the extracellular fraction. The soluble ScFv-containing extracts (such as periplasmatic fraction) may be used directly for some immunoassays. Alternatively, the soluble ScFv may be purified from E. coli proteins if the components of the bacterial extract interfere with the immunoassay or/and when the ScFv is for in vivo use. In conclusion, the preparation of soluble ScFv of MC3 may provide a promising targeting vehicle for in vitro and potential in vivo imaging and therapeutic applications to colorectal and gastric carcinomas, and also offer the antibody a stable genetic source.
Footnotes
Edited by Wu XN
References
- 1.DeNardo SJ, Kramer EL, O'Donnell RT, Richman CM, Salako QA, Shen S, Noz M, Glenn SD, Ceriani RL, DeNardo GL. Radioimmunotherapy for breast cancer using indium-111/yttrium-90 BrE-3: results of a phase I clinical trial. J Nucl Med. 1997;38:1180–1185. [PubMed] [Google Scholar]
- 2.Alvarez RD, Partridge EE, Khazaeli MB, Plott G, Austin M, Kilgore L, Russell CD, Liu T, Grizzle WE, Schlom J, et al. Intraperitoneal radioimmunotherapy of ovarian cancer with 177Lu-CC49: A phase I/II study. Gynecol Oncol. 1997;65:94–101. doi: 10.1006/gyno.1996.4577. [DOI] [PubMed] [Google Scholar]
- 3.Tempero M, Leichner P, Dalrymple G, Harrison K, Augustine S, Schlam J, Anderson J, Wisecarver J, Colcher D. High-dose therapy with iodine-131-labeled monoclonal antibody CC49 in patients with gastrointestinal cancers: A phase I trial. J Clin Oncol. 1997;15:1518–1528. doi: 10.1200/JCO.1997.15.4.1518. [DOI] [PubMed] [Google Scholar]
- 4.Buchsbaum D, Khazaeli MB, Liu T, Bright S, Richardson K, Jones M, Meredith R. Fractionated radioimmunotherapy of human colon carcinoma xenografts with 131I-labeled monoclonal antibody CC49. Cancer Res. 1995;55:5881s–5887s. [PubMed] [Google Scholar]
- 5.Liu HF, Liu WW, Fang DC, Men RP. Expression and significance of proapoptotic gene Bax in gastric carcinoma. World J Gastroenterol. 1999;5:15–17. doi: 10.3748/wjg.v5.i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goel A, Augustine S, Baranowska-Kortylewicz J, Colcher D, Booth BJ, Pavlinkova G, Tempero M, Batra SK. Single-Dose versus fractionated radioimmunotherapy of human colon carcinoma xenografts using 131I-labeled multivalent CC49 single-chain fvs. Clin Cancer Res. 2001;7:175–184. [PubMed] [Google Scholar]
- 7.Goel A, Baranowska-Kortylewicz J, Hinrichs SH, Wisecarver J, Pavlinkova G, Augustine S, Colcher D, Booth BJ, Batra SK. 99mTc-labeled divalent and tetravalent CC49 single-chain Fv's: novel imaging agents for rapid in vivo localization of human colon carcinoma. J Nucl Med. 2001;42:1519–1527. [PubMed] [Google Scholar]
- 8.Khare PD, Shao-Xi L, Kuroki M, Hirose Y, Arakawa F, Nakamura K, Tomita Y, Kuroki M. Specifically targeted killing of carcinoembryonic antigen (CEA)-expressing cells by a retroviral vector displaying single-chain variable fragmented antibody to CEA and carrying the gene for inducible nitric oxide synthase. Cancer Res. 2001;61:370–375. [PubMed] [Google Scholar]
- 9.Schmidt M, McWatters A, White RA, Groner B, Wels W, Fan Z, Bast RC. Synergistic interaction between an anti-p185HER-2 pseudomonas exotoxin fusion protein [scFv (FRP5)-ETA] and ionizing radiation for inhibiting growth of ovarian cancer cells that overexpress HER-2. Gynecol Oncol. 2001;80:145–155. doi: 10.1006/gyno.2000.6040. [DOI] [PubMed] [Google Scholar]
- 10.Mayer A, Tsiompanou E, O'Malley D, Boxer GM, Bhatia J, Flynn AA, Chester KA, Davidson BR, Lewis AA, Winslet MC, et al. Radioimmunoguided surgery in colorectal cancer using a genetically engineered anti-CEA single-chain Fv antibody. Clin Cancer Res. 2000;6:1711–1719. [PubMed] [Google Scholar]
- 11.Barth S, Huhn M, Matthey B, Tawadros S, Schnell R, Schinköthe T, Diehl V, Engert A. Ki-4 (scFv)-ETA', a new recombinant anti-CD30 immunotoxin with highly specific cytotoxic activity against disseminated Hodgkin tumors in SCID mice. Blood. 2000;95:3909–3914. [PubMed] [Google Scholar]
- 12.Barth S, Huhn M, Matthey B, Schnell R, Tawadros S, Schinköthe T, Lorenzen J, Diehl V, Engert A. Recombinant anti-CD25 immunotoxin RFT5 (SCFV)-ETA' demonstrates successful elimination of disseminated human Hodgkin lymphoma in SCID mice. Int J Cancer. 2000;86:718–724. doi: 10.1002/(sici)1097-0215(20000601)86:5<718::aid-ijc18>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 13.Onda M, Olafsen T, Tsutsumi Y, Bruland OS, Pastan I. Cytotoxicity of antiosteosarcoma recombinant immunotoxins composed of TP-3 Fv fragments and a truncated Pseudomonas exotoxin A. J immunother. 2001;24:144–150. [PubMed] [Google Scholar]
- 14.Hoffmann P, Mueller N, Shively JE, Fleischer B, Neumaier M. Fusion proteins of B7.1 and a carcinoembryonic antigen (CEA)-specific antibody fragment opsonize CEA-expressing tumor cells and coactivate T-cell immunity. Int J Cancer. 2001;92:725–732. doi: 10.1002/1097-0215(20010601)92:5<725::aid-ijc1252>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 15.Roovers RC, van der Linden E, de Bruïne AP, Arends JW, Hoogenboom HR. In vitro characterisation of a monovalent and bivalent form of a fully human anti Ep-CAM phage antibody. Cancer immunol immunother. 2001;50:51–59. doi: 10.1007/s002620000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mersmann M, Schmidt A, Rippmann JF, Wüest T, Brocks B, Rettig WJ, Garin-Chesa P, Pfizenmaier K, Moosmayer D. Human antibody derivatives against the fibroblast activation protein for tumor stroma targeting of carcinomas. Int J Cancer. 2001;92:240–248. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1170>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 17.Kashentseva EA, Seki T, Curiel DT, Dmitriev IP. Adenovirus targeting to c-erbB-2 oncoprotein by single-chain antibody fused to trimeric form of adenovirus receptor ectodomain. Cancer Res. 2002;62:609–616. [PubMed] [Google Scholar]
- 18.Tur MK, Sasse S, Stöcker M, Djabelkhir K, Huhn M, Matthey B, Gottstein C, Pfitzner T, Engert A, Barth S. An anti-GD2 single chain Fv selected by phage display and fused to Pseudomonas exotoxin A develops specific cytotoxic activity against neuroblastoma derived cell lines. Int J Mol Med. 2001;8:579–584. doi: 10.3892/ijmm.8.5.579. [DOI] [PubMed] [Google Scholar]
- 19.Fan DM, Zhang XY, Chen XT, Mou ZX, Chen BJ, Qiao TD, Yang HB, Yang ZD. Establishment of four monoclonal antibodies to colonic cancer cells and immunohistochemical study on their corresponding antigens. Disi Junyi Daxue Xuebao. 1988;9:74–77. [Google Scholar]
- 20.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 21.McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990;348:552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- 22.Marks JD, Hoogenboom HR, Bonnert TP, McCafferty J, Griffiths AD, Winter G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991;222:581–597. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- 23.Winter G, Milstein C. Man-made antibodies. Nature. 1991;349:293–299. doi: 10.1038/349293a0. [DOI] [PubMed] [Google Scholar]
- 24.Hoogenboom HR, Griffiths AD, Johnson KS, Chiswell DJ, Hudson P, Winter G. Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains. Nucleic Acids Res. 1991;19:4133–4137. doi: 10.1093/nar/19.15.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He F, Chen B, Nie Y, Han Z, Qiao T, Fan D. [Production of phage-displayed single chain variable fragments of monoclonal antibody MGb1] Zhonghua Neike Zazhi. 2000;39:585–587. [PubMed] [Google Scholar]
- 26.He F, Nie Y, Han Z. [Production of phage-displayed anti-idiotypic antibody single chain variable fragments to MG7 monoclonal antibody directed against gastric carcinoma] Zhonghua Yixue Zazhi. 2001;81:33–36. [PubMed] [Google Scholar]
- 27.Schlebusch H, Reinartz S, Kaiser R, Grünn U, Wagner U. Production of a single-chain fragment of the murine anti-idiotypic antibody ACA125 as phage-displayed and soluble antibody by recombinant phage antibody technique. Hybridoma. 1997;16:47–52. doi: 10.1089/hyb.1997.16.47. [DOI] [PubMed] [Google Scholar]
- 28.George J, Meroni PL, Gilburd B, Raschi E, Harats D, Shoenfeld Y. Anti-endothelial cell antibodies in patients with coronary atherosclerosis. Immunol Lett. 2000;73:23–27. doi: 10.1016/s0165-2478(00)00192-9. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, Gurlo T, von Grafenstein H. Cell-ELISA using beta-galactosidase conjugated antibodies. J immunol Methods. 2000;234:P153–P167. doi: 10.1016/s0022-1759(99)00216-1. [DOI] [PubMed] [Google Scholar]
- 30.Devereux G, Hall AM, Barker RN. Measurement of T-helper cytokines secreted by cord blood mononuclear cells in response to allergens. J immunol Methods. 2000;234:13–22. doi: 10.1016/s0022-1759(99)00185-4. [DOI] [PubMed] [Google Scholar]
- 31.Salih AM, Nixon NB, Dawes PT, Mattey DL. Antibodies to neuroblastoma cells in rheumatoid arthritis: A potential marker for neuropathy. Clin Exp Rheumatol. 2000;18:23–30. [PubMed] [Google Scholar]
- 32.Pereira S, Maruyama H, Siegel D, Van Belle P, Elder D, Curtis P, Herlyn D. A model system for detection and isolation of a tumor cell surface antigen using antibody phage display. J immunol Methods. 1997;203:11–24. doi: 10.1016/s0022-1759(97)00005-7. [DOI] [PubMed] [Google Scholar]
- 33.Heitner T, Moor A, Garrison JL, Marks C, Hasan T, Marks JD. Selection of cell binding and internalizing epidermal growth factor receptor antibodies from a phage display library. J immunol Methods. 2001;248:17–30. doi: 10.1016/s0022-1759(00)00340-9. [DOI] [PubMed] [Google Scholar]
- 34.Kupsch JM, Tidman NH, Kang NV, Truman H, Hamilton S, Patel N, Newton Bishop JA, Leigh IM, Crowe JS. Isolation of human tumor-specific antibodies by selection of an antibody phage library on melanoma cells. Clin Cancer Res. 1999;5:925–931. [PubMed] [Google Scholar]
- 35.Tordsson JM, Ohlsson LG, Abrahmsén LB, Karlström PJ, Lando PA, Brodin TN. Phage-selected primate antibodies fused to superantigens for immunotherapy of malignant melanoma. Cancer immunol immunother. 2000;48:691–702. doi: 10.1007/s002620050018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang CD, Chen YL, Wu T, Liu YR. Association between lowe expression of somatostatin receptor II gene and lymphoid metastasis in patients with gastric cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:864–866. [Google Scholar]
- 37.Han FC, Yan XJ, Hou Yu, Xiao LY, Guo YH, Su CZ. Gold immunochromatographic assay for anti-Helicobacter pylori antibody. Shijie Huaren Xiaohua Zazhi. 1999;7:743–746. [Google Scholar]
- 38.Cui DX, Yan XJ, Zhang L, Zhao JR, Jiang M, Guo YH. Screening and its clinical significance of 6 fragments of highly expressing genes in gastric cancer and precancerous mucosa. Shijie Huaren Xiaohua Zazhi. 1999;7:770–773. [Google Scholar]
- 39.Winter G, Griffiths AD, Hawkins RE, Hoogenboom HR. Making antibodies by phage display technology. Annu Rev immunol. 1994;12:433–455. doi: 10.1146/annurev.iy.12.040194.002245. [DOI] [PubMed] [Google Scholar]
- 40.Chowdhury PS, Pastan I. Improving antibody affinity by mimicking somatic hypermutation in vitro. Nat Biotechnol. 1999;17:568–572. doi: 10.1038/9872. [DOI] [PubMed] [Google Scholar]
- 41.Chowdhury PS, Viner JL, Beers R, Pastan I. Isolation of a high-affinity stable single-chain Fv specific for mesothelin from DNA-immunized mice by phage display and construction of a recombinant immunotoxin with anti-tumor activity. Proc Natl Acad Sci USA. 1998;95:669–674. doi: 10.1073/pnas.95.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mao S, Gao C, Lo CH, Wirsching P, Wong CH, Janda KD. Phage-display library selection of high-affinity human single-chain antibodies to tumor-associated carbohydrate antigens sialyl Lewisx and Lewisx. Proc Natl Acad Sci USA. 1999;96:6953–6958. doi: 10.1073/pnas.96.12.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roovers RC, van der Linden E, de Bruïne AP, Arends JW, Hoogenboom HR. Identification of colon tumour-associated antigens by phage antibody selections on primary colorectal carcinoma. Eur J Cancer. 2001;37:542–549. doi: 10.1016/s0959-8049(00)00432-9. [DOI] [PubMed] [Google Scholar]
- 44.Palmer DB, Crompton T, Marandi MB, George AJ, Ritter MA. Intrathymic function of the human cortical epithelial cell surface antigen gp200-MR6: single-chain antibodies to evolutionarily conserved determinants disrupt mouse thymus development. Immunology. 1999;96:236–245. doi: 10.1046/j.1365-2567.1999.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He J, Zhou G, Liu KD, Qin XY. Construction and preliminary screening of a human phage single-chain antibody library associated with gastric cancer. J Surg Res. 2002;102:150–155. doi: 10.1006/jsre.2001.6298. [DOI] [PubMed] [Google Scholar]
- 46.Graus YF, Verschuuren JJ, Degenhardt A, van Breda Vriesman PJ, De Baets MH, Posner JB, Burton DR, Dalmau J. Selection of recombinant anti-HuD Fab fragments from a phage display antibody library of a lung cancer patient with paraneoplastic encephalomyelitis. J Neuroimmunol. 1998;82:200–209. doi: 10.1016/s0165-5728(97)00199-9. [DOI] [PubMed] [Google Scholar]
- 47.Figini M, Obici L, Mezzanzanica D, Griffiths A, Colnaghi MI, Winter G, Canevari S. Panning phage antibody libraries on cells: isolation of human Fab fragments against ovarian carcinoma using guided selection. Cancer Res. 1998;58:991–996. [PubMed] [Google Scholar]
- 48.Watkins JD, Beuerlein G, Pecht G, McFadden PR, Glaser SM, Huse WD. Determination of the relative affinities of antibody fragments expressed in Escherichia coli by enzyme-linked immunosorbent assay. Anal Biochem. 1997;253:37–45. doi: 10.1006/abio.1997.2335. [DOI] [PubMed] [Google Scholar]
- 49.Tordsson J, Abrahmsén L, Kalland T, Ljung C, Ingvar C, Brodin T. Efficient selection of scFv antibody phage by adsorption to in situ expressed antigens in tissue sections. J immunol Methods. 1997;210:11–23. doi: 10.1016/s0022-1759(97)00165-8. [DOI] [PubMed] [Google Scholar]
- 50.Govorko D, Cohen G, Solomon B. Single-chain antibody against the common epitope of mutant p53: isolation and intracytosolic expression in mammalian cells. J immunol Methods. 2001;258:169–181. doi: 10.1016/s0022-1759(01)00495-1. [DOI] [PubMed] [Google Scholar]
- 51.Shadidi M, Sioud M. An anti-leukemic single chain Fv antibody selected from a synthetic human phage antibody library. Biochem Biophys Res Commun. 2001;280:548–552. doi: 10.1006/bbrc.2000.4158. [DOI] [PubMed] [Google Scholar]


