Abstract
AIM: To study the dose-dependent of progesterone (P) effect and the interaction between the oxytocin (OT) and P on gastrointestinal motility.
METHODS: In order to monitor the gastric emptying and intestinal transit, the SD male rats were intubated via a catheter with normal saline (3 mL/kg) containing Na251CrO4 (0.5 μCi/mL) and 10% charcoal. OT was dissolved into normal saline and P was dissolved into 75% alcohol.
RESULTS: Low does of P (1 mg/kg, i.p.) enhanced the gastric emptying (75% ± 3%, P < 0.05) and high dose of P (5 mg/kg, i.p.) inhibit it (42% ± 11.2%, P < 0.01). P (1 mg/kg) increased the intestinal transit (4.2 ± 0.3, P < 0.05) while the higher dose (10-20 mg/kg) had no effect. OT (0.8 mg/kg, i.p.) inhibited the gastric emptying (23.5% ± 9.8%, P < 0.01). The inhibitory effects of P (20 mg/kg) (32% ± 9.7%, P < 0.05) and OT (0.8 mg/kg) on gastric emptying enhanced each other when the two chemicals were administrated simultaneously (17% ± 9.4%, P < 0.01).
CONCLUSION: Low dose of P increased GI motility while high dose of P decreased it. During the later period of pregnancy, elevated plasma level of OT may also participate in the gastrointestinal inhibition.
INTRODUCTION
Gastrointestinal motility is disturbed in pregnant women because of the changes of some hormone levels in the plasma, such as estrogen (E), progesterone (P), and other gastrointestinal hormones[1,2]. Steroids, especially P and E, participate the regulation of gastrointestinal motility[3-5] and are involved in the pathogenesis of some functional disorders in the gut[6]. We reported that administration of estrogen inhibited the rat gastric emptying and intestinal transit while P enhanced the gut motility[7]. The effects of E were testified by other scholars while the inhibitory effect of P was observed[8]. Oxytocin (OT) is also a hormone related to pregnancy, and its plasma concentration is higher during the late phase[9,10]. It is reported that OT inhibited gastric motility and secretion[11-13]. We recently found that administration of OT inhibited the rat gastrointestinal motility through inducing the release of cholecystokinin (CCK) (unpublished data). Progesterone affects the OT effect on uterine smooth muscle[14], but it is unknown if P and OT interact with each other on the effect of gut motility. In this study, three experiments were conducted to investigate the dose-dependent effect of P and the interaction between P and OT on gastric emptying and intestinal transit in male rats.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats weighing 250-350 g were housed at 22 °C and light-controlled environment (6 am-8 pm) and fed with rat chow. Tap water was given ad libitum.
Experiment protocol
Experiment 1. The male rats were randomly divided into four groups (n = 6 each). They were fasted but with access to water for 24 h before use. On the day of experiment, the four groups of animals were injected intraperitoneally (i.p.) with 0, 0.3, 1.0 and 3.0 mg/kg of P, respectively 15 min before gastric intubation of a non-nutrient liquid meal. Fifteen min after the administration of the liquid meal, the rats were decapitated and the gastric emptying and intestinal transit were measured.
Experiment 2. The procedure was identical to that in experiment 1, except the dose of P was 0, 5, 10 and 20 mg/kg, respectively in the four groups of animals (n = 7).
Experiment 3. Male rats were divided into four groups with 7 animals each and fasted for 24 h before use. Fifteen min before gastric intubation of a non-nutrient liquid meal, the animals were injected i.p. with the normal saline and 75% alcohol (1 mL/kg) in group 1, OT (0.8 mg/kg) and 75% alcohol (1 mL/kg) in group 2, normal saline (1 mL/kg) and P (20 mg/kg) in group 3, and OT (0.8 mg/kg) and P (20 mg/kg) in group 4. OT was dissolved in the normal saline and P was dissolved in the alcohol (75%).
Measurement of gastric emptying and GI transit
Gastric emptying and GI transit were measured as described by Doong et al[15]. Rats were intubated via a catheter (PE-205, ID 1.67 mm, OD 2.42 mm, Clay-Adam, Parsippany, NJ, USA) with normal saline (3 mL/kg) containing Na251CrO4 (0.5 μCi/mL) and 10% charcoal. The test meal was continuously stirred before intubation. Air (0.5 mL) was used to flush the residual charcoal suspension in the catheter into the rats. Fifteen min later, the rats were decapitated and the stomach and attached small intestine immediately exposed by laparotomy. After ligation of the esophagogastric, gastroduodenal, and ileocecal junctions, the whole stomach and small intestine were carefully removed and placed on a wooden board to observe the leading edge of the charcoal in the intestine. The small intestine was then divided into 10 equal segments and the radioactivity in the stomach and each segment of small intestine was measured in an automatic gamma counter (1470 Vizard, Pharmacia, Turku, Finland). Gastric emptying was measured by determining the amount of labeled chromium contained in the small intestine 15 min after intubation, expressed as a percentage of the amount given. Intestinal transit was assessed by calculating the geometric center of distribution of the radioactivity with the 10 segments by summation of the radioactivity in each segment multiplied by the segment number.
Statistical analysis
The data were expressed as the mean value ± SEM. The treatment means were tested for homogeneity using one-way analysis of variance, and the significance of any difference between means was tested using Duncan's multiple range test. A difference between two means was considered to be statistically significant when P was less than 0.05.
RESULTS
Low dose of P on gastric emptying and intestinal transit
Gastric emptying was enhanced slightly after P (0.3 mg/kg) was injected i.p., and was elevated significantly by administration of P (1 mg/kg) in the same way (P < 0.05, n = 6). P (3 mg/kg) inhibited the gastric emptying, although the difference was not significant (Figure 1A). Administration of P, 1 mg/kg or 3 mg/kg, increased the intestinal transit significantly (P < 0.05, n = 6), but the dose of 0.3 mg/kg did not influence it (Figure 1B).
Figure 1.
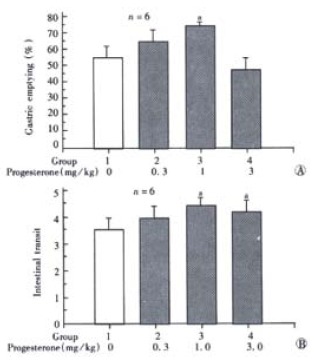
A: Effect of low dose of progesterone (i.p.) on gastric emptying in male rats. aP < 0.05 vs group 1; B: Effect of low dose of progesterone (i.p.) on intestinal transit in male rats. aP < 0.05 vs group 1
High dose of P on gastric emptying and intestinal transit
Administration of P (5-20 mg/kg, i.p.) dose dependently decreased the gastric emptying (Figure 2A). In this experiment, only the lowest dose of P (5 mg/kg, i.p.) significantly increased the intestinal transit, P with much higher dose (10 and 20 mg/kg) did not influence the intestinal transit (Figure 2B).
Figure 2.
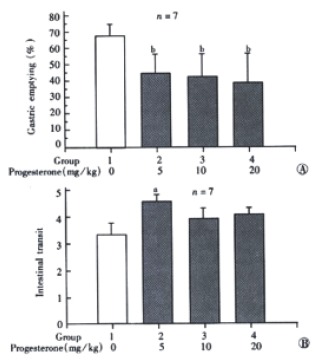
A: Effect of high dose of progesterone (i.p.) on gastric emptying in male rats. bP < 0.01 vs group 1; B: Effect of high dose of progesterone (i.p.) on intestinal transit in male rats. aP < 0.05 vs group 1
Interaction of OT and P on gastric emptying and intestinal transit
Compared with control, both OT (0.8 mg/kg, i.p.) and P (20 mg/kg, i.p.) significantly inhibited the gastric emptying. When both OT and P were administrated, the gastric emptying was further inhibited (Figure 3). Administration of OT (0.8 mg/kg) and/or P (20 mg/kg) did not influence the intestinal transit.
Figure 3.
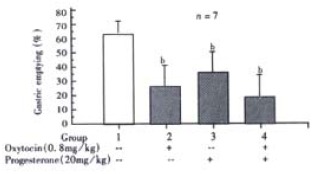
Interaction of progesterone and oxytocin (i.p.) on gastric emptying in male rats. bP < 0.01 vs group 1
DISCUSSION
It is known that the liquid emptying slows as the calorie content increases. For nutrient liquid that stimulates intestinal feedback inhibition, there is an initial rapid emptying phase followed by a linear phase. In this study, we used non-nutrient liquid meal, and therefore the emptying rate was rapid, representing the initial rapid emptying of nutrient liquid. Because the volume in the initial rapid emptying contributes greatly to the nutrient liquid being half emptied, it is reasonable to assume that the changes of liquid emptying caused by the hormones from our study could influence the half-emptying time of nutrient liquid.
The result in this study indicated that the lower dose of P enhanced the gastrointestinal motility while the higher dose inhibited it. This data could explain why different effects of P on gastric emptying and intestinal transit were observed. Different routes of administration could make the chemicals absorbed at different velocity, thus making the plasma level of this hormone different. Besides the central nervous system, other organs such as the uterus also secrete OT into blood[16,17]. In the pregnant women with or without pain, the OT concentration in plasma is much higher than that in the cerebrospinal fluid, so the higher level of OT during the late period of pregnancy may mainly come from the peripheral organs[18]. Although there is no direct evidence that there exists OT receptor in the gut, our data indicated that OT could inhibit the gastric emptying.
Disturbed gastrointestinal motility was observed in the pregnant women. In the early period, the high level of estrogen and progesterone may contribute to this pathophysiological phenomenon. During the late period, the OT level in plasma was elevated[9,10]. In the present study, when administrated simultaneously, the inhibitory effects of P and OT on gastrointestinal were strengthened each other. Thus, OT may also participate in the inhibition of gastrointestinal motility during the late period of pregnancy.
In the uterus, The OT receptor is upregulated in the secretary phase during the oestrous cycle and downregulated during the early pregnancy[19-21]. Two levels of interaction between OT and steroid hormones were reported, the genomic and non - genomic mechanisms[22]. Estrogen induces the OT receptor (OTR) mRNA expression, and then increases the OTR density on the membrane of the uterus smooth muscle[22-35]. This effect of estrogen was also observed in the central nervous system[36]. The effect of P on OTR expression is controversial. It has been suggested that, through the nuclear receptors, P inhibits the OTR mRNA expression, and makes the target cell less sensitive to OT stimulation[25,26,37-43]. Giacalone also reported that, RU-486, a P receptor antagonist, increases the incidences of tachysystole, hypertonia and fetal heart rat abnormality[44]. However, Al-Matubsi reported that pretreatment with P enhanced the OTR mRNA expression in the ovine corpus luteum[45]. OT excites the uterus smooth muscle mainly through two mechanisms: binding the membrane receptor directly and increasing the secretion of prostaglandin (PG)[46-48]. P enhanced uterine PGF 2α secretion in response to OT in ovaritctomized sows[49].
Direct interaction, but not through the regulation of RNA expression, between P and OT was also reported. Grazzini et al[14,50] found that P specially binded to the OTR and inhibited its ligand binding and signal functions. Burger also reported that P inhibited the calcium signaling evoked by ligand stimulation of G protein-coupled receptor. This kind of P effect was fast, reversible and was not prevented by cycloheximide, indicating a non-genomic nature[51].
In this experiment, administration of P (20 mg/kg) and OT (0.8 mg/kg) in combination inhibited the gastric emptying, and the gastric motility was further attenuated. Because this effect was found within 30 min after the chemical treatment, it is clear that the nature of the potentiation between P and OT is non-genomic.
In conclusion, the data indicated that high dose of P inhibited the GI motility while low dose of P enhanced it. During pregnancy, especially during the later period, high levels of P and OT enhance each other to disturb the gastrointestinal motility.
Footnotes
Edited by Ma JY
Supported by Chinese Developing Funds (provided by Taiwan) and Scientific Initiating Grants of Shandong University
References
- 1.Chiloiro M, Darconza G, Piccioli E, De Carne M, Clemente C, Riezzo G. Gastric emptying and orocecal transit time in pregnancy. J Gastroenterol. 2001;36:538–543. doi: 10.1007/s005350170056. [DOI] [PubMed] [Google Scholar]
- 2.Chang FY, Lee SD, Yeh GH, Lu CC, Wang PS, Wang SW. Disturbed small intestinal motility in the late rat pregnancy. Gynecol Obstet Invest. 1998;45:221–224. doi: 10.1159/000009971. [DOI] [PubMed] [Google Scholar]
- 3.Bradesi S, Eutamene H, Fioramonti J, Bueno L. Acute restraint stress activates functional NK1 receptor in the colon of female rats: involvement of steroids. Gut. 2002;50:349–354. doi: 10.1136/gut.50.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheen-Chen SM, Ho HT, Chen WJ, Sheen CW, Eng HL, Chou FF. Progesterone receptor in patients with hepatolithiasis. Dig Dis Sci. 2001;46:2374–2377. doi: 10.1023/a:1012347130235. [DOI] [PubMed] [Google Scholar]
- 5.Tierney S, Nakeeb A, Wong O, Lipsett PA, Sostre S, Pitt HA, Lillemoe KD. Progesterone alters biliary flow dynamics. Ann Surg. 1999;229:205–209. doi: 10.1097/00000658-199902000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathias JR, Clench MH. Relationship of reproductive hormones and neuromuscular disease of the gastrointestinal tract. Dig Dis. 1998;16:3–13. doi: 10.1159/000016844. [DOI] [PubMed] [Google Scholar]
- 7.Chen TS, Doong ML, Chang FY, Lee SD, Wang PS. Effects of sex steroid hormones on gastric emptying and gastrointestinal transit in rats. Am J Physiol. 1995;268:G171–G176. doi: 10.1152/ajpgi.1995.268.1.G171. [DOI] [PubMed] [Google Scholar]
- 8.Coşkun T, Sevinç A, Tevetoğlu I, Alican I, Kurtel H, Yeğen BC. Delayed gastric emptying in conscious male rats following chronic estrogen and progesterone treatment. Res Exp Med ( Berl) 1995;195:49–54. doi: 10.1007/BF02576773. [DOI] [PubMed] [Google Scholar]
- 9.Mizutani S, Hayakawa H, Akiyama H, Sakura H, Yoshino M, Oya M, Kawashima Y. Simultaneous determinations of plasma oxytocin and serum placental leucine aminopeptidase (P-LAP) during late pregnancy. Clin Biochem. 1982;15:141–145. doi: 10.1016/s0009-9120(82)90582-3. [DOI] [PubMed] [Google Scholar]
- 10.Kumaresan P, Subramanian M, Anandarangam PB, Kumaresan M. Radioimmunoassay of plasma and pituitary oxytocin in pregnant rats during various stages of pregnancy and parturition. J Endocrinol Invest. 1979;2:65–70. doi: 10.1007/BF03349277. [DOI] [PubMed] [Google Scholar]
- 11.Wang F, Luo JQ, Zheng TZ, Qu SY, Li W, He DY. Effect of oxytocin on the contractile activity of isolated gastric strips in rats. Zhongguo Yaolixue Yu Dulixue Zazhi. 1999;13:285–287. [Google Scholar]
- 12.Duridanova DB, Nedelcheva MD, Gagov HS. Oxytocin-induced changes in single cell K+ currents and smooth muscle contraction of guinea-pig gastric antrum. Eur J Endocrinol. 1997;136:531–538. doi: 10.1530/eje.0.1360531. [DOI] [PubMed] [Google Scholar]
- 13.Asad M, Shewade DG, Koumaravelou K, Abraham BK, Vasu S, Ramaswamy S. Gastric antisecretory and antiulcer activity of oxytocin in rats and guinea pigs. Life Sci. 2001;70:17–24. doi: 10.1016/s0024-3205(01)01376-5. [DOI] [PubMed] [Google Scholar]
- 14.Grazzini E, Guillon G, Mouillac B, Zingg HH. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature. 1998;392:509–512. doi: 10.1038/33176. [DOI] [PubMed] [Google Scholar]
- 15.Doong ML, Lu CC, Kau MM, Tsai SC, Chiao YC, Chen JJ, Yeh JY, Lin H, Huang SW, Chen TS, et al. Inhibition of gastric emptying and intestinal transit by amphetamine through a mechanism involving an increased secretion of CCK in male rats. Br J Pharmacol. 1998;124:1123–1130. doi: 10.1038/sj.bjp.0701937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell BF, Chibbar R. Synthesis and metabolism of oxytocin in late gestation in human decidua. Adv Exp Med Biol. 1995;395:365–380. [PubMed] [Google Scholar]
- 17.Shojo H, Kaneko Y. Characterization and expression of oxytocin and the oxytocin receptor. Mol Genet Metab. 2000;71:552–558. doi: 10.1006/mgme.2000.3094. [DOI] [PubMed] [Google Scholar]
- 18.Takagi T, Tanizawa O, Otsuki Y, Sugita N, Haruta M, Yamaji K. Oxytocin in the cerebrospinal fluid and plasma of pregnant and nonpregnant subjects. Horm Metab Res. 1985;17:308–310. doi: 10.1055/s-2007-1013526. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs AR, Behrens O, Maschek H, Kupsch E, Einspanier A. Oxytocin and vasopressin receptors in human and uterine myomas during menstrual cycle and early pregnancy. Hum Reprod Update. 1998;4:594–604. doi: 10.1093/humupd/4.5.594. [DOI] [PubMed] [Google Scholar]
- 20.Robinson RS, Mann GE, Lamming GE, Wathes DC. Expression of oxytocin, oestrogen and progesterone receptors in uterine biopsy samples throughout the oestrous cycle and early pregnancy in cows. Reproduction. 2001;122:965–979. [PubMed] [Google Scholar]
- 21.Einspanier A, Bielefeld A, Kopp JH. Expression of the oxytocin receptor in relation to steroid receptors in the uterus of a primate model, the marmoset monkey. Hum Reprod Update. 1998;4:634–646. doi: 10.1093/humupd/4.5.634. [DOI] [PubMed] [Google Scholar]
- 22.Zingg HH, Grazzini E, Breton C, Larcher A, Rozen F, Russo C, Guillon G, Mouillac B. Genomic and non-genomic mechanisms of oxytocin receptor regulation. Adv Exp Med Biol. 1998;449:287–295. doi: 10.1007/978-1-4615-4871-3_36. [DOI] [PubMed] [Google Scholar]
- 23.Engstrom T, Bratholm P, Christensen NJ, Vilhardt H. Up-regulation of oxytocin receptors in non-pregnant rat myometrium by isoproterenol: effects of steroids. J Endocrinol. 1999;161:403–411. doi: 10.1677/joe.0.1610403. [DOI] [PubMed] [Google Scholar]
- 24.Zingg HH, Grazzini E, Breton C, Larcher A, Rozen F, Russo C, Guillon G, Mouillac B. Genomic and non-genomic mechanisms of oxytocin receptor regulation. Adv Exp Med Biol. 1998;449:287–295. doi: 10.1007/978-1-4615-4871-3_36. [DOI] [PubMed] [Google Scholar]
- 25.Behrendt-Adam CY, Adams MH, Simpson KS, McDowell KJ. Oxytocin-neurophysin imRNA abundance in equine uterine endometrium. Domest Anim Endocrinol. 1999;16:183–192. doi: 10.1016/s0739-7240(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 26.Leung ST, Wathes DC. Regulatory effect of steroid hormones and fetal tissues on expression of oxytocin receptor in the endometrium of late pregnant ewes. J Reprod Fertil. 1999;115:243–250. doi: 10.1530/jrf.0.1150243. [DOI] [PubMed] [Google Scholar]
- 27.Vasudevan N, Davidkova G, Zhu YS, Koibuchi N, Chin WW, Pfaff D. Differential interaction of estrogen receptor and thyroid hormone receptor isoforms on the rat oxytocin receptor promoter leads to differences in transcriptional regulation. Neuroendocrinology. 2001;74:309–324. doi: 10.1159/000054698. [DOI] [PubMed] [Google Scholar]
- 28.Leung ST, Wathes DC. Oestradiol regulation of oxytocin receptor expression in cyclic bovine endometrium. J Reprod Fertil. 2000;119:287–292. [PubMed] [Google Scholar]
- 29.Ivell R, Walther N. The role of sex steroids in the oxytocin hormone system. Mol Cell Endocrinol. 1999;151:95–101. doi: 10.1016/s0303-7207(99)00025-8. [DOI] [PubMed] [Google Scholar]
- 30.Mittaud P, Labourdette G, Zingg H, Guenot-Di Scala D. Neurons modulate oxytocin receptor expression in rat cultured astrocytes: involvement of TGF-beta and membrane components. Glia. 2002;37:169–177. doi: 10.1002/glia.10029. [DOI] [PubMed] [Google Scholar]
- 31.Terenzi MG, Jiang QB, Cree SJ, Wakerley JB, Ingram CD. Effect of gonadal steroids on the oxytocin-induced excitation of neurons in the bed nuclei of the stria terminalis at parturition in the rat. Neuroscience. 1999;91:1117–1127. doi: 10.1016/s0306-4522(98)00687-3. [DOI] [PubMed] [Google Scholar]
- 32.Amico JA, Davis AM, McCarthy MM. An ovarian steroid hormone regimen that increases hypothalamic oxytocin expression alters [3H] muscimol binding in the hypothalamic supraoptic nucleus of the female rat. Brain Res. 2000;857:279–282. doi: 10.1016/s0006-8993(99)02373-2. [DOI] [PubMed] [Google Scholar]
- 33.Brussaard AB, Devay P, Leyting-Vermeulen JL, Kits KS. Changes in properties and neurosteroid regulation of GABAergic synapses in the supraoptic nucleus during the mammalian female reproductive cycle. J Physiol. 1999;516(Pt 2):513–524. doi: 10.1111/j.1469-7793.1999.0513v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Israel JM, Poulain DA. 17-Oestradiol modulates in vitro electrical properties and responses to kainate of oxytocin neurones in lactating rats. J Physiol. 2000;524 Pt 2:457–470. doi: 10.1111/j.1469-7793.2000.t01-2-00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leng G. Steroidal influences on oxytocin neurones. J Physiol. 2000;524 Pt 2:315. doi: 10.1111/j.1469-7793.2000.t01-1-00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivell R, Kimura T, Müller D, Augustin K, Abend N, Bathgate R, Telgmann R, Balvers M, Tillmann G, Fuchs AR. The structure and regulation of the oxytocin receptor. Exp Physiol. 2001;86:289–296. doi: 10.1113/eph8602185. [DOI] [PubMed] [Google Scholar]
- 37.Murata T, Murata E, Liu CX, Narita K, Honda K, Higuchi T. Oxytocin receptor gene expression in rat uterus: regulation by ovarian steroids. J Endocrinol. 2000;166:45–52. doi: 10.1677/joe.0.1660045. [DOI] [PubMed] [Google Scholar]
- 38.Mann GE. Hormone control of prostaglandin F (2 alpha) production and oxytocin receptor concentrations in bovine endometrium in explant culture. Domest Anim Endocrinol. 2001;20:217–226. doi: 10.1016/s0739-7240(01)00091-1. [DOI] [PubMed] [Google Scholar]
- 39.Bouchard P. Progesterone and the progesterone receptor. J Reprod Med. 1999;44:153–157. [PubMed] [Google Scholar]
- 40.Sladek CD, Swenson KL, Kapoor R, Sidorowicz HE. The role of steroid hormones in the regulation of vasopressin and oxytocin release and mRNA expression in hypothalamo-neurohypophysial explants from the rat. Exp Physiol. 2000;85 Spec No:171S–177S. doi: 10.1111/j.1469-445x.2000.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 41.Mann GE, Payne JH, Lamming GE. Hormonal regulation of oxytocin-induced prostaglandin F (2alpha) secretion by the bovine and ovine uterus in vivo. Domest Anim Endocrinol. 2001;21:127–141. doi: 10.1016/s0739-7240(01)00105-9. [DOI] [PubMed] [Google Scholar]
- 42.Breton C, Di Scala-Guenot D, Zingg HH. Oxytocin receptor gene expression in rat mammary gland: structural characterization and regulation. J Mol Endocrinol. 2001;27:175–189. doi: 10.1677/jme.0.0270175. [DOI] [PubMed] [Google Scholar]
- 43.Leung ST, Reynolds TS, Wathes DC. Regulation of oxytocin receptor in the placentome capsule throughout pregnancy in the ewe: the possible role of oestradiol receptor, progesterone receptor and aromatase. J Endocrinol. 1998;158:173–181. doi: 10.1677/joe.0.1580173. [DOI] [PubMed] [Google Scholar]
- 44.Giacalone PL, Daurés JP, Faure JM, Boulot P, Hedon B, Laffargue F. The effects of mifepristone on uterine sensitivity to oxytocin and on fetal heart rate patterns. Eur J Obstet Gynecol Reprod Biol. 2001;97:30–34. doi: 10.1016/s0301-2115(00)00506-6. [DOI] [PubMed] [Google Scholar]
- 45.Al-Matubsi HY, Frazer S, Fairclough RJ, Jenkin G. Effects of progesterone on expression of messenger RNA encoding oxytocin-neurophysin, oxytocin receptor and prostaglandin G/H synthase-1 and -2 during the early oestrous cycle in the ovine corpus luteum. Reprod Fertil Dev. 1999;11:435–442. doi: 10.1071/rd99093. [DOI] [PubMed] [Google Scholar]
- 46.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 47.Engstrøm T, Bratholm P, Christensen NJ, Vilhardt H. Effect of oxytocin receptor blockade on rat myometrial responsiveness to prostaglandin f (2) (alpha) Biol Reprod. 2000;63:1443–1449. doi: 10.1095/biolreprod63.5.1443. [DOI] [PubMed] [Google Scholar]
- 48.Wu WX, Verbalis JG, Hoffman GE, Derks JB, Nathanielsz PW. Characterization of oxytocin receptor expression and distribution in the pregnant sheep uterus. Endocrinology. 1996;137:722–728. doi: 10.1210/endo.137.2.8593823. [DOI] [PubMed] [Google Scholar]
- 49.Edgerton LA, Kaminski MA, Silvia WJ. Effects of progesterone and estradiol on uterine secretion of prostaglandin f (2alpha)in response to oxytocin in ovariectomized sows. Biol Reprod. 2000;62:365–369. doi: 10.1095/biolreprod62.2.365. [DOI] [PubMed] [Google Scholar]
- 50.Gimpl G, Burger K, Politowska E, Ciarkowski J, Fahrenholz F. Oxytocin receptors and cholesterol: interaction and regulation. Exp Physiol. 2000;85 Spec No:41S–49S. doi: 10.1111/j.1469-445x.2000.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 51.Burger K, Fahrenholz F, Gimpl G. Non-genomic effects of progesterone on the signaling function of G protein-coupled receptors. FEBS Lett. 1999;464:25–29. doi: 10.1016/s0014-5793(99)01668-3. [DOI] [PubMed] [Google Scholar]


