Abstract
AIM: The objective of this study is to elucidate the potential role of poly-morphonuclear neutrophils (PMN) in the development of such a sinusoidal endothelial cell (SEC) injury during early acute obstructive cholangitis (AOC) in rats.
METHODS: Twenty one Wistar rats were divided into three groups: the AOC group, the bile duct ligated group (BDL group), and the sham operation group (SO group). The common bile duct (CBD) of rats in AOC group was dually ligated and 0.2 mL of the E. coli O111 B4 (5 × 109 cfu/mL) suspension was injected into the upper segment, in BDL group, only the CBD was ligated and in SO group, neither injection of E. coli suspension nor CBD ligation was done, but the same operative procedure. Such group consisted of seven rats, all animals were killed 6 h after the operation. Morphological changes of the liver were observed under light and electron microscope. Expression of intercellular adhesion molecule-1 (ICAM-1) mRNA in hepatic tissue was determined with reverse transcription polymerase chain reaction (RT-PCR). The serum levels of alanine aminotransferase (ALT) were determined with anutoanalyger and cytokine-induced neutrophil chemoattractant (CINC) was determined by enzyme-linked immunosorbent assay (ELISA).
RESULTS: Neutrophils was accumulated in the hepatic sinusoids and sinusoidal endothelial cell injury existed in AOC group. In contrast, in rats of BDL group, all the features of SEC damage were greatly reduced. Expression of ICAM-1 mRNA in hepatic tissue in three groups were 7.54 ± 0.82, 2.87 ± 0.34, and 1.01 ± 0.12, respectively. There were significant differences among three groups (P < 0.05). The serum CINC levels in the three groups were 188 ± 21 ng•L⁻¹, 94 ± 11 ng•L⁻¹, and 57 ± 8 ng•L⁻¹, respectively. There were also significant differences among the three groups (P < 0.05). Activity of the serum ALT was 917 ± 167 nkat•L⁻¹, 901 ± 171 nkat•L⁻¹, and 908 ± 164 nkat•L⁻¹, respectively, (P > 0.05).
CONCLUSION: Hepatic SEC injury occurs earlier than hepatic parenchymal cells during AOC. Recruitments of circulating neutrophils in the hepatic sinusoidal space might mediate the SEC injury, and ICAM-1 in the liver may modulate the PMN of accumulation.
INTRODUCTION
Biliary tract infection, especially acute obstructive cholangitis (AOC) is common[1,2]. Despite a multitude of advances in treatment of surgical infection, this remains a significant cause of morbidity and mortality[3,4], where sepsis and endotoxemia from AOC are important causes of multiple organ failure (MOF) and deaths[5-9]. Ohtsuka et al[10] reported that polymorphonuclear neutrophils (PMN) accumulated in the hepatic sinusoidal space increased obviously in rats with obstructive jaundice and there were interaction between PMN and sinusoidal endothelial cells (SEC) causing injury during AOC. This study was study the potential role of PMN in the development of SEC injury and the mechanism of accumulation of PMN during early period of AOC.
MATERIALS AND METHODS
Animal Experiment
Male Wistar rats weighing 200-230 g were purchased from Laboratory Animal Center of Chongqing University of Medical Science. These animals were divided into three groups: the AOC group, bile duct ligated group of (BDL group), and sham operation group (SO group). All the animals were killed 6 h after operation, the surgical procedures were carried out under light diethyl ether anesthesia. The animal models were performed as follows. In AOC group, a 1.5 cm medium incision was made over the upper abdomen, the common bile duct (CBD) was mobilized and dually ligated, and 0.2 mL of the E. coli O111 B4 (5 × 1012 cfu•L⁻¹) (Sigma, USA) suspension was injected into the upper segment. In BDL group, the CBD was doubly ligated but without injection of E. coli O111 B4 suspension. In SO group, neither E. coli injection of suspention nor CBD ligation was done, but only routine operative procedure was performed. Seven rats constituted each group.
Serum ALT and CINC
Hepatic injury was assessed by measuring the serum alanine aminotransferase (ALT) which was performed with autoanalyger. The serum cytokine-induced neutrophil chemoattractant (CINC) concentration was measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's direction with a lower limit of 10 ng•L⁻¹. For CINC, microtitre plates were coated with anti CINC mAb overnight at room temperature on a plate shaker, after the blockage, samples were then added. The detected antibody was biotinylated anti-CINC. Standard curves were made with a natural CINC calibrated against the WHO interim International Standard.
Expression of ICAM-1 mRNA in Hepatic Tissue
Total RNA was isolated from rat liver tissue by using the Trizol Reagent (Life Technologies, USA). The quality of RNA was controlled by the intactness of ribosomal RNA bands. A total of 0.5 mg of each intact total RNA sample was reverse-transcribed to complementary DNA (cDNA) by using reverse transcription polymerase chain reaction (RT-PCR) kit (Roche, USA). cDNA was stored at -70 °C until PCR analysis. The PCR primers used were ICAM-1: sense (5'-CTTCTCCTGCTCTGCAACCC-3'), antisense (5'-GGGAGAGCACATTCAGGTC-3'); β-actin: sense (5'-ACCACAGCTGAGAGGGAAATCG-3'), antisense (5'-AGAGGTCTTTACGGATGTCAACG-3'). The sizes of the amplified PCR products were 326 bp for ICAM-1 and 281 bp for β-actin. The procedure was as follows: denaturation at 95 °C for 30 s, annealing at 55 °C for 1 min, and extension at 71 °C for 1 min for 28 cycles. The PCR products were electrophoresed in 20 g•L⁻¹ agarose gels, and the gels were ethidium bromide stained and video photographed on an ultraviolet transilluminator. The bands representing reactive product were scanned by densitometer of a Bio-Image Analysis System (Doc Gel 2000). The relative optical density (ROD) values were expressed as the level of ICAM-1 mRNA in hepatic tissue.
Morphologic Observations of Hepatic Tissue
Liver samples from different liver lobes were fixed with 100 mL•L⁻¹ buffered formalin or 25 g•L⁻¹ glutaraldehyde immediately. For light microscopy, the tissue blocks were embedded in paraffin, and the sections were stained with hematoxylin and eosin (H&E). For transmission electron microscopy (TEM), the tissue blocks were embedded in Epon 618 resin and ultrathin sections were stained with uranyl acetate and lead citrate. A H-2000 transmission electron microscope was used.
Statistical Analysis
Results were presented as ¯x ± s. Statistical difference was analysed by means of the analysis of Variance (ANOVA). P < 0.05 is considered significant.
RESULTS
Accumulation of PMN in hepatic sinusoidal space
Accumulation of PMN in the hepatic sinusoidal space was found, under light microscopy, PMN counts in hepatic sinusoidal space increased significantly after 6 h in AOC group in comparison with BDL group or SO group. Under electron microscopy, PMN were seen easily in hepatic sinusoidal space in AOC group (Figure 1A).
Figure 1.
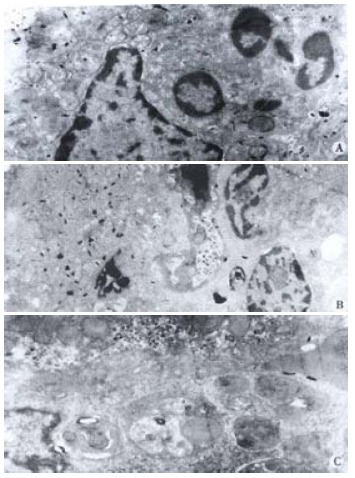
A: In AOC group, PMN was seen easily in hepatic sinusoidal. TEM × 4000; B: In AOC group, two PMNs were seen in hepatic sinusoid with decreased electronic density of cytoplasm, and swollen or even vacuolated mitochondria in SEC. TEM × 3000; C: In AOC group, KC was also seen easily in hepatic sinusoid with active phagocytosis. TEM × 4000
Sinusoidal endothelial cell injury
Under light microscopy, no distinct change in SEC could be shown in any of the above groups. Electron microscopically, however, focal detachment, decreased electron density of cytoplasm, and swollen or even vacuolated mitochondria in SEC could often be observed in the AOC group (Figure 1B). In this group, the Kupffer cells were enlarged, but normal surface structures were retained and no degenerative changes of the nucleus or cytoplasm were shown (Figure 1C). In contrast such changes could be occasionally seen in the SEC of BDL group. No evident morphological changes of SEC could be observed in SO group.
Expression of ICAM-1 mRNA in hepatic tissue
Expression of ICAM-1 mRNA in hepatic tissues was distinctly enhanced after 6 h in AOC group when compared to other two groups (P < 0.05). There was less expression of ICAM-1 gene in BDL group and no expression of ICAM-1 gene in SO group (Figure 2).
Figure 2.
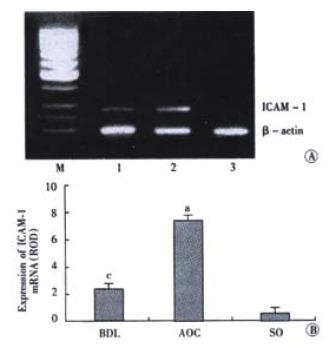
A: Expression of ICAM-1 mRNA. M, Marker. Lane 1: BDL; Lane 2: AOC; Lane 3: SO; B: Expression ofICAM-1 mRNA.aP < 0.05, vs other two groups, cP < 0.05, vs control group
The serum ALT level and CINC concentration
The serum ALT level and CINC concentration were shown in Table 1. The serum ALT activity in the three groups was evidently unchanged in the same period (P > 0.05). but, the serum CINC concentration in the AOC group was significantly higher than that in the BDL group or the SO group (P < 0.05).
Table 1.
The changes of serum ALT level and CINC concentration in the three groups (¯x ± s, n = 7)
| Serum parameters | AOC | BDL | SO |
| ALT (nkat•L⁻¹) | 917 ± 167 | 901 ± 171 | 908 ± 164 |
| CINC ( ng•L⁻¹) | 188 ± 21a | 94 ± 11c | 57 ± 8 |
P < 0.05, vs other two groups.
P < 0.05, vs SO group.
DISCUSSION
Neutrophils and macrophages play a central role in the host defence against microbial infections. However, they also produce damage to normal tissue by releasing oxygen free radicals, elastase, and various cytokines[11,12]. The hepatic sinusoidal endothelia are fenestrated allowing exchange of substance between the circulating blood and hepatocytes[13]. The Kupffer cells are located at the luminal side of the SEC and is able to phagocytize pathogens and release cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1, IL-6, IL-8, and adhesion molecules ICAM-1 (CD54), etc.[14-16], these inflammatory cytokines and chemokines can be upregulated in inflammatory processes within the liver[17-20]. Recent reports have revealed the interaction between neutrophils and SEC in sepsis, and neutrophils have the potential to cause endothelial cell injury by producting protease and superoxides[13,16,21]. Overreaction of neutrophils may be responsible for organ failure in cholestatic rats[10,12,22]. We found that accumulation of PMNs in the hepatic sinusoidal space was accompanied by SEC injury with decreased electron density of cytoplasm, and swollen or even vacuolated mitochondria in the early period of AOC. Our results indicated that damage of SEC ocurred earlier than that of hepatic parenchymal cells and the vascular endothelium which was a critical and initial event in AOC and organ failure processes. SEC injury might develop microcirculatory disturbance in the liver, resulted in hepatocytic damage and hepatic dysfunction.
Although obvious parenchymal cell necrosis was not observed in our study, it is likely that the microcirculatory disturbance could provoke liver dysfunction during AOC. CINC, a member of IL-8 family and a major neutrophil chemotactic factor in rats, increased in the liver during sepsis[23]. Substantial neutrophil accumulation in the liver and the role of these cells in the pathophysiology of liver injury was found in models of endotoxin shock and hepatic ischemia-reperfusion injury[24-32]. But, the relationship between PMN accumulation, ICAM-1 expression and SEC injury during AOC is unclear. The injury to SECs was induced by the interaction of the activated PMNs and SECs via ICAM-1 and CD18[33-37]. Ohtsuka et al[38-52] reported ICAM-1 expression on SEC started to rise 6-12 h after partial hepatectomy, reaching a peak after 24-48 h. ICAM-1 is particularly expressed on Kupffer cells, endothelial cells, and leukocytes and it promotes accumulation of PMN in the hepatic sinusoids and is linked to endothelial cell injury. The mechanisms of upregulated ICAM-1 gene expression during AOC may included (1) inflammatory cytokines upregulate ICAM-1 expression in endotoxemia[28]; (2)synthesis of ICAM-1 is increased and/or its elimination is decreased through the bile in bile duct ligated animals.
In conclusion, hepatic SEC injury occurs earlier than hepatic parenchymal cells damage during AOC. Recruitment of circulating neutrophils in the hepatic sinusoidal space enhance the SEC injury, and ICAM-1 in the liver can modulate the accumulation of PMN.
Footnotes
Edited by Wu XN
Supported by the National Natural Science Foundation of China, No. 39970719, 30170919
References
- 1.Zhi QH. New development of biliary surgery in China. World J Gastroenterol. 2000;6:187–192. doi: 10.3748/wjg.v6.i2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimmings AN, van Deventer SJ, Rauws EAJ K, Gouma DJ. Systemic inflammatory response in acute cholangitis and after subsequent treatment. Eur J Surg. 2000;166:700–705. doi: 10.1080/110241500750008457. [DOI] [PubMed] [Google Scholar]
- 3.Lillemoe KD. Surgical treatment of biliary tract infections. Am Surg. 2000;66:138–144. [PubMed] [Google Scholar]
- 4.Gong JP, Liu CA, Wu CX, Li SW, Shi YJ, Li XH. Nuclear factor κB activity in patients with acute severe cholangitis. World J Gastroenterol. 2002;8:346–349. doi: 10.3748/wjg.v8.i2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling YL, Meng AH, Zhao XY, Shan BE, Zhang JL, Zhang XP. Effect of cholecystokinin on cytokines during endotoxic shock in rats. World J Gastroenterol. 2001;7:667–671. doi: 10.3748/wjg.v7.i5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomioka M, Iinuma H, Okinaga K. Impaired Kupffer cell function and effect of immunotherapy in obstructive jaundice. J Surg Res. 2000;92:276–282. doi: 10.1006/jsre.2000.5868. [DOI] [PubMed] [Google Scholar]
- 7.Kordzaya DJ, Goderdzishvili VT. Bacterial translocation in obstructive jaundice in rats: role of mucosal lacteals. Eur J Surg. 2000;166:367–374. doi: 10.1080/110241500750008907. [DOI] [PubMed] [Google Scholar]
- 8.Kimmings AN, van Deventer SJ, Obertop H, Rauws EA, Huibregtse K, Gouma DJ. Endotoxin, cytokines, and endotoxin binding proteins in obstructive jaundice and after preoperative biliary drainage. Gut. 2000;46:725–731. doi: 10.1136/gut.46.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Téllez Gil L, Roselló AM, Collado Torres A, Moreno RL, Antonio Ferrón Orihuela J. Modulation of soluble phases of endothelial/Leukocyte adhesion molecule 1, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1 with interleukin-1beta after experimental endotoxic challenge. Crit Care Med. 2001;29:776–781. doi: 10.1097/00003246-200104000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Ohtsuka M, Miyazaki M, Kubosawa H, Kondo Y, Ito H, Shimizu H, Shimizu Y, Nozawa S, Furuya S, Nakajima N. Role of neutrophils in sinusoidal endothelial cell injury after extensive hepatectomy in cholestatic rats. J Gastroenterol Hepatol. 2000;15:880–886. doi: 10.1046/j.1440-1746.2000.02224.x. [DOI] [PubMed] [Google Scholar]
- 11.Xu MQ, Gong JP, Xue L, Han BL, Xu F. Effects of Kupffer cell NF-κB activation on liver regeneration after partial hepatectomy in biliary obstructive rats. Disan Junyi Daxue Xuebao. 2001;23:1143–1145. [Google Scholar]
- 12.Ito Y, Machen NW, Urbaschek R, McCuskey RS. Biliary obstruction exacerbates the hepatic microvascular inflammatory response to endotoxin. Shock. 2000;14:599–604. doi: 10.1097/00024382-200014060-00005. [DOI] [PubMed] [Google Scholar]
- 13.Braet F, Zanger RD, Spector I, Wisse E. Structure and dynamics of hepatic endothelial fenestrae. World J Gastroenterol. 2000;6(Suppl):1. [Google Scholar]
- 14.Bone-Larson CL, Simpson KJ, Colletti LM, Lukacs NW, Chen SC, Lira S, Kunkel SL, Hogaboam CM. The role of chemokines in the immunopathology of the liver. Immunol Rev. 2000;177:8–20. doi: 10.1034/j.1600-065x.2000.17703.x. [DOI] [PubMed] [Google Scholar]
- 15.Han DW. The clinical sine of subsequent liver injury induced by gut derived endotoxemia. Shijie Huaren Xiaohua Zazhi. 1999;7:1055–1058. [Google Scholar]
- 16.Liu BH, Chen HS, Zhou JH, Xiao N. Effects of endotoxin on endothelin receptor in hepatic and intestinal tissues after endotoxemia in rats. World J Gastroenterol. 2000;6:298–300. doi: 10.3748/wjg.v6.i2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang SC, Dai Q, Wang JY, He BM, Zhou K. Gut-derived endotoxemia: one of the factors leading to prodution of cytokines in liver diseases. World J Gastroenterol. 2000;6(Suppl):16. [Google Scholar]
- 18.Lin E, Calvano SE, Lowry SF. Inflammatory cytokines and cell response in surgery. Surgery. 2000;127:117–126. doi: 10.1067/msy.2000.101584. [DOI] [PubMed] [Google Scholar]
- 19.Gong JP, Xu MQ, Zhu J, Han BL, Li K. Expression of CD14 in Kupffer cells induced by lipopolysaccharide. Disan Junyi Daxue Xuebao. 2001;23:425–428. [Google Scholar]
- 20.Koo DJ, Chaudry IH, Wang P. Kupffer cells are responsible for producing inflammatory cytokines and hepatocellular dysfunction during early sepsis. J Surg Res. 1999;83:151–157. doi: 10.1006/jsre.1999.5584. [DOI] [PubMed] [Google Scholar]
- 21.Hardaway RM. A review of septic shock. Am Surg. 2000;66:22–29. [PubMed] [Google Scholar]
- 22.Roggin KK, Papa EF, Kurkchubasche AG, Tracy TF. Kupffer cell inactivation delays repair in a rat model of reversible biliary obstruction. J Surg Res. 2000;90:166–173. doi: 10.1006/jsre.2000.5879. [DOI] [PubMed] [Google Scholar]
- 23.Bautista AP. Impact of alcohol on the ability of Kupffer cells to produce chemokines and its role in alcoholic liver disease. J Gastroenterol Hepatol. 2000;15:349–356. doi: 10.1046/j.1440-1746.2000.02174.x. [DOI] [PubMed] [Google Scholar]
- 24.Deaciuc IV, D'Souza NB, Sarphie TG, Schmidt J, Hill DB, McClain CJ. Effects of exogenous superoxide anion and nitric oxide on the scavenging function and electron microscopic appearance of the sinusoidal endothelium in the isolated, perfused rat liver. J Hepatol. 1999;30:213–221. doi: 10.1016/s0168-8278(99)80064-6. [DOI] [PubMed] [Google Scholar]
- 25.Fan K. Regulatory effects of lipopolysaccharide in murine macrophage proliferation. World J Gastroenterol. 1998;4:137–139. doi: 10.3748/wjg.v4.i2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Le Roy D, Di Padova F, Adachi Y, Glauser MP, Calandra T, Heumann D. Critical role of lipopolysaccharide-binding protein and CD14 in immune responses against gram-negative bacteria. J immunol. 2001;167:2759–2765. doi: 10.4049/jimmunol.167.5.2759. [DOI] [PubMed] [Google Scholar]
- 28.Wu RQ, Xu YX, Song XH, Chen LJ, Meng XJ. Adhesion molecule and proinflammatory cytokine gene expression in hepatic sinusoidal endothelial cells following cecal ligation and puncture. World J Gastroenterol. 2001;7:128–130. doi: 10.3748/wjg.v7.i1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson GD, Dai Y, Sewell WA. Bile mediates intestinal pathology in endotoxemia in rats. Infect immun. 2000;68:4714–4719. doi: 10.1128/iai.68.8.4714-4719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- 31.Gong JP, Han BL. Effects of CD14 in LPS mediating activation of Kupffer cells. Shijie Huaren Xiaohua Zazhi. 1999;7:875–877. [Google Scholar]
- 32.Bai XY, Jia XH, Cheng LZ, Gu YD. Influence of IFN alpha-2b and BCG on the release of TNF and IL-1 by Kupffer cells in rats with hepatoma. World J Gastroenterol. 2001;7:419–421. doi: 10.3748/wjg.v7.i3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo X, Dudman NP. Homocysteine induces expressions of adhesive molecules on leukocytes in whole blood. Chin Med J ( Engl) 2001;114:1235–1239. [PubMed] [Google Scholar]
- 34.Mäck C, Jungermann K, Götze O, Schieferdecker HL. Anaphylatoxin C5a actions in rat liver: synergistic enhancement by C5a of lipopolysaccharide-dependent alpha (2)-macroglobulin gene expression in hepatocytes via IL-6 release from Kupffer cells. J immunol. 2001;167:3972–3979. doi: 10.4049/jimmunol.167.7.3972. [DOI] [PubMed] [Google Scholar]
- 35.Hedin KE, Kaczynski JA, Gibson MR, Urrutia R. Transcription factors in cell biology, surgery, and transplantation. Surgery. 2000;128:1–5. doi: 10.1067/msy.2000.106426. [DOI] [PubMed] [Google Scholar]
- 36.Lehmann C, König JP, Dettmann J, Birnbaum J, Kox WJ. Effects of iloprost, a stable prostacyclin analog, on intestinal leukocyte adherence and microvascular blood flow in rat experimental endotoxemia. Crit Care Med. 2001;29:1412–1416. doi: 10.1097/00003246-200107000-00019. [DOI] [PubMed] [Google Scholar]
- 37.Soler-Rodriguez AM, Zhang H, Lichenstein HS, Qureshi N, Niesel DW, Crowe SE, Peterson JW, Klimpel GR. Neutrophil activation by bacterial lipoprotein versus lipopolysaccharide: differential requirements for serum and CD14. J immunol. 2000;164:2674–2683. doi: 10.4049/jimmunol.164.5.2674. [DOI] [PubMed] [Google Scholar]
- 38.Neubauer K, Ritzel A, Saile B, Ramadori G. Decrease of platelet-endothelial cell adhesion molecule 1-gene-expression in inflammatory cells and in endothelial cells in the rat liver following CCl (4)-administration and in vitro after treatment with TNFalpha. Immunol Lett. 2000;74:153–164. doi: 10.1016/s0165-2478(00)00203-0. [DOI] [PubMed] [Google Scholar]
- 39.Madorin WS, Cepinskas G, Kvietys PR. Peritonitis induces rat cardiac myocytes to promote polymorphonuclear leukocyte emigration and activate endothelial cells: effect of lipopolysaccharide pretreatment. Crit Care Med. 2001;29:1774–1779. doi: 10.1097/00003246-200109000-00020. [DOI] [PubMed] [Google Scholar]
- 40.Emmanuilidis K, Weighardt H, Maier S, Gerauer K, Fleischmann T, Zheng XX, Hancock WW, Holzmann B, Heidecke CD. Critical role of Kupffer cell-derived IL-10 for host defense in septic peritonitis. J immunol. 2001;167:3919–3927. doi: 10.4049/jimmunol.167.7.3919. [DOI] [PubMed] [Google Scholar]
- 41.Funda DP, Tucková L, Farré MA, Iwase T, Moro I, Tlaskalová-Hogenová H. CD14 is expressed and released as soluble CD14 by human intestinal epithelial cells in vitro: lipopolysaccharide activation of epithelial cells revisited. Infect immun. 2001;69:3772–3781. doi: 10.1128/IAI.69.6.3772-3781.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Assy N, Jacob G, Spira G, Edoute Y. Diagnostic approach to patients with cholestatic jaundice. World J Gastroenterol. 1999;5:252–262. doi: 10.3748/wjg.v5.i3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuo GQ, Gong JP, Liu CA, Li SW, Wu XC, Yang K, Li Y. Expression of lipopolysaccharide binding protein and its receptor CD14 in experimental alcoholic liver disease. World J Gastroenterol. 2001;7:836–840. doi: 10.3748/wjg.v7.i6.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seki S, Habu Y, Kawamura T, Takeda K, Dobashi H, Ohkawa T, Hiraide H. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol Rev. 2000;174:35–46. doi: 10.1034/j.1600-0528.2002.017404.x. [DOI] [PubMed] [Google Scholar]
- 45.Arai M, Peng XX, Currin RT, Thurman RG, Lemasters JJ. Protection of sinusoidal endothelial cells against storage/reperfusion injury by prostaglandin E2 derived from Kupffer cells. Transplantation. 1999;68:440–445. doi: 10.1097/00007890-199908150-00017. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe M, Chijiiwa K, Kameoka N, Yamaguchi K, Kuroki S, Tanaka M. Gadolinium pretreatment decreases survival and impairs liver regeneration after partial hepatectomy under ischemia/reperfusion in rats. Surgery. 2000;127:456–463. doi: 10.1067/msy.2000.104744. [DOI] [PubMed] [Google Scholar]
- 47.Enomoto N, Ikejima K, Yamashina S, Enomoto A, Nishiura T, Nishimura T, Brenner DA, Schemmer P, Bradford BU, Rivera CA, et al. Kupffer cell-derived prostaglandin E (2) is involved in alcohol-induced fat accumulation in rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;279:G100–G106. doi: 10.1152/ajpgi.2000.279.1.G100. [DOI] [PubMed] [Google Scholar]
- 48.Wang LS, Zhu HM, Zhou DY, Wang YL, Zhang WD. Influence of whole peptidoglycan of bifidobacterium on cytotoxic effectors produced by mouse peritoneal macrophages. World J Gastroenterol. 2001;7:440–443. doi: 10.3748/wjg.v7.i3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Islam AF, Moss ND, Dai Y, Smith MS, Collins AM, Jackson GD. Lipopolysaccharide-induced biliary factors enhance invasion of Salmonella enteritidis in a rat model. Infect immun. 2000;68:1–5. doi: 10.1128/iai.68.1.1-5.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gong JP, Wu CX, Liu CA, Li SW, Shi YJ, Yang K, Li Y, Li XH. Intestinal damage mediated by Kupffer cells in rats with endotoxemia. World J Gastroenterol. 2002;8:923–927. doi: 10.3748/wjg.v8.i5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sindram D, Porte RJ, Hoffman MR, Bentley RC, Clavien PA. Synergism between platelets and leukocytes in inducing endothelial cell apoptosis in the cold ischemic rat liver: A Kupffer cell-mediated injury. FASEB J. 2001;15:1230–1232. doi: 10.1096/fj.00-0554fje. [DOI] [PubMed] [Google Scholar]
- 52.Li SW, Gong JP, Wu CX, Shi YJ, Liu CA. Lipopolysaccharide induced synthesis of CD14 proteins and its gene expression in hepatocytes during endotoxemia. World J Gastroenterol. 2002;8:124–127. doi: 10.3748/wjg.v8.i1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]


