Abstract
AIM: To investigate the effects of areca on the contractile activity of isolated colonic muscle strips in rats and mechanism involved.
METHODS: Each strip (LMPC, longitudinal muscle of proximal colon; CMPC, circular muscle of proximal colon; LMDC, longitudinal muscle of distal colon; CMDC, circular muscle of distal colon.) was suspended in a tissue chamber containing 5 mL Krebs solution (37 °C), bubbled continuously with 950 mL•L⁻¹ O2 and 50 mL•L⁻¹ CO2. The mean contractile amplitude (A), the resting tension (T), and the contractile frequency (F) were simultaneously recorded on recorders.
RESULTS: Areca dose dependently increased the mean contractile amplitude, the resting tension of proximal and distal colonic smooth muscle strips in rats (P < 0.05). It also partly increased the contractile frequency of colonic smooth muscle strips in rats (P < 0.05). The effects were partly inhibited by atropine (the resting tension of LMPC decreased from 0.44 ± 0.12 to 0.17 ± 0.03; the resting tension of LMDC decreased from 0.71 ± 0.14 to 0.03 ± 0.01; the mean contractile amplitude of LMPC increased from -45.8 ± 7.2 to -30.5 ± 2.9; the motility index of CMDC decreased from 86.6 ± 17.3 to 32.8 ± 9.3; P < 0.05 vs areca), but the effects were not inhibited by hexamethonium (P > 0.05).
CONCLUSION: Areca stimulated the motility of isolated colonic smooth muscle strips in rats. The stimulation of areca might be relevant with M receptor partly.
INTRODUCTION
Areca (Areca catechu L.) had already been shown to relieve indigestion, unblocked stagnation of the circulation of vital energy. It had been used to treat abdominal distention and constipation, which were caused by stagnation of the circulation of vital energy in taste. But the actions and mechanisms of areca on the colonic smooth muscle motility are not reported. In this study, we observed the effect of areca on the different colonic smooth muscle strips in rats and investigated the mechanism involved.
MATERIALS AND METHODS
Animal preparation
Wistar rats of either sex (grade I, purchased from Animal Center of Lanzhou Medical College), weighing 200-250 g, were sacrificed, and the proximal colon and distal colon were removed[1]. The segments of the colon were opened along the mesentery. Muscle strips (8 × 3 mm) were cut, parallel to either the circular or the longitudinal fibers, and named circular muscle of proximal colon (CMPC), longitudinal muscle of proximal colon (LMPC), circular muscle of distal colon (CMDC), and longitudinal muscle of distal colon (LMDC). The mucosa on each strip was carefully removed.
Experiments
The muscle strip was suspended in a tissue chamber containing 5 mL Krebs solution (37 °C) and bubbled continuously with 950 mL•L⁻¹ O2 and 50 mL•L⁻¹ CO2[2]. One end of the strip was fixed to a hook on the bottom of the chamber. The other end was connected to an external isometric force transducer (JZ-BK, BK). Motility of colonic strips (under an initial tension of 1 g) in 4 tissue chambers were simultaneously recorded on ink-writing recorders (LMS-ZB, Cheng-Du). After 1 h equilibration, areca (10, 100, 1000 g•L⁻¹) was added in the tissue chamber to observe their effects on colon; atropine (0.01 μmol•L⁻¹) or hexamethonium (10 μmol•L⁻¹), given 3 min before the administration of areca (100 g•L⁻¹), was added separately to investigate whether the actions of areca were relevant with M receptor or N receptor. The resting tension, the frequency, and the mean contractile amplitude of LMPC, CMPC and LMDC, as well as the motility index of CMDC were measured. Motility index = ∑ (amplitude × duration).
Drugs preparation
Areca was broken into pieces, boiled, filtrated, and diluted to 1000 g•L⁻¹ (the drug was appraised and prepared by Drug Control Institute of Gansu Province). The following agents were used: Atropine (Pharmaceutical Factory in Yancheng, Jiangsu Province), hexamethonium (Sigma Chemical Company).
Data analysis
The results were presented as ¯x ± s, and statistically analyzed by paired t test, P < 0.05 was considered to be significant.
RESULTS
Effect of areca on the spontaneous contraction of colonic smooth muscle strips
Areca (10, 100, 1000 g•L⁻¹) dose dependently increased the mean contractile amplitude of CMPC and LMDC, the motility index of CMDC, and the resting tension of LMPC, LMDC and CMDC; but it decreased the mean contractile amplitude of LMPC (Figure 1). It increased the contractile frequency of CMPC and LMDC (Table 1). It had no significant effects on the resting tension of CMPC and the contractile frequency of LMPC and CMDC.
Figure 1.
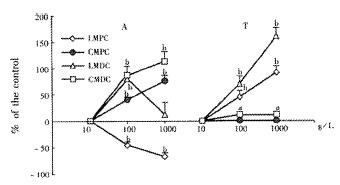
Effect of areca on the mean contractile (the motility index of CMDC) and the resting tension (¯x ± s, n = 12). LMPC: longitudinal muscle of proximal colon; CMPC: circular muscle of proximal colon; LMDC: longitudinal muscle of distal colon; CMDC: circular muscle of distal colon. A, the mean contractile amplitude; T, the resting tension. aP < 0.05, bP < 0.01 vs control.
Table 1.
Effect of areca on the contractile frequency of colonic contractile in rats (¯x ± s, waves·min-1, n = 12)
|
Areca (g•L⁻¹) |
||||||
| 0 | 10 | 0 | 100 | 0 | 1000 | |
| LMPC | 1.8 ± 0.2 | 1.9 ± 0.2 | 2.2 ± 0.2 | 2.5 ± 0.3 | 1.8 ± 0.2 | 1.8 ± 0.4 |
| CMPC | 1.5 ± 0.1 | 1.5 ± 0.1 | 1. 6 ± 0.1 | 2.1 ± 0.2a | 1.6 ± 0.1 | 2.3 ± 0.1b |
| LMDC | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.5 ± 0.1 | 2.3 ± 0.2a | 1.5 ± 0.2 | 2.7 ± 0.5b |
| CMDC | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 |
LMPC: longitudinal muscle of proximal colon; CMPC: circular muscle of proximal colon; LMDC: longitudinal muscle of distal colon; CMDC: circular muscle of distal colon.
P < 0.05,
P < 0.01 vs control (0).
Effect of atropine on the responses caused by areca
Atropine (0.01 μmol•L⁻¹) itself had no significant effects on rat colon. But when given 3 min before the administration of areca (100 g•L⁻¹), it reduced the increasing action of areca on the resting tension of LMPC and LMDC, the motility index of CMDC, and the mean contractile amplitude of LMPC. It had no significant effects on the other action of areca (Table 2).
Table 2.
Effect of areca on the mean contractile amplitude and the resting tension of colon, and the motilityindex of distal colon after atropine pretreatment in rats (¯x ± s, n = 12)
|
LMPC |
CMPC |
LMDC |
CMDC |
|||||
| T/g | A/mm | T/g | A/mm | T/g | A/mm | T/g | MI/mm·s-1 | |
| Areca | 0.44 ± 0.12b | -45.8 ± 7.2b | 0 | 40.0 ± 3.5b | 0.71 ± 0.14b | 79.7 ± 12.8b | 0.11 ± 0.05a | 86.6 ± 17.3b |
| Atropine | 0 | 0.1 ± 0.1 | 0 | 0.6 ± 1.4 | 0 | 1.3 ± 3.0 | 0 | 0.9 ± 1.3 |
| Atropine + Areca | 0.17 ± 0.03bc | -30.5 ± 2.9 | 0 | 36.9 ± 2.5b | 0.03 ± 0.01ab | 70.9 ± 13.6b | 0.03 ± 0.02 | 32. ± 98.3bc |
T, the resting tension; A, the mean contractile amplitude; MI, the motility index.
P < 0.05,
P < 0.001 vs control.
P < 0.05, dP < 0.001 vs areca.
Effect of hexamethonium on the responses caused by areca
Hexamethonium (10 μmol•L⁻¹) had no significant effect on the contractile activity of each colonic smooth muscle strip. Hexamethonium given 3 minute before administration of areca (100 g•L⁻¹) had no significant effects on the action of areca.
DISCUSSION
There are many diseases which are caused by colonic motility disorder or accompany with colonic motility abnormality, such as constipation, diarrhea, irritable bowel syndrome and so on[3-11]. There are some reports on the study of normal colonic motility and intestinal diseases that are connected with colonic motility[12-25]. The studies on how to treat the diseases that are caused by colonic motility disorder have also been reported[26-35]. But it still needs a long time for us to recognize the colonic motility completely.
Recently, the effects of Chinese herbals on the gastrointestinal motility have been reported[36-46]. Areca had been used to treat abdominal distention, constipation, abdominal pain and non-ulcer dyspepsia, which were considered to be connected with intestinal motility disorder[47-49]. Whether the clinical use is connected with its effects on colonic motility The present study revealed that areca dose dependently stimulated the contractions of proximal and distal colonic smooth muscle strips of rats. The exciting actions suggested that areca might caused the colonic contents to be mixed, stirred, promoted, and even excreted. These results can partly explained why areca was used to treat intestinal motility disorder.
Areca has been showed to stimulate both cholinergic M and N receptors. Our results showed that the stimulating effects of areca were partly blocked by atropine but not by hexamethonium. Our results suggested that the stimulating effects of areca on rat colonic smooth muscle strips were relevant with M receptor but irrelevant with N receptor. When M receptor was stimulated, the potential sensitive Ca2+ channel was opened, which will cause the influx of extracellular Ca2+ and then cause the contraction of smooth muscle[50]. Areca might stimulate M receptor and then cause the concentration of intracellular Ca2+ increased, areca might also act on the Ca2+ channel receptor directly, which still need to be further studied. In conclusion, areca stimulates the contractile activity of colonic smooth muscle of rats in vitro. The effect of areca is partly relevant with M receptor, but irrelevant with N receptor.
Footnotes
Edited by Wang YQ
Supported by the Natural Scientific Foundation of Shandong Province, No.Y2001C06
References
- 1.Xie DP, Li W, Qu SY, Zheng TZ, Yang YL, Ding YH, Wei YL. Effects of ranitidine and cimetidine on the contractile activity of colonic smooth muscle strips in rats. Zhongguo Yaolixue Yu Dulixue Zazhi. 2001;15:12–16. [Google Scholar]
- 2.Qu SY, Zheng TZ, Li W. Comparative study of ranitidine and cimetidine on contractile activity of isolated gastric muscle strips in rats. Xin Xiaohuabingxue Zazhi. 1997;5:75–76. [Google Scholar]
- 3.Rajapakse R, Warman J, Korelitz BI. Colchicine for persistent constipation after total abdominal colectomy with ileorectostomy for colonic inertia. J Clin Gastroenterol. 2001;33:81–84. doi: 10.1097/00004836-200107000-00022. [DOI] [PubMed] [Google Scholar]
- 4.O'Sullivan MA, O'Morain CA. Bacterial supplementation in the irritable bowel syndrome. A randomised double-blind placebo-controlled crossover study. Dig Liver Dis. 2000;32:294–301. doi: 10.1016/s1590-8658(00)80021-3. [DOI] [PubMed] [Google Scholar]
- 5.Chey WY, Jin HO, Lee MH, Sun SW, Lee KY. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol. 2001;96:1499–1506. doi: 10.1111/j.1572-0241.2001.03804.x. [DOI] [PubMed] [Google Scholar]
- 6.Coulie B, Camilleri M, Bharucha AE, Sandborn WJ, Burton D. Colonic motility in chronic ulcerative proctosigmoiditis and the effects of nicotine on colonic motility in patients and healthy subjects. Aliment Pharmacol Ther. 2001;15:653–663. doi: 10.1046/j.1365-2036.2001.00959.x. [DOI] [PubMed] [Google Scholar]
- 7.Bonapace ES, Maurer AH, Davidoff S, Krevsky B, Fisher RS, Parkman HP. Whole gut transit scintigraphy in the clinical evaluation of patients with upper and lower gastrointestinal symptoms. Am J Gastroenterol. 2000;95:2838–2847. doi: 10.1111/j.1572-0241.2000.03195.x. [DOI] [PubMed] [Google Scholar]
- 8.Borum ML. Irritable bowel syndrome. Prim Care. 2001;28:523–538, vi. doi: 10.1016/s0095-4543(05)70051-8. [DOI] [PubMed] [Google Scholar]
- 9.Knowles CH, Scott SM, Lunniss PJ. Slow transit constipation: A disorder of pelvic autonomic nerves. Dig Dis Sci. 2001;46:389–401. doi: 10.1023/a:1005665218647. [DOI] [PubMed] [Google Scholar]
- 10.Knowles CH, Nickols CD, Scott SM, Bennett NI, de Oliveira RB, Chimelli L, Feakins R, Williams NS, Martin JE. Smooth muscle inclusion bodies in slow transit constipation. J Pathol. 2001;193:390–397. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH797>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 11.Ringel Y, Sperber AD, Drossman DA. Irritable bowel syndrome. Annu Rev Med. 2001;52:319–338. doi: 10.1146/annurev.med.52.1.319. [DOI] [PubMed] [Google Scholar]
- 12.Eglen RM. Muscarinic receptors and gastrointestinal tract smooth muscle function. Life Sci. 2001;68:2573–2578. doi: 10.1016/s0024-3205(01)01054-2. [DOI] [PubMed] [Google Scholar]
- 13.Plattner V, Leray V, Leclair MD, Aubé AC, Cherbut C, Galmiche JP. Interleukin-8 increases acetylcholine response of rat intestinal segments. Aliment Pharmacol Ther. 2001;15:1227–1232. doi: 10.1046/j.1365-2036.2001.01009.x. [DOI] [PubMed] [Google Scholar]
- 14.Percy WH, Brunz JT, Burgers RE, Fromm TH, Merkwan CL, van Dis J. Interrelationship between colonic muscularis mucosae activity and changes in transmucosal potential difference. Am J Physiol Gastrointest Liver Physiol. 2001;281:G479–G489. doi: 10.1152/ajpgi.2001.281.2.G479. [DOI] [PubMed] [Google Scholar]
- 15.Lin VW, Hsiao I, Goodwin D, Perkash I. Functional magnetic stimulation facilitates colonic transit in rats. Arch Phys Med Rehabil. 2001;82:969–972. doi: 10.1053/apmr.2001.23290. [DOI] [PubMed] [Google Scholar]
- 16.Shafik A, El-Sibai O, Ahmed A. Study of the mechanism underlying the difference in motility between the large and small intestine: the "single" and "multiple" pacemaker theory. Front Biosci. 2001;6:B1–B5. doi: 10.2741/a584. [DOI] [PubMed] [Google Scholar]
- 17.Onori L, Aggio A, Taddei G, Ciccocioppo R, Severi C, Carnicelli V, Tonini M. Contribution of NK3 tachykinin receptors to propulsion in the rabbit isolated distal colon. Neurogastroenterol Motil. 2001;13:211–219. doi: 10.1046/j.1365-2982.2001.00261.x. [DOI] [PubMed] [Google Scholar]
- 18.Herve S, Leroi AM, Mathiex-Fortunet H, Garnier P, Karoui S, Menard JF, Ducrotte P, Denis P. Effects of polyethylene glycol 4000 on 24-h manometric recordings of left colonic motor activity. Eur J Gastroenterol Hepatol. 2001;13:647–654. doi: 10.1097/00042737-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Al-Saffar A, Hellström PM. Contractile responses to natural tachykinins and selective tachykinin analogs in normal and inflamed ileal and colonic muscle. Scand J Gastroenterol. 2001;36:485–493. [PubMed] [Google Scholar]
- 20.Sun WM, Hasler WL, Lien HC, Montague J, Owyang C. Nizatidine enhances the gastrocolonic response and the colonic peristaltic reflex in humans. J Pharmacol Exp Ther. 2001;299:159–163. [PubMed] [Google Scholar]
- 21.Portincasa P, Moschetta A, Giampaolo M, Palasciano G. Diffuse gastrointestinal dysmotility by ultrasonography, manometry and breath tests in colonic inertia. Eur Rev Med Pharmacol Sci. 2000;4:81–87. [PubMed] [Google Scholar]
- 22.Carini F, Lecci A, Tramontana M, Giuliani S, Maggi CA. Tachykinin NK (2) receptors and enhancement of cholinergic transmission in the inflamed rat colon: An in vivo motility study. Br J Pharmacol. 2001;133:1107–1113. doi: 10.1038/sj.bjp.0704164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye JH, Ponnudurai R, Schaefer R. Ondansetron: A selective 5-HT (3) receptor antagonist and its applications in CNS-related disorders. CNS Drug Rev. 2001;7:199–213. doi: 10.1111/j.1527-3458.2001.tb00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lecci A, Carini F, Tramontana M, D'Aranno V, Marinoni E, Crea A, Bueno L, Fioramonti J, Criscuoli M, Giuliani S, et al. Nepadutant pharmacokinetics and dose-effect relationships as tachykinin NK2 receptor antagonist are altered by intestinal inflammation in rodent models. J Pharmacol Exp Ther. 2001;299:247–254. [PubMed] [Google Scholar]
- 25.Bassotti G, Fratini M. Of tubes and men: studying manometrically the effects of laxatives on colonic motility. Eur J Gastroenterol Hepatol. 2001;13:631–633. doi: 10.1097/00042737-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Crowell MD. The role of serotonin in the pathophysiology of irritable bowel syndrome. Am J Manag Care. 2001;7:S252–S260. [PubMed] [Google Scholar]
- 27.Menzies JR, McKee R, Corbett AD. Differential alterations in tachykinin NK2 receptors in isolated colonic circular smooth muscle in inflammatory bowel disease and idiopathic chronic constipation. Regul Pept. 2001;99:151–156. doi: 10.1016/s0167-0115(01)00244-0. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz D, Stollman N. University of Miami Division of Clinical Pharmacology therapeutic rounds: irritable bowel syndrome-pathophysiology, diagnosis, and treatment. Am J Ther. 2000;7:265–272. doi: 10.1097/00045391-200007040-00007. [DOI] [PubMed] [Google Scholar]
- 29.Talley NJ. Drug therapy options for patients with irritable bowel syndrome. Am J Manag Care. 2001;7:S261–S267. [PubMed] [Google Scholar]
- 30.Niedzielin K, Kordecki H, Birkenfeld B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2001;13:1143–1147. doi: 10.1097/00042737-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Villanueva A, Domínguez-Muñoz JE, Mearin F. Update in the therapeutic management of irritable bowel syndrome. Dig Dis. 2001;19:244–250. doi: 10.1159/000050687. [DOI] [PubMed] [Google Scholar]
- 32.Beglinger C. Tegaserod: A novel, selective 5-HT4 receptor partial agonist for irritable bowel syndrome. Int J Clin Pract. 2002;56:47–51. [PubMed] [Google Scholar]
- 33.De Ponti F, Tonini M. Irritable bowel syndrome: new agents targeting serotonin receptor subtypes. Drugs. 2001;61:317–332. doi: 10.2165/00003495-200161030-00001. [DOI] [PubMed] [Google Scholar]
- 34.De Schryver AM, Samsom M. New developments in the treatment of irritable bowel syndrome. Scand J Gastroenterol Suppl. 2000;232:38–42. [PubMed] [Google Scholar]
- 35.Bouras EP, Camilleri M, Burton DD, Thomforde G, McKinzie S, Zinsmeister AR. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001;120:354–360. doi: 10.1053/gast.2001.21166. [DOI] [PubMed] [Google Scholar]
- 36.Tian XL, Mourelle M, Li YL, Guarner F, Malagelada JR. The role of Chinese herbal medicines in a rat model of chronic colitis. World J Gastroenterol. 2000;6:40. [Google Scholar]
- 37.Zheng BJ. Understanding of gastrointestinal prokinetic Chinese drugs. Zhongyi Zazhi. 1996;37:697–698. [Google Scholar]
- 38.Yang YL, Zheng TZ, Qu SY, Li W, Xie DP. Action of Binglang on contractile activity of isolated small intestinal strips in rats. Lanzhou Daxue Xuebao. 2000;36:43–46. [Google Scholar]
- 39.Yang YL, Zheng TZ, Qu SY, Li W, Xie DP, Ding YH, Wei YL. Action of Zhishi on the smooth muscle of isolated small intestine in rats. Xibei Shifan Daxue Xuebao. 1998;34:69–72. [Google Scholar]
- 40.Xie DP, Li W, Qu SY, Zheng TZ, Yang YL, Ding YH, Wei YL, Zhang MY. Effect of Fructus Aurantii immaturus on contractile activity of colonic muscle strips in rats. Shandong Yike Daxue Xuebao. 2001;39:437–438. [Google Scholar]
- 41.Xie DP, Li W, Qu SY, Zheng TZ, Yang YL, Ding YH, Wei YL. Effect of Qing Pi on contractile of colonic muscle strips in rats. Lanzhou Yixueyuan Xuebao. 1998;24:1–3. [Google Scholar]
- 42.Song YJ. Treatment of chronic colitis by integration of traditional and western medicine method in 92 cases. Huaren Xiaohua Zazhi. 1998;6:454. [Google Scholar]
- 43.Li Y, Sun SY, Zhou Z. Effect of Chinese herbal medicines Xiaoshixingqi on gastrointestinal motility in mice. Xin Xiaohuabingxue Zazhi. 1997;5:153. [Google Scholar]
- 44.Chen RS, Zhang YF, Deng LM, Guo DQ. The Influences of Jiechang-Kangtai on the Movement Function of Intestine. Zhongguo Zhongxiyi Jiehe Piwei Zazhi. 1998;6:157–159. [Google Scholar]
- 45.Zhang HX, Ren P, Huang X, Li Y. Regulation of Chinese herbal on gastrointestinal hormones and gastrointestinal motility. Shijie Huaren Xiaohua Zazhi. 2000;8:1141–1144. [Google Scholar]
- 46.Dou DP, Cai G. Regulation of gastric motility. Shijie Huaren Xiaohua Zazhi. 1999;7:353–354. [Google Scholar]
- 47.Zhao XP, Zhang BL. Therapy of qingfutongchangchongji for 46 patients with intestinal disorder after abdomen operated. Shanxi Zhongyi. 1999;20:57–58. [Google Scholar]
- 48.Qin ZD. Abstracts about therapy of muxiangbinglangwan for patients with rectostenosis in 23 cases. Guangxi Zhongyiyao. 1999;22:33. [Google Scholar]
- 49.Xiao ChQ, Huo GQ. Therapy of simowubeitang for 60 patients with non-ulcer dyspepsia. Shijie Huaren Xiaohua Zazhi. 2000;8:98. [Google Scholar]
- 50.Zhou L, Ke MY. Textbook of Gastrointestinal Motility-Basic and Clinical Aspects. Beijing: Science Press; 1999. p. 202. [Google Scholar]


