Abstract
AIM: To investigate the expression of the transforming growth factor beta 1 (TGF- beta 1) mRNA in different stages of alcoholic liver disease (ALD) and its clinical value.
METHODS: One hundred and seven male alcoholics were grouped by clinical findings into four groups: Alcohol abusers without liver impairment (n = 22), alcoholic steatosis (n = 30); alcoholic hepatitis (n = 31); and alcoholic cirrhosis (n = 24). Using peripheral blood mononuclear cells (PBMC) as samples the gene expression of TGF-beta 1 was examined quantitatively by reverse transcription polymerase chain reaction (RT-PCR) and dot blot. There are 34 healthy subjects served as control.
RESULTS: The expression of TGF-beta 1 from all ALD patients was significantly greater than that in controls (1.320 ± 1.162 vs 0.808 ± 0.276, P < 0.001). The differences of the expressions were significant between the patients from each groups (alcoholic steatosis, alcoholic hepatitis and alcoholic cirrhosis) and the controls (1.168 ± 0.852, 1.462 ± 1.657, 1.329 ± 0.610 vs 0.808 ± 0.276, P < 0.050). No significant differences of TGF-beta 1 mRNA expression were observed between alcohol abusers without liver impairment and controls. The expressions in patients with alcoholic hepatitis and alcoholic cirrhosis were significantly greater than that in alcohol abusers respectively (1.462 ± 1.657, 1.329 ± 0.610 vs 0.841 ± 0.706, P < 0.050). No significant differences of TGF-beta 1 mRNA expression were observed between alcoholic fatty liver men and alcohol abusers.
CONCLUSION: TGF-beta 1 expression level can be a risk factor for alcoholic liver disease and might be related to the inflammatory activity and fibrosis of the liver in patients.
INTRODUCTION
Alcoholic liver disease (ALD) is the most common cause of liver disease in late stage in the developed world[1], and ranked as the second cause of hepatic cirrhosis in China now. The increased deposition of extracellular matrix in the liver is a key factor in the developing of the disease.
In recent yeas, the role of TGF-beta 1 in ALD has been much emphasized[2,3], but the study of expression of TGF-beta 1 in peripheral blood mononuclear cells (PBMC) from ALD patients has scarcely been performed. This study was aimed at quantitative analysis of the expression of TGF-beta 1 mRNA in PBMC of ALD patients through reverse transcription-polymerase chain reaction (RT-PCR) technique and dot blot.
MATERIALS AND METHODS
Subjects
The subjects was from the epidemiologic survey of ALD in Zhejiang province including 107 male alcoholics, aged 25-70 yeas (χ = 43.32 ± 10.39), who had ingested more than 40 g of alcohol a day for more than 5 years. According to the diagnostic criteria of Nanjing conference, there were 22 alcohol abuser without hepatic impairment, 30 alcoholic steatosis, 31 alcoholic hepatitis and 31 alcoholic cirrhosis. Percutaneous liver biopsy was performed in all cases, C and B hepatitis were ruled out by appropriate serological tests. 34 healthy subjects served as control, with age range from 26 to 68 years (χ = 45.60 ± 10.08). There were no significant differences in their ages among the alcoholics and healthy controls.
Design and synthesis of primers
TGF-β1primers and probe were synthesized by Gibco BRL Co. The forward primer, reverse primer and probe were 5'-GGACACCAACTATTGCTTCAG-3', 5'-TCCAGGCTCCAAATGTAGG-3' and 5'-CAGCTGTACATTGACTTCCGCAAGGACCT-3' respectively. β-actin primers were synthesized by the Academy of Microrganism Science of Lubeck University, and kindly provided by Prof. Chen Zhi. The sequence of the primes were 5'-TTCCAGCCTTCCTTCCTGG-3' (forward primer) and 5'-TTGCGCTCAGGAGGAGCAAT-3' (reverse primer). The Primers were designed according to the previously reported sequence as shown in Roulot's report[4].
Samples preparation and RNA extraction
Blood samples 3 mL were mixed with Ficoll fluid to isolate the PBMC and then 150 μL Trizol Reagent (Gibco BRL Co.) was added. Total cellular RNA was extracted by the guanidinium thiocyanate/phenol chloroform single-step method. RNA was washed in ethanol and dissolved in diethyl pyrocarbonate (DEPC)-treated water.
RT-PCR
The RT-PCR mixture consisted of RNA of each sample 5 μL, 2 mM dNTP 5 μL, 5 × first stand buffer 5 μL, 100 mM TT 2.5 μL, 100 pmol/μL oligod (T) 15 μL, MMLV-RT100 unit, Rnase 1 μL (Pharmacia Biotech) to a total volume of 20 μL. The reaction was allowed to proceed at 41 °C for 1 h.
The PCR mixture consisted of RT-PCR products 5 μL, 10 × PCR buffer 5 μL, 20 pmol of each primer, 2 mM dNTP 5 μL and Taq polymerase 1.25 unit (Gibco BRL Co.) to a total volume of 40 μL. This reactioo mixture was overlaid with 1 drop of mineral oil.
PCR was carried out in a DNA thermal cycler (Perkin-Elmer Cetus) for 30 cycles; after initial denaturation by heating to 95 °C for 2 min, each cycle consisted of denaturation at 94 °C for 60 s, annealing at 56 °C for 60 s and extension at 72 °C for 45 s. An extra extension at 72 °C for 10 min was performed after the final cycle.
Dot blot
The probe was labelled using a DIG oligonucleotide 3'-end labeling kit (Boehringer Mannheim). The efficiency of the labeling reaction was checked by comparing with the labelled control-oligonucleotide by direct detection.5 μL PCR products was dropped to on a nylon membrane. The membranes were hybridized at 60 °C for 6 h after being denatured, fixed, prehybridized and then washed and stained.
Quantitative analysis
Quantification of TGF-beta 1 mRNA was carried out using β-actin mRNA as an internal standard because the transcription levels of β-actin were very stable in all types of tissues. The dots on the membranes were quantitatively analyzed and pictures were taken using IS-1000 multifunction agarose imaging analysis system (Alpha Innotech Co.). The relative index (RI) of mRNA was calculated using a formula as RI = scan value of TGF-beta 1 mRNA dot/scan value of β-actin mRNA dot[5]. Here RI might represent the relative levels of TGF-β1 mRNA in PBMC.
Statistics
The data were presented as means ± s, all the statstics were done with the software Statistical Package for the Social Sciences (SPSS) and the P value was considered significant when it was less than 0.05.
RESULTS
The expression of TGF-beta 1 from all ALD patients was significantly greater than that in the controls (1.320 ± 1.162 vs 0.808 ± 0.276, t = 3.811, P = 0.0001, Figure 1).
Figure 1.
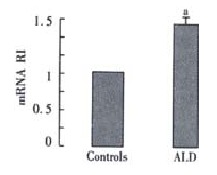
TGF-β1 mRNA RI values in ALD and Controls' aP < 0.05 vs controls
The expressions in patients with the alcoholic steatosis, alcoholic hepatitis and alcoholic cirrhosis were significantly greater than the expression in the controls respectively (1.168 ± 0.852, 1.462 ± 1.657, 1.329 ± 0.610 vs 0.808 ± 0.276, t = 2.213, P = 0.017, t = 2.171, P = 0.019, t = 3.915, P = 0.0002). No significant differences of TGF-beta 1 mRNA expression were observed between alcohol abusers and the controls (Figure 2).
Figure 2.
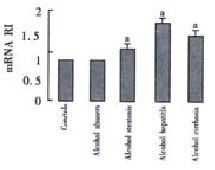
The TGF-beta 1 mRNA in alcohol abusers, alcohol steatosis, alcohol hepatitis, alcohol cirrhosis and controls.aP < 0.05 vs controls
The expression in patients with alcoholic hepatitis and alcoholic hepatic cirrhosis was significantly greater than that in alcohol abusers respectively (1.462 ± 1.657, 1.329 ± 0.610 vs 0.841 ± 0.706, t = 1.859, P = 0.035, t = 2.495, P = 0.008), No significant differences of TGF-beta 1 mRNA expression were observed between alcoholic fatty liver males and alcohol abusers ( (Figure 3).
Figure 3.
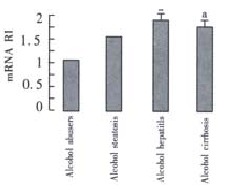
The TGF-beta 1 mRNA in alcohol abusers, alcohol steatosis, alcohol hepatitis, alcohol cirrhosis and alcohol abusers. aP < 0.05 vs alcohol abusers
DISCUSSION
In Western societies roughly 50% of all cases of liver cirrhosis are related with alcohol abuse. The increased deposition of extracellular matrix in the liver is a key factor in the morbidity and mortality of alcoholic liver disease[6]. This increased fibrosis may be due to a superabundance of profibrogenic factors such as transforming growth factor-beta 1 and/or relative decreacement of the factors that inhibit fibrogenesis such as collagenase or interferon[7-10].Transforming growth beta 1 (TGF-beta 1) is believed to play a key role in enhancing fibrogenesis and inhibiting extracellular matrix degradation[11]. Acetaldehyde, the oxidative metabolite of ethanol damages cell membranes, initiates lipid peroxidation and forms noxious protein adducts, which result in the activation of Kupffer cells and perisinusoidal lipocytes/portal fibroblasts[12-18]. The activation of lipocytes and fibroblasts to a proliferative and collagen-producing myofibroblast-like phenotype is triggered by the release of TGF-beta 1 from the activated Kupffer cells. In the liver, TGF-beta 1 is primarily responsible for activation of lipocytes, which are the main source of extracellular matrix proteins. Their deposition play a key role in the development of alcoholic liver cirrhosis[19-21].
A number of investigators have shown increase of TGF-beta 1 mRNA and protein levels in animal models of hepatic fibrosis[22-27]. A few studies also demonstrated increased levels of TGF-beta 1 mRNA and protein in animal models of ALD. Matsuoka et al[28] examined Kupffer cells and hepatic lipocytes isolated from a rat model of alcoholic liver fibrosis. The collagen formation was increased significantly in alcohol-fed group more than the control, which was completely inhibited by anti-transforming growth factor beta IgG. The major peak of the molecular weight was about 25000 which was revealed by high-performance liquid chromatography and demonstrated with Northern blotting and hybridization. Kamimura et al[29] examined Kupffer cell gene expression of TGF beta 1 in the rat model of ALD. Kupffer cells were isolated from the model after 10 and 17 wk of intragastric ethanol infusion, the protein and mRNA levels of TGF beta 1 were significantly increased by 143% and 204% at 10 wk and 238% and 295% at 17 wk respectively in the ethanol-fed rats.
A number of studies have suggested a role for TGF-beta 1 in human liver disease[30].In our study, the expression of TGF-beta 1 was significantly greater in all the ALD patients than in the controls and the expression in the patients with the alcoholic steatosis, alcoholic hepatitis and alcoholic cirrhosis was significantly greater than their expressions in the controls respectively. No significant differences of TGF-beta 1 mRNA expression were observed between alcohol abusers and the controls. The results demonstrated that the expression of TGF-beta 1 was related with ALD. Our study also showed that the expression of patients with alcoholic hepatitis and alcoholic cirrhosis was significantly greater than their expression in alcohol abusers respectively, and no significant differences of TGF-beta 1 mRNA expression were observed between alcoholic steatosis men and alcohol abusers. These results indicated that the expression of TGF-beta 1 was highes in more active and advanced ALD patients. Santos et al[31] also demonstrated similar results in liver specimens. The presence of TGF-beta1 expression could be recognized as a risk factor for active and advanced alcoholic liver disease.
Footnotes
Edited by Wu XN and Ma JY
References
- 1.Kumar S, Stauber RE, Gavaler JS, Basista MH, Dindzans VJ, Schade RR, Rabinovitz M, Tarter RE, Gordon R, Starzl TE. Orthotopic liver transplantation for alcoholic liver disease. Hepatology. 1990;11:159–164. doi: 10.1002/hep.1840110202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malizia G, Brunt EM, Peters MG, Rizzo A, Broekelmann TJ, McDonald JA. Growth factor and procollagen type I gene expression in human liver disease. Gastroenterology. 1995;108:145–156. doi: 10.1016/0016-5085(95)90019-5. [DOI] [PubMed] [Google Scholar]
- 3.Czaja MJ, Weiner FR, Flanders KC, Giambrone MA, Wind R, Biempica L, Zern MA. In vitro and in vivo association of transforming growth factor-beta 1 with hepatic fibrosis. J Cell Biol. 1989;108:2477–2482. doi: 10.1083/jcb.108.6.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roulot D, Durand H, Coste T, Rautureau J, Strosberg AD, Benarous R, Marullo S. Quantitative analysis of transforming growth factor beta 1 messenger RNA in the liver of patients with chronic hepatitis C: Absence of correlation between high levels and severity of disease. Hepatology. 1995;21:298–304. [PubMed] [Google Scholar]
- 5.Yang B, Li F. Quantitative analysis of TGF-alpha and EGFR mRNA in laryngeal carcinoma tissues. Chin Med J ( Engl) 1999;112:1088–1092. [PubMed] [Google Scholar]
- 6.Breitkopf K, Lahme B, Tag CG, Gressner AM. Expression and matrix deposition of latent transforming growth factor beta binding proteins in normal and fibrotic rat liver and transdifferentiating hepatic stellate cells in culture. Hepatology. 2001;33:387–396. doi: 10.1053/jhep.2001.21996. [DOI] [PubMed] [Google Scholar]
- 7.Bai WY, Yao XX, Feng LY. Current situation in studies of hepatic fibrosis. Shijie Huaren Xiaohua Zazhi. 2000;8:1267–1268. [Google Scholar]
- 8.Blazejewski S, Preaux AM, Mallat A, Brocheriou I, Mavier P, Dhumeaux D, Hartmann D, Schuppan D, Rosenbaum J. Human myofibroblastlike cells obtained by outgrowth are representative of the fibrogenic cells in the liver. Hepatology. 1995;22:788–797. [PubMed] [Google Scholar]
- 9.Milani S, Herbst H, Schuppan D, Grappone C, Pellegrini G, Pinzani M, Casini A, Calabró A, Ciancio G, Stefanini F. Differential expression of matrix-metalloproteinase-1 and -2 genes in normal and fibrotic human liver. Am J Pathol. 1994;144:528–537. [PMC free article] [PubMed] [Google Scholar]
- 10.Graham MF, Bryson GR, Diegelmann RF. Transforming growth factor beta 1 selectively augments collagen synthesis by human intestinal smooth muscle cells. Gastroenterology. 1990;99:447–453. doi: 10.1016/0016-5085(90)91028-5. [DOI] [PubMed] [Google Scholar]
- 11.Liou F, Liou JX. The role of transforming growth factor-beta 1 in hepatic fibrosis. Shijie Huaren Xiaohua Zazhi. 2000;8:86–88. [Google Scholar]
- 12.Chen J, Robinson NC, Schenker S, Frosto TA, Henderson GI. Formation of 4-hydroxynonenal adducts with cytochrome c oxidase in rats following short-term ethanol intake. Hepatology. 1999;29:1792–1798. doi: 10.1002/hep.510290611. [DOI] [PubMed] [Google Scholar]
- 13.Xu D, Thiele GM, Beckenhauer JL, Klassen LW, Sorrell MF, Tuma DJ. Detection of circulating antibodies to malondialdehyde-acetaldehyde adducts in ethanol-fed rats. Gastroenterology. 1998;115:686–692. doi: 10.1016/s0016-5085(98)70148-9. [DOI] [PubMed] [Google Scholar]
- 14.Li CJ, Nanji AA, Siakotos AN, Lin RC. Acetaldehyde-modified and 4-hydroxynonenal-modified proteins in the livers of rats with alcoholic liver disease. Hepatology. 1997;26:650–657. doi: 10.1002/hep.510260317. [DOI] [PubMed] [Google Scholar]
- 15.Israel Y. Antibodies against ethanol-derived protein adducts: pathogenic implications. Gastroenterology. 1997;113:353–355. [PubMed] [Google Scholar]
- 16.Clot P, Parola M, Bellomo G, Dianzani U, Carini R, Tabone M, Aricò S, Ingelman-Sundberg M, Albano E. Plasma membrane hydroxyethyl radical adducts cause antibody-dependent cytotoxicity in rat hepatocytes exposed to alcohol. Gastroenterology. 1997;113:265–276. doi: 10.1016/s0016-5085(97)70104-5. [DOI] [PubMed] [Google Scholar]
- 17.Tuma DJ, Thiele GM, Xu D, Klassen LW, Sorrell MF. Acetaldehyde and malondialdehyde react together to generate distinct protein adducts in the liver during long-term ethanol administration. Hepatology. 1996;23:872–880. doi: 10.1002/hep.510230431. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama H, Nagata S, Moriya S, Kato S, Ito T, Kamegaya K, Ishii H. Hepatic fibrosis produced in guinea pigs by chronic ethanol administration and immunization with acetaldehyde adducts. Hepatology. 1995;21:1438–1442. [PubMed] [Google Scholar]
- 19.Fan K. Regulatory effects of lipopolysaccharide in murine macrophage proliferation. World J Gastroenterol. 1998;4:137–139. doi: 10.3748/wjg.v4.i2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang GC, Zhang JS, Zhang YE. Effects of retinoic acid on proliferation, phenotype and expression of cyclin-dependent kinase inhibitors in TGF-beta1-stimulated rat hepatic stellate cells. World J Gastroenterol. 2000;6:819–823. doi: 10.3748/wjg.v6.i6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gressner AM, Bachem MG. Molecular mechanisms of liver fibrogenesis--a homage to the role of activated fat-storing cells. Digestion. 1995;56:335–346. doi: 10.1159/000201257. [DOI] [PubMed] [Google Scholar]
- 22.Raghow B, Irish P, Kang AH. Coordinate regulation of transforming growth factor beta gene expression and cell proliferation in hamster lungs undergoing bleomycin-induced pulmonary fibrosis. J Clin Invest. 1989;84:1836–1842. doi: 10.1172/JCI114369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kresina TF, He Q, Degli Esposti S, Zern MA. Gene expression of transforming growth factor beta 1 and extracellular matrix proteins in murine Schistosoma mansoni infection. Gastroenterology. 1994;107:773–780. doi: 10.1016/0016-5085(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 24.Milani S, Herbst H, Schuppan D, Stein H, Surrenti C. Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am J Pathol. 1991;139:1221–1229. [PMC free article] [PubMed] [Google Scholar]
- 25.Krull NB, Zimmermann T, Gressner AM. Spatial and temporal patterns of gene expression for the proteoglycans biglycan and decorin and for transforming growth factor-beta 1 revealed by in situ hybridization during experimentally induced liver fibrosis in the rat. Hepatology. 1993;18:581–589. [PubMed] [Google Scholar]
- 26.Xiang DD, Wei YL, Li QF. Molecular mechanism of transforming growth factor β1 on Ito cell. Shijie Huaren Xiaohua Zazhi. 1999;7:980–981. [Google Scholar]
- 27.Fang C, Lindros KO, Badger TM, Ronis MJ, Ingelman-Sundberg M. Zonated expression of cytokines in rat liver: effect of chronic ethanol and the cytochrome P450 2E1 inhibitor, chlormethiazole. Hepatology. 1998;27:1304–1310. doi: 10.1002/hep.510270516. [DOI] [PubMed] [Google Scholar]
- 28.Matsuoka M, Tsukamoto H. Stimulation of hepatic lipocyte collagen production by Kupffer cell-derived transforming growth factor beta: implication for a pathogenetic role in alcoholic liver fibrogenesis. Hepatology. 1990;11:599–605. doi: 10.1002/hep.1840110412. [DOI] [PubMed] [Google Scholar]
- 29.Kamimura S, Tsukamoto H. Cytokine gene expression by Kupffer cells in experimental alcoholic liver disease. Hepatology. 1995;22:1304–1309. [PubMed] [Google Scholar]
- 30.Bedossa P, Peltier E, Terris B, Franco D, Poynard T. Transforming growth factor-beta 1 (TGF-beta 1) and TGF-beta 1 receptors in normal, cirrhotic, and neoplastic human livers. Hepatology. 1995;21:760–766. [PubMed] [Google Scholar]
- 31.Santos RM, Norton P, Degli Esposti S, Zern MA. TGF-beta isoforms in alcoholic liver disease. J Gastroenterol. 1998;33:383–389. doi: 10.1007/s005350050100. [DOI] [PubMed] [Google Scholar]


