Abstract
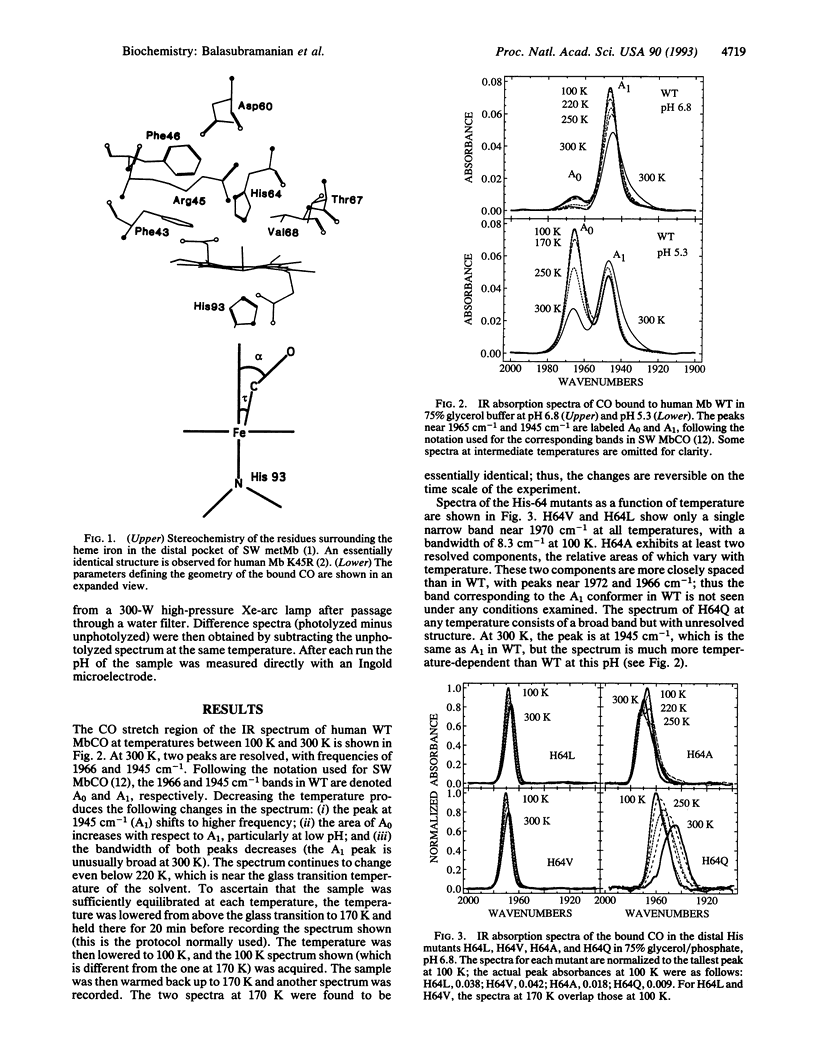
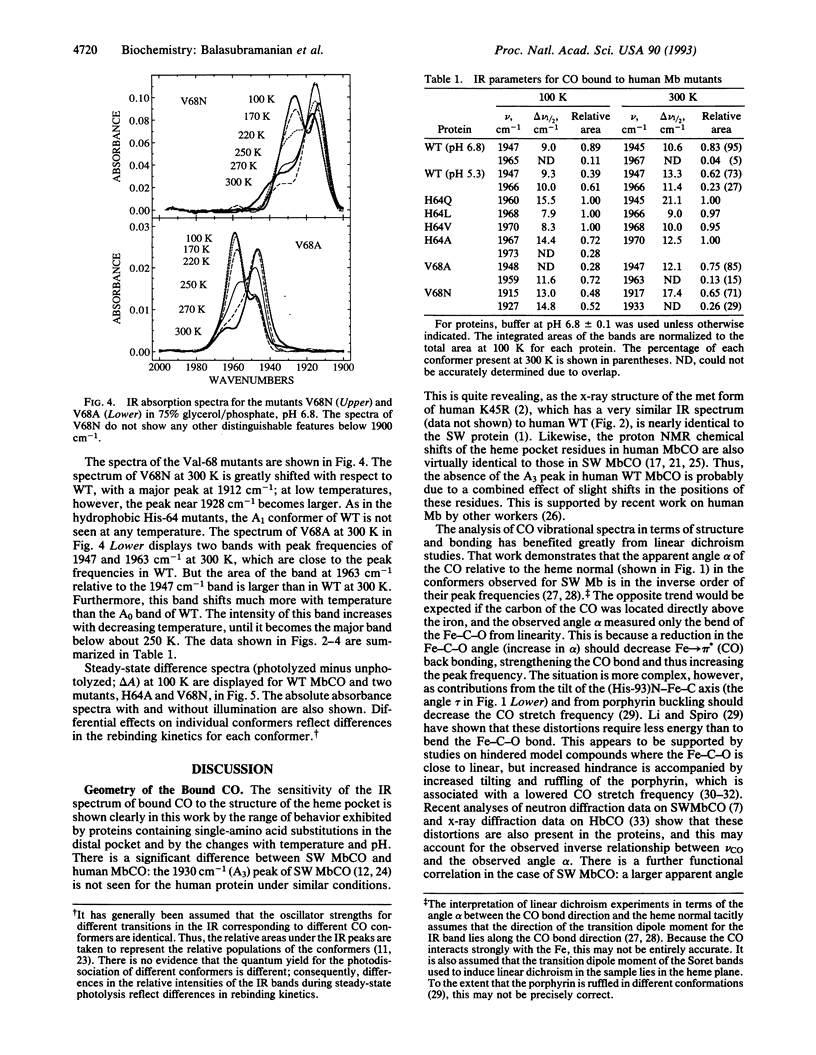
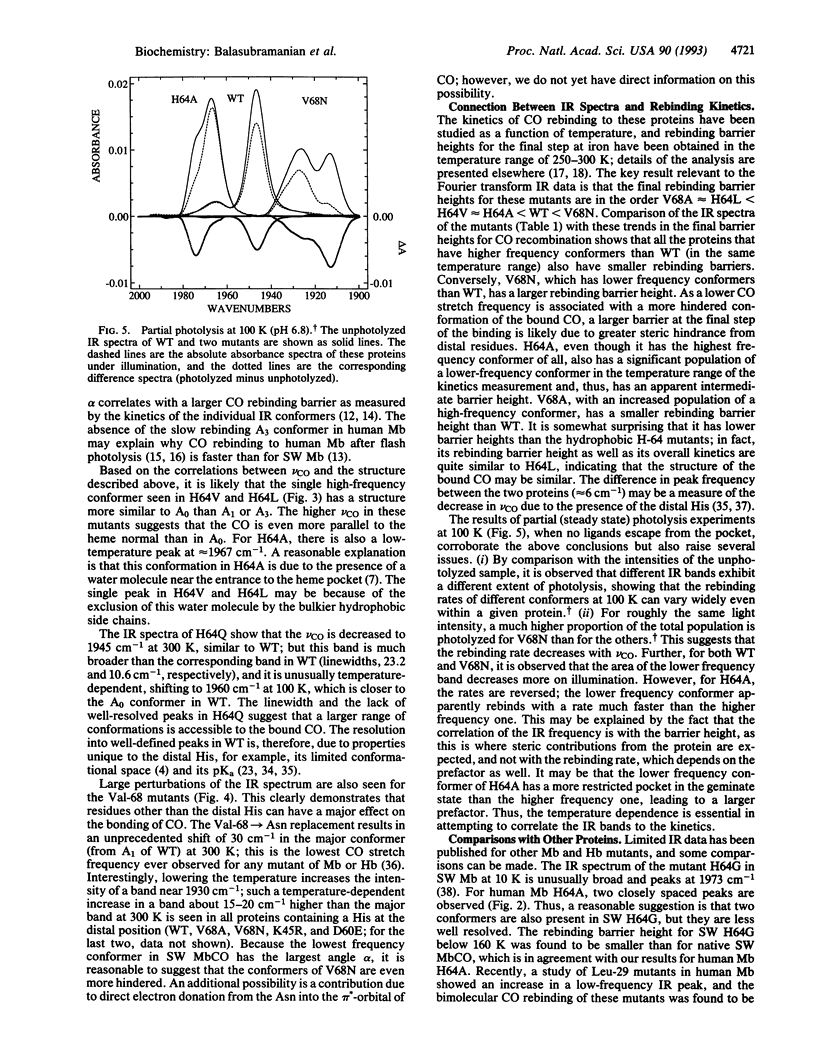
The infrared spectra of CO bound to human myoglobin and myoglobin mutants at positions His-64, Val-68, Asp-60, and Lys-45 on the distal side have been measured between 100 and 300 K. Large differences are observed with mutations at His-64 and Val-68 as well as with temperature and pH. Although distal His-64 is found to affect CO bonding, Val-68 also plays a major role. The variations are analyzed qualitatively in terms of a simple model involving steric interaction between the bound CO and the distal residues. A strong correlation is found between the final barrier height to CO recombination and the CO stretch frequency: as compared to wild type, the barrier is smaller in those mutants that have a higher CO stretch frequency (vCO) and vice versa. Possible reasons for this correlation are discussed. It is emphasized that the temperature and pH dependence of both the kinetics and the infrared spectra must be measured to obtain a consistent picture.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi S., Sunohara N., Ishimori K., Morishima I. Structure and ligand binding properties of leucine 29(B10) mutants of human myoglobin. J Biol Chem. 1992 Jun 25;267(18):12614–12621. [PubMed] [Google Scholar]
- Alben J. O., Beece D., Bowne S. F., Doster W., Eisenstein L., Frauenfelder H., Good D., McDonald J. D., Marden M. C., Moh P. P. Infrared spectroscopy of photodissociated carboxymyoglobin at low temperatures. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3744–3748. doi: 10.1073/pnas.79.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A., Berendzen J., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Johnson J. B., Ormos P., Sauke T. B. Rebinding and relaxation in the myoglobin pocket. Biophys Chem. 1987 May 9;26(2-3):337–355. doi: 10.1016/0301-4622(87)80034-0. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S., Lambright D. G., Marden M. C., Boxer S. G. CO recombination to human myoglobin mutants in glycerol-water solutions. Biochemistry. 1993 Mar 9;32(9):2202–2212. doi: 10.1021/bi00060a011. [DOI] [PubMed] [Google Scholar]
- Braunstein D., Ansari A., Berendzen J., Cowen B. R., Egeberg K. D., Frauenfelder H., Hong M. K., Ormos P., Sauke T. B., Scholl R. Ligand binding to synthetic mutant myoglobin (His-E7----Gly): role of the distal histidine. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8497–8501. doi: 10.1073/pnas.85.22.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier C., Momenteau M., Dartyge E., Fontaine A., Tourillon G., Bianconi A., Verdaguer M. X-ray absorption spectroscopy of carbonyl basket handle Fe(II) porphyrins: the distortion of the tetrapyrrolic macrocycle. Biochim Biophys Acta. 1992 Feb 26;1119(2):169–174. doi: 10.1016/0167-4838(92)90387-s. [DOI] [PubMed] [Google Scholar]
- Carver T. E., Olson J. S., Smerdon S. J., Krzywda S., Wilkinson A. J., Gibson Q. H., Blackmore R. S., Ropp J. D., Sligar S. G. Contributions of residue 45(CD3) and heme-6-propionate to the biomolecular and geminate recombination reactions of myoglobin. Biochemistry. 1991 May 14;30(19):4697–4705. doi: 10.1021/bi00233a009. [DOI] [PubMed] [Google Scholar]
- Chance M. R., Campbell B. F., Hoover R., Friedman J. M. Myoglobin recombination at low temperature. Two phases revealed by Fourier transform infrared spectroscopy. J Biol Chem. 1987 May 25;262(15):6959–6961. [PubMed] [Google Scholar]
- Cheng X. D., Schoenborn B. P. Neutron diffraction study of carbonmonoxymyoglobin. J Mol Biol. 1991 Jul 20;220(2):381–399. doi: 10.1016/0022-2836(91)90020-7. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Brauman J. I., Doxsee K. M. Carbon monoxide binding to iron porphyrins. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6035–6039. doi: 10.1073/pnas.76.12.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collman J. P., Brauman J. I., Halbert T. R., Suslick K. S. Nature of O2 and CO binding to metalloporphyrins and heme proteins. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3333–3337. doi: 10.1073/pnas.73.10.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalvit C., Wright P. E. Assignment of resonances in the 1H nuclear magnetic resonance spectrum of the carbon monoxide complex of sperm whale myoglobin by phase-sensitive two-dimensional techniques. J Mol Biol. 1987 Mar 20;194(2):313–327. doi: 10.1016/0022-2836(87)90378-0. [DOI] [PubMed] [Google Scholar]
- Derewenda Z., Dodson G., Emsley P., Harris D., Nagai K., Perutz M., Renaud J. P., Reynaud J. P. Stereochemistry of carbon monoxide binding to normal human adult and Cowtown haemoglobins. J Mol Biol. 1990 Feb 5;211(3):515–519. doi: 10.1016/0022-2836(90)90262-k. [DOI] [PubMed] [Google Scholar]
- Dlott D. D., Frauenfelder H., Langer P., Roder H., DiIorio E. E. Nanosecond flash photolysis study of carbon monoxide binding to the beta chain of hemoglobin Zürich [beta 63(E7)His leads to Arg]. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6239–6243. doi: 10.1073/pnas.80.20.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchsman W. H., Appleby C. A. CO and O2 complexes of soybean leghemoglobins: pH effects upon infrared and visible spectra. Comparisons with CO and O2 complexes of myoglobin and hemoglobin. Biochemistry. 1979 Apr 3;18(7):1309–1321. doi: 10.1021/bi00574a030. [DOI] [PubMed] [Google Scholar]
- Hubbard S. R., Hendrickson W. A., Lambright D. G., Boxer S. G. X-ray crystal structure of a recombinant human myoglobin mutant at 2.8 A resolution. J Mol Biol. 1990 May 20;213(2):215–218. doi: 10.1016/S0022-2836(05)80181-0. [DOI] [PubMed] [Google Scholar]
- Ikeda-Saito M., Lutz R. S., Shelley D. A., McKelvey E. J., Mattera R., Hori H. EPR characterization of the stereochemistry of the distal heme pocket of the engineered human myoglobin mutants. J Biol Chem. 1991 Dec 15;266(35):23641–23647. [PubMed] [Google Scholar]
- Kuriyan J., Wilz S., Karplus M., Petsko G. A. X-ray structure and refinement of carbon-monoxy (Fe II)-myoglobin at 1.5 A resolution. J Mol Biol. 1986 Nov 5;192(1):133–154. doi: 10.1016/0022-2836(86)90470-5. [DOI] [PubMed] [Google Scholar]
- Lambright D. G., Balasubramanian S., Boxer S. G. Ligand and proton exchange dynamics in recombinant human myoglobin mutants. J Mol Biol. 1989 May 5;207(1):289–299. doi: 10.1016/0022-2836(89)90456-7. [DOI] [PubMed] [Google Scholar]
- Lin S. H., Yu N. T., Tame J., Shih D., Renaud J. P., Pagnier J., Nagai K. Effect of the distal residues on the vibrational modes of the Fe-CO bond in hemoglobin studied by protein engineering. Biochemistry. 1990 Jun 12;29(23):5562–5566. doi: 10.1021/bi00475a021. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Houtchens R. A., Caughey W. S. Structure of carboxymyoglobin in crystals and in solution. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6042–6046. doi: 10.1073/pnas.76.12.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. N., Hansen P. A., Hochstrasser R. M. Iron-carbonyl bond geometries of carboxymyoglobin and carboxyhemoglobin in solution determined by picosecond time-resolved infrared spectroscopy. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5062–5066. doi: 10.1073/pnas.85.14.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikis D., Champion P. M., Springer B. A., Sligar S. G. Resonance raman investigations of site-directed mutants of myoglobin: effects of distal histidine replacement. Biochemistry. 1989 May 30;28(11):4791–4800. doi: 10.1021/bi00437a041. [DOI] [PubMed] [Google Scholar]
- Nagai M., Yoneyama Y., Kitagawa T. Unusual CO bonding geometry in abnormal subunits of hemoglobin M Boston and hemoglobin M Saskatoon. Biochemistry. 1991 Jul 2;30(26):6495–6503. doi: 10.1021/bi00240a021. [DOI] [PubMed] [Google Scholar]
- Ormos P., Braunstein D., Frauenfelder H., Hong M. K., Lin S. L., Sauke T. B., Young R. D. Orientation of carbon monoxide and structure-function relationship in carbonmonoxymyoglobin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8492–8496. doi: 10.1073/pnas.85.22.8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S. M., Ibers J. A. Stereochemistry of carbonylmetalloporphyrins. The structure of (pyridine)(carbonyl)(5, 10, 15, 20-tetraphenylprophinato)iron(II). J Am Chem Soc. 1976 Dec 8;98(25):8032–8036. doi: 10.1021/ja00441a025. [DOI] [PubMed] [Google Scholar]
- Ramsden J., Spiro T. G. Resonance Raman evidence that distal histidine protonation removes the steric hindrance to upright binding of carbon monoxide by myoglobin. Biochemistry. 1989 Apr 18;28(8):3125–3128. doi: 10.1021/bi00434a001. [DOI] [PubMed] [Google Scholar]
- Shimada H., Caughey W. S. Dynamic protein structures. Effects of pH on conformer stabilities at the ligand-binding site of bovine heart myoglobin carbonyl. J Biol Chem. 1982 Oct 25;257(20):11893–11900. [PubMed] [Google Scholar]
- Shimada H., Dong A., Matsushima-Hibiya Y., Ishimura Y., Caughey W. S. Distal His----Arg mutation in bovine myoglobin results in a ligand binding site similar to the abnormal beta site of hemoglobin Zurich (beta 63 His----Arg). Biochem Biophys Res Commun. 1989 Jan 16;158(1):110–114. doi: 10.1016/s0006-291x(89)80184-6. [DOI] [PubMed] [Google Scholar]
- Steinbach P. J., Ansari A., Berendzen J., Braunstein D., Chu K., Cowen B. R., Ehrenstein D., Frauenfelder H., Johnson J. B., Lamb D. C. Ligand binding to heme proteins: connection between dynamics and function. Biochemistry. 1991 Apr 23;30(16):3988–4001. doi: 10.1021/bi00230a026. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. II. Structure of deoxymyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):569–584. doi: 10.1016/s0022-2836(77)80112-5. [DOI] [PubMed] [Google Scholar]
- Varadarajan R., Lambright D. G., Boxer S. G. Electrostatic interactions in wild-type and mutant recombinant human myoglobins. Biochemistry. 1989 May 2;28(9):3771–3781. doi: 10.1021/bi00435a022. [DOI] [PubMed] [Google Scholar]
- Varadarajan R., Szabo A., Boxer S. G. Cloning, expression in Escherichia coli, and reconstitution of human myoglobin. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5681–5684. doi: 10.1073/pnas.82.17.5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N. T., Kerr E. A., Ward B., Chang C. K. Resonance Raman detection of Fe-CO stretching and Fe-C-O bending vibrations in sterically hindered carbonmonoxy "strapped hemes". A structural probe of Fe-C-O distortion. Biochemistry. 1983 Sep 13;22(19):4534–4540. doi: 10.1021/bi00288a028. [DOI] [PubMed] [Google Scholar]



