Abstract
Purpose
Long-term survival rates for older patients with newly diagnosed acute myeloid leukemia (AML) are extremely low. Previous observational studies suggest that allogeneic hematopoietic stem-cell transplantation (HSCT) may improve overall survival (OS) because of lower rates of relapse. We sought to prospectively determine the value of HSCT for older patients with AML in first complete remission.
Patients and Methods
We conducted a prospective multicenter phase II study to assess the efficacy of reduced-intensity conditioning HSCT for patients between the ages of 60 and 74 years with AML in first complete remission. The primary end point was disease-free survival at 2 years after HSCT. Secondary end points included nonrelapse mortality (NRM), graft-versus-host disease (GVHD), relapse, and OS.
Results
In all, 114 patients with a median age of 65 years received transplantations. The majority (52%) received transplantations from unrelated donors and were given antithymocyte globulin for GVHD prophylaxis. Disease-free survival and OS at 2 years after transplantation were 42% (95% CI, 33% to 52%) and 48% (95% CI, 39% to 58%), respectively, for the entire group and 40% (95% CI, 29% to 55%) and 50% (95% CI, 38% to 64%) for the unrelated donor group. NRM at 2 years was 15% (95% CI, 8% to 21%). Grade 2 to 4 acute GVHD occurred in 9.6% (95% CI, 4% to 15%) of patients, and chronic GVHD occurred in 28% (95% CI, 19% to 36%) of patients. The cumulative incidence of relapse at 2 years was 44% (95% CI, 35% to 53%).
Conclusion
Reduced-intensity conditioning HSCT to maintain remission in selected older patients with AML is relatively well tolerated and appears to provide superior outcomes when compared with historical patients treated without HSCT. GVHD and NRM rates were lower than expected. Future transplantation studies in these patients should focus on further reducing the risk of relapse.
INTRODUCTION
The prognosis for patients with acute myeloid leukemia (AML) who are age 60 years or older at the time of initial diagnosis is poor.1,2 Despite first complete remission (CR1) rates of up to 50% to 60%, prospects for long-term survival after chemotherapy are dismal because of the high risk of relapse (> 80%).2–7 Myeloablative conditioning followed by allogeneic hematopoietic stem-cell transplantation (HSCT) is associated with significantly lower rates of relapse compared with conventional chemotherapy when performed in younger patients with AML in CR1 and improves survival in intermediate- and high-risk patients.8 Historically, the toxicity of this approach in older patients was considered prohibitive.9 Reduced-intensity conditioning (RIC) regimens were developed to decrease regimen-related toxicity in older patients and in those with comorbidities.10–12 Although preliminary results with this approach in older patients with AML were encouraging and suggested improved disease-free survival (DFS) rates, most published reports were retrospective and were based on single-institution experiences with a wide variety of regimens.13,14 We sought to determine the effectiveness of a uniform RIC transplantation regimen in older patients with AML in CR1 and available HLA-matched related or unrelated donors on a prospective multicenter phase II trial conducted by the Alliance for Clinical Trials in Oncology (formerly Cancer and Leukemia Group B [CALGB]) and the Blood and Marrow Transplant Clinical Trial Network. We show RIC-based HSCT to be an effective strategy for suitable older patients with an available matched donor.
PATIENTS AND METHODS
Patients and Donors
Patients from age 60 to 74 years with a diagnosis of AML in CR1 according to International Working Group criteria were eligible.15 Patients with acute promyelocytic leukemia and AML following a myeloproliferative disorder were not eligible. Patients with a prior history of myelodysplastic syndrome were eligible if they progressed to AML without any prior therapy. Patients with therapy-related AML were eligible provided the other treated malignancy was in remission. CR had to be achieved after no more than two cycles of induction chemotherapy. Up to two cycles of consolidation therapy could be administered before transplantation. Induction and consolidation chemotherapy regimens were not defined by the study. Transplantation was required within 6 months from initial documentation of morphologic CR. Additional eligibility criteria are included in the Appendix (online only).
From January 2004 to April 2006, the initial study design included only HLA-identical sibling donors, but in April 2006, the study was amended to allow for enrollment of patients with volunteer unrelated donors confirmed to be matched at HLA-A, -B, -C, -DRB1, or -DQB1 by high-resolution molecular typing.
Both patients and sibling donors gave written informed consent in accordance with the Helsinki protocol on a study approved by the institutional review board at each participating institution. All unrelated donors included in the study provided written informed consent for participation in Center for International Blood and Marrow Transplant Research studies approved by the National Marrow Donor Program institutional review board. The study was monitored by the CALGB Data Safety and Monitoring Board.
Conditioning Regimen
All patients received a conditioning regimen consisting of fludarabine at 30 mg/m2 per day intravenously (IV) over 30 minutes for 5 days on days −7 through −3 and busulfan 0.8 mg/kg IV over 2 hours every 6 hours for 8 doses on days −4 and −3. Included in the April 2006 amendment to allow unrelated donors was the administration of rabbit antithymocyte globulin (ATG; thymoglobulin; sanofi-aventis, Cambridge, MA) at 2.5 mg/kg per day IV over 6 hours for 3 doses on days −4 through −2 to all patients regardless of donor type. Eight recipients of HLA-matched sibling allografts were treated before the amendment and did not receive ATG.
Donor Mobilization and Target Allograft Composition
Donors received granulocyte colony-stimulating factor 10 μg/kg subcutaneously on days −5 through −2 (and on day −1, if necessary) and began leukapheresis on transplantation days −1 and 0 (and on day +1, if needed) to achieve a CD34+ cell dose of 2 to 8 × 106 cells per kilogram (actual weight of the recipient).
Supportive Care and Patient Assessments
Tacrolimus (target level, 5 to 10 ng/mL) and methotrexate (5 mg/m2 IV days +1, +3, +6, and +11) were given for graft-versus-host disease (GVHD) prophylaxis. Tacrolimus was to be tapered starting on day +90 in the absence of active GVHD with a goal of tapering off completely by day +180. Granulocyte colony-stimulating factor was given at 5 μg/kg subcutaneously starting on day +12 and was continued until neutrophil engraftment. Additional supportive care details are included in the Appendix.
Acute GVHD (aGVHD) and chronic GVHD (cGVHD) were graded according to established criteria.16,17 Patients were considered evaluable for GVHD if they engrafted. Organ toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
Definitions
Neutrophil engraftment was defined as an increase in the absolute neutrophil count to 500/μL or greater for 3 consecutive days after a conditioning regimen–induced nadir. Platelet engraftment was defined as the first day of three consecutive platelet count measurements greater than 20,000/μL without the aid of transfusion for 7 consecutive days. Nonrelapse mortality (NRM) was defined as death in continuous complete remission (CR). Primary graft failure was defined as failure of neutrophil engraftment by day +30. Secondary graft failure was defined as primary engraftment followed by a subsequent decline in the neutrophil count to less than 500/μL without any apparent cause (eg, drugs or opportunistic infection) and unresponsiveness to hematopoietic growth factor therapy. Cytogenetic risk category was assigned based on the CALGB criteria.18
Statistical Considerations
The primary end point of this single-arm phase II multicenter trial was 2-year DFS in the unrelated donor recipient group. We focused primarily on the unrelated donor group because the majority of older patients require unrelated donors. DFS time was measured as time from transplantation to relapse or death as a result of any cause, censoring at loss to follow-up. A 2-year DFS probability of 0.35 was considered sufficiently promising to proceed to a phase III trial, and a probability of ≤ 0.20 was considered not promising, based on CALGB historical data.19 Kaplan and Meier estimation was used to determine survival probability.20 Power for rejecting Ho: DFS ≤ 0.20, calculated for a sample size of 61 patients under H1: DFS = 0.35, was estimated as 84% at the α = .05 level.
Secondary end points were rate of aGVHD at 100 days from transplantation, relapse, NRM, rate of cGVHD, and overall survival (OS) at 2 years from date of transplantation. Cumulative incidence curves were computed for time to relapse, with NRM as a competing risk. Cumulative incidence of NRM was computed with death as a result of relapse as a competing risk. Cumulative incidence for GVHD was computed with death as a result of any cause as a competing risk. OS was analyzed by using Kaplan and Meier estimation, including death as a result of any cause. For all end points, median time to event was estimated by using the Kaplan and Meier method.
After the study was amended to allow inclusion of patients receiving transplantations from unrelated donors, univariable Cox proportional hazards regression was used to test for differences in time-to-event end points between patients receiving transplantations from related versus unrelated donors (donor type).21 For the comparison analyses, patients experiencing a competing risk event were treated as censored.
Univariable Cox proportional hazards models were also used to test for the influence of a series of covariates on time-to-event end points by using cause-specific hazards for outcomes with competing risks. Patient age, sex, WBC count at diagnosis, cytogenetic risk category, time from diagnosis to transplantation, time from achievement of CR to transplantation, receipt of consolidation (yes/no), patient-donor sex matching, ABO mismatch (match, minor mismatch, major mismatch), CD34 dose in graft, CD3 dose in graft, receipt of ATG (yes/no), and donor type were all considered. A detailed description of the methods for testing the association of chimerism with time-to-event end points is provided in the Appendix.
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. All analyses were based on the study database (frozen on December 10, 2014). Data were downloaded and preprocessed by using SAS version 9.3 (SAS Institute, Cary, NC).22 All statistical calculations were performed by using version 3.1.3 of the R software environment for statistical computing and its extension packages.23–27 Additional information concerning these statistical approaches is provided in the Appendix.
RESULTS
Patient and Donor Characteristics
Patient and donor characteristics are provided in Table 1. In all, 121 patients were registered and received transplantations at 21 centers between November 2004 and November 2011. Seven of the patients who received transplantations were excluded from the primary analysis after they were deemed ineligible for the following reasons: received three cycles of consolidation (one patient), beyond CR1 at time of transplantation (one patient), lack of documentation of CR1 before transplantation (two patients), low diffusing capacity of lungs for carbon monoxide (one patient), history of polycythemia vera (one patient), and development of AML after therapy for myelodysplastic syndrome (one patient). The remainder of the results pertain to the 114 eligible recipients. The median patient age was 65 years (range, 60 to 74 years) and was similar for recipients who had either related or unrelated donors. The median time from diagnosis to transplantation was 138 days (range, 61 to 265 days), and the median time from achievement of CR1 to transplantation was 85 days (range, 9 to 184 days).
Table 1.
Recipient and Donor Characteristics (N = 114)
| Characteristic | Result |
|
|---|---|---|
| No. (%) | Median (range) | |
| Recipient age, years | 65 (60-74) | |
| Recipient sex | ||
| Female | 43 (38) | |
| Male | 71 (62) | |
| Cytogenetic risk at diagnosis | ||
| Favorable | 1 (1) | |
| Intermediate | 80 (70) | |
| Adverse | 32 (28) | |
| Complex (including 17p del)* | 26 | |
| del 7 only† | 2 | |
| 11q23 (not t(9;11)) | 2 | |
| Other | 2 | |
| Unknown | 1 (1) | |
| WBC at diagnosis, × 103/mL | 3.9 (0.4-333) | |
| Antecedent hematologic disorder | 20 (18) | |
| One induction course | 70 (61) | |
| No. of consolidation courses | ||
| None | 29 (25) | |
| One | 60 (53) | |
| Two | 24 (21) | |
| Unknown | 1 (1) | |
| Type of consolidation | ||
| High-dose cytarabine based | 48 (56) | |
| Standard-dose cytarabine based or other | 37 (44) | |
| Time from diagnosis to transplantation, days | 138 (61-265) | |
| Time from CR1 documentation to transplantation, days | 85 (9-184) | |
| Donor type | ||
| MRD | 55 (48) | |
| MUD | 59 (52) | |
| Donor age, years | ||
| MRD | 63 (43-81) | |
| MUD | 31 (19-55) | |
| Recipient/donor sex match | ||
| Male/female | 30 (26) | |
| Male/male | 41 (36) | |
| Female/female | 18 (16) | |
| Female/male | 25 (22) | |
| Recipient/donor CMV | ||
| Positive/positive | 33 (29) | |
| Positive/negative | 29 (25) | |
| Negative/positive | 17 (15) | |
| Negative/negative | 30 (26) | |
| Unknown | 5 (4) | |
| ABO compatibility | ||
| Match | 66 (58) | |
| Minor mismatch | 22 (19) | |
| Major mismatch | 24 (21) | |
| Unknown | 2 (2) | |
| Allograft composition | ||
| CD34+ dose/kg (× 106) | 6.2 (0.8-30.5) | |
| CD3+ dose/kg (× 108) | 1.9 (0-5.1) | |
Abbreviations: CMV, cytomegalovirus; CR1, first complete response; MRD, matched related donor; MUD, matched unrelated donor.
Complex, three or more cytogenetic abnormalities.
17p del, deletion of the short arm of chromosome 17.
Engraftment and Chimerism
Twenty-two patients (19%) never developed a neutrophil nadir below 500/μL, and 54 (47%) never developed a platelet nadir below 20,000/μL. Among patients who did develop neutropenia and/or thrombocytopenia, the median time to neutrophil engraftment was 14 days (range, 1 to 22 days) and to platelet engraftment was 11 days (range, 1 to 25 days) post-transplantation. No patients experienced primary graft failure. Two patients developed secondary graft failure and both required second transplantations. Beginning with the first planned sample on day +30, the median proportion of donor cells in samples of peripheral blood analyzed for myeloid chimerism was consistently higher than 98% (range, 50% to 100%) at all time points analyzed. In contrast, mixed CD3+ chimerism was frequently observed. Median CD3+ chimerism values gradually increased over time in the surviving patients without relapse and were 96% (range, 0% to 100%) at day +30, 95% (range, 7% to 100%) at day +90, 98% (range, 8% to 100%) at day +180, and 100% (range, 10% to 100%) at day +365. Table 2 summarizes the observed CD3+ cell chimerism levels at each time point in survivors without relapse (graphically illustrated in Appendix Fig A1, online only). There was no association between level of CD3+ chimerism at any time point with relapse, DFS, or OS.
Table 2.
CD3+ Chimerism After Transplantation
| Time Point (days) | No. of Samples Analyzed | Minimum | First Quartile | Median | Third Quartile | Maximum |
|---|---|---|---|---|---|---|
| 30 | 26 | 0 | 80 | 96 | 100 | 100 |
| 90 | 18 | 7 | 57 | 95 | 100 | 100 |
| 180 | 22 | 8 | 67 | 98 | 100 | 100 |
| 365 | 23 | 10 | 82 | 100 | 100 | 100 |
aGVHD and cGVHD
The cumulative incidences of grades 2 to 4 and 3 to 4 aGVHD at 100 days were 9.6% (95% CI, 4% to 15%) and 2.6% (95% CI, 0% to 6%), respectively, and did not differ by donor type (Fig 1). The median time to onset of grade 2 to 4 aGVHD was 41 days (range, 4 to 79 days). The cumulative incidence of any cGVHD by 2 years was 28% (95% CI, 19% to 36%); cumulative incidence of extensive cGVHD was 11% (95% CI, 5% to 16%) at 2 years. For all patients experiencing limited or extensive cGVHD, the median time to onset of cGVHD was 216 days (range, 99 to 806 days) post-transplantation. Patients receiving ATG had a lower risk of cGVHD (hazard ratio, 0.26; 95% CI, 0.11 to 0.64; P = .002). There was no association between the other clinical variables analyzed, including age and donor type and the risk of grades 2 to 4 aGVHD or all stages of cGVHD (Table 3).
Fig 1.
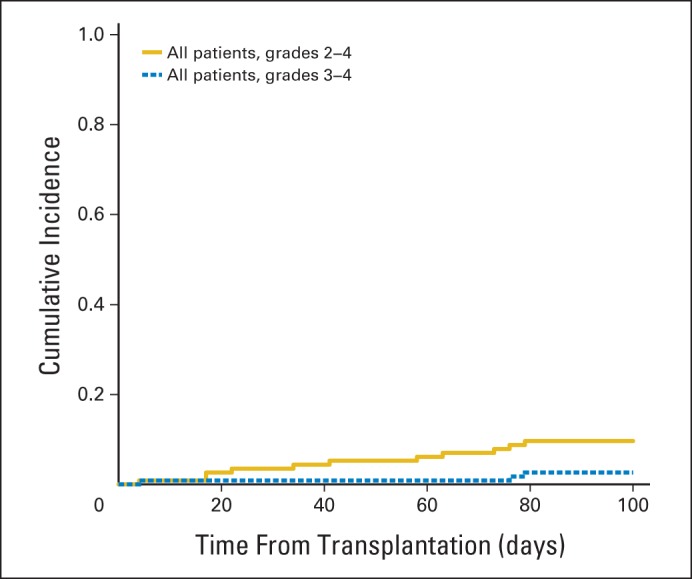
Cumulative incidence of grades 2 to 4 and grades 3 to 4 acute graft-versus-host disease in all patients regardless of donor type.
Table 3.
Univariable Analysis of Clinical Variables and Their Influence on Outcomes After Transplantation
| Covariate | Outcome |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aGVHD Grade 2 to 4 |
cGVHD |
Relapse |
NRM |
DFS |
OS |
|||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Patient age, years | 0.99 (0.84 to 1.16) | .89 | 1.08 (0.99 to 1.18) | .08 | 0.99 (0.92 to 1.06) | .71 | 1.05 (0.94 to 1.18) | .38 | 1.00 (0.95 to 1.06) | .92 | 1.0 (0.94 to 1.06) | .94 |
| Male sex | 1.54 (0.41 to 5.80) | .52 | 0.93 (0.45 to 1.90) | .84 | 0.90 (0.53 to 1.52) | .68 | 1.56 (0.56 to 4.38) | .39 | 1.01 (0.64 to 1.62) | .95 | 0.88 (0.55 to 1.42) | .61 |
| Sex match (yes) | 1.63 (0.48 to 5.58) | .43 | 1.33 (0.65 to 2.69) | .43 | 0.81 (0.48 to 1.35) | .41 | 0.82 (0.33 to 2.08) | .68 | 0.81 (0.51 to 1.27) | .36 | 0.89 (0.56 to 1.42) | .62 |
| Donor type (MUD) | 2.52 (0.67 to 9.50) | .16 | 0.79 (0.39 to 1.57) | .49 | 0.97 (0.58 to 1.65) | .92 | 1.55 (0.60 to 3.99) | .36 | 1.09 (0.69 to 1.72) | .72 | 1.06 (0.66 to 1.70) | .80 |
| ATG (yes) | 7.4 × 107 (0.00 to inf) | .35 | 0.26 (0.11 to 0.64) | .002 | 2.64 (0.63 to 11.08) | .17 | 0.29 (0.10 to 0.88) | .02 | 1.04 (0.45 to 2.43) | .93 | 0.83 (0.35 to 1.95) | .67 |
| Consolidation (yes) | 1.62 (0.35 to 7.49) | .53 | 1.06 (0.47 to 2.35) | .89 | 0.79 (0.45 to 1.40) | .42 | 2.57 (0.59 to 11.20) | .19 | 0.98 (0.58 to 1.66) | .95 | 1.06 (0.62 to 1.82) | .82 |
| WBC at diagnosis, × 103/mL | 1.00 (0.99 to 1.01) | .95 | 0.98 (0.96 to 1.01) | .12 | 1.00 (1.00 to 1.01) | .32 | 0.99 (0.97 to 1.01) | .34 | 1.00 (1.00 to 1.01) | .66 | 1.00 (0.99 to 1.00) | .95 |
| Adverse cytogenetic risk | 0.64 (0.14 to 3.03) | .57 | 0.68 (0.28 to 1.66) | .40 | 1.51 (0.87 to 2.63) | .14 | 1.51 (0.57 to 4.03) | .40 | 1.51 (0.93 to 2.45) | .09 | 1.26 (0.76 to 2.09) | .37 |
| Time from diagnosis to transplantation, days | 1.00 (0.99 to 1.02) | .75 | 1.00 (0.99 to 1.01) | .55 | 1.00 (0.99 to 1.00) | .17 | 1.00 (0.99 to 1.01) | .81 | 1.00 (0.99 to 1.00) | .18 | 1.00 (0.99 to 1.00) | .10 |
| Time from CR1 to transplantation, days | 1.01 (0.99 to 1.02) | .49 | 0.99 (0.98 to 1.00) | .10 | 0.99 (0.99 to 1.00) | .08 | 1.00 (0.99 to 1.01) | .50 | 1.00 (0.99 to 1.00) | .25 | 1.00 (0.99 to 1.00) | .22 |
| ABO mismatch (relative to match) | ||||||||||||
| Minor | 2.21 (0.50 to 9.89) | .48 | 2.27 (0.99 to 5.17) | .14 | 0.68 (0.34 to 1.38) | .24 | 1.33 (0.44 to 3.97) | .61 | 0.81 (0.45 to 1.46) | .24 | 0.86 (0.47 to 1.56) | .18 |
| Major | 2.09 (0.47 to 9.32) | .48 | 1.45 (0.62 to 3.40) | .14 | 0.58 (0.29 to 1.18) | .4 | 0.65 (0.17 to 2.39) | .61 | 0.60 (0.32 to 1.10) | .24 | 0.55 (0.29 to 1.04) | .18 |
| CD34 (dose/kg × 106) | 1.06 (0.96 to 1.17) | .22 | 0.90 (0.80 to 1.01) | .08 | 0.93 (0.86 to 1.01) | .10 | 0.94 (0.82 to 1.08) | .40 | 0.93 (0.87 to 1.00) | .07 | 0.96 (0.90 to 1.03) | .23 |
| CD3 (dose/kg × 108) | 1.29 (0.73 to 2.25) | .38 | 0.78 (0.56 to 1.09) | .15 | 1.32 (1.04 to 1.68) | .02 | 0.90 (0.57 to 1.43) | .66 | 1.21 (0.98 to 1.49) | .07 | 1.23 (1.00 to 1.52) | .05 |
Abbreviations: aGVHD, acute graft-versus-host disease; ATG, antithymocyte globulin; cGVHD, chronic graft-versus-host disease; CR1, first complete remission; DFS, disease-free survival; HR, hazard ratio; inf, infinity; MUD, matched unrelated donor; NRM, nonrelapse mortality; OS, overall survival.
Nonhematologic Toxicity and Opportunistic Infections
Nonhematologic toxicity attributable to the conditioning regimen was generally mild to moderate and reversible. Although cumulatively, 68% of patients experienced at least one grade 3 to 5 toxicity, the incidences of grade 3 to 5 organ toxicity (mucositis, other GI, hepatic, pulmonary, renal, cardiac, and neurologic) were 5% or less in each category. No cases of hepatic sinusoidal obstruction syndrome were observed.
Reactivation of cytomegalovirus (viremia) occurred in 33 (41%) of 81 donor/recipient pairs at risk, although nine patients (8%) experienced Epstein-Barr virus reactivation requiring treatment. No cytomegalovirus organ disease was observed, but two patients developed Epstein-Barr virus–related post-transplantation lymphoproliferative disorder and died of causes secondary to related complications.
NRM, Relapse, DFS, and OS
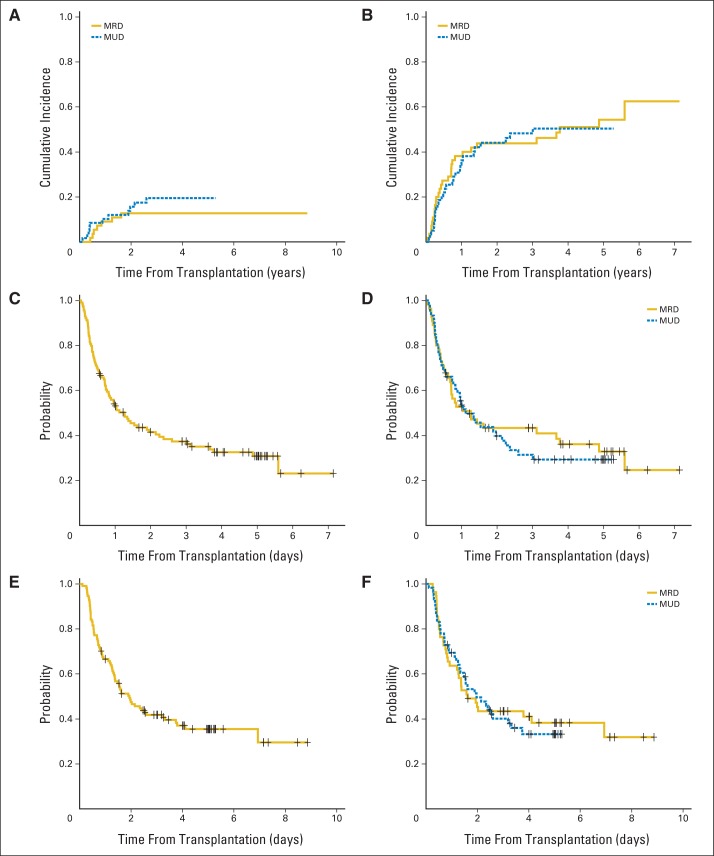
The median follow-up of the 43 surviving eligible patients was 1,602 days (range, 306 to 3,233 days). Eighteen patients (16%) died from causes other than relapse at a median of 279 days (range, 41 to 949 days) after transplantation. The cumulative incidence of NRM at 2 years was 15% (95% CI, 8% to 21%; Fig 2A) and did not differ by donor type; it was 13% (95% CI, 4% to 22%) for related versus 16% (95% CI, 6% to 26%) for unrelated donor transplantations. Fifty-seven patients relapsed at a median of 194 days (range, 15 to 2,041 days) after transplantation. The cumulative incidence of relapse for the entire group was 44% (95% CI, 35% to 53%) at 2 years and did not differ by donor type (Fig 2B). The DFS probability at 2 years after transplantation was 42% (95% CI, 33% to 52%) for the entire group. The DFS probabilities at 2 years for recipients of related and unrelated transplantations were 43% (95% CI, 32% to 59%) and 40% (95% CI, 29% to 55%), respectively (P = .72; Figs 2C and 2D). Receipt of ATG did not affect relapse or DFS. The observed 40% DFS (95% CI, 29% to 55%) in the unrelated donor group satisfied our prespecified criteria for success. OS probability at 2 years was 48% (95% CI, 39% to 58%) for the entire group, 45% (95% CI, 34% to 61%) for recipients of related donor transplantations, and 50% (95% CI, 38% to 64%) for recipients of unrelated donor transplantations (P = .80; Figs 2E and 2F). When we reanalyzed the data and included the seven patients who were deemed to be ineligible post hoc, there were no significant differences in DFS or OS between the 121 total versus 114 eligible patients (Appendix Table A1, online only). Of the 43 surviving patients, at least 31 (72%) were known to not be receiving any type of immunosuppressive therapy at last follow-up. The median OS for those patients who relapsed post-transplantation was 136 days (95% CI, 107 to 198 days). Cytogenetic risk category did not significantly influence the risk of relapse, DFS, or OS (Table 3 and Appendix Fig A2, online only). Additional information about the relapsing patients is provided in the Appendix.
Fig 2.
Cumulative incidence of (A) nonrelapse mortality by donor type and (B) relapse by donor type. Kaplan-Meier estimates of (C) disease-free survival for all patients, (D) disease-free survival by donor type, (E) overall survival for all patients, and (F) overall survival by donor type. MRD, matched related donor; MUD, matched unrelated donor.
Seventy-one patients have died. As shown in Table 4, relapse was the most common cause of death (n = 53), representing 75% of all deaths.
Table 4.
Causes of Death
| Cause | No. (%) |
|---|---|
| Relapse | 53 (74.6) |
| aGVHD related | 3 (4.2) |
| cGVHD related | 6 (8.5) |
| PTLD | 2 (2.8) |
| Sepsis | 1 (1.4) |
| Secondary graft failure | 1 (1.4) |
| Rectal cancer | 1 (1.4) |
| Pneumonia | 1 (1.4) |
| Pneumonitis | 1 (1.4) |
| HHV-6 encephalitis | 1 (1.4) |
| Sudden death | 1 (1.4) |
Abbreviations: aGVHD, acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease; HHV-6, human herpes virus 6; PTLD, post-transplantation Epstein-Barr virus–related lymphoproliferative disorder.
DISCUSSION
This prospective trial demonstrates that RIC HSCT from HLA-matched donors is feasible and effective in older patients with AML in CR1. The rate of NRM is comparable to that reported in studies involving younger patients and suggests that assessment of physiologic status is a more informative criterion for transplantation eligibility than chronologic age.28–30 Of critical importance, for the first time (to the best of our knowledge), favorable results in transplantation of older patients have been obtained in a multicenter cooperative group setting, which makes the results more likely to be generalizable. The results are similar to those in previous single-institution prospective and retrospective studies involving older patients with AML.19,31–35 The 2-year DFS and OS rates in this group compare favorably to those in studies of conventional chemotherapy–based approaches to remission consolidation in which DFS and OS rates beyond 2 years are typically below 20%. We note that this was not a comparative trial, and the rate of accrual was relatively slow (two to three patients per month) while the study was open to accrual, suggesting that patients involved in this study were a selected group. The possible selection biases inherent in such a trial should be considered when analyzing these results.
Relapse remains the major barrier to successful outcome after transplantation, regardless of patient age. The 44% relapse rate at 2 years was high, although relapse rates approaching 80% to 90% have been observed in older patients after conventional chemotherapy, suggesting a potential graft-versus-leukemia effect.2–6 Interpretation of our trial results is limited somewhat by lack of consistent knowledge of the mutational status of the patients at diagnosis or of disease burden at CR by minimal residual disease assessment. Interestingly, cytogenetic results did not appear to influence clinical outcomes in this data set. Our trial may have included better-risk patients, although 28% harbored a poor-risk karyotype at diagnosis, and 18% had an antecedent hematologic disorder or treatment-related AML.36,37 Future transplantation studies should make every effort to incorporate molecular characterization at diagnosis in these patients to better understand the role of transplantation in specific molecular subgroups. Nonetheless, relapse remains the primary cause of death in these patients, and efforts to prevent its development after transplantation are imperative for building on these encouraging results.
The rates of aGVHD and cGVHD observed on this trial were actually lower than originally anticipated. The study was initiated before the 2005 National Institutes of Health consensus panel on cGVHD, so the older cGVHD classification scheme was used.17 Only 13% of patients experienced extensive cGVHD by using this classification, which is somewhat lower than rates reported in other studies.38,39 The incorporation of rabbit ATG into the conditioning regimen for all patients, including recipients with matched sibling donors, may have contributed to the relatively low rates of GVHD and NRM, as has been observed in previous studies.38,40 However, ATG use at this relatively high dose may also have contributed to more relapses after the RIC regimen, consistent with retrospective data reported by the Center for International Blood and Marrow Transplant Research.41 Whether ATG should be incorporated into the GVHD prophylaxis regimen for these patients remains an open question.
How should these results be interpreted in comparison to conventional consolidation therapy? Given multiple potential biases among leukemia therapists and their patients, a truly randomized study comparing transplantation to chemotherapy seems unlikely because of the lack of equipoise. The preliminary results of a randomized phase III trial performed in Germany (NCT00766779; HCT Versus CT in Elderly AML) were recently reported in abstract form and showed a significant advantage in leukemia-free survival for patients receiving an allograft.42 The results of that study and ours suggest that another randomized study in this setting may not be feasible or even necessary. Another National Cancer Institute–sponsored trial in older patients with AML that incorporated HSCT into the study (ECOG-E2906/NCT02085408; Clofarabine or Daunorubicin Hydrochloride and Cytarabine Followed By Decitabine or Observation in Treating Older Patients With Newly Diagnosed Acute Myeloid Leukemia) is ongoing, and the results are pending. Patients who receive transplantation are certainly a selected group. A donor-versus-no-donor analysis is the only way to fairly compare the two approaches, but the successful use of unrelated donors and availability of alternative donors (eg, umbilical cord blood, haploidentical related) would collapse the size of the no-donor group and make such a design difficult to complete.
The results of this trial create a platform on which future trials can build. Studies to more precisely define the physiologic characteristics that determine a patient's suitability for transplantation are needed. Methods to assess minimal residual disease before and after transplantation should be explored, as should strategies to prevent relapse after transplantation. Notwithstanding, these results demonstrate that for the foreseeable future, RIC HSCT has an established role as a consolidative strategy for selected older patients with AML in CR1. Studies are needed to better define the group of older patients with AML most likely to benefit from this approach.
Glossary Terms
- Allogeneic stem cell transplant:
the transplantation of multipotent hematopoietic stem cells, usually derived from bone marrow, peripheral blood, or the umbilical cord blood of a genetically nonidentical human donor.
- CD34:
an antigen selectively expressed on human lymphoid and myeloid hematopoietic progenitor cells. CD34 is also expressed on vascular endothelium.
- Cumulative incidence:
a statistical measure of an event of interest (eg, relapse, death, second malignant neoplasm, a specific disease) occurring in a specified period of time in the population at risk. It is calculated by using the formula: (number of new cases of the event of interest)/(total population at risk).
- Disease free survival:
the survival period spanning the time from surgery to a recurrence of cancer.
Appendix
Methods
Patient Eligibility
In addition to the patient eligibility criteria outlined in the article, eligible patients had to have an Eastern Cooperative Oncology Group performance status between 0 and 2, a diffusing capacity of the lungs for carbon monoxide of more than 40% on pulmonary function testing, a left ventricular ejection fraction of ≥ 30% on echocardiogram or multiple gated acquisition scan, no active serious infection requiring antibiotics, no uncontrolled diabetes mellitus, no evidence of exposure to HIV, bilirubin less than 2 mg/dL, AST level less than 3× upper limit of normal, and a calculated creatinine clearance ≥ 40 mL/min.
Donor Mobilization and Target Allograft Composition
If the yield of CD34+ cells during donor mobilization was less than 2 × 106/kg on day −1, an additional leukapheresis was performed on day 0 to achieve a targeted total dose of 2 to 8 × 106/kg CD34+ cells.
Supportive Care and Patient Assessments
Blood cytomegalovirus surveillance was performed once per week through day +100, and preemptive treatment was given according to institutional guidelines. Surveillance for Epstein-Barr virus (EBV) infection was performed once every 2 weeks from neutrophil engraftment by using a real-time quantitative EBV DNA polymerase chain reaction plasma-based assay through day +100. Patients who developed blood EBV DNA concentration of more than 1,000 copies per milliliter on any two consecutive tests were recommended to receive preemptive rituximab at 375 mg/m2 for at least one dose.
Donor hematopoietic chimerism was assessed on samples of separated T cells (CD3+) and myeloid cells (CD15+ or CD33+) from peripheral blood obtained on or around days +30, +90, +180, and +365 by using methods that were available at each institution.
Statistical Considerations and Study Design
In all, 121 patients were registered and received transplantations at 21 centers between November 2004 and November 2011. The study was amended in April 2006 to allow for the inclusion of patients receiving transplantation from unrelated donors. In the original design, the primary end point was to estimate disease-free survival (DFS) in recipients of both matched sibling and matched unrelated donor transplantation. The power estimates projected that a total of 61 patients would be required. When that accrual total was met, the study was temporarily suspended on December 29, 2008, and amended so that the primary analysis would be to estimate DFS in the recipients of unrelated donor transplantations only. The rationale at the time was that most of these older patients with acute myeloid leukemia would require volunteer unrelated donors to receive a transplantation. The study was reopened on November 2, 2009, and completed accrual in November 2011 after a sufficient number of unrelated donor recipients had been registered.
Acute Graft-Versus-Host Disease Censoring
For cumulative incidence and cause-specific hazard analyses of acute graft-versus-host disease (aGVHD), censoring was imposed at 100 days post-transplantation. Any patients not experiencing aGVHD (or one of the competing risks) within 100 days of transplantation was treated as censored at 100 days in the analysis, regardless of whether or not they experienced aGVHD at a later time. All other time-to-event and cumulative incidence analyses were based on censoring resulting from loss to follow-up.
Chimerism Models
For the chimerism analyses, the missing data on patient chimerism measurements was assumed to be noninformative. Association of chimerism with time-to-event end points was tested by using cause-specific hazards. Univariable Cox proportional hazards regression was used to test for association of time-to-event end points with the chimerism level at 30, 90, 180, or 365 days.
In addition, joint models for longitudinal and time-to-event data were used to test for association between outcomes and chimerism as a time-dependent variable (Rizopoulos D: Joint models for longitudinal and time-to-event data: With applications in R. Boca Raton, FL, CRC Press, 2012). First, a linear mixed effect model was fit to the longitudinal chimerism data with days since transplantation and the interaction of donor type and days since transplantation as fixed effects and days since transplantation as a random effect. Next, a Cox model was fit to the time-to-event outcome with donor type as a covariate by using cause-specific hazards for outcomes with competing risks. Finally, a survival model with a piece-wise constant baseline risk function was fit to the time-to-event outcome, with donor type as a time-independent covariate and chimerism level as estimated in the longitudinal model as a time-dependent variable. The baseline risk was approximated for intervals between six internal knots at evenly spaced percentiles across the observed event times. The analyses were conducted first for all patients with chimerism data and again limited to only those patients with chimerism data at two or more time points.
Covariate Analysis
Univariable Cox proportional hazards models were used to test for the influence of a series of covariates on time-to-event end points by using cause-specific hazards for outcomes with competing risks. For categorical variables, values of “unknown” were treated as missing. For the cytogenetic risk category, only one patient was categorized as “favorable,” so the cells for “favorable” and “intermediate” were combined. Antithymocyte globulin (ATG) administration status was determined by patient registration date, with patients registered before the amendment allowing for unrelated donors assumed not to have received ATG, and those registered following the amendment assumed to have received it.
Overall Survival and DFS
In addition to the analyses described in the article, the overall survival (OS) and DFS analyses were repeated for all patients who received transplantations, including those deemed ineligible.
Results
Low Doses of Transplanted CD34+ Cells and Secondary Graft Failure
There were three patients who received transplanted CD34+ cell doses below the target of 2.0 × 106/kg. All three engrafted and did not develop late graft failure. Secondary graft failure was observed in two patients who received CD34+ cell doses of 4.9 and 6.6 ×106/kg, respectively.
OS and DFS
Appendix Table A1 shows that the OS and DFS results for the analysis population are similar to those for all patients who received a transplantation. The inclusion of the seven ineligible patients changes OS and DFS at 2 years by only approximately 1% and changes median survival times by less than 2 months.
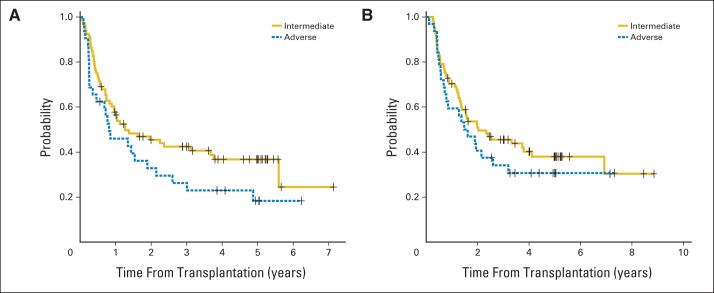
We also analyzed DFS and OS by cytogenetic risk category. The one patient with favorable cytogenetics did not relapse. Of the 80 patients with intermediate risk and 32 with adverse risk cytogenetics, 37 and 19 relapsed, respectively. DFS and OS by cytogenetic category are depicted in Appendix Fig A2. The differences are not statistically significant.
Treatment and Survival of Relapsed Patients
Data were reported on 42 patients who received nonprotocol therapy for relapsed acute myeloid leukemia. Of these, 16 received conventional chemotherapy–based treatment, 16 hypomethylating agent–based treatment, six investigational agents, two intrathecal chemotherapy only, one donor lymphocyte infusion (DLI) only, and one unknown. Five of the patients who received some form of chemotherapy for relapse also received a DLI. Twenty relapsed patients survived 6 months or longer after relapse, 10 patients survived at least 1 year after relapse, and three patients were surviving 2 years after relapse. In total, four of the 57 relapsed patients were still alive at last follow-up, and one of these survived beyond 2 years of last follow-up.
Eight patients, all of whom had received ATG, received a DLI either alone or following re-induction chemotherapy. Three patients received a DLI for poor donor chimerism without overt evidence of relapse. The median survival of these eight patients was 201 days (range, 63 to 1,017 days). All have died.
Of the original eight recipients of matched sibling donor transplantation who did not receive ATG, seven had intermediate-risk and one had adverse risk cytogenetics. Of these eight, two relapsed, four died from nonrelapse mortality as a result of complications of aGVHD or chronic GVHD, and two became long-term disease-free survivors.
Institutions That Participated in the Study
The following institutions participated in this study: Christiana Care Health Services Community Clinical Oncology Program, Wilmington, DE (Stephen Grubbs, MD, supported by CA45418); Dana-Farber Cancer Institute, Boston, MA (Harold J. Burstein, MD, PhD, supported by CA32291); Massachusetts General Hospital, Boston, MA (Jeffrey W. Clark, MD, supported by CA32291); Mount Sinai School of Medicine, New York, NY (Lewis R. Silverman, MD, supported by CA04457); Monter Cancer Center of North Shore-Long Island Jewish Health Systems, Lake Success, NY (Daniel Budman, MD, supported by CA35279); Roswell Park Cancer Institute, Buffalo, NY (Ellis Levine, MD, supported by CA59518); The Ohio State University Medical Center, Columbus, OH (Clara D. Bloomfield, MD, supported by CA77658); University of California at San Francisco, San Francisco, CA (Charles J. Ryan, MD, supported by CA60138); University of Maryland Greenebaum Cancer Center, Baltimore, MD (Martin Edelman, MD, supported by CA31983); University of Minnesota, Minneapolis, MN (Bruce A. Peterson, MD, supported by CA16450); University of North Carolina at Chapel Hill, Chapel Hill, NC (Thomas C. Shea, MD, supported by CA47559); Washington University School of Medicine, St. Louis, MO (Nancy Bartlett, MD, supported by CA77440); and Weill Medical College of Cornell University, New York, NY (John Leonard, MD, supported by CA07968).
Table A1.
OS and DFS
| Analysis | No. of Patients | No. of Events | Percent Survival at 2 Years | Median of Years for OS or DFS | 95% CI |
|---|---|---|---|---|---|
| OS | 114 | 71 | 47.5 | 1.93 | 1.37 to 3.32 |
| OS MRD | 55 | 34 | 45.3 | 1.62 | 1.26 to NA |
| OS MUD | 59 | 37 | 49.6 | 1.97 | 1.34 to 3.74 |
| OS, all | 121 | 76 | 47.3 | 1.89 | 1.36 to 3.32 |
| OS MRD, all | 58 | 36 | 46.4 | 1.78 | 1.26 to NA |
| OS MUD, all | 63 | 40 | 48.0 | 1.89 | 1.31 to 3.74 |
| DFS | 114 | 75 | 41.5 | 1.26 | 0.83 to 2.25 |
| DFS MRD | 55 | 36 | 43.3 | 1.26 | 0.71 to 4.87 |
| DFS MUD | 59 | 39 | 39.7 | 1.12 | 0.83 to 2.36 |
| DFS, all | 121 | 80 | 40.4 | 1.12 | 0.81 to 2.14 |
| DFS MRD, all | 58 | 38 | 42.2 | 1.26 | 0.71 to 4.87 |
| DFS MUD, all | 63 | 42 | 38.8 | 1.04 | 0.77 to 2.24 |
NOTE. The term “all” signifies all patients who received a transplantation.
Abbreviations: DFS, disease-free survival; MRD, matched related donor; MUD, matched unrelated donor; NA, not applicable; OS, overall survival.
Fig A1.
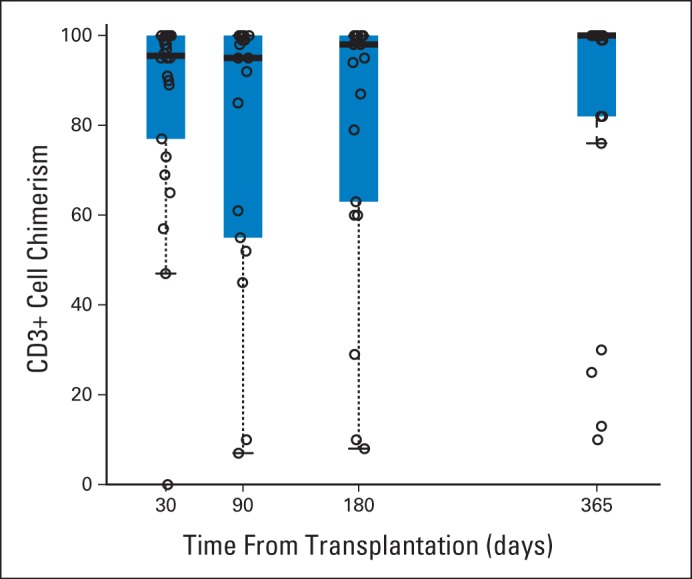
CD3+ cell chimerism levels in surviving patients without relapse by time point. Thick bars represent median values; thin bars indicate 95% CIs.
Fig A2.
(A) Disease-free survival and (B) overall survival by cytogenetic risk category.
Footnotes
Supported in part by Grants No. U10CA180821 and U10CA180882 (Alliance for Clinical Trials in Oncology) and 1U10CA180850, 1U10CA180867, 1U10CA180858, 1U10CA180833, 1U10CA180790, 1U10CA180836, 1U10CA180838, and 1U10CA180791 from the National Cancer Institute, National Institutes of Health (NIH), by Grant No. U10HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute (NIH), and by Genzyme.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Presented in part as an oral presentation at the 54th American Society of Hematology Annual Meeting and Exposition, Atlanta, GA, December 8-11, 2012.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00070135.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Steven M. Devine, Kouros Owzar, Richard A. Larson, Thomas C. Shea, Sergio Giralt, Shelly Carter, Mary M. Horowitz, Charles Linker, Edwin P. Alyea
Provision of study materials or patients: Richard E. Champlin, Richard A. Larson
Collection and assembly of data: Steven M. Devine, Kouros Owzar, Flora Mulkey, Vera Hars, Alexander B. Sibley, Shelly Carter, Mary M. Horowitz
Data analysis and interpretation: Steven M. Devine, Kouros Owzar, William Blum, Flora Mulkey, Richard M. Stone, Jack W. Hsu, Richard E. Champlin, Yi-Bin Chen, Ravi Vij, James Slack, Robert J. Soiffer, Richard A. Larson, Thomas C. Shea, Vera Hars, Alexander B. Sibley, Sergio Giralt, Mary M. Horowitz, Charles Linker, Edwin P. Alyea
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Study of Allogeneic Transplantation for Older Patients With Acute Myeloid Leukemia in First Complete Remission Using a Reduced-Intensity Conditioning Regimen: Results From Cancer and Leukemia Group B 100103 (Alliance for Clinical Trials in Oncology)/Blood and Marrow Transplant Clinical Trial Network 0502
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Steven M. Devine
Research Funding: Genzyme
Kouros Owzar
Patents, Royalties, Other Intellectual Property: Biomarkers of High-Grade Serious Ovarian Carcinomas Pending Application (Inst), Biomarkers for Locally Advanced Breast Cancer (Labc) and Inflammatory Breast Cancer (Ibc) (Inst), Tumor Markers of Efficacy and Resistance for Targeted Cancer Therapy (Inst)
William Blum
Consulting or Advisory Role: Celator Pharmaceuticals, Boehringer Ingelheim, Celgene, AbbVie
Research Funding: AbbVie, Boehringer Ingelheim, Karyopharm Therapeutics, Astellas Pharma
Flora Mulkey
No relationship to disclose
Richard M. Stone
Consulting or Advisory Role: Amgen, AbbVie, Agios Pharmaceuticals, Bristol-Myers Squibb, Celator Pharmaceuticals, Roche/Genentech, Merck, Karyopharm Therapeutics, Sunesis Pharmaceuticals, Celgene, Juno Therapeutics, Seattle Genetics, Xenetic Biosciences, Pfizer
Research Funding: Novartis (Inst), Agios Pharmaceuticals (Inst), Karyopharm Therapeutics (Inst), Celator Pharmaceuticals (Inst)
Jack W. Hsu
No relationship to disclose
Richard E. Champlin
No relationship to disclose
Yi-Bin Chen
Honoraria: Millennium Pharmaceuticals, Bayer HealthCare Pharmaceuticals
Consulting or Advisory Role: Millennium Pharmaceuticals, Seattle Genetics, ARIAD Pharmaceuticals
Research Funding: Seattle Genetics, Novartis, Otsuka, Bayer HealthCare Pharmaceuticals, Celgene
Ravi Vij
Consulting or Advisory Role: Celgene, Onyx Pharmaceuticals, Takeda Pharmaceuticals, Sanofi Oncology, Bristol-Myers Squibb, Novartis, Janssen Oncology, Merck
Research Funding: Celgene (Inst), Takeda Pharmaceuticals (Inst), Onyx Pharmaceuticals (Inst)
James Slack
No relationship to disclose
Robert J. Soiffer
Consulting or Advisory Role: Novartis, Kiadis Pharma, Juno Therapeutics, Merck, Jazz Pharmaceuticals
Research Funding: Neovii Biotech (Inst)
Richard A. Larson
No relationship to disclose
Thomas C. Shea
No relationship to disclose
Vera Hars
Research Funding: Celgene
Alexander B. Sibley
Patents, Royalties, Other Intellectual Property: Methods of Predicting Responsiveness of a Cancer to an Agent and Methods for Determining a Prognosis for a Cancer Patient. US Patent Application No. 14/703535, filed May 4, 2015, pending (Inst)
Sergio Giralt
Honoraria: Celgene, Takeda Pharmaceuticals, Johnson & Johnson, Amgen, Novartis, Jazz Pharmaceuticals, Sanofi
Consulting or Advisory Role: Amgen, Sanofi, Johnson & Johnson, Takeda Pharmaceuticals, Jazz Pharmaceuticals, Celgene
Research Funding: Celgene, Takeda Pharmaceuticals, Johnson & Johnson, Mitenyi Biotec
Shelly Carter
No relationship to disclose
Mary M. Horowitz
Research Funding: Swedish Orphan Biovitrum, Otsuka, Novartis, Therakos, Telomere Diagnostics, Gamida Cell
Travel, Accommodations, Expenses: Novartis
Charles Linker
No relationship to disclose
Edwin P. Alyea
No relationship to disclose
REFERENCES
- 1.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: A population-based study. Haematologica. 2012;97:1916–1924. doi: 10.3324/haematol.2012.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowe JM, Tallman MS. How I treat acute myeloid leukemia. Blood. 2010;116:3147–3156. doi: 10.1182/blood-2010-05-260117. [DOI] [PubMed] [Google Scholar]
- 3.Goldstone AH, Burnett AK, Wheatley K, et al. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: The results of the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1302–1311. doi: 10.1182/blood.v98.5.1302. [DOI] [PubMed] [Google Scholar]
- 4.Rowe JM, Neuberg D, Friedenberg W, et al. A phase 3 study of three induction regimens and of priming with GM-CSF in older adults with acute myeloid leukemia: A trial by the Eastern Cooperative Oncology Group. Blood. 2004;103:479–485. doi: 10.1182/blood-2003-05-1686. [DOI] [PubMed] [Google Scholar]
- 5.Schoch C, Kern W, Schnittger S, et al. The influence of age on prognosis of de novo acute myeloid leukemia differs according to cytogenetic subgroups. Haematologica. 2004;89:1082–1090. [PubMed] [Google Scholar]
- 6.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Löwenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361:1235–1248. doi: 10.1056/NEJMoa0901409. [DOI] [PubMed] [Google Scholar]
- 8.Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: Systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaconescu R, Flowers CR, Storer B, et al. Morbidity and mortality with nonmyeloablative compared with myeloablative conditioning before hematopoietic cell transplantation from HLA-matched related donors. Blood. 2004;104:1550–1558. doi: 10.1182/blood-2004-03-0804. [DOI] [PubMed] [Google Scholar]
- 10.Giralt S, Estey E, Albitar M, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: Harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997;89:4531–4536. [PubMed] [Google Scholar]
- 11.Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 12.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: Replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 13.Cancer and Leukemia Group B 8461. Farag SS, Archer KJ, et al. Pretreatment cytogenetics add to other prognostic factors predicting complete remission and long-term outcome in patients 60 years of age or older with acute myeloid leukemia: Results from Cancer and Leukemia Group B 8461. Blood. 2006;108:63–73. doi: 10.1182/blood-2005-11-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878–1887. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 16.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 17.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man: A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 18.Byrd JC, Mrózek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: Results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 19.Farag SS, Maharry K, Zhang MJ, et al. Comparison of reduced-intensity hematopoietic cell transplantation with chemotherapy in patients age 60-70 years with acute myelogenous leukemia in first remission. Biol Blood Marrow Transplant. 2011;17:1796–1803. doi: 10.1016/j.bbmt.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 22.SAS. Base SAS 9.3 Procedures Guide (ed 2) SAS Institute. 2011 [Google Scholar]
- 23.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 24.Gerds TA. Package “prodlim”: Product-limit estimation for censored event history analysis. 2014 [Google Scholar]
- 25.Gray B. Package “cmprsk”: Subdistribution Analysis of Competing Risks. 2014 [Google Scholar]
- 26.Therneau TM. survival: A Package for Survival Analysis in S. R package version 2.37-7. 2014 [Google Scholar]
- 27.Rizopoulos D. JM: An R Package for the Joint Modelling of Longitudinal and Time-to-Event Data. J Stat Software. 2010;35:1–33. [Google Scholar]
- 28.Artz AS. Older patients/older donors: Choosing wisely. Hematology Am Soc Hematol Educ Program. 2013;2013:70–75. doi: 10.1182/asheducation-2013.1.70. [DOI] [PubMed] [Google Scholar]
- 29.Klepin HD, Geiger AM, Tooze JA, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121:4287–4294. doi: 10.1182/blood-2012-12-471680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wildes TM, Stirewalt DL, Medeiros B, et al. Hematopoietic stem cell transplantation for hematologic malignancies in older adults: Geriatric principles in the transplant clinic. J Natl Compr Canc Netw. 2014;12:128–136. doi: 10.6004/jnccn.2014.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertz H, Potthoff K, Finke J. Allogeneic stem-cell transplantation from related and unrelated donors in older patients with myeloid leukemia. J Clin Oncol. 2003;21:1480–1484. doi: 10.1200/JCO.2003.09.110. [DOI] [PubMed] [Google Scholar]
- 32.de Lima M, Anagnostopoulos A, Munsell M, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: Dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:865–872. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]
- 33.Valcárcel D, Martino R, Caballero D, et al. Sustained remissions of high-risk acute myeloid leukemia and myelodysplastic syndrome after reduced-intensity conditioning allogeneic hematopoietic transplantation: Chronic graft-versus-host disease is the strongest factor improving survival. J Clin Oncol. 2008;26:577–584. doi: 10.1200/JCO.2007.11.1641. [DOI] [PubMed] [Google Scholar]
- 34.Koreth J, Aldridge J, Kim HT, et al. Reduced-intensity conditioning hematopoietic stem cell transplantation in patients over 60 years: Hematologic malignancy outcomes are not impaired in advanced age. Biol Blood Marrow Transplant. 2010;16:792–800. doi: 10.1016/j.bbmt.2009.12.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Estey E, de Lima M, Tibes R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS) Blood. 2007;109:1395–1400. doi: 10.1182/blood-2006-05-021907. [DOI] [PubMed] [Google Scholar]
- 36.Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 37.Mrózek K, Marcucci G, Nicolet D, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30:4515–4523. doi: 10.1200/JCO.2012.43.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Socié G, Schmoor C, Bethge WA, et al. Chronic graft-versus-host disease: Long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117:6375–6382. doi: 10.1182/blood-2011-01-329821. [DOI] [PubMed] [Google Scholar]
- 39.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: A randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–864. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 41.Soiffer RJ, Lerademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117:6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niederwieser D, Al-Ali HK, Krahl R, et al. Higher Leukemia free survival after post-induction hematopoietic cell transplantation compared to consolidation therapy in patients > 60 years with acute myelogenous leukemia (AML): Report from the AML 2004 East German Study Group (OSHO) Blood. 2014;124(21) abstr 280. [Google Scholar]