Abstract
Background: Little information is available on B vitamin concentrations in human milk or on how they are affected by maternal B vitamin deficiencies, antiretroviral therapy, or maternal supplementation.
Objective: The objective was to evaluate the effects of antiretroviral therapy and/or lipid-based nutrient supplements (LNSs) on B vitamin concentrations in breast milk from HIV-infected women in Malawi.
Design: Breast milk was collected from 537 women recruited within the Breastfeeding, Antiretrovirals, and Nutrition study at 2 or 6 wk and 24 wk postpartum. Women were assigned to receive antiretrovirals and LNSs, antiretrovirals only, LNSs only, or a control. Antiretrovirals and LNSs were given to the mothers from weeks 0 to 28. The antiretrovirals were zidovudine/lamivudine and nelfinavir or lopinavir/ritonavir. LNSs provided 93–118% of the Recommended Dietary Allowances of thiamin, riboflavin, niacin, pyridoxine, and vitamin B-12. Infants were exclusively breastfed.
Results: LNSs increased milk concentrations of all vitamins except thiamin, whereas antiretrovirals lowered concentrations of nicotinamide, pyridoxal, and vitamin B-12. Although antiretrovirals alone had no significant effect on riboflavin concentrations, they negatively affected the LNS-induced increase in this vitamin. Thiamin was not influenced by the study interventions. Concentrations of all B vitamins were much lower than usually accepted values.
Conclusions: All B vitamins were low in milk, and all but thiamin were increased by maternal supplementation with LNSs. Antiretrovirals alone decreased concentrations of some B vitamins in milk. When LNS was given in addition to antiretrovirals, the negative effect of antiretrovirals offset the positive effect of LNSs for all vitamins except thiamin. This trial was registered at clinicaltrials.gov as NCT00164762.
Keywords: antiretrovirals, B vitamins, breast milk, human milk, ultraperformance liquid chromatography tandem mass spectrometry
INTRODUCTION
The WHO recommends exclusive breastfeeding (EBF)10 for the first 6 mo of life based on its positive benefits for infant health and development, and the assumption that it provides adequate amounts of all nutrients, including micronutrients (1). However, breastfeeding increases the risk of mother-to-child HIV transmission. Several studies have indicated that postnatal HIV-1 transmission from mother to child can be reduced by antiretroviral drugs given to the mother or infant (2–9). These findings led to the current WHO recommendations for HIV-infected mothers to breastfeed exclusively for 6 mo and to continue breastfeeding to 12 mo in conjunction with taking antiretrovirals (either the mother or infant) for the duration of breastfeeding (1).
HIV infection increases energy and nutrient requirements, which makes it more challenging to meet the needs of both the mother and the infant (10, 11). No information is available concerning whether antiretroviral therapy provided to HIV-infected mothers during lactation affects micronutrient concentrations in breast milk. This would be of greatest concern in areas where maternal micronutrient status is poor because inadequate intake or the status of many micronutrients, especially B vitamins, adversely affects the amounts secreted in milk (12). Lipid-based nutrient supplements (LNSs) could increase the supply of energy and micronutrients to the HIV-positive mother, and the secretion of some micronutrients in her milk; however, the efficacy of this approach has not been evaluated (13, 14).
This study examines the effects of antiretrovirals and LNSs on thiamin, riboflavin, nicotinamide, pyridoxal, and vitamin B-12 concentrations in the breast milk of HIV-infected Malawian mothers. Values for the concentration of each B vitamin were compared with those published for milk from well-nourished women and used to set Adequate Intakes (AIs) for infants aged 0–6 mo (15).
METHODS
Subjects and sample collection
The Breastfeeding, Antiretrovirals, and Nutrition study population consisted of HIV-infected women recruited from 4 antenatal clinics in Lilongwe, Malawi, between 2004 and 2009. The recruitment methods and flow of participants have been described in detail elsewhere (8, 11, 16, 17). Mothers in this study were counseled to exclusively breastfeed for 24 wk and then to rapidly wean from 24 to 28 wk, in accordance with WHO recommendations at the time the study began (16, 18).
At delivery, women and their infants were randomly assigned by using the permuted block method to a 2-arm (LNSs or no LNSs) by 3-arm (maternal antiretrovirals, infant antiretrovirals, or neither) factorial design (Supplemental Figure 1). For the current analysis of the effects on breast milk, no differentiation was made between infants treated or not treated with antiretrovirals, which resulted in 4 intervention groups. After screening and enrollment by study nurses, a data manager generated the random allocation sequence. Study pharmacists assigned participants to interventions via sequentially numbered envelopes and distributed antiretrovirals and LNSs during the scheduled visits at 0, 1, 2, 4, 6, 8, 12, 18, 21, 24, and 28 wk postpartum. The LNS was manufactured by Nutriset in France (www.nutriset.fr). Each sachet contained 70 g, and the women were instructed to consume 2 sachets/d, which provided 746 kcal/d and approximately the Recommended Dietary Allowance for most micronutrients (Table 1). However, the LNS provided no vitamin A because of concerns that supplements could increase HIV transmission through breast milk (19, 20). The antiretroviral drug regimen for mothers consisted of zidovudine and lamivudine (Combivir) plus either nevirapine (until January 2005), nelfinavir (from January 2005 to February 2006), or lopinavir and ritonavir (Kaletra, Abbott; from February 2006 until study completion). In addition, 2 kg maize flour was provided weekly to all participants for family consumption.
TABLE 1.
Composition of the lipid-based nutrient supplement formulated for use by lactating women aged 19–30 y1
| Nutrient | RDA for lactating women | Amount per 2 packets (140 g) |
| Energy | ||
| (kcal) | — | 746 |
| (kJ) | — | 3120 |
| Protein, g | — | 20.8 |
| Iron, mg | 9 | 15 |
| Zinc, mg | 12 | 19 |
| Phosphorus, μg | 700 | 1200 |
| Selenium, μg | 70 | 75 |
| Thiamin, mg | 1.4 | 1.6 |
| Riboflavin, mg | 1.6 | 1.8 |
| Niacin, mg equivalent | 17 | 20 |
| Pyridoxine, mg | 2.0 | 2.2 |
| Vitamin B-12, μg | 2.8 | 2.6 |
| Ascorbic acid, mg | 120 | 100 |
| α-Tocopherol, mg | 19 | 12 |
| Folic acid, μg | 500 | 300 |
| Iodine, μg | 290 | 200 |
| Potassium, g | 5.1 | 1.1 |
| Magnesium, mg | 310 | 124 |
| Copper, mg | 1.3 | 0.3 |
| Calcium, mg | 1000 | 294 |
The supplement consisted of ground peanuts, dried skim milk, vegetable fat, sugar, multivitamin-mineral premix (Nutriset, France; www.nutriset.fr). RDA, Recommended Dietary Allowance [Institute of Medicine (15)].
Maternal reports of LNS consumption were taken at 1, 4, 8, 12, and 21 wk. Breast milk was manually expressed by the participants, producing opportunistic samples during the regular study visits at weeks 2, 6, and 24. The milk was immediately frozen after collection. The 6-wk samples were obtained when a 2-wk sample was unavailable or the infant had insufficient plasma at 2 wk. The sample size was predetermined by the number of participants providing milk, maternal, and infant plasma samples; participants were prioritized for selection in the micronutrient subsample if they had anthropometric and dietary intake data. They were excluded from the subsample if the infants became HIV-positive or were multiple births. The larger study’s sample size calculations were previously described in detail (16). As with all food-based nutrition supplement trials, participants and physicians could not be blinded to the study arm. Outcome assessors and data analysts were not informed about the allocation strategy.
No milk samples were collected before supplementation with LNS; therefore, no true baseline concentrations were available for control in the statistical analyses. Samples were shipped on dry ice to the CDC in Atlanta and stored at −80°C until analyzed at the USDA/Agricultural Research Service, Western Human Nutrition Research Center in Davis, CA.
This research was approved by the Malawi National Health Science Research Committee, the Institutional Review Boards at the University of North Carolina Chapel Hill, the CDC, and the University of California, Davis. The trial was monitored for safety and efficacy by the National Institute of Allergy and Infectious Diseases Vaccine and Prevention Data and Safety Monitoring Board.
Biochemical analyses
Free thiamin, riboflavin, flavin adenine dinucleotide (FAD), nicotinamide, and pyridoxal in milk were analyzed simultaneously by ultraperformance liquid chromatography–tandem mass spectrometry (21). Briefly, samples were subjected to protein precipitation, and nonpolar constituents were removed by liquid-liquid extraction before analysis. Thiamin monophosphate (TMP), thiamin pyrophosphate (TPP), and free thiamin were measured by HPLC-fluorescence detection after precolumn derivatization by using the thiochrome method as described previously (22, 23) with a few modifications. An Agilent Zorbax Eclipse Plus C18 column (4.6 × 150 mm, 5 μm) was used with an 8-min gradient of 0.15 mol K2HPO4/L (pH 7.0; A) and methanol (B) as follows: 0 min, 15% B; 1–3 min, 20% B; 3–6 min, 50% B; and 7–8 min, 15% B at a flow rate of 1.5 mL/min, which allowed the simultaneous detection of TPP, TMP, and thiamin derivatives. Vitamin B-12 concentrations were measured by using the IMMULITE solid-phase, competitive chemiluminescent enzyme immunoassay (24). Thiamin was calculated as the sum of combined free thiamin, TMP, and TPP concentrations based on molecular weights: thiamin = free thiamin + (TMP × 0.871) + (TPP × 0.707). Riboflavin was calculated as the sum of the combined free riboflavin and FAD concentrations, also based on molecular weights: riboflavin = free riboflavin + (FAD × 0.479) (25, 26).
Statistical analysis
The outcomes were concentrations of thiamin, riboflavin, nicotinamide, pyridoxal, and vitamin B-12 in breast milk within the 4 defined groups (antiretrovirals + LNSs, antiretrovirals, LNSs, and control). SAS statistical software 9.4 (SAS Institute) was used for all statistical analyses. Logarithmic transformations were performed on thiamin, riboflavin, nicotinamide, and vitamin B-12, and square root transformations were performed on pyridoxal concentrations to normalize the distributions. The original hypothesis considered only outcomes due to LNS supplementation. However, preliminary data showed significant effects of antiretrovirals for some of the micronutrients; therefore, possible negative interactions with antiretrovirals were also analyzed. Mixed-model repeated-measures ANOVA (MIXED procedure) was used to fit a 3-factor model, which included LNSs and antiretrovirals as between-subject main effects, time as a within-subject main effect, all 2- and 3-factor interactions, and a random effect of subject, assuming an unstructured covariance matrix. Because no significant interactions with time were observed, the 2 time points were pooled to assess the effect of treatment. For outcomes with a significant interaction (P < 0.05) between LNSs and antiretrovirals, pairwise comparisons were performed, adjusted for multiple comparisons with Tukey-Kramer’s test; otherwise, only main effects were examined. Breast-milk B vitamin concentrations were also compared with the values used by the Institute of Medicine to set recommended intakes for infants from 0 to 6 mo of age (15). P values <0.05 were considered to be statistically significant.
RESULTS
Maternal characteristics at initial visit
No differences were observed in the characteristics of study participants among the groups at the initial time point (Table 2). The average BMI was within the normal range. Overall compliance with antiretroviral treatment and supplementation was high. Mothers self-reported that they took their prescribed antiretroviral treatment 89% of the time and LNS supplement 92% of the time, based on adherence reports collected over 5 follow-up visits. The self-reported frequency of EBF was 96% at 21 wk postpartum (8, 11).
TABLE 2.
Characteristics of participants in the Breastfeeding, Antiretrovirals, and Nutrition study at the initial time point1
| Treatment group |
||||||||
| Control |
LNS |
ARV |
ARV + LNS |
|||||
| Characteristic | n | Value | n | Value | n | Value | n | Value |
| Age, y | 176 | 25.5 (22.9–29.8)2 | 183 | 26.2 (22.2–29.9) | 85 | 27.0 (24.0–29.4) | 90 | 25.0 (22.9–30.0) |
| Postprimary education, % | 177 | 36.7 | 185 | 37.8 | 85 | 35.3 | 90 | 32.2 |
| Literacy, % | 172 | 78.5 | 179 | 75.4 | 81 | 76.5 | 85 | 77.6 |
| Married, % | 177 | 90.4 | 185 | 90.3 | 85 | 89.4 | 90 | 92.2 |
| Vaginal delivery, % | 177 | 96.6 | 185 | 95.7 | 85 | 96.5 | 90 | 94.4 |
| Anthropometric measurements | ||||||||
| Height, cm | 177 | 157 (154–160) | 185 | 155 (152–159) | 85 | 157 (154–160) | 90 | 157 (154–161) |
| Weight, kg | 177 | 55.5 (50.0–60.3) | 185 | 54.4 (50.3–59.1) | 85 | 54.6 (50.5–60.2) | 90 | 54.5 (50.2–60.4) |
| BMI, kg/m2 | 177 | 22.4 (20.7–24.1) | 185 | 22.3 (20.9–24.2) | 85 | 22.3 (20.6–24.3) | 90 | 22.2 (20.8–23.7) |
| Laboratory measurements | ||||||||
| Hemoglobin, g/L | 177 | 122 (111–131) | 183 | 121 (111–131) | 85 | 121 (107–126) | 90 | 120 (111–131) |
| CD4 count, cells/μL | 159 | 465 (319–665) | 170 | 500 (337–738) | 78 | 616 (439–780) | 85 | 607 (412–796) |
ARV, antiretroviral; LNS, lipid-based nutrient supplement.
Median; IQR in parentheses (all such values).
Overall treatment effects and time interaction
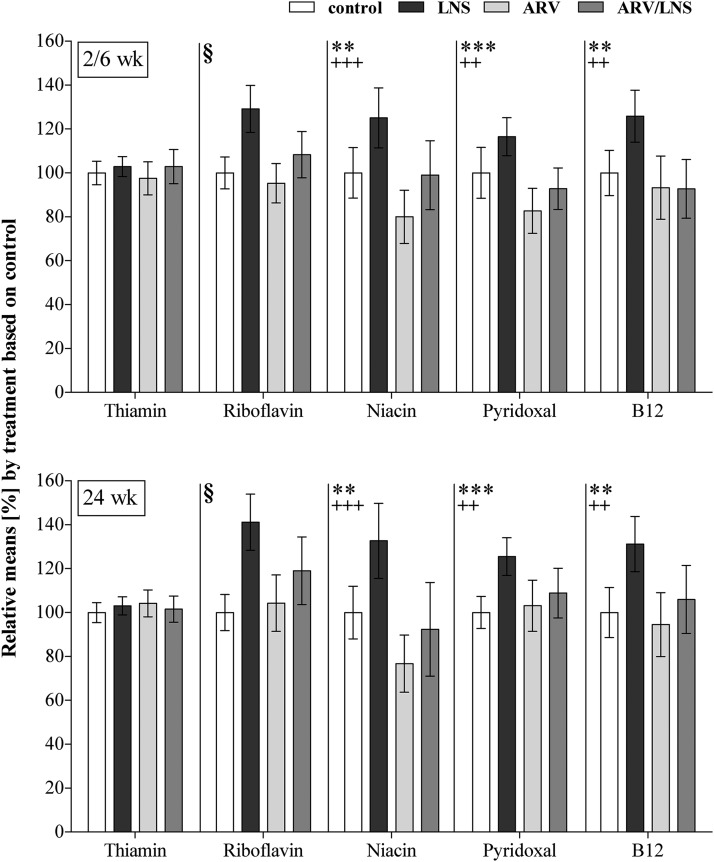
The results of the mixed model showed that the concentrations of all vitamins changed over time (P < 0.012 for all). However, none of the interactions involving time were significant, which indicated that the effects of antiretrovirals and LNSs at 2 or 6 wk were not different from the effects at 24 wk; therefore, we pooled the data from the 2 time points for the statistical analysis. A positive overall effect of LNSs on all vitamins, except thiamin, and a negative overall effect of antiretrovirals on nicotinamide, pyridoxal, and vitamin B-12 were observed. The LNS × antiretroviral interaction was significant for riboflavin only (P = 0.015; Table 3).
TABLE 3.
Median concentrations (and IQRs) of thiamin, riboflavin, nicotinamide, pyridoxal, and vitamin B-12 in breast milk from HIV-infected women assigned to 1 of 4 treatment arms in the Breastfeeding, Antiretrovirals, and Nutrition study1
| Treatment group |
P2 |
||||||
| Vitamin | Control (n = 177) | LNS (n = 185) | ARV (n = 85) | ARV/LNS (n = 90) | LNS | ARV | LNS × ARV |
| Thiamin,3 μg/L | 0.44 | 0.82 | 0.53 | ||||
| 2 or 6 wk | 176 (130–220)4 | 178 (144–212) | 169 (123–215) | 173 (134–229) | |||
| 24 wk | 199 (160–238) | 208 (174–241) | 202 (176–238) | 201 (165–242) | |||
| Riboflavin,5 μg/L | <.001 | 0.071 | 0.015 | ||||
| 2 or 6 wk | 100 (71–147) | 133 (87–184) | 104 (74–132) | 114 (81–148) | |||
| 24 wk | 94 (69–124) | 129 (88–168) | 95 (66–127) | 102 (67–163) | |||
| Nicotinamide, μg/L | 0.001 | <0.001 | 0.12 | ||||
| 2 or 6 wk | 430 (244–689) | 566 (374–793) | 386 (248–519) | 404 (253–708) | |||
| 24 wk | 219 (124–358) | 281 (169–457) | 161 (102–268) | 188 (97–315) | |||
| Pyridoxal, μg/L | <0.001 | 0.006 | 0.12 | ||||
| 2 or 6 wk | 62 (40–99) | 84 (57–109) | 56 (39–80) | 64 (50–89) | |||
| 24 wk | 113 (82–150) | 142 (100–190) | 110 (77–152) | 120 (83–161) | |||
| Vitamin B-12, μg/L | 0.0018 | 0.0036 | 0.16 | ||||
| 2 or 6 wk | 0.33 (0.22–0.59) | 0.41 (0.26–0.81) | 0.29 (0.20–0.55) | 0.30 (0.23–0.49) | |||
| 24 wk | 0.24 (0.19–0.35) | 0.32 (0.23–0.49) | 0.23 (0.19–0.33) | 0.28 (0.21–0.39) | |||
Main effects and interactions of LNS and ARV were tested by ANOVA. A mixed-model repeated-measures analysis was used to test for the main effect of time and interactions between treatment variables and time (no interactions with time were significant). ARV, antiretroviral; LNS, lipid-based nutrient supplement; n, number of samples.
P values represent both time points because of no significant LNS × ARV × time interaction.
Thiamin = thiamin + (thiamin monophosphate × 0.871) + (thiamin pyrophosphate × 0.707).
Median; IQR in parentheses (all such values).
Riboflavin = riboflavin + (flavin adenine dinucleotide × 0.479).
Treatment effects at 2 or 6 and 24 wk
Concentrations of the nutrients in milk varied widely among women in every treatment group, independently of the time of collection. All women assigned to receive LNSs started at or very close to delivery. Interactions between antiretrovirals and LNSs were observed only for riboflavin (Table 3). The main effects of LNSs and antiretrovirals were significant for nicotinamide, pyridoxal, and vitamin B-12, but not for thiamin (Figure 1). Whereas LNS consumption resulted in significantly higher concentrations, antiretroviral treatment lowered the amounts of these 3 vitamins in breast milk. Riboflavin concentrations increased with LNSs when compared with antiretrovirals, antiretrovirals + LNSs, and the control treatment (P < 0.016 for all), but were not influenced by antiretrovirals regimen alone. No significant differences were observed between the antiretrovirals, antiretrovirals + LNSs, and control groups. Neither LNS nor antiretroviral treatment affected thiamin concentrations. The consumption of antiretrovirals with LNSs eliminated the positive effects of LNS supplementation on all vitamins but thiamin, i.e., the resulting milk concentrations were reduced to the concentration of the control group.
FIGURE 1.
Relative mean concentrations and 95% CIs of B vitamins in the treatment groups. Main effects (LNS − no LNS; ARV – no ARV) and interactions between LNS, ARV, and time were tested by mixed-model repeated-measures ANOVA. No interactions with time were significant. Significant main effects of LNS: **P < 0.01, ***P < 0.001. Significant main effects of ARVs: ++P < 0.01, +++P < 0.001. §Significant ARV × LNS interaction: pairwise comparisons were adjusted for multiple comparisons with Tukey-Kramer’s test, LNS significantly increased riboflavin concentrations (P < 0.0001 when compared with the control and ARV groups; P = 0.015 when compared with the ARV + LNS group), and no significant differences were observed between the 3 remaining groups. Control group: n = 177; LNS group: n = 185; ARV group: n = 85; ARV + LNS group: n = 91. ARV, antiretroviral; LNS, lipid-based nutrient supplement.
Comparison of milk B vitamin concentrations at 24 wk with values used to set the AI for infants aged 0–6 mo
In comparison with the concentrations used to estimate the AI for infants aged 0–6 mo (Table 4) (15), only 50–56% of the samples met the estimated value for thiamin concentration in milk regardless of treatment group. For pyridoxal, 60% of the samples of the LNS group reached the values used to set the AIs, whereas only 36–44% of the samples were adequate in the remaining treatment groups. In contrast, <2% of samples met the AI milk values for riboflavin (0.6–1.6%) and nicotinamide (0–1.6%) in any group; 16–22% met vitamin B-12 assumptions, which increased to 34% when mothers were given LNSs alone.
TABLE 4.
Percentage of samples meeting the values used to set the AI for each vitamin, by collection time and treatment group1
| Treatment group |
|||||
| Vitamin | AI, μg/L | Control (n = 177) | LNS (n = 185) | ARV (n = 85) | ARV + LNS (n = 90) |
| Thiamin,2 % | |||||
| 2 or 6 wk | 200 | 35 | 35 | 31 | 36 |
| 24 wk | 200 | 50 | 56 | 52 | 50 |
| Riboflavin,3 % | |||||
| 2 or 6 wk | 350 | 0 | 3.2 | 0 | 0 |
| 24 wk | 350 | 0.6 | 1.6 | 1.2 | 1.1 |
| Nicotinamide, % | |||||
| 2 or 6 wk | 1800 | 2.8 | 5.4 | 1.2 | 1.1 |
| 24 wk | 1800 | 0 | 1.6 | 0 | 1.1 |
| Pyridoxal, % | |||||
| 2 or 6 wk | 130 | 9.0 | 17 | 5.9 | 7.8 |
| 24 wk | 130 | 36 | 60 | 44 | 41 |
| Vitamin B-12, % | |||||
| 2 or 6 wk | 0.42 | 35 | 47 | 33 | 33 |
| 24 wk | 0.42 | 16 | 34 | 17 | 22 |
AI, Adequate Intake; ARV, antiretroviral; LNS, lipid-based nutrient supplement; n, number of samples.
Thiamin = thiamin + (thiamin monophosphate × 0.871) + (thiamin pyrophosphate × 0.707).
Riboflavin = riboflavin + (flavin adenine dinucleotide × 0.479).
DISCUSSION
On the basis of strong evidence of the benefits of EBF during the first 6 mo of life, it is crucial to understand how factors such as maternal micronutrient intake, status, supplementation, and antiretroviral therapy affect breast-milk concentrations of nutrients during the period of lactation. In areas where dietary intake alone may not be sufficient to guarantee an adequate supply of vitamins in milk, maternal supplementation may help to ensure adequate nutrition of the infant. LNSs have been used historically in the clinical setting for the treatment of severe acute malnutrition in children and are currently being evaluated in trials aimed at improving birth and health outcomes in infants and children with moderate malnutrition (18, 27). In this trial, LNSs mitigated maternal weight loss, even in women receiving antiretrovirals (11).
These results suggest that the usual maternal intake of all of the vitamins measured is insufficient, given the observation that <1% of control samples reached the concentration used to set the AI values for riboflavin and nicotinamide and only 16–50% of samples met the AI values for any of the other B vitamins. However, given that AIs are based on studies with small sample sizes collected under unclear and variable conditions (12), the true reference nutrient contents of breast milk and subsequently the requirements of infants during the first 6 mo of life remain somewhat uncertain.
Maternal LNS supplementation increased breast-milk concentrations of riboflavin, nicotinamide, pyridoxal, and vitamin B-12. However when concentrations were compared with the values used to set the AI (15, 28), only 2–60% of the participants receiving LNSs alone met the AI estimates across all the vitamins measured. Therefore, only a limited amount of the micronutrients in maternal LNSs get transferred to the infant via breast milk and may not fulfill the infant’s requirements. Duggan et al. also observed significantly higher vitamin B-12 concentrations in breast milk collected at 6 wk, but not at 3 or 6 mo postpartum after a daily maternal supplement consumption of 50 μg/d during pregnancy through 6 wk postpartum (29). This supplementation regimen resulted in a median breast-milk vitamin B-12 concentration of only 136 pmol/L (0.18 μg/L) vs. 87 pmol/L, less than half of the value assumed to set the AI. Even a daily maternal supplementation of 250 μg/d during pregnancy up to 3 mo postpartum (30) resulted in a median vitamin B-12 concentration at 3 mo of 235 pmol/L (0.32 μg/L) vs. 170 pmol/L, which indicated a limitation of vitamin transfer into breast milk. Recommendations regarding antiretroviral use during lactation were introduced in the 2010 WHO guidelines in response to evidence of a reduced risk of postnatal transmission of HIV through breastfeeding when antiretrovirals are given to either the HIV-infected mother or the HIV-exposed infant (1). Thus, it is important to understand the effect of these treatments on the concentrations of nutrients in breast milk and the potential effect on the exclusively breastfed infant. Some antiretrovirals given to the mother do appear in breast milk. In a study conducted in Botswana, the concentrations of nevirapine, lamivudine, and zidovudine were 0.67, 3.34, and 3.21 times, respectively, the concentrations in maternal serum (31). Whereas these drugs were also found in breast milk analyzed within the Breastfeeding, Antiretrovirals, and Nutrition study, only lamivudine was detected in infant plasma (32). However, it has been shown that infants exposed to the maternal antiretroviral regimen through breast milk developed HIV-1 drug resistance, which indicated the need for close monitoring of the infants’ HIV status and to consider the maternal regimen when deciding on infant treatment (33). These findings are important in the context of Option B+, which provides lifelong antiretroviral therapy to pregnant and/or lactating women as soon as HIV is diagnosed, regardless of their baseline CD4 count, as recommended by the WHO (34, 35). Although Option B+ aids considerably in the Prevention of Mother-to-Child Transmission program in resource-limited countries with a high prevalence of HIV, it has been shown that micronutrients such as vitamin D and B-12 can be negatively affected by antiretrovirals based on lower plasma concentrations (36–38). These observations call attention to the need to measure effects of Option B+ on maternal and infant vitamin status.
LNSs increased all B vitamin concentrations in breast milk, except thiamin. Antiretroviral treatment without LNS supplementation negatively affected the concentrations of nicotinamide, pyridoxal, and vitamin B-12. When LNS was taken with antiretroviral treatment, the positive effect of supplementation was offset by the negative effect of the antiretrovirals on nicotinamide, pyridoxal, and vitamin B-12, which resulted in vitamin concentrations that were comparable with those in the control group. Even though antiretroviral treatment alone did not affect milk riboflavin concentrations, the combination of antiretrovirals with LNSs prevented LNS-induced increases in this vitamin. These results suggest that antiretroviral treatment negatively influences the uptake or transfer of certain B vitamins in breast milk and that LNS supplementation of lactating women receiving antiretrovirals may be beneficial to breast-milk vitamin concentrations and subsequently to infants who are exclusively breastfed. It remains to be determined whether the timing of taking supplements and antiretrovirals might improve the benefits of micronutrient supplementation.
Even though the number of milk samples analyzed in the current study is extensive, they may not be representative of 24-h milk production, because the samples were not obtained by emptying the breast. No information is available about the timing of the last infant feed or LNS consumption, but usually there is relatively little variability in B vitamin concentrations during a feed. In addition, because our initial samples were from 2 or 6 wk, the actual effect of the treatments from 0 to 2 wk and 0 to 6 wk is not reflected in the analysis. Nevertheless, we concluded that LNS supplementation had a positive effect on breast-milk concentrations of all B vitamins analyzed, except thiamin, whereas antiretroviral treatment affected B vitamin uptake into breast milk. Additional studies are necessary to gain a better understanding of the interactions between maternal antiretroviral treatment and micronutrient supplementation and their long-term effects on breast-milk vitamin concentrations and maternal and infant status. In addition, more information is desirable about the effect of different levels of supplemental nutrients, and the optimal timing of supplementation during pregnancy or lactation, in healthy women and in the context of HIV infection.
Acknowledgments
We thank Charles M van der Horst, principal investigator of the Breastfeeding, Antiretrovirals, and Nutrition study. We thank Janet M Peerson for guidance on the statistical analysis.
The authors’ responsibilities were as follows—LHA: was responsible for the laboratory analyses conducted at the Western Human Nutrition Research Center, oversaw the data analyses, and was responsible for the final version of the manuscript; DH: wrote the manuscript; DH, SS-F, and ERY: conducted the breast-milk and statistical analyses and helped prepare the manuscript; VLF: contributed to the data and statistical analyses; MEB and LSA: contributed to the trial design and obtained funding for the study; and all authors: reviewed the manuscript revisions and contributed to the intellectual content of the manuscript. None of the authors declared a conflict of interest. USDA is an equal opportunity employer and provider.
Footnotes
Abbreviations used: AI, Adequate Intake; EBF, exclusive breastfeeding; FAD, flavin adenine dinucleotide; LNS, lipid-based nutrient supplement; TMP, thiamin monophosphate; TPP, thiamin pyrophosphate.
REFERENCES
- 1.World Health Organization. Guidelines on HIV and infant feeding 2010: principles and recommendations for infant feeding in the context of HIV and a summary of evidence. Geneva (Switzerland): WHO; 2010. [PubMed] [Google Scholar]
- 2.Thior I, Lockman S, Smeaton LM, Shapiro RL, Wester C, Heymann SJ, Gilbert PB, Stevens L, Peter T, Kim S. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana. JAMA 2006;296:794–805. [DOI] [PubMed] [Google Scholar]
- 3.Palombi L, Marazzi MC, Voetberg A, Magid NA. Treatment acceleration program and the experience of the DREAM program in prevention of mother-to-child transmission of HIV. AIDS 2007;21:S65–71. [DOI] [PubMed] [Google Scholar]
- 4.Kilewo C, Karlsson K, Ngarina M, Massawe A, Lyamuya E, Swai A, Lipyoga R, Mhalu F, Biberfeld G. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr 2009;52:406–16. [DOI] [PubMed] [Google Scholar]
- 5.Peltier CA, Ndayisaba GF, Lepage P, Van Griensven J, Leroy V, Omes C, Ndimubanzi PC, Courteille O. Breastfeeding with maternal antiretroviral therapy or formula feeding to prevent HIV postnatal mother-to-child transmission in Rwanda. AIDS 2009;23:2415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shapiro RL, Hughes M, Ogwu A, Kitch D, Lockman S, Moffat C, Makhema J, Moyo S, Thior I, McIntosh K. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med 2010;362:2282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumwenda NI, Hoover DR, Mofenson LM, Thigpen MC, Kafulafula G, Li Q, Mipando L, Nkanaunena K, Mebrahtu T, Bulterys M. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med 2008;359:119–29. [DOI] [PubMed] [Google Scholar]
- 8.Chasela CS, Hudgens MG, Jamieson DJ, Kayira D, Hosseinipour MC, Kourtis AP, Martinson F, Tegha G, Knight RJ, Ahmed YI. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med 2010;362:2271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bedri A, Gudetta B, Isehak A, Kumbi S, Lulseged S, Mengistu Y. Six Week Extended-Dose Nevirapine (SWEN) Study Team. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet 2008;372:300–13. [DOI] [PubMed] [Google Scholar]
- 10.de Pee S, Semba RD. Role of nutrition in HIV infection: review of evidence for more effective programming in resource-limited settings. Food Nutr Bull 2010;31:313S–44S. [PubMed] [Google Scholar]
- 11.Kayira D, Bentley ME, Wiener J, Mkhomawanthu C, King CC, Chitsulo P, Chigwenembe M, Ellington S, Hosseinipour MC, Kourtis AP. A lipid-based nutrient supplement mitigates weight loss among HIV-infected women in a factorial randomized trial to prevent mother-to-child transmission during exclusive breastfeeding. Am J Clin Nutr 2012;95:759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen LH. B vitamins in breast milk: relative importance of maternal status and intake, and effects on infant status and function. Adv Nutr 2012;3:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lartey A, Manu A, Brown KH, Dewey KG. Predictors of micronutrient status among six-to twelve-month-old breast-fed Ghanaian infants. J Nutr 2000;130:199–207. [DOI] [PubMed] [Google Scholar]
- 14.Allen LH, Graham JM. Assuring micronutrient adequacy in the diets of young infants Delange FM, West KP Jr, editors. Micronutrient deficiencies in the first months of life. Basel (Switzerland): S. Karger AG; 2003. p. 55–88. [Google Scholar]
- 15.Institute of Medicine. Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academy Press; 1998. [PubMed] [Google Scholar]
- 16.van der Horst C, Chasela C, Ahmed Y, Hoffman I, Hosseinipour M, Knight R, Fiscus S, Hudgens M, Kazembe P, Bentley M. Modifications of a large HIV prevention clinical trial to fit changing realities: a case study of the Breastfeeding, Antiretroviral, and Nutrition (BAN) protocol in Lilongwe, Malawi. Contemp Clin Trials 2009;30:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamieson DJ, Chasela CS, Hudgens MG, King CC, Kourtis AP, Kayira D, Hosseinipour MC, Kamwendo DD, Ellington SR, Wiener JB, et al. Maternal and infant antiretroviral regimens to prevent postnatal HIV-1 transmission: 48-week follow-up of the BAN randomised controlled trial. Lancet 2012;379:2449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phuka JC, Maleta K, Thakwalakwa C, Cheung YB, Briend A, Manary MJ, Ashorn P. Postintervention growth of Malawian children who received 12-mo dietary complementation with a lipid-based nutrient supplement or maize-soy flour. Am J Clin Nutr 2009;89:382–90. [DOI] [PubMed] [Google Scholar]
- 19.Fawzi WW, Msamanga GI, Hunter D, Renjifo B, Antelman G, Bang H, Manji K, Kapiga S, Mwakagile D, Essex M. Randomized trial of vitamin supplements in relation to transmission of HIV-1 through breastfeeding and early child mortality. AIDS 2002;16:1935–44. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. HIV and infant feeding: guidelines for decision-makers. Geneva (Switzerland): WHO; 2003. [Google Scholar]
- 21.Hampel D, York ER, Allen LH. Ultra-performance liquid chromatography tandem mass-spectrometry (UPLC-MS/MS) for the rapid, simultaneous analysis of thiamin, riboflavin, flavin adenine dinucleotide, nicotinamide and pyridoxal in human milk. J Chromatogr B Analyt Technol Biomed Life Sci 2012;903:7–13. [DOI] [PubMed] [Google Scholar]
- 22.Stuetz W, Carrara VI, McGready R, Lee SJ, Biesalski HK, Nosten FH. Thiamine diphosphate in whole blood, thiamine and thiamine monophosphate in breast-milk in a refugee population. PLoS One 2012;7:e36280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stuetz W, Carrara VI, McGready R, Lee SJ, Erhardt JG, Breuer J, Biesalski HK, Nosten FH. Micronutrient status in lactating mothers before and after introduction of fortified flour: cross-sectional surveys in Maela refugee camp. Eur J Nutr 2012;51:425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hampel D, Shahab-Ferdows S, Domek JM, Siddiqua T, Raqib R, Allen LH. Competitive chemiluminescent enzyme immunoassay for vitamin B12 analysis in human milk. Food Chem 2014;153:60–5. [DOI] [PubMed] [Google Scholar]
- 25.Roughead ZK, McCormick DB. Flavin composition of human milk. Am J Clin Nutr 1990;52:854–7. [DOI] [PubMed] [Google Scholar]
- 26.Sakurai T, Furukawa M, Asoh M, Kanno T, Kojima T, Yonekubo A. Fat-soluble and water-soluble vitamin contents of breast milk from Japanese women. J Nutr Sci Vitaminol (Tokyo) 2005;51:239–47. [DOI] [PubMed] [Google Scholar]
- 27.Thakwalakwa C, Ashorn P, Phuka J, Cheung YB, Briend A, Puumalainen T, Maleta K. A lipid-based nutrient supplement but not corn-soy blend modestly increases weight gain among 6-to 18-month-old moderately underweight children in rural Malawi. J Nutr 2010;140:2008–13. [DOI] [PubMed] [Google Scholar]
- 28.Young VR. Setting Dietary Reference Intakes for micronutrients for healthy North American infants: a process of trials and errors. In:Delange FM, West KP, eds. Micronutrient deficiencies in the first months of life. Basel (Switzerland): S. Karger AG; 2003. p. 35–53. [Google Scholar]
- 29.Duggan C, Srinivasan K, Thomas T, Samuel T, Rajendran R, Muthayya S, Finkelstein JL, Lukose A, Fawzi W, Allen LH, et al. Vitamin B-12 supplementation during pregnancy and early lactation increases maternal, breast milk, and infant measures of vitamin B-12 status. J Nutr 2014;144:758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siddiqua TJ, Ahmad SM, Ahsan KB, Rashid M, Roy A, Rahman SM, Shahab-Ferdows S, Hampel D, Ahmed T, Allen LH, et al. Vitamin B12 supplementation during pregnancy and postpartum improves B12 status of both mothers and infants but vaccine response in mothers only: a randomized clinical trial in Bangladesh. Eur J Nutr 2015 Feb 4 (Epub ahead of print; DOI:10.1007/s00394-015-0845-x). [DOI] [PubMed] [Google Scholar]
- 31.Shapiro RL, Holland DT, Capparelli E, Lockman S, Thior I, Wester C, Stevens L, Peter T, Essex M, Connor JD. Antiretroviral concentrations in breast-feeding infants of women in Botswana receiving antiretroviral treatment. J Infect Dis 2005;192:720–7. [DOI] [PubMed] [Google Scholar]
- 32.Corbett AH, Kayira D, White NR, Davis NL, Kourtis AP, Chasela C, Martinson F, Phiri G, Musisi B, Kamwendo D, et al. Antiretroviral pharmacokinetics in mothers and breastfeeding infants from 6 to 24 weeks post partum: results of the BAN Study. Antivir Ther 2014;19:587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeh C, Weidle PJ, Nafisa L, Lwamba HM, Okonji J, Anyango E, Bondo P, Masaba R, Fowler MG, Nkengasong JN. HIV-1 drug resistance emergence among breastfeeding infants born to HIV-infected mothers during a single-arm trial of triple-antiretroviral prophylaxis for prevention of mother-to-child transmission: a secondary analysis. PLoS Med 2011;8:e1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed S, Kim MH, Abrams EJ. Risks and benefits of lifelong antiretroviral treatment for pregnant and breastfeeding women: a review of the evidence for the Option B+ approach. Curr Opin HIV AIDS 2013;8:474–89. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Use of antiretroviral drugs for treating pregnant women and preventing HIV infections in infants: executive summary. Geneva (Switzerland): WHO; 2012. [Google Scholar]
- 36.Childs K, Welz T, Samarawickrama A, Post FA. Effects of vitamin D deficiency and combination antiretroviral therapy on bone in HIV-positive patients. AIDS 2012;26:253–62. [DOI] [PubMed] [Google Scholar]
- 37.Paltiel O, Falutz J, Veilleux M, Rosenblatt DS, Gordon K. Clinical correlates of subnormal vitamin B12 concentrations in patients infected with the human immunodeficiency virus. Am J Hematol 1995;49:318–22. [DOI] [PubMed] [Google Scholar]
- 38.Woods MN, Tang AM, Forester J, Jones C, Hendricks K, Ding B, Knox TA. Effect of dietary intake and protease inhibitors on serum vitamin B12 levels in a cohort of human immunodeficiency virus – positive patients. Clin Infect Dis 2003;37:S124–31. [DOI] [PubMed] [Google Scholar]