Abstract
Background: The effects of nuts on major cardiovascular disease (CVD) risk factors, including dose-responses and potential heterogeneity by nut type or phytosterol content, are not well established.
Objectives: We examined the effects of tree nuts (walnuts, pistachios, macadamia nuts, pecans, cashews, almonds, hazelnuts, and Brazil nuts) on blood lipids [total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein, and triglycerides], lipoproteins [apolipoprotein A1, apolipoprotein B (ApoB), and apolipoprotein B100], blood pressure, and inflammation (C-reactive protein) in adults aged ≥18 y without prevalent CVD.
Design: We conducted a systematic review and meta-analysis following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Two investigators screened 1301 potentially eligible PubMed articles in duplicate. We calculated mean differences between nut intervention and control arms, dose-standardized to one 1-oz (28.4 g) serving/d, by using inverse-variance fixed-effects meta-analysis. Dose-response for nut intake was examined by using linear regression and fractional polynomial modeling. Heterogeneity by age, sex, background diet, baseline risk factors, nut type, disease condition, duration, and quality score was assessed with meta-regression. Publication bias was evaluated by using funnel plots and Egger’s and Begg’s tests.
Results: Sixty-one trials met eligibility criteria (n = 2582). Interventions ranged from 3 to 26 wk. Nut intake (per serving/d) lowered total cholesterol (−4.7 mg/dL; 95% CI: −5.3, −4.0 mg/dL), LDL cholesterol (−4.8 mg/dL; 95% CI: −5.5, −4.2 mg/dL), ApoB (−3.7 mg/dL; 95% CI: −5.2, −2.3 mg/dL), and triglycerides (−2.2 mg/dL; 95% CI: −3.8, −0.5 mg/dL) with no statistically significant effects on other outcomes. The dose-response between nut intake and total cholesterol and LDL cholesterol was nonlinear (P-nonlinearity < 0.001 each); stronger effects were observed for ≥60 g nuts/d. Significant heterogeneity was not observed by nut type or other factors. For ApoB, stronger effects were observed in populations with type 2 diabetes (−11.5 mg/dL; 95% CI: −16.2, −6.8 mg/dL) than in healthy populations (−2.5 mg/dL; 95% CI: −4.7, −0.3 mg/dL) (P-heterogeneity = 0.015). Little evidence of publication bias was found.
Conclusions: Tree nut intake lowers total cholesterol, LDL cholesterol, ApoB, and triglycerides. The major determinant of cholesterol lowering appears to be nut dose rather than nut type. Our findings also highlight the need for investigation of possible stronger effects at high nut doses and among diabetic populations.
Keywords: nuts, cholesterol, lipids, apolipoprotein, cardiovascular
INTRODUCTION
Accumulating evidence from prospective observational studies and a large clinical trial suggests that nut intake lowers the risk of cardiovascular disease (CVD)6 (1, 2). Tree nuts are rich in unsaturated fats, soluble fiber, antioxidants, and phytosterols (3), which separately or together may produce beneficial effects on serum lipids, blood pressure, and inflammation (4, 5). Prior meta-analyses of controlled trials have shown that tree nut intake lowers total and LDL cholesterol (6–8). However, effects of nut consumption on other key CVD risk factors, including specific lipoproteins, blood pressure, and inflammation, are not established. In addition, 2 of these prior meta-analyses evaluated only one type of nuts—almonds (6) (n = 5 trials) and walnuts (7) (n = 13 trials)—and potential effects of other tree nuts remain unclear. Furthermore, previous analyses (6–9) have not standardized pooled effects to a common dose or tested for nonlinearity of dose-responses, preventing conclusions about the magnitude of effects for a given intake of nuts or potential for nonlinear effects. Therefore, key questions remain on the major cardiovascular mechanisms influenced by tree nuts, on whether some types of nuts are preferential for improving risk, and on dose-response relations of these effects.
To address these knowledge gaps, we performed a systematic review and meta-analysis of controlled interventional trials to examine the effects of tree nuts (walnuts, pistachios, macadamia nuts, pecans, cashews, almonds, hazelnuts, pine nuts, and Brazil nuts) on major CVD risk factors, including blood lipids (total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides), lipoproteins [apolipoprotein A1, apolipoprotein (ApoB), and apolipoprotein B100], blood pressure (systolic and diastolic), and inflammation (C-reactive protein, CRP) in adults aged ≥18 y without prevalent CVD. We hypothesized that tree nuts would lower concentrations of LDL cholesterol and its primary lipoprotein, ApoB. As a secondary hypothesis, we evaluated potential differences in effects by nut type.
METHODS
We followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (10) during all stages of implementation, analysis, and reporting of this meta-analysis. A review protocol has not been published.
Eligibility criteria
We searched for all published controlled trials that reported the effect of tree nut consumption on blood lipids (total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides), lipoproteins (apolipoprotein A1, ApoB, and apolipoprotein B100), blood pressure (systolic and diastolic), or inflammation (CRP). We did not include body weight or adiposity as outcomes because a meta-analysis of nut intake and body weight was recently reported (11). Trials had to be controlled but could be randomized or nonrandomized (with plans to evaluate only randomized trials and all trials combined) and provided mean levels of the outcome in each group with an accompanying measure of statistical uncertainty (e.g., 95% CI, SE) or other data to calculate variance.
We excluded trials testing nonnut parts of the plant, nut oils, nuts other than tree nuts (e.g., areca, betel), or legumes (e.g., peanuts) and trials testing mixed dietary interventions for which the specific effect of nuts could not be evaluated. We also excluded trials among children (aged <18 y), participants with known CVD (myocardial infarction, angina, stroke, severe heart failure, coronary revascularization, or peripheral vascular disease), and participants receiving medication treatment of diabetes, obesity, metabolic syndrome, hypertension, or hyperlipidemia. For crossover trials without a washout period, we excluded trials with an intervention period <3 wk to minimize carryover effects (12). Trials with ≥20% dropout rates or having imbalanced dropout between intervention and control groups were also excluded. Articles presenting only observational data, editorials/commentaries, letters, and reviews were not eligible.
Search and selection of articles
Potentially eligible articles were identified by means of a systematic search in PubMed from the earliest available online indexing year to March 2013, without language restrictions. Query terms were as follows: (Apolipoproteins B[MeSH]) OR Apolipoprotein A-1[MeSH]) OR (Cholesterol, HDL [MeSH] OR Cholesterol, LDL [MeSH])) OR Triglycerides [MeSH]) OR Lipoprotein(a) [MeSH]) OR C-Reactive Protein [MeSH] OR Factor VIII [MeSH]) OR Fibrinogen [MeSH] OR von Willebrand Factor [MeSH]) OR Carotid Intima-Media Thickness [MeSH]) OR Blood Pressure [MeSH]) OR Heart Rate [MeSH] OR (diabetes or cardiovascular) AND (Nuts [MeSH] or Tree nuts or almonds or pecans or brazil nuts or hazelnuts or macadamia or pine nuts or pistachios or walnuts).
Two investigators (MF, KL) screened the titles and abstracts of all potentially eligible articles in duplicate, as well as the full text of all articles identified for further review. In addition, citation lists and the first 20 “related citations” on PubMed of all final included articles were hand-searched for additional eligible trials.
Data extraction
Data were screened and extracted independently and in duplicate by 2 investigators (MF, KL) by using a standardized electronic form, including information on study randomization (yes, no), design (parallel, crossover), nut type, age (mean), sex (percent male), baseline disease condition, treatment duration, dose (g/d), and description of the placebo or control condition. Differences in data extraction between investigators were infrequent and were resolved by consensus. For each outcome, we extracted its mean value (concentration/amount), variance measure, and the number of participants in the treatment and control arms for all reported periods (e.g., baseline, end treatment).
Study quality was assessed by using the Academy of Nutrition and Dietetics (formerly American Dietetic Association) Evidence Analysis Process (13), which evaluates relevance and validity by using a 14-question quality control checklist, including questions on comparability of control and intervention groups, handling of dropouts, blinding, appropriateness of statistical methods, and potential biases (see “Assessment” on last page of Supplemental Material). Studies meeting criteria for ≥6 of the 10 validity questions, including questions 2, 3, 6, and 7, were given a positive quality rating; studies meeting ≥6 of the 10 validity questions, but not questions 2, 3, 6, and 7, were given a quality rating of neutral; and studies not meeting at least 6 of 10 validity questions were considered of lower quality (13).
Statistical analysis
For parallel trials, the primary effect measure was the mean difference in change from baseline to follow-up in the intervention vs. control group (14). For crossover trials, the primary effect measure was the mean difference at follow-up in the intervention vs. control periods. The SE of the difference measure was extracted (when directly reported), calculated by using a related statistical measure of uncertainty, or estimated by using the IQR of the difference measure provided in studies. To address within-individual correlation in crossover trials, the median reported correlation across all crossover trials (r = 0.60) was used in calculating the SE of the difference when the study-specific correlation coefficient was not otherwise provided. In trials with repeated measures, we included the estimate closest to the median duration of follow-up across trials (4 wk). For trials with more than one comparison group, we included estimates from the control diet most like the intervention diet other than the inclusion of nuts.
For each trial, the effect size and corresponding variance were standardized to one 1-oz daily serving (28.4 g) of nuts. Meta-analyses were performed by using fixed-effects inverse-variance weighting, evaluating randomized trials, nonrandomized trials, and all trials combined. Heterogeneity was quantified by using the I2 statistic (15), with >30% considered at least moderate heterogeneity. Heterogeneity was evaluated by prespecified sources, including randomized vs. nonrandomized trials, age, sex, background diet, baseline risk factor level, nut type, comorbidity, intervention duration, and quality score by using meta-regression. For categorical sources of heterogeneity with ≥3 subgroups, P-heterogeneity from meta-regression was obtained for each indicator category relative to the primary reference category (16).
To test dose-response relations, we plotted the relation between absolute nut intake (g/d) and the absolute mean difference in each outcome, with nonlinearity evaluated by using the F test of linear lack of fit. Fractional polynomial models were used to evaluate nonlinear dose-response relations, with the best-fitting model considered the one with the lowest deviance.
Publication bias was evaluated by visual inspection of funnel plots and by Egger’s (17) and Begg’s (18) tests. All analyses were performed with STATA 12 (StataCorp LP), with 2-tailed α = 0.05.
RESULTS
Study characteristics
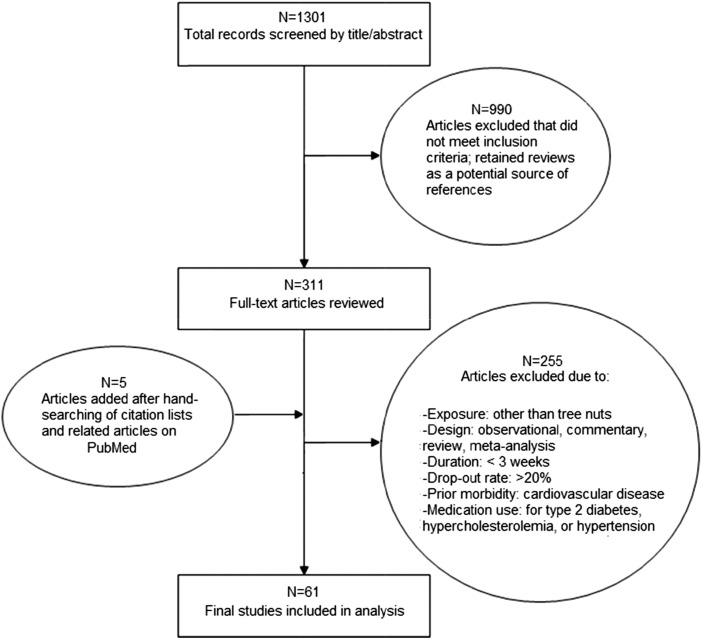
Of 1301 articles, 61 trials met eligibility criteria (19–80) (Figure 1), totaling 2582 unique participants in 42 randomized and 18 nonrandomized trials (Table 1). Trials directly provided nuts to the intervention group, rather than relying only on dietary advice to consume nuts. Compliance was most often assessed by using self-reported dietary recalls or direct supervision of nut consumption. Median participant age was 45 y, and two-thirds of trials (41/61) included both men and women (see Supplemental Table 1 for individual study details).
FIGURE 1.
Screening and selection of randomized (n = 42) and nonrandomized controlled trials (n = 19) on tree nut intake and lipids/apolipoproteins, blood pressure, and C-reactive protein (19–80).
TABLE 1.
Summary of 61 trials included in meta-analysis of the effect of tree nut intake on lipids/apolipoproteins, blood pressure, and C-reactive protein, stratified by randomization status and tree nut type1
| Study type/nut type | Trials, n | Participants (maximum), n | Median age, y | Male, % | Cardiovascular (CVD) comorbidities2 | Median duration, wk | Median nut dose,3 g/d | Quality score, n trials4 |
| Randomized controlled trials | ||||||||
| Walnut | 17 | 939 | 54 | 47 | 5 trials (n = 1 with diabetes) | 5 | 49 | 10(+), 5(Ø), 2(−) |
| Pistachio | 6 | 229 | 48 | 50 | 2 trials (n = 1 with prostate disease) | 4 | 60 | 4(+), 2(Ø) |
| Macadamia | 2 | 68 | 48 | 70 | 1 trial (overweight/obese) | 4.5 | 59 | 2(Ø) |
| Pecan | 2 | 65 | 41 | 45 | 2 trials (high cholesterol, MetS) | 6 | 70 | 1(+), 1(Ø) |
| Cashew | 2 | 54 | 64 | 83 | 2 trials (n = 1 with diabetes) | 8 | 85.5 | 1(+), 1(Ø) |
| Almond | 9 | 429 | 50 | 56 | 3 trials (obese, high cholesterol, diabetes) | 4 | 60 | 6(+), 3(Ø) |
| Hazelnut | 2 | 201 | 46 | 68 | 2 trials (high cholesterol) | 8 | 36 | 2(Ø) |
| Mixed nuts | 2 | 106 | 51 | 51 | 2 trials (obese, MetS) | 9 | 30 | 1(+), 1(Ø) |
| Overall | 42 | 2101 | 53 | 53 | 19/42 trials (45%) with CVD comorbidities | 5.5 | 59.5 | 23(+), 17(Ø), 2(−) |
| Nonrandomized trials | ||||||||
| Walnut | 4 | 78 | 60 | 43 | 0 trials | 6 | 45 | 3(Ø), 1(−) |
| Pistachio | 1 | 17 | 48 | 100 | 0 trials | 3 | 100 | 1(Ø) |
| Macadamia | 2 | 41 | 37 | 50 | 0 trials | 3.5 | 43 | 2(−) |
| Almond | 7 | 199 | 46 | 45 | 2 trials (obese, high cholesterol) | 4 | 84 | 2(+), 5(Ø) |
| Hazelnut | 4 | 109 | 45 | 64 | 1 trials (high cholesterol) | 4 | 54 | 1(+), 3(Ø) |
| Brazil | 1 | 37 | 35 | 0 | 0 trials | 8 | 5 | 1(−) |
| Overall | 19 | 481 | 45 | 50 | 3/19 trials (16%) with CVD comorbidities | 4 | 49.5 | 3(+), 12(Ø), 4(−) |
| All trials | ||||||||
| Overall | 61 | 2582 | 45 | 50 | 22/61 trials (36%) with CVD comorbidities | 4 | 56 | 26(+), 29(Ø), 6(−) |
Total cholesterol and LDL cholesterol were measured as outcomes in 61 trials; HDL cholesterol in 60 trials; triglycerides in 59 trials; apolipoprotein A1 and apolipoprotein B in 23 and 20 trials, respectively; blood pressure in 21 trials; C-reactive protein in 12 trials; and apolipoprotein B100 in 5 trials (19–80). Descriptive information for individual studies is given in Supplemental Table 1. For a summary of the number of studies and effect sizes by outcome, see Table 2. Outcomes included total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, apolipoprotein A1, apolipoprotein B, apolipoprotein B100, systolic blood pressure, diastolic blood pressure, and C-reactive protein. CVD, cardiovascular disease; MetS, metabolic syndrome.
CVD comorbidities refers to trials of patients with diabetes or those that enrolled at least some participants with high cholesterol, metabolic syndrome, or overweight/obesity. Other conditions are specified. Participants were either not receiving medication for CVD comorbidities, or medication use was not specified in the trial.
For meta-analysis, nut dose (g/d) was standardized to 1 serving (28.4 g) of nuts/d.
A quality control checklist comprising 14 questions on relevance and validity was used to award studies a positive (+), neutral (Ø), or negative score (−). Further details on the questions and scoring system are given in the Supplemental Appendix.
Most trials examined walnuts (n = 21) or almonds (n = 16); others examined pistachios (n = 7), hazelnuts (n = 6), macadamia nuts (n = 4), pecans (n = 2), cashews (n = 2), mixed tree nuts (n = 2), and Brazil nuts (n = 1). The dose of nuts varied from 5 to 100 g/d (median: 56 g/d), and the duration of intervention was from 3 to 26 wk (median: 4 wk). Participants had existing disease conditions in 45% (19/42) of randomized trials and 16% (3/19) of nonrandomized trials; these were most commonly hypertension, hyperlipidemia, and metabolic syndrome (Table 1). In 14 trials, participants received detailed advice to maintain total energy constant between intervention arms; in the remaining 47 trials, participants were provided nuts on top of a common background diet. The most common background diet (i.e., recommended to both intervention and control arms) was habitual diet (n = 30 trials); other background diets included American Heart Association, low-fat, high-fat, and Mediterranean-type diets. Most trials obtained a positive (n = 26) or neutral (n = 29) quality score; 6 trials had a negative score.
Main outcomes
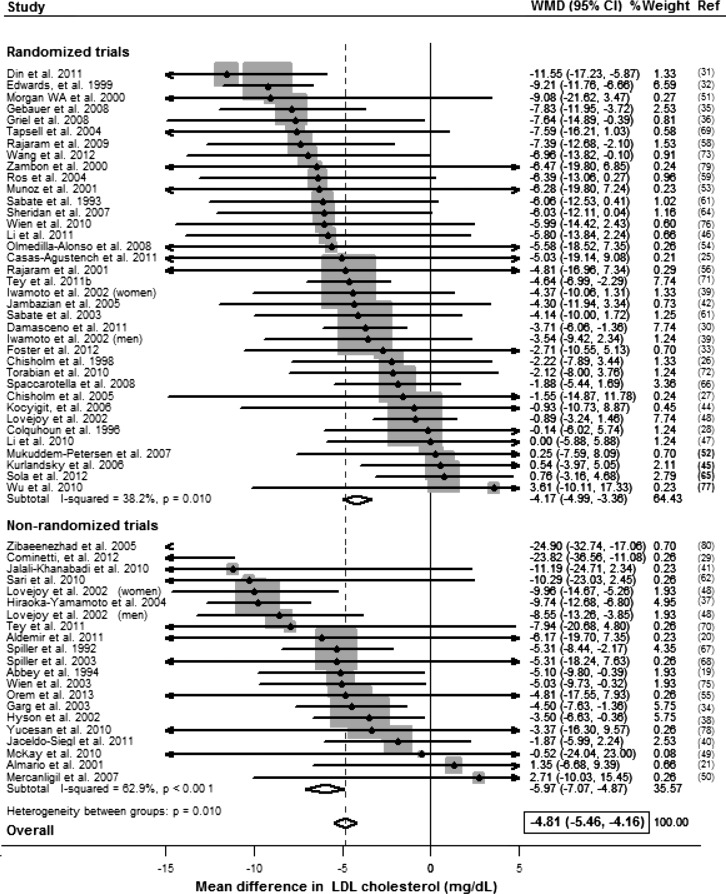
Compared with control, consumption of tree nuts significantly lowered concentrations (mg/dL) of total cholesterol (weighted mean difference per 28 g serving/d: −4.7; 95% CI: −5.3, −4.0), LDL cholesterol (−4.8; 95% CI: −5.5, −4.2), ApoB (−3.7; 95% CI: −5.2, −2.3), and triglycerides (−2.2; 95% CI: −3.8, −0.5) (Table 2). Reductions in total cholesterol were seen in both randomized trials (−3.6; 95% CI: −4.4, −2.9) and nonrandomized trials (−6.7; 95% CI: −7.8, −5.6); effects in the latter were significantly larger (P-interaction < 0.001) (Supplemental Figure 1). Similar findings were seen for LDL cholesterol: randomized trials, −4.2 (95% CI: −5.0, −3.4); nonrandomized trials, −6.0 (95% CI: −7.1, −4.9); P-interaction = 0.01 (Figure 2). For ApoB, no significant differences in effects were observed in randomized trials (−4.2; 95% CI: −5.7, −2.6) vs. nonrandomized trials (−1.1; 95% CI: −5.1, 3.0) (P-interaction = 0.17) (Supplemental Figure 2). Effects on triglycerides were also not statistically significant in nonrandomized trials (−4.6; 95% CI: −8.4, −0.8) vs. randomized trials (−1.6; 95% CI: −3.5, 0.24) (P-interaction = 0.16) (Supplemental Figure 3).
TABLE 2.
WMDs in lipids/apolipoproteins, blood pressure, and CRP per 1 serving of tree nuts/d (28.4 g/d) in randomized and nonrandomized controlled trials (19–80)1
| Randomized controlled trials |
Nonrandomized trials |
All trials |
|||||||
| Outcome | Trials, n | WMD (95% CI) | I2 | Trials, n | WMD (95% CI) | I2 | Trials, n | WMD (95% CI) | P value2 |
| Total cholesterol | 38 | −3.6 (−4.4, −2.9) | 53.8 | 23 | −6.7 (−7.8, −5.6) | 76.8 | 61 | −4.7 (−5.3, −4.0) | 0.001 |
| LDL cholesterol | 38 | −4.2 (−5.0, −3.4) | 38.2 | 23 | −6.0 (−7.1, −4.9) | 62.9 | 61 | −4.8 (−5.5, −4.2) | 0.01 |
| HDL cholesterol | 38 | −0.04 (−0.8, 0.7) | 0 | 22 | −0.7 (−1.7, 0.4) | 35.9 | 60 | −0.3 (−0.9, 0.4) | 0.33 |
| TG | 37 | −1.6 (−3.5, 0.24) | 0 | 22 | −4.6 (−8.4, −0.8) | 0 | 59 | −2.2 (−3.8, −0.5) | 0.16 |
| ApoA1 | 15 | −0.8 (−2.1, 0.6) | 12.8 | 8 | 1.0 (−2.7, 4.7) | 0 | 23 | −0.6 (−1.9, 0.7) | 0.38 |
| ApoB | 13 | −4.2 (−5.7, −2.6) | 20.3 | 7 | −1.1 (−5.1, 3.0) | 0 | 20 | −3.7 (−5.2, −2.3) | 0.17 |
| ApoB100 | 3 | −1.5 (−5.8, 2.8) | 0 | 2 | −5.2 (11.0, 0.6) | 0 | 5 | −2.8 (−6.2, 0.7) | 0.31 |
| SBP | 17 | 1.3 (−0.03, 2.6) | 0 | 4 | −3.3 (−5.7, 0.9) | 0 | 21 | 0.3 (−0.8, 1.4) | 0.001 |
| DBP | 17 | 0.6 (−0.7, 1.8) | 0 | 3 | −1.6 (−5.8, 2.5) | 0 | 20 | 0.4 (−0.8, 1.6) | 0.32 |
| CRP | 8 | 0.2 (−1.7, 2.0) | 0 | 4 | −0.4 (−5.7, 4.8) | 0 | 12 | 0.1 (−1.6, 1.8) | 0.84 |
Values for lipids/apolipoproteins and CRP are presented in mg/dL; blood pressure is presented in mmHg. The WMD represents the amount by which the tree nut intervention changed the outcome on average compared with the control group or period. Estimates were pooled by using fixed-effects, inverse-variance meta-analysis. Outcomes included total cholesterol, LDL cholesterol, HDL cholesterol, TG, ApoA1, ApoB, ApoB100, SBP, DBP, and CRP. The I2 index indicates the percentage of total variability in the effect sizes due to between-study heterogeneity, with I2 > 30% considered at least moderate heterogeneity. ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoB100, apolipoprotein B100; CRP, C-reactive protein; DBP, diastolic blood pressure; SBP, systolic blood pressure; TG, triglycerides; WMD, weighted mean difference.
P-heterogeneity between WMD of randomized controlled trials and nonrandomized trials is shown.
FIGURE 2.
WMD in LDL cholesterol (mg/dL) per 1 serving of nuts/d (28.4 g/d) in randomized and nonrandomized controlled trials, pooled by using fixed-effects meta-analysis (19–80). To convert mg/dL to mmol/L, multiply by 0.0259. WMD, weighted mean difference.
No significant effects of tree nut consumption were identified for HDL cholesterol, apolipoprotein A1, apolipoprotein B100, systolic or diastolic blood pressure, or CRP (Supplemental Figures 4–9). These findings were similar when randomized and nonrandomized trials were separately evaluated.
Dose-responses between nut intake and outcomes
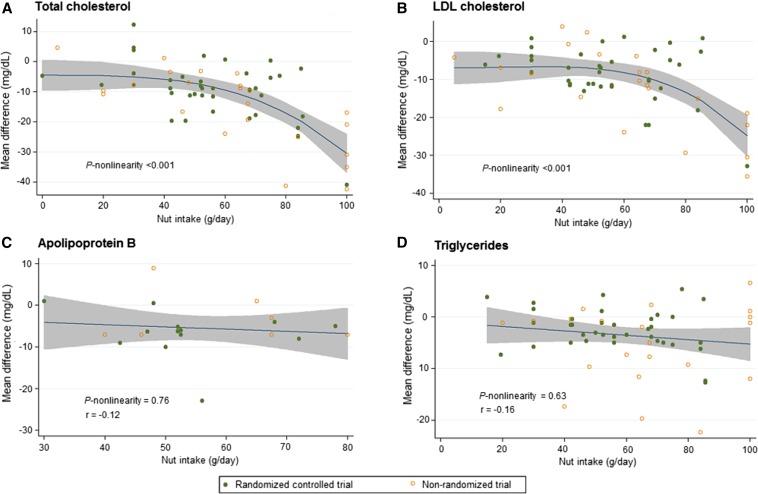
When we evaluated dose-responses, tree nut intake lowered total cholesterol and LDL cholesterol in a nonlinear fashion (P-nonlinearity < 0.001); stronger effects were observed in trials providing doses of ≥60 g nuts/d (Figure 3). In contrast, there was little evidence for nonlinear dose-response relations between nut intake and ApoB or triglycerides (P-nonlinearity > 0.05 each).
FIGURE 3.
Dose-response relations between tree nut intake (g/d) and absolute (unstandardized) mean difference (mg/dL) in total cholesterol (n = 61 trials) (A), LDL cholesterol (n = 61 trials) (B), apolipoprotein B (n = 19 trials) (C), and triglycerides (n = 59 trials) (D) (19–80). Nut intake lowers total cholesterol and LDL cholesterol in a nonlinear fashion (P-nonlinearity = 0.001 for both), with stronger effects observed above a nut dose of ∼60 g nuts/d. Linear dose-response relations were observed between nut intake and apolipoprotein B (r = −0.12) and triglycerides (r = −0.16). The 95% CI is depicted in the shaded regions.
Heterogeneity
Heterogeneity was at least moderate (I2 > 30%) among trials of total cholesterol and LDL cholesterol and nonrandomized trials of triglycerides and HDL cholesterol, as well as low (I2 < 30%) among trials of apolipoproteins, blood pressure, and CRP (Table 2). No significant differences in effects by nut type were observed (Supplemental Table 2), although relatively few trials were available for certain nut types. Heterogeneity by quality score, with greater effect sizes found in lower quality trials, was observed for total cholesterol and LDL cholesterol (P-heterogeneity = 0.09 and 0.005, respectively); however, these differences were no longer statistically significant in analyses including only randomized controlled trials (Supplemental Table 3). Visual inspection of funnel plots suggested that nonrandomized trials more frequently reported larger effect sizes for total cholesterol and LDL cholesterol (Supplemental Figure 10). For ApoB, significant heterogeneity by comorbidity was found, with stronger effects observed in studies including participants with type 2 diabetes (weighted mean difference: −11.5; 95% CI: −16.2, −6.8) than among healthy populations (−2.5; 95% CI: −4.7, −0.3) (P-heterogeneity = 0.015) (Supplemental Tables 2 and 3). No significant heterogeneity by other disease conditions, age, sex, background diet, baseline outcome level, or intervention duration was observed.
Evaluation of publication bias
Visual inspection of funnel plots did not suggest publication bias. Statistical evidence of publication bias was also not detected by using Egger’s or Begg’s tests (Supplemental Table 4).
DISCUSSION
In this systematic review and meta-analysis of controlled trials including 2582 participants, nut consumption lowered total cholesterol, LDL cholesterol, and its primary apolipoprotein, ApoB. Effects on total cholesterol and LDL cholesterol were generally larger in nonrandomized vs. randomized trials but statistically evident in each. For ApoB, stronger effects were also observed in populations with type 2 diabetes. These benefits were not significantly different across diverse types of tree nuts or when added to a variety of background diets. Nut consumption also lowered triglyceride concentrations, although effects were small in magnitude and only statistically significant in nonrandomized trials. Significant effects of nut consumption on HDL cholesterol, ApoA, blood pressure, or CRP were not identified. This meta-analysis provides the most comprehensive estimates to date of the effects of tree nut intake on major cardiovascular disease risk factors, including dose-response relations and presentation of effects by different nut types.
Accumulating evidence indicates that nut intake lowers risk of CVD events, including consistent findings from prospective observational studies (1, 81) and the Prevención con Dieta Mediterránea trial (2). Our findings showing that nut intake significantly improves the lipid profile, lowering LDL cholesterol, ApoB, and triglycerides, provide critical mechanistic evidence to support a causal link between nut intake and lowered CVD risk.
In dose-response analyses, the relations between tree nut intake and total cholesterol and LDL cholesterol were nonlinear, with stronger effects at consumption amounts at ≥60 g (about 2 oz, or 2 servings) per day. Trials providing 100 g nuts/d lowered concentrations of LDL cholesterol by up to 35 mg/dL, an effect size comparable to some statin regimens (82). As a point of caution, only 5 trials (4 nonrandomized, 1 randomized) provided nuts in this quantity, however, and additional trials comparing the effects of multiple nut doses on LDL cholesterol within the same study, particularly at high amounts (e.g., 100 g nuts/d) are needed. In comparison, effects of nuts on ApoB appeared more linear, which could relate to differential effects of tree nuts on LDL cholesterol particle size vs. particle number at different doses, a smaller number of studies of high-dose nut consumption and ApoB, or chance. Further randomized studies of high-dose nut consumption will help clarify whether benefits on blood lipids and apolipoproteins are nonlinear.
We did not observe significant heterogeneity in outcomes across different types of tree nuts. In addition, our meta-regression demonstrated that the major determinant of cholesterol lowering appears to be the total dose of tree nut consumption rather than nut type. Significant heterogeneity in effects was also not observed for most other factors, including age, sex, background diet, baseline outcome level, and intervention duration; an exception was that tree nut intake lowered ApoB to a 3- to 4-fold greater degree in trials of diabetic populations in comparison to trials including only nondiabetic participants. In diabetic patients, ApoB provides more accurate information about atherogenic particles than LDL cholesterol concentrations (83). These findings suggest that nut consumption may be particularly important for lowering CVD risk in patients with diabetes.
On the basis of the magnitude of effects of nut intake on lowering LDL cholesterol and ApoB observed in this meta-analysis, together with the established relation between LDL cholesterol and ApoB and CVD events (84), we calculated the predicted changes in risk of CVD events if one daily serving of nuts was incorporated into the diet. For an LDL cholesterol reduction of 4.2 mg/dL and an ApoB reduction of 4.1 mg/dL per daily serving of nuts observed in randomized trials of this meta-analysis, a 4% (HR: 0.96; 95% CI: 0.93, 0.99) and a 6% lower risk of coronary events are predicted, respectively. These calculated effects are smaller than associations between nut intake and CVD events observed in both prospective cohorts (81, 85) and the Prevención con Dieta Mediterránea trial (2). For instance, in prospective observational studies (85), a daily serving (28.4 g) of nuts was associated with 29% lower risk of CVD (HR: 0.71; 95% CI: 0.59, 0.85), whereas in the Prevención con Dieta Mediterránea trial, a Mediterranean diet supplemented with one daily serving (30 g) of mixed nuts reduced CVD events by 28% (HR: 0.72; 95% CI: 0.54, 0.96) over 4.8 y of follow-up (2). These consistent effect sizes in prospective studies and controlled clinical trials suggest that tree nuts have additional cardiovascular benefits beyond LDL cholesterol and ApoB lowering, for example, improving blood glucose and endothelial function (59). Similarly, specific constituents in tree nuts, such as polyunsaturated fats, are thought to influence CVD risk through both lipid and nonlipid mechanisms (86–88).
Our study has several strengths. Our systematic search makes it unlikely that large reports were missed, and error and bias were minimized by independent, duplicate decisions on study inclusion and data extraction. Effect sizes were standardized to a common dose, avoiding combining of heterogeneous comparisons (e.g., “high vs. low” intake) and, importantly, allowing quantitative assessment of dose-response relations. The duration of trials was adequate to achieve changes and stabilization of lipid values (12). We evaluated multiple cardiovascular disease risk factors, including apolipoproteins; separately evaluated different types of tree nuts; and assessed several sources of heterogeneity. The identified trial populations were relatively diverse, including differences in age, sex, disease status, and background diet, increasing generalizability of our findings.
Potential limitations should be considered. Compliance was often assessed by self-report, and low compliance would cause underestimation of effects. Greater effect sizes were observed in lower quality, nonrandomized trials, yet significant effects on total cholesterol, LDL cholesterol, and ApoB were still seen in high-quality, randomized trials. The relatively few trials in some subgroups examined in heterogeneity analyses limited statistical power to detect potential interaction; for example, few estimates (n ≤ 2) were available for some nut types, such as Brazil nuts, cashews, and pecans. Although larger effects on lowering LDL cholesterol were observed at higher nut doses in our study, we did not examine the effects of nuts on weight change. A recent meta-analysis of controlled trials on this topic (11) found that nut intake had nonsignificant, inverse effects on adiposity, but doses in most included trials were modest (<56 g/d, or 2 servings, of nuts). Furthermore, nut intake was associated with less weight gain over time in US cohorts of male and female health professionals (89, 90). Taken together, the inverse associations with weight gain observed in both controlled trials and free-living populations suggest that nut intake might augment satiety and displace other, less healthful foods in the diet, potentially resulting in less weight gain over time.
In conclusion, this systematic review and meta-analysis of controlled trials demonstrates that tree nut consumption lowers total cholesterol, LDL cholesterol, ApoB, and triglycerides. Our findings also highlight the need for additional investigation of potentially stronger effects at high doses of nuts and among diabetic populations.
Acknowledgments
The authors’ responsibilities were as follows—LCDG, MCF, RF, and KL: conducted research; LCDG: analyzed data and performed statistical analysis; LCDG and DM: wrote the manuscript; LCDG: had primary responsibility for final content; and all authors: designed research and read and approved the final manuscript. DM reports ad hoc honoraria from Bunge, Pollock Institute, and Quaker Oats; ad hoc consulting for Foodminds, Nutrition Impact, Amarin, Astra Zeneca, Winston, and Strawn LLP; membership, Unilever North America Scientific Advisory Board; and chapter royalties from UpToDate. LCDG and DM received modest ad hoc consulting fees from the Life Sciences Research Organization (LSRO) in Bethesda, MD, to support this study. MCF, RF, and KL received payment through LSRO (<5% of gross income) to conduct a review of nuts and cardiovascular health outcomes, which was funded through a contract with the International Tree Nut Council (ITNC). No author has stock or ownership in the INTC.
Footnotes
Abbreviations used: ApoB, apolipoprotein B; CRP, C-reactive protein; CVD, cardiovascular disease.
REFERENCES
- 1.Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Association of nut consumption with total and cause-specific mortality. N Engl J Med 2013;369:2001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- 3.Kris-Etherton PM, Hu FB, Ros E, Sabaté J. The role of tree nuts and peanuts in the prevention of coronary heart disease: multiple potential mechanisms. J Nutr 2008;138:1746S–51S. [DOI] [PubMed] [Google Scholar]
- 4.Ros E. Health benefits of nut consumption. Nutrients 2010;2:652–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabate J, Wien M. Consumption of nuts in the prevention of cardiovascular disease. Curr Nutr Rep 2013;2:258–66. [Google Scholar]
- 6.Demonty I, Ras RT, van der Knaap HC, Duchateau GS, Meijer L, Zock PL, Geleijnse JM, Trautwein EA. Continuous dose-response relationship of the LDL-cholesterol-lowering effect of phytosterol intake. J Nutr 2009;139:271–84. [DOI] [PubMed] [Google Scholar]
- 7.Phung OJ, Makanji SS, White CM, Coleman C. Almonds have a neutral effect on serum lipid profiles: a meta-analysis of randomized trials. J Am Diet Assoc 2009;109:865–73. [DOI] [PubMed] [Google Scholar]
- 8.Banel DK, Hu FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am J Clin Nutr 2009;90:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabaté J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med 2010;170:821–7. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flores-Mateo G, Rojas-Rueda D, Basora J, Ros E, Salas-Salvado J. Nut intake and adiposity: meta-analysis of clinical trials. Am J Clin Nutr 2013;97:1346–55. [DOI] [PubMed] [Google Scholar]
- 12.Kris-Etherton PM, Dietschy J. Design criteria for studies examining individual fatty acid effects on cardiovascular disease risk factors: human and animal studies. Am J Clin Nutr 1997;65:1590S–6S. [DOI] [PubMed] [Google Scholar]
- 13.Research and strategic business development evidence analysis manual: steps in the academy evidence analysis process [Internet]. Chicago: Academy of Nutrition and Dietetics. 2002. [cited 2015 Jan 10]. Available from: http://www.andeal.org/files/Docs/2012_Jan_EA_Manual.pdf.
- 14.The mean difference (or difference in means), section 9.2.3.1. In: Cochrane handbook for systematic reviews and interventions: version 5.1.0 [Internet]. 2011 [cited 2015 Jan 15]. Available from: http://handbook.cochrane.org/chapter_9/9_2_3_1_the_mean_difference_or_difference_in_means.htm.
- 15.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meta-regression, section 9.6.4. In: Cochrane handbook for systematic reviews and interventions: version 5.1.0 [Internet]. 2011 [cited 2015 Jan 15]. Available from: http://handbook.cochrane.org/chapter_9/9_6_4_meta_regression.htm.
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- 19.Abbey M, Noakes M, Belling GB, Nestel PJ. Partial replacement of saturated fatty acids with almonds or walnuts lowers total plasma cholesterol and low-density-lipoprotein cholesterol. Am J Clin Nutr 1994;59:995–9. [DOI] [PubMed] [Google Scholar]
- 20.Aldemir M, Okulu E, Neşelioglu S, Erel O, Kayigil O. Pistachio diet improves erectile function parameters and serum lipid profiles in patients with erectile dysfunction. Int J Impot Res 2011;23:32–8. [DOI] [PubMed] [Google Scholar]
- 21.Almario RU, Vonghavaravat V, Wong R, Kasim-Karakas SE. Effects of walnut consumption on plasma fatty acids and lipoproteins in combined hyperlipidemia. Am J Clin Nutr 2001;74:72–9. [DOI] [PubMed] [Google Scholar]
- 22.Aronis KN, Vamvini MT, Chamberland JP, Sweeney LL, Brennan AM, Magkos F, Mantzoros CS. Short-term walnut consumption increases circulating total adiponectin and apolipoprotein A concentrations, but does not affect markers of inflammation or vascular injury in obese humans with the metabolic syndrome: data from a double-blinded, randomized, placebo-controlled study. Metabolism 2012;61:577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canales A, Benedí J, Nus M, Librelotto J, Sánchez-Montero JM, Sánchez-Muniz FJ. Effect of walnut-enriched restructured meat in the antioxidant status of overweight/obese senior subjects with at least one extra CHD-risk factor. J Am Coll Nutr 2007;26:225–32. [DOI] [PubMed] [Google Scholar]
- 24.Canales A, Sánchez-Muniz FJ, Bastida S, Librelotto J, Nus M, Corella D, Guillen M, Benedí J. Effect of walnut-enriched meat on the relationship between VCAM, ICAM, and LTB4 levels and PON-1 activity in ApoA4 360 and PON-1 allele carriers at increased cardiovascular risk. Eur J Clin Nutr 2011;65:703–10. [DOI] [PubMed] [Google Scholar]
- 25.Casas-Agustench P, López-Uriarte P, Bullo M, Ros E, Cabre-Vila JJ, Salas-Salvadó J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr Metab Cardiovasc Dis 2011;21:126–35. [DOI] [PubMed] [Google Scholar]
- 26.Chisholm A, Mann J, Skeaff M, Frampton C, Sutherland W, Duncan A, Tiszavari S. A diet rich in walnuts favourably influences plasma fatty acid profile in moderately hyperlipidaemic subjects. Eur J Clin Nutr 1998;52:12–6. [DOI] [PubMed] [Google Scholar]
- 27.Chisholm A, Mc Auley K, Mann J, Williams S, Skeaff M. Cholesterol lowering effects of nuts compared with a Canola oil enriched cereal of similar fat composition. Nutr Metab Cardiovasc Dis 2005;15:284–92. [DOI] [PubMed] [Google Scholar]
- 28.Colquhoun DM, Humphries JA, Moores D, Somerset SM. Effects of macadamia nut enriched diet on serum lipids and lipoproteins compared to a low fat diet. Food Aust 1996;48:216–22. [Google Scholar]
- 29.Cominetti C, de Bortoli MC, Garrido AB Jr, Cozzolino SM. Brazilian nut consumption improves selenium status and glutathione peroxidase activity and reduces atherogenic risk in obese women. Nutr Res 2012;32:403–7. [DOI] [PubMed] [Google Scholar]
- 30.Damasceno NR, Pérez-Heras A, Serra M, Cofán M, Sala-Vila A, Salas-Salvadó J, Ros E. Crossover study of diets enriched with virgin olive oil, walnuts or almonds: effects on lipids and other cardiovascular risk markers. Nutr Metab Cardiovasc Dis 2011;21:S14–20. [DOI] [PubMed] [Google Scholar]
- 31.Din JN, Aftab SM, Jubb AW, Carnegy FH, Lyall K, Sarma J, Newby DE, Flapan AD. Effect of moderate walnut consumption on lipid profile, arterial stiffness and platelet activation in humans. Eur J Clin Nutr 2011;65:234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards K, Kwaw I, Matud J, Kurtz I. Effect of pistachio nuts on serum lipid levels in patients with moderate hypercholesterolemia. J Am Coll Nutr 1999;18:229–32. [DOI] [PubMed] [Google Scholar]
- 33.Foster GD, Shantz KL, Vander Veur SS, Oliver TL, Lent MR, Virus A, Szapary PO, Rader DJ, Zemel BS, Gilden-Tsai A. A randomized trial of the effects of an almond-enriched, hypocaloric diet in the treatment of obesity. Am J Clin Nutr 2012;96:249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garg ML, Blake RJ, Wills RB. Macadamia nut consumption lowers plasma total and LDL cholesterol levels in hypercholesterolemic men. J Nutr 2003;133:1060–3. [DOI] [PubMed] [Google Scholar]
- 35.Gebauer SK, West SG, Kay CD, Alaupovic P, Bagshaw D, Kris-Etherton PM. Effects of pistachios on cardiovascular disease risk factors and potential mechanisms of action: a dose-response study. Am J Clin Nutr 2008;88:651–9. [DOI] [PubMed] [Google Scholar]
- 36.Griel AE, Cao Y, Bagshaw DD, Cifelli AM, Holub B, Kris-Etherton PM. A macadamia nut-rich diet reduces total and LDL-cholesterol in mildly hypercholesterolemic men and women. J Nutr 2008;138:761–7. [DOI] [PubMed] [Google Scholar]
- 37.Hiraoka-Yamamoto J, Ikeda K, Negishi H, Mori M, Hirose A, Sawada S, Onobayashi Y, Kitamori K, Kitano S, Tashiro M, et al. Serum lipid effects of a monounsaturated (palmitoleic) fatty acid–rich diet based on macadamia nuts in healthy, young Japanese women. Clin Exp Pharmacol Physiol 2004;31:S37–8. [DOI] [PubMed] [Google Scholar]
- 38.Hyson DA, Schneeman BO, Davis PA. Almonds and almond oil have similar effects on plasma lipids and LDL oxidation in healthy men and women. J Nutr 2002;132:703–7. [DOI] [PubMed] [Google Scholar]
- 39.Iwamoto M, Imaizumi K, Sato M, Hirooka Y, Sakai K, Takeshita A, Kono M. Serum lipid profiles in Japanese women and men during consumption of walnuts. Eur J Clin Nutr 2002;56:629–37. [DOI] [PubMed] [Google Scholar]
- 40.Jaceldo-Siegl K, Sabaté J, Batech M, Fraser GE. Influence of body mass index and serum lipids on the cholesterol-lowering effects of almonds in free-living individuals. Nutr Metab Cardiovasc Dis 2011;21:S7–13. [DOI] [PubMed] [Google Scholar]
- 41.Jalali-Khanabadi BA, Mozaffari-Khosravi H, Parsaeyan N. Effects of almond dietary supplementation on coronary heart disease lipid risk factors and serum lipid oxidation parameters in men with mild hyperlipidemia. J Altern Complement Med 2010;16:1279–83. [DOI] [PubMed] [Google Scholar]
- 42.Jambazian PR, Haddad E, Rajaram S, Tanzman J, Sabaté J. Almonds in the diet simultaneously improve plasma a-tocopherol concentrations and reduce plasma lipids. J Am Diet Assoc 2005;105:449–54. [DOI] [PubMed] [Google Scholar]
- 43.Jia X, Li N, Zhang W, Zhang X, Lapsley K, Huang G, Blumberg J, Ma G, Chen J. A pilot study on the effects of almond consumption on DNA damage and oxidative stress in smokers. Nutr Cancer 2006;54:179–83. [DOI] [PubMed] [Google Scholar]
- 44.Kocyigit A, Koylu AA, Keles H. Effects of pistachio nuts consumption on plasma lipid profile and oxidative status in healthy volunteers. Nutr Metab Cardiovasc Dis 2006;16:202–9. [DOI] [PubMed] [Google Scholar]
- 45.Kurlandsky SB, Stote KS. Cardioprotective effects of chocolate and almond consumption in healthy women. Nutr Res 2006;26:509–16. [Google Scholar]
- 46.Li SC, Liu YH, Liu JF, Chang WH, Chen CM, Chen CY. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism 2011;60:474–9. [DOI] [PubMed] [Google Scholar]
- 47.Li Z, Song R, Nguyen C, Zerlin A, Karp H, Naowamondhol K, Thames G, Gao K, Li L, Tseng CH, et al. Pistachio nuts reduce triglycerides and body weight by comparison to refined carbohydrate snack in obese subjects on a 12-week weight loss program. J Am Coll Nutr 2010;29:198–203. [DOI] [PubMed] [Google Scholar]
- 48.Lovejoy JC, Most MM, Lefevre M, Greenway FL, Rood JC. Effect of diets enriched in almonds on insulin action and serum lipids in adults with normal glucose tolerance or type 2 diabetes. Am J Clin Nutr 2002;76:1000–6. [DOI] [PubMed] [Google Scholar]
- 49.McKay DL, Chen CY, Yeum KJ, Matthan NR, Lichtenstein AH, Blumberg JB. Chronic and acute effects of walnuts on antioxidant capacity and nutritional status in humans: a randomized, cross-over pilot study. Nutr J 2010;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mercanligil SM, Arslan P, Alasalvar C, Okut E, Akgul E, Pinar A, Geyik PO, Tokgozoglu L, Shahidi F. Effects of hazelnut-enriched diet on plasma cholesterol and lipoprotein profiles in hypercholesterolemic adult men. Eur J Clin Nutr 2007;61:212–20. [DOI] [PubMed] [Google Scholar]
- 51.Morgan WA, Clayshulte BJ. Pecans lower low-density lipoprotein cholesterol in people with normal lipid levels. J Am Diet Assoc 2000;100:312–8. [DOI] [PubMed] [Google Scholar]
- 52.Mukuddem-Petersen J, Stonehouse OW, Jerling JC, Hanekom SM, White Z. Effects of a high walnut and high cashew nut diet on selected markers of the metabolic syndrome: a controlled feeding trial. Br J Nutr 2007;97:1144–53. [DOI] [PubMed] [Google Scholar]
- 53.Muñoz S, Merlos M, Zambón D, Rodriguez C, Sabaté J, Ros E, Laguna JC. Walnut-enriched diet increases the association of LDL from hypercholesterolemic men with human HepG2 cells. J Lipid Res 2001;42:2069–76. [PubMed] [Google Scholar]
- 54.Olmedilla-Alonso B, Granado-Lorencio F, Herrero-Barbudo C, Blanco-Navarro I, Blázquez-García S, Pérez-Sacristán B. Consumption of restructured meat products with added walnuts has a cholesterol-lowering effect in subjects at high cardiovascular risk: a randomised, crossover, placebo-controlled study. J Am Coll Nutr 2008;27:342–8. [DOI] [PubMed] [Google Scholar]
- 55.Orem A, Yucesan FB, Orem C, Akcan B, Kural BV, Alasalvar C, Shahidi F. Hazelnut-enriched diet improves cardiovascular risk biomarkers beyond a lipid-lowering effect in hypercholesterolemic subjects. J Clin Lipidol 2013;7:123–31. [DOI] [PubMed] [Google Scholar]
- 56.Rajaram S, Burke K, Connell B, Myint T, Sabaté J. A monounsaturated fatty acid–rich pecan-enriched diet favorably alters the serum lipid profile of healthy men and women. J Nutr 2001;131:2275–9. [DOI] [PubMed] [Google Scholar]
- 57.Rajaram S, Connell KM, Sabaté J. Effect of almond-enriched high-monounsaturated fat diet on selected markers of inflammation: a randomised, controlled, crossover study. Br J Nutr 2010;103:907–12. [DOI] [PubMed] [Google Scholar]
- 58.Rajaram S, Haddad EH, Mejia A, Sabaté J. Walnuts and fatty fish influence different serum lipid fractions in normal to mildly hyperlipidemic individuals: a randomized controlled study. Am J Clin Nutr 2009;89:1657S–63S. [DOI] [PubMed] [Google Scholar]
- 59.Ros E, Núñez I, Pérez-Heras A, Serra M, Gilabert R, Casals E, Deulofeu R. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation 2004;109:1609–14. [DOI] [PubMed] [Google Scholar]
- 60.Sabaté J, Fraser GE, Burke K, Knutsen SF, Bennett H, Lindsted KD. Effects of walnuts on serum lipid levels and blood pressure in normal men. N Engl J Med 1993;328:603–7. [DOI] [PubMed] [Google Scholar]
- 61.Sabaté J, Haddad E, Tanzman JS, Jambazian P, Rajaram S. Serum lipid response to the graduated enrichment of a Step I diet with almonds: a randomized feeding trial. Am J Clin Nutr 2003;77:1379–84. [DOI] [PubMed] [Google Scholar]
- 62.Sari I, Baltaci Y, Bagci C, Davutoglu V, Erel O, Celik H, Ozer O, Aksoy N, Aksoy M. Effect of pistachio diet on lipid parameters, endothelial function, inflammation, and oxidative status: a prospective study. Nutrition 2010;26:399–404. [DOI] [PubMed] [Google Scholar]
- 63.Schutte AE, Van Rooyen JM, Huisman HW, Mukuddem-Petersen J, Oosthuizen W, Hanekom SM, Jerling JC. Modulation of baroreflex sensitivity by walnuts versus cashew nuts in subjects with metabolic syndrome. Am J Hypertens 2006;19:629–36. [DOI] [PubMed] [Google Scholar]
- 64.Sheridan MJ, Cooper JN, Erario M, Cheifetz CE. Pistachio nut consumption and serum lipid levels. J Am Coll Nutr 2007;26:141–8. [DOI] [PubMed] [Google Scholar]
- 65.Solà R, Valls RM, Godàs G, Perez-Busquets G, Ribalta J, Girona J, Heras M, Cabré A, Castro A, Domenech G, et al. Cocoa, hazelnuts, sterols and soluble fiber cream reduces lipids and inflammation biomarkers in hypertensive patients: a randomized controlled trial. PLoS One 2012;7:e31103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spaccarotella KJ, Kris-Etherton PM, Stone WL, Bagshaw DM, Fishell VK, West SG, Lawrence FR, Hartman TJ. The effect of walnut intake on factors related to prostate and vascular health in older men. Nutr J 2008;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spiller GA, Jenkins DJ, Cragen LN, Gates JE, Bosello O, Berra K, Rudd C, Stevenson M, Superko R. Effect of a diet high in monounsaturated fat from almonds on plasma cholesterol and lipoproteins. J Am Coll Nutr 1992;11:126–30. [PubMed] [Google Scholar]
- 68.Spiller GA, Miller A, Olivera K, Reynolds J, Miller B, Morse SJ, Dewell A, Farquhar JW. Effects of plant-based diets high in raw or roasted almonds, or roasted almond butter on serum lipoproteins in humans. J Am Coll Nutr 2003;22:195–200. [DOI] [PubMed] [Google Scholar]
- 69.Tapsell LC, Gillen LJ, Patch CS, Batterham M, Owen A, Bare M, Kennedy M. Including walnuts in a low-fat/modified-fat diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes Care 2004;27:2777–83. [DOI] [PubMed] [Google Scholar]
- 70.Tey SL, Brown RC, Chisholm AW, Delahunty CM, Gray AR, Williams SM. Effects of different forms of hazelnuts on blood lipids and α-tocopherol concentrations in mildly hypercholesterolemic individuals. Eur J Clin Nutr 2011;65:117–24. [DOI] [PubMed] [Google Scholar]
- 71.Tey SL, Brown R, Gray A, Chisholm A, Delahunty C. Nuts improve diet quality compared to other energy-dense snacks while maintaining body weight. J Nutr Metab 2011;2011:357350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Torabian S, Haddad E, Cordero-MacIntyre Z, Tanzman J, Fernandez ML, Sabaté J. Long-term walnut supplementation without dietary advice induces favorable serum lipid changes in free-living individuals. Eur J Clin Nutr 2010;64:274–9. [DOI] [PubMed] [Google Scholar]
- 73.Wang X, Li Z, Liu Y, Lv X, Yang W. Effects of pistachios on body weight in Chinese subjects with metabolic syndrome. Nutr J 2012;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.West SG, Gebauer SK, Kay CD, Bagshaw DM, Savastano DM, Diefenbach C, Kris-Etherton PM. Diets containing pistachios reduce systolic blood pressure and peripheral vascular responses to stress in adults with dyslipidemia. Hypertension 2012;60:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wien MA, Sabaté JM, Iklé DN, Cole SE, Kandeel FR. Almonds vs complex carbohydrates in a weight reduction program. Int J Obes Relat Metab Disord 2003;27:1365–72. [DOI] [PubMed] [Google Scholar]
- 76.Wien M, Bleich D, Raghuwanshi M, Gould-Forgerite S, Gomes J, Monahan-Couch L, Oda K. Almond consumption and cardiovascular risk factors in adults with prediabetes. J Am Coll Nutr 2010;29:189–97. [DOI] [PubMed] [Google Scholar]
- 77.Wu H, Pan A, Yu Z, Qi Q, Lu L, Zhang G, Yu D, Zong G, Zhou Y, Chen X, et al. Lifestyle counseling and supplementation with flaxseed or walnuts influence the management of metabolic syndrome. J Nutr 2010;140:1937–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yücesan FB, Orem A, Kural BV, Orem C, Turan I. Hazelnut consumption decreases the susceptibility of LDL to oxidation, plasma oxidized LDL level and increases the ratio of large/small LDL in normolipidemic healthy subjects. Anadolu Kardiyol Derg 2010;10:28–35. [DOI] [PubMed] [Google Scholar]
- 79.Zambón D, Sabaté J, Muñoz S, Campero B, Casals E, Merlos M, Laguna JC, Ros E. Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women: a randomized crossover trial. Ann Intern Med 2000;132:538–46. [DOI] [PubMed] [Google Scholar]
- 80.Zibaeenezhad MJ, Shamsnia SJ, Khorasani M. Walnut consumption in hyperlipidemic patients. Angiology 2005;56:581–3. [DOI] [PubMed] [Google Scholar]
- 81.Afshin A, Micha R, Khatibzadeh S, Mozaffarian D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: a systematic review and meta-analysis. Am J Clin Nutr 2014;100:278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cholesterol Treatment Trialists’ Collaborators, Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012;380:581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martin SS, Qasim AN, Mehta NN, Wolfe M, Terembula K, Schwartz S, Iqbal N, Schutta M, Bagheri R, Reilly MP. Apolipoprotein B but not LDL cholesterol is associated with coronary artery calcification in type 2 diabetic whites. Diabetes 2009;58:1887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Di Angelantonio E; Emerging Risk Factors Collaboration. Major lipids, apolipoprotiens, and risk of vascular disease. JAMA 2009;302:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luo C, Zhang Y, Ding Y, Shan Z, Chen S, Yu M, Hu FB, Liu L. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a systematic review and meta-analysis. Am J Clin Nutr 2014;100:256–69. [DOI] [PubMed] [Google Scholar]
- 86.Pan A, Chen M, Chowdhury R, Wu JH, Sun Q, Campos H, Mozaffarian D, Hu FB. α-Linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr 2012;96:1262–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med 2010;7:e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao G, Etherton TD, Martin KR, Gillies PJ, West SG, Kris-Etherton PM. Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am J Clin Nutr 2007;85:385–91. [DOI] [PubMed] [Google Scholar]
- 89.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith JD, Hou T, Ludwig DS, Rimm EB, Willett W, Hu FB, Mozaffarian D. Changes in intake of protein foods, carbohydrate amount and quality, and long-term weight gain: results from three prospective cohorts. Am J Clin Nutr 2015. Apr 8 (Epub ahead of print; DOI: 10.3945/ajcn.114.100867). [DOI] [PMC free article] [PubMed] [Google Scholar]