Abstract
Background: Manganese, an essential metal for normal growth and development, is neurotoxic on excessive exposure. Standard trace element–supplemented neonatal parenteral nutrition (PN) has a high manganese content and bypasses normal gastrointestinal absorptive control mechanisms, which places infants at risk of manganese neurotoxicity. Magnetic resonance (MR) relaxometry demonstrating short T1 relaxation time (T1R) in the basal ganglia reflects excessive brain manganese accumulation.
Objective: This study tested the hypothesis that infants with greater parenteral manganese exposure have higher brain manganese accumulation, as measured by MR imaging, than do infants with lower parenteral manganese exposure.
Design: Infants exposed to parenteral manganese were enrolled in a prospective cohort study. Infants classified as having high manganese exposure received >75% of their nutrition in the preceding 4 wk as PN. All others were classified as having low exposure. Daily parenteral and enteral manganese intakes were calculated. Whole-blood manganese was measured by high-resolution inductively coupled plasma mass spectrometry. Brain MR relaxometry was interpreted by a masked reviewer. Linear regression models, adjusted for gestational age (GA) at birth, estimated the association of relaxometry indexes with total and parenteral manganese exposures.
Results: Seventy-three infants were enrolled. High-quality MR images were available for 58 infants, 39 with high and 19 with low manganese exposure. Four infants with a high exposure had blood manganese concentrations >30 μg/L. After controlling for GA, higher parenteral and total manganese intakes were associated with a lower T1R (P = 0.01) in the globus pallidus and putamen but were not associated with whole-blood manganese (range: 3.6–56.6 μg/L). Elevated conjugated bilirubin magnified the association between parenteral manganese and decreasing T1R.
Conclusion: A short T1R for GA identifies infants at risk of increased brain manganese deposition associated with PN solutions commonly used to nourish critically ill infants. These trials were registered at clinicaltrials.gov as NCT00392977 and NCT00392730.
Keywords: infants, manganese, neuroimaging, parenteral nutrition, trace metals
INTRODUCTION
Manganese is an essential metal required for normal growth and development. Excessive manganese exposure in adults results in a constellation of psychological and neurological symptoms resembling Parkinson disease, with destructive lesions in the basal ganglia (1, 2). However, little is known about how excessive manganese exposure affects the brain and development of critically ill infants.
The manganese concentration in human milk is low relative to cow milk–based and soy-based infant formulas (Table 1). Adaptive changes to a high dietary manganese intake reduce gastrointestinal absorption, enhance liver metabolism, and increase biliary excretion of manganese to maintain normal serum concentrations (3–7). These mechanisms are effective, even in preterm infants, over a wide range of enteral manganese intakes (8).
TABLE 1.
Manganese content of enteral and parenteral diets for the infants1
| Mn content, μg/L | Mn intake,2 μg · kg−1 · d−1 | Absorbed, % | Systemic Mn uptake,2 μg · kg−1 · d−1 | |
| Human milk | 3–103 | 0.45–1.5 | 84 | 0.04–0.12 |
| Bovine milk-based formula | 30–503 | 4.5–7.5 | 24 | 0.09–0.15 |
| Soy formula | ∼180–3003 | 27–45 | <14 | 0.3–0.45 |
| PN (absent TES) | 7.6 (6.9–11.9)5 | ∼1.0 | 100 | ∼1.0 |
| Multitrace-4 neonatal | 2500 | 7.56 | 100 | 7.5 |
| TES-containing PN | 60.0 (38.4–77.7) | ∼8.5 | 100 | 8.5 |
PN, parenteral nutrition; TES, trace element supplement.
Based on intake of 150 mL · kg−1 · d−1.
Based on Lönnerdal et al. (6).
Based on Davidsson et al. (7).
Median; IQR in parentheses (all such values). Values based on original data obtained by high-resolution–inductively coupled plasma mass spectrometry.
Based on recommended addition of 0.2 mL Multitrace-4 neonatal to every 100 mL of PN solution.
Parenteral nutrition (PN)15 solutions are routinely used in critically ill infants and preterm infants who cannot tolerate sufficient enteral nutrition. PN solutions may contribute to excessive manganese exposure because intravenous administration of manganese-containing solutions circumvents normal gastrointestinal control mechanisms. Standard PN solutions contain manganese as a contaminant (8, 9). Furthermore, routine addition of a multiple trace element supplement (TES), considered standard of care, dramatically increases the manganese content of PN solutions. The total manganese delivered in trace element–supplemented PN may be ≥100 times the daily manganese that would be absorbed by an infant receiving a human milk diet (11). Hepatic dysfunction and cholestasis, common complications of prolonged PN in infants, may further magnify the risks of excessive manganese exposure and its potential neurotoxicity as ≥90% of manganese is eliminated in bile (12, 13).
Manganese is paramagnetic, appearing as a hyperintense signal in the basal ganglia on T1-weighted magnetic resonance (MR) images of humans and animals with environmental or experimental manganese poisoning (14–16). Other metals, such as copper and chromium, do not manifest this appearance, whereas iron predominantly shortens T2 relaxation time (T2R) (17). Numerous case reports document altered T1-weighted signals in older children and adults exposed to prolonged PN (18–20), but prospective neuroimaging studies in infants have not been reported.
We conducted a prospective study to test the hypothesis that infants with greater parenteral manganese exposure have greater brain manganese deposition, as measured by shorter T1 relaxation times (T1Rs) in the basal ganglia, than do infants with lower parenteral manganese exposure. Secondary goals were to examine the associations between decreasing T1R and whole-blood manganese concentrations, enteral manganese exposure, and conjugated bilirubin concentrations at the time of neuroimaging.
METHODS
We conducted a prospective observational study of infants hospitalized in the neonatal intensive care unit (NICU) at the Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, Tennessee, between October 2006 and September 2010. Subjects in the high-exposure group met the following inclusion criteria: 1) postnatal age >30 d, 2) received >75% of their nutrition as PN in the preceding 4 wk, 3) deemed clinically stable to undergo MRI. Study participants in the low-exposure group were recruited from the Vanderbilt NICU (n = 26) or the pediatric neuroimaging department (n = 3) and had a clinical indication for MRI. Patients in the low-exposure group received <14 d of PN in total—a duration corresponding to the achievement of full enteral feeds applying the NICU feeding protocols for preterm infants—and they had been off PN for ≥2 d at the time of MRI. Exclusion criteria for both groups were diagnoses of severe hypoxic ischemic encephalopathy, congenital brain malformations, or the expectation that the infant would not survive to hospital discharge. An additional exclusion criterion in the low-exposure group only was the presence of liver disease as determined by review of the medical record and laboratory values of liver function tests for patients in the NICU and by parental report on a structured questionnaire for outpatients.
Daily enteral, parenteral, and total manganese intakes from birth and in the 4 wk before MRI were calculated for all hospitalized study subjects by using medical and PN pharmacy records. The daily quantity and type of infant formula or human milk, nutritional supplements, and the volume and content of PN received daily by each patient were entered into an electronic database developed specifically for this study. For each type of nutrition, the manganese content was ascertained from the manufacturer, the PN pharmacy, or published data (for human milk), and an electronic calculator computed the enteral, parenteral, and total manganese actually received based on intake volumes and the specific manganese content of all nutritional components (Table 1).
For outpatients, a structured parent questionnaire ascertained the infant’s gestational age (GA) at birth; chronological age; overall health, including evidence of liver disease; history of jaundice beyond the first week of life; nutritional history, including current diet; and indication for MRI. All infants in the high-exposure group had a standardized neurological examination before discharge (21, 22).
Ethical approval for this research was obtained from the Vanderbilt Institutional Review Board (protocol nos. 060058 and 040881). Parents of all participating infants provided written informed consent. The procedures followed were in accordance with the ethical standards of the institutional committee on human experimentation.
Image acquisition and analysis
Brain manganese deposition was assessed by MR relaxometry, as previously described (23). Briefly, infants were placed in an MRI-compatible papoose (Universal Medical), and T1- and T2-weighted images were obtained on a Philips Achieva 1.5T MRI scanner. Infants were swaddled and fed before MRI when possible. Most were scanned without sedation, but those who were fussy received oral chloral hydrate. None of the infants were intubated or given narcotics for the purposes of the scan. T2 images served as an internal control because manganese accumulation substantially shortens T1R but has a minimal effect on T2R (17). All brain images were obtained by using the same MRI instrument throughout the course of the study. Technical details of the imaging protocols developed for this study to measure T1R and T2R are described in the article by Maitre et al. (23).
Globus pallidus (GP) T2R was calculated for each image voxel, assuming monoexponential signal decay. GP T1R was calculated by using a 2-parameter fit for T1 and equilibrium magnetization. Complex image data were phase-corrected by using the scan with the shortest inversion delay as a phase reference. The signal amplitude was then fit to a standard inversion recovery function. All calculations were performed by using MATLAB (The Mathworks) programming language.
Maps of T1 and T2 were aligned in a common space to improve the accuracy of comparisons across subjects; one subject was chosen to define a reference set of brain images. Remaining T1 and T2 data for this subject were discarded to avoid bias in the final results. The T1 and T2 maps for other subjects were spatially registered to this reference by using linear transformations and maximizing mutual information.
Regions of interest in both hemispheres of the reference brain image set were defined in the GP internus and putamen—2 distinct regions of the basal ganglia known to accumulate manganese on excessive exposure in adults, older children, and experimental animals (10). The centrum semiovale served as a control region of interest, because manganese accumulation has not been reported in this white matter region of the brain. Mean T1 and T2 values were calculated for each region and subject as previously described (23).
MRIs were performed as soon as possible after the enrolled infants were no longer receiving respiratory support (a requirement of the Vanderbilt Institutional Review Board). In infants with evidence of increased deposition of brain manganese (T1R in the GP >2 SDs shorter than the mean T1R of a control group of NICU infants enrolled in a pilot study), manganese supplementation from TES was withheld for those on PN by adding other trace elements (copper, chromium, zinc, selenium) individually to achieve the same dosing as would have been administered with the addition of routine TES.
Whole-blood manganese and conjugated bilirubin measurements
In the high-exposure group, whole blood and samples of PN solutions were obtained within 48 h of MRI for measurement of manganese by high-resolution inductively coupled plasma mass spectrometry, as previously described (24). Blood samples for measurement of manganese were drawn at the time of the next clinically indicated blood draw after MRI. Total and conjugated bilirubin were measured in the Vanderbilt clinical chemistry laboratory by using a standard diazonium salt reaction.
Statistics
The study sample size was based on the variability in absolute GP T1 and T2 values among 10 infants enrolled in a pilot study between November 2004 and January 2006. All but 3 of these infants (who only had high-quality axial images) were later included in the analyses. Differences in demographic variables by high compared with low manganese exposure were tested by using Wilcoxon’s rank-sum (continuous variables) and Pearson's chi-square (categorical variables) tests. Separate linear regression models estimated the association of T1Rs or T2Rs (outcomes) with total and parenteral manganese exposure (in μg) from birth and in the 28 d before MRI. Exposures were included as continuous predictors, and each model contained only one exposure type (e.g., total, parenteral or whole blood manganese) due to co-linearity. We fit unadjusted models and models that adjusted for GA at birth to evaluate confounding. Results were reported as the expected change in each outcome per 100-unit increase in exposure with a corresponding 95% CI. A CI that does not cross 0 is synonymous with P < 0.05 (25). All analyses were conducted by using R statistical software (version 2.13.2).
RESULTS
Study population characteristics
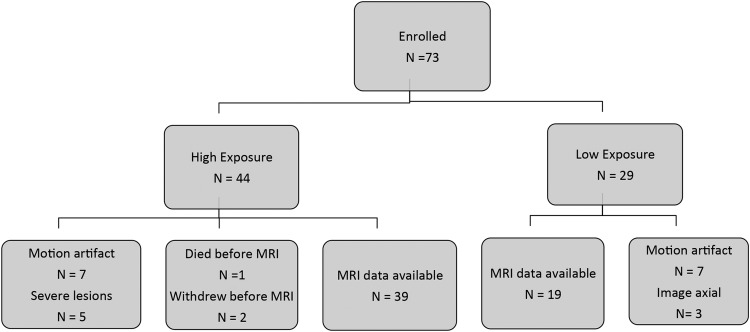
Of the 73 subjects enrolled in the study, 2 were withdrawn by their parents and 1 died before MRI, which left 70 available for MRI. Of these, 58 subjects (83%; n = 39 and 19 in the high- and low-exposure groups, respectively) had sufficiently high-quality MRI data to be included in the analysis (Figure 1). Poor quality was due primarily to motion artifact in unsedated neonates. Postmenstrual age (GA plus chronological age) at the time of MRI was similar in the 2 groups, although median GA at birth was lower in the high-exposure group (27 compared with 37 wk) (Table 2). Per the study design, days of PN at MRI were greater in the high-exposure than in the low-exposure group. In the low-exposure group, 10 of 19 subjects had received small amounts of TPN (Table 2); the median time between last PN and MRI for these infants was 6.5 d (IQR: 1.75–10.75). In the high-exposure group, 13 of 39 infants were still receiving TPN. Likewise, per the study design, total manganese intake from birth and in the 28 d before the MRI was greater in the high-exposure group, primarily due to the contribution of parenteral manganese (Table 2).
FIGURE 1 .
Flow diagram of study subject enrollment.
TABLE 2.
Study group characteristics and manganese intake1
| High exposure (n = 39) | Low exposure(n = 19) | |
| GA, wk | 27 (25–35)2 | 37 (35–39)3 |
| Postmenstrual age at MRI, wk | 40 (38–43) | 39 (37–43) |
| Male sex, % | 58 | 55 |
| Total time on PN before MRI, d | 48 (34–62) | 2 (0–12)3 |
| Total manganese intake from birth to MRI, μg | 853 (669–1397) | 96 (12–584)3 |
| Total manganese intake in 28 d before MRI, μg | 595 (329–746) | 96 (9–426)3 |
| Parenteral manganese from birth to MRI, μg | 415 (312–760) | 5 (0–101)3 |
| Parenteral manganese in 28 d before MRI, μg | 187 (102–407) | 0 (0–67)3 |
GA, gestational age; MRI, manganese resonance imaging; PN, parenteral nutrition.
Median; 25th–75th quartiles in parentheses (all such values).
Significantly different from high exposure, P < 0.05 [Wilcoxon’s rank-sum (continuous variables) and Pearson's χ-square (categorical variables) tests].
MRI results
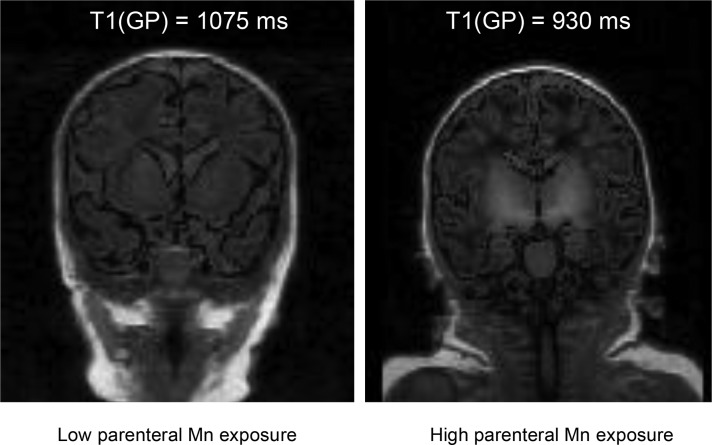
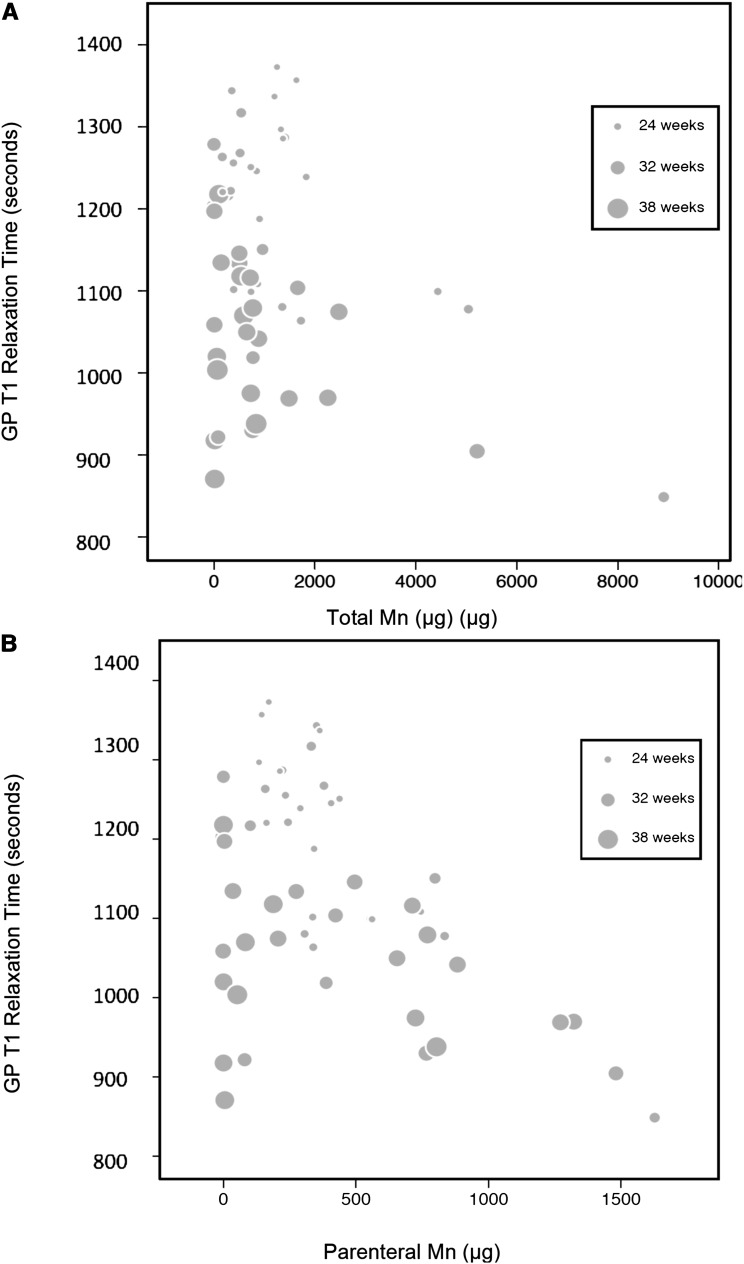
On the basis of our previous report that T1R in the GP increases with higher GA but is minimally affected by postnatal age (23), the effects of total (enteral plus parenteral) manganese intake and parenteral manganese intake on T1R were adjusted for GA. In T1-weighted brain MR images, the GP and remainder of the basal ganglia typically appeared brighter in the high-exposure group, which reflected the shortened T1R (Figure 2). The overlapping range of T1R between the low-exposure (median: 1075; IQR: 1015–1199) and high-exposure (median: 1109; IQR: 1046–1260) groups indicates that T1R for GA was normal in some subjects in the high-exposure group. To determine whether a higher manganese intake is associated with altered GP T1R, the high- and low-exposure groups were combined, and all regression models considered manganese intake as a continuum of exposure. Higher total manganese (Figure 3A) and parenteral manganese (Figure 3B) from birth until MRI were associated with lower GP T1R. Similar associations were observed between total manganese intake and parenteral manganese intake in the 28 d before MRI and T1R in the GP (data not shown). Regression models incorporating correction for GA (Table 3) showed that, for every 100-μg increase in parenteral manganese intake in the 28 d preceding the MRI, T1R decreased by 14 ms (95% CI: −27, −1.6). This relation was maintained for parenteral manganese exposure from birth to time of MRI. No significant association was observed between T1R and enteral manganese intake.
FIGURE 2.
T1-weighted MR images of 2 study infants. The GP and entire basal ganglia appear bright in the T1-weighted coronal image (right) of an infant in the high-exposure group with short T1 relaxation time (gestational age: 37 wk; postmenstrual age at time of MR imaging: 39 wk). The T1-weighted image of an infant in the low-exposure group (gestational age: 36 wk; postmenstrual age at time of MR imaging: 41 wk) appears homogeneous (left). Total parenteral manganese from birth to MR imaging was 205 μg for the infant in the left panel and was 766 μg for the infant in the right panel. GP, globus pallidus; MR, magnetic resonance.
FIGURE 3.
Relation between GP T1 relaxation time and total manganese intake (A) and parenteral manganese intake (B). After controlling for GA at birth, shorter T1 relaxation time was associated with higher total manganese (P = 0.02; A) and parenteral manganese (P = 0.02; B) from birth to magnetic resonance imaging. The size of the circles is proportional to GA at birth (A and B); the smallest circles represent a GA of 24 wk, and the largest circles represent a GA of 42 wk. The symbols in the legend show the representative symbol size for 3 specific gestational ages within the continuum. GA, gestational age; GP, globus pallidus.
TABLE 3.
Regression models: adjusted and unadjusted associations between manganese intake and globus pallidus T1R and T2R1
| n | Unadjusted regression coefficients (95% CI) | Adjusted for GA regression coefficients (95% CI) | |
| Predictor: T1R | |||
| Total manganese from birth | 57 | −2.6 (−4.2, −0.29)* | −3.3 (−4.4, −2.1)* |
| Parenteral manganese from birth | 57 | −14 (−21, −7.5)* | −14 (−20, −7.9)* |
| Total manganese 28 d before MRI | 57 | −3.1 (−7, −0.7) | −4.5 (−7, −2.1)* |
| Parenteral manganese 28 d before MRI | 57 | −24 (−36, −11)* | −14 (−27, −1.6)* |
| Whole-blood manganese | 31 | −2.4 (−7.9, 3) | −1.8 (−5.6, 2) |
| Predictor: T2R | |||
| Total manganese from birth | 54 | −1.1 (−1.5, −0.6)* | −1.6 (−2.9, −0.2)* |
| Parenteral manganese from birth | 54 | −3 (−6.6, 0.6) | −3 (−6.6, 0.6) |
| Total manganese 28 d before MRI | 54 | −1.7 (−2.9, −0.4)* | −1.6 (−2.9, −0.2)* |
| Parenteral manganese 28 d before MRI | 54 | 2.4 (−7.1, 12) | 1.5 (−8.2, 11) |
| Whole-blood manganese | 31 | 0.1 (−2, 2.2) | 0.1 (−2, 2.1) |
The regression coefficients from multivariable regression models represent the unit decrease in globus pallidus T1R and T2R for each 100-unit increase in the predictor. *Statistically significant at P < 0.05. GA, gestational age; MRI, magnetic resonance imaging; T1R, T1 relaxation time; T2R, T2 relaxation time.
The effects of manganese intake were consistent in separate but related areas of the basal ganglia, because correlation coefficients in the GP and putamen were similar (Table 4). No association was observed between parenteral or total manganese intake and T1R in the white matter of the centrum semiovale. Parenteral, enteral, and total manganese intakes had weak or no associations with T2R in the GP and the putamen, which supports a T1-selective manganese paramagnetic effect.
TABLE 4.
Regression models: adjusted and unadjusted associations between manganese intake and putamen T1R and T2R1
| n | Unadjusted regression coefficients (95% CI) | Adjusted for GA regression coefficients (95% CI) | |
| Predictor: T1 | |||
| Total manganese from birth | 57 | 3.3 (−4.5, −2.2) | −3.9 (−5, −2.9)* |
| Parenteral manganese from birth | 57 | −17 (−23, −11) | −17 (−22, −12)* |
| Total manganese 28 d before MRI | 57 | −6.5 (−10, −2.6) | −7.7 (−10, −5)* |
| Parenteral manganese 28 d before MRI | 57 | −26 (−37, −15) | −19 (−30, −7.1)* |
| Blood manganese | 31 | −1.6 (−7.6, 4.3) | −1.1 (−6, 3.9) |
| Predictor: T2 | |||
| Total manganese from birth | 54 | −0.54 (−0.71, −0.37) | −0.59 (−0.74, −0.43)* |
| Parenteral manganese from birth | 54 | −2.5 (−3.3, −1.8) | −2.2 (−3.9, −0.54)* |
| Total manganese 28 d before MRI | 54 | −0.9 (−1.5, −0.28) | −0.98 (−1.5, −0.46)* |
| Parenteral manganese 28 d before MRI | 54 | −2.8 (−4.3, −1.3) | 1.5 (−8.2, 11) |
| Blood manganese | 31 | 0.22 (−0.8, 1.2) | 0.28 (−0.61, 1.2) |
The regression coefficients from multivariable regression models represent the unit decrease in putamen T1R and T2R for each 100-unit increase in the predictor. *Statistically significant at P < 0.05. GA, gestational age; T1R, T1 relaxation time; T2R, T2 relaxation time.
Of the 39 infants in the high-exposure group, 6 had neuroimaging evidence of brain injury (2 with periventricular leukomalacia, 2 with cerebellar hemorrhage, and 2 with periventricular hemorrhagic infarction). Only one infant in the high-exposure group had a severely abnormal neurological examination at hospital discharge, as defined by standardized testing (22). This preterm infant with short bowel syndrome had a short GP T1R in the context of an otherwise normal brain on MRI.
Whole-blood manganese and conjugated bilirubin concentrations
The median whole-blood manganese concentration measured in 31 of the 39 subjects in the high-exposure group was 13.2 (IQR: 9.7–17.0; normal range: 8–12) μg/L; 4 patients had concentrations >30 μg/L. No significant associations were observed between whole-blood manganese concentrations and T1R or T2R in any studied brain region of interest.
Manganese concentrations in PN solutions containing TES (median: 60.0 μg/L; IQR: 38.4–77.7 μg/L) were on average 8-fold higher than those in PN solutions without additional trace elements (median: 7.6 μg/L; IQR: 6.9–11.9 μg/L; P < 0.001) (Table 1).
In the high-exposure group, the median conjugated bilirubin concentration at the time of MRI was 2.7 (IQR: 1.6–4.0) mg/dL. The normal laboratory range for conjugated bilirubin in newborns is <2 mg/dL. Higher conjugated bilirubin concentrations at the time of MRI magnified the effects of parenteral manganese exposure such that every 100-μg increase in parenteral manganese corresponded to a 17-ms decrease in T1R (95% CI: −23, −11). Conjugated bilirubin did not alter the relations between total manganese or enteral manganese exposure and T1R to the same extent.
DISCUSSION
We show in this prospective study that infants in the NICU receiving standard-of-care PN support have evidence of manganese accumulation in the basal ganglia that is related to their intravenous manganese intake. Similar radiographic findings have been reported in adults and older children on long-term PN, with serious neurological consequences (26). In case reports of older children on PN, neurological symptoms associated with deposition of manganese in the basal ganglia include movement disorders (27) and seizures (19), some of which resolved after discontinuation of manganese supplementation.
Standard PN administered to pediatric patients is prepared with a commercial TES containing fixed ratios of zinc, copper, chromium, and manganese with or without selenium. For an infant receiving 150 mL · kg−1 · d−1 (typical daily volume) supplemented PN, the metal content of TES that provides the recommended daily dose of zinc results in delivery of 7–8 μg Mn · kg−1 · d−1. Intravenous exposure poses a higher risk of potential manganese toxicity because the bioavailability of manganese in parenteral fluids is higher than that after oral exposure (∼100% and 5–8%, respectively) (7, 28). Manganese intoxication has been associated with PN solutions providing ≥0.1 mg Mn/d, or ∼1.5–2.0 μg Mn · kg−1 · d−1 for an average adult (29–31). The 2004 American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Special Report and the 2012 A.S.P.E.N. position paper recommend 1 μg Mn · kg−1 · d−1 for preterm neonates weighing <3 kg and term neonates weighing 3–10 kg (32, 33). However, these recommendations are not evidence based. The presence of manganese as a contaminant of other PN components and the bioavailability of parenteral manganese may obviate the need for additional parenteral manganese supplements, even in infants with normal hepatobiliary function. The A.S.P.E.N recommendation that TES be withheld or reduced in infants with evidence of cholestasis (32, 33) is sound and should be uniformly followed.
Recent shortages of TES have appropriately reignited concerns about zinc deficiency in parenterally fed infants (34). A similar concern about manganese deficiency is not justified, because manganese contamination occurs through other components of the PN, particularly KPO4 and MgSO4 (10), and manganese deficiency has not been reported during long-term PN in humans who do not receive supplemental manganese. As in prior studies (8, 9), we found that PN solutions without TES contain ∼7 μg Mn/L as a contaminant (Table 1)—an amount sufficient to meet the daily manganese requirements of parenterally fed infants, without need for additional supplementation.
Our study also supports the limited value of blood manganese measurements as a screening tool for excessive manganese exposure. We observed no correlation between whole-blood manganese concentration and parenteral exposure to manganese or brain manganese deposition assessed by T1R. Blood and plasma manganese concentrations are often less reliable biomarkers of manganese tissue concentrations than are red blood cell manganese concentrations or MRI evidence of manganese tissue deposition (35). In our study, calculated cumulative parenteral manganese exposure correlated with T1R, which suggests that T1-weighted MRI may serve as an alternative screening approach for excessive tissue manganese in patients exposed to long-term PN. PN calculators integrated into computerized physician order entry systems have been successful in preventing common PN ordering errors (36); they may provide a less costly safeguard against excessive manganese exposure in NICU patients or others dependent on prolonged PN.
Young children with cholestasis are at the highest risk of developing increased serum or tissue manganese concentrations from prolonged PN (9). We found that higher conjugated bilirubin enhanced the association between higher parenteral manganese exposure and lower GP T1R. Thus, in infants as in adults, hepatic metabolism and biliary excretion appear to influence the brain accumulation of manganese. Per NICU policy, infants in this study with conjugated bilirubin concentrations >2 mg/dL received manganese-containing TES in their PN twice weekly rather than daily. Nonetheless, some study infants had evidence of elevated brain manganese. Therefore, use of a single TES product with fixed ratios of multiple components cannot achieve recommended doses of zinc and other metals without the risk that some infants will receive excessive parenteral manganese. A more flexible and individualized approach to TES may be needed for NICU infants to avoid excessive manganese brain deposition and potential associated neurotoxicity.
The effect of total manganese on T1R was considerably smaller than that of parenteral manganese, which suggests that our observations were driven by parenteral rather than enteral manganese intake. In contrast, the effects of total and parenteral manganese on T2R in the GP and putamen were inconsistent, and, even when statistically significant associations were observed, the magnitude of the effect was small. Because manganese has minimal effects on T2R, other paramagnetic metals—such as iron—could be driving the effect on T2R by interacting or competing with manganese for transport or cellular uptake (17). The relations between manganese and other divalent metals on MR relaxation times in biological fluids in vitro is difficult to model in vivo because of the complexity of biological fluids (37) and was a limitation of this study.
Our sample size limited the number of variables we could analyze, but the large effects of parenteral manganese exposure on T1R in the GP and putamen likely occur in other areas of the basal ganglia critical to neurodevelopment, such as the caudate nucleus. Furthermore, our study was unable to document functional consequences of excess brain manganese deposition due to the relatively small sample size and numerous confounding factors that affect neurodevelopmental outcomes. Preterm infants, who have an immature and more permeable blood-brain barrier and cortical dysmaturity, may have an enhanced risk of manganese-induced neurotoxicity. Only one infant had a severely abnormal neurological finding in the absence of injury on neuroimaging at the time of hospital discharge; this infant had an increased accumulation of manganese, as evidenced by decreased GP T1R. However, we cannot attribute the cause of this infant’s abnormal neurological examination results to manganese, especially because the length of exposure to manganese in our study population was far shorter than in prior published reports.
In adults, the manganese exposure required to produce altered signal intensities on MRI is lower than the threshold necessary to cause overt clinical signs of manganism (38). Manganese exposures causing overt symptoms can lead to selective γ-aminobutyric acid minergic and dopaminergic neuronal loss in the GP (10). Acute manifestations of manganese poisoning in adults with Parkinson-like symptoms occur after a 60–80% loss of dopamine-producing neurons and include cognitive and emotional disturbances, mood changes, compulsive behaviors, loss of fine motor coordination, and intellectual deficits. How such injury would present clinically in an infant is uncertain. Exposure to manganese and deposition in the GP and other basal ganglia nuclei in early life may result in depleted dopaminergic neuronal populations, below the threshold needed to acutely alter normal function. However, in the face of expected neuronal attrition associated with aging, this subclinical exposure in infancy could lead to early-onset Parkinson disease in genetically susceptible individuals.
In conclusion, T1-weighted MRI in infants who received routine trace element–supplemented PN showed increased deposition of manganese in the basal ganglia, correlated with parenteral manganese exposure. A short T1R for GA in the basal ganglia, but not whole-blood manganese concentrations, identifies infants at risk of increased brain manganese accumulation. The use of a standard TES with fixed ratios of multiple components limits the flexibility to allow adjustment of manganese intake under various clinical conditions, such as cholestasis or prematurity. Our study highlights the need for new guidelines and practice safeguards to protect hospitalized neonates from excess manganese exposure.
Acknowledgments
We thank Donna K Daily for her invaluable contributions to patient follow-up and neurological testing.
The authors’ responsibilities were as follows—JLA: conceptualized and designed the research, provided study oversight, conducted the research, wrote the manuscript, and had primary responsibility for the final content; AA: designed the imaging data-acquisition protocols, algorithms for imaging data analysis and carried them out, and participated in the data analysis; JCS: analyzed the data and performed the statistical analysis and reviewed and revised the manuscript; MA and HMF: contributed to the initial study design and literature review and to the data collection and the protocols of the pilot study; SS, AB, and AM: coordinated and participated in patient enrollment, data collection, and sample collection in the NICU and the follow-up clinic, interpreted the results, and coordinated the MRI studies; NLM: conceptualized the design of data collection, designed the algorithms for imaging data analysis, designed the analysis, and participated in the draft of the initial manuscript and all subsequent versions; and AA, JCS, MA, HMF, SS, AB, AM, and NLM: approved the final manuscript. No funding agency had a role in the design, implementation, analysis, or interpretation of the data. The authors had no potential conflicts of interest to report.
Footnotes
Abbreviations used: GA, gestational age; GP, globus pallidus; MR, magnetic resonance; NICU, neonatal intensive care unit; PN, parenteral nutrition; T1R, T1 relaxation time; T2R, T2 relaxation time; TES, trace element supplement.
REFERENCES
- 1.Dietz MC, Ihrig A, Wrazidlo W, Bader M, Jansen O, Triebig G. Results of magnetic resonance imaging in long-term manganese dioxide-exposed workers. Environ Res 2001;85:37–40. [DOI] [PubMed] [Google Scholar]
- 2.Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS. Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurology 2001;56:8–13. [DOI] [PubMed] [Google Scholar]
- 3.Britton AA, Cotzias GC. Dependence of manganese turnover on intake. Am J Physiol 1966;211:203–6. [DOI] [PubMed] [Google Scholar]
- 4.Finley JW, Davis CD. Manganese deficiency and toxicity: are high or low dietary amounts of manganese cause for concern? Biofactors 1999;10:15–24. [DOI] [PubMed] [Google Scholar]
- 5.Dorman DC, Struve MF, James RA, McManus BE, Marshall MW, Wong BA. Influence of dietary manganese on the pharmacokinetics of inhaled manganese sulfate in male CD rats. Toxicol Sci 2001;60:242–51. [DOI] [PubMed] [Google Scholar]
- 6.Lönnerdal B. Nutritional aspects of soy formula. Acta Paediatr Suppl 1994;402:105–8. [DOI] [PubMed] [Google Scholar]
- 7.Davidsson L, Cederblad A, Lönnerdal B, Sandström B. Manganese absorption from human milk, cow’s milk, and infant formulas in humans. Am J Dis Child 1989;143:823–7. [DOI] [PubMed] [Google Scholar]
- 8.Wilson DC, Tubman TRJ, Halliday HL, McMaster D. Plasma manganese levels in the very low birth weight infant are high in early life. Biol Neonate 1992;61:42–6. [DOI] [PubMed] [Google Scholar]
- 9.Hambidge KM, Sokol RJ, Fidanza SJ, Goodall MA. Plasma manganese concentrations in infants and children receiving parenteral nutrition. JPEN J Parenter Enteral Nutr. 1989;13:168–71. [DOI] [PubMed] [Google Scholar]
- 10.Kurkus J, Alcock NW, Shils ME. Manganese content of large-volume parenteral solutions and of nutrient additives. JPEN J Parenter Enteral Nutr. 1984;8:254–7. [DOI] [PubMed] [Google Scholar]
- 11.Aschner JL, Maitre NL. Manganese and parenteral nutrition In: LaCosta LG, Aschner M, editors. Manganese in health and disease. Cambridge (United Kingdom): Royal Society of Chemistry; 2014. [Google Scholar]
- 12.Malecki EA, Radzanowski GM, Radzanowski TJ, Gallaher DD, Greger JL. Biliary manganese excretion in conscious rats is affected by acute and chronic manganese intake but not by dietary fat. J Nutr 1996;126:489–98. [DOI] [PubMed] [Google Scholar]
- 13.Ballatori N, Miles E, Clarkson TW. Homeostatic control of manganese excretion in the neonatal rat. Am J Physiol 1987;252:R842–7. [DOI] [PubMed] [Google Scholar]
- 14.Racette BA, Aschner M, Guilarte TR, Dydak U, Criswell SR, Zheng W. Pathophysiology of manganese-associated neurotoxicity. Neurotoxicology 2012;33:881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dydak U, Jiang YM, Long LL, Zhu H, Chen J, Li WM, Edden RA, Hu S, Fu X, Long Z, et al. In vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese. Environ Health Perspect 2011;119:219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitsanakis VA, Zhang N, Anderson JG, Erikson KM, Avison MJ, Gore JC, Aschner M. Measuring brain manganese and iron accumulation in rats following 14 weeks of low-dose manganese treatment using atomic absorption spectroscopy and magnetic resonance imaging. Toxicol Sci 2008;103:116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang N, Fitsanakis VA, Erikson KM, Aschner M, Avison MJ, Gore JC. A model for the analysis of competitive relaxation effects of manganese and iron in vivo. NMR Biomed 2009;22:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdalian R, Saqui O, Fernandes G, Allard JP. Effects of manganese from a commercial multi-trace element supplement in a population sample of Canadian patients on long-term parenteral nutrition. JPEN J Parenter Enteral Nutr. 2013;37:538–43. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh C-T, Liang J-S, Peng SS-F, Lee W-T. Seizure associated with total parenteral nutrition–related hypermanganesemia. Pediatr Neurol 2007;36:181–3. [DOI] [PubMed] [Google Scholar]
- 20.Mizoguchi N, Nishimura Y, Ono H, Sakura N. Manganese elevations in blood of children with congenital portosystemic shunts. Eur J Pediatr 2001;160:247–50. [DOI] [PubMed] [Google Scholar]
- 21.Daily DK, Ellison PH. The Premie-Neuro: a clinical neurologic examination of premature infants. Neonatal Netw 2005;24:15–22. [DOI] [PubMed] [Google Scholar]
- 22.Sheridan-Pereira M, Ellison PH, Helgeson V. The construction of a scored neonatal neurological examination for assessment of neurological integrity in full-term neonates. J Dev Behav Pediatr 1991;12:25–30. [PubMed] [Google Scholar]
- 23.Maitre NL, Slaughter JC, Stark AR, Aschner JL, Anderson AW. Validation of a brain MRI relaxometry protocol to measure effects of preterm birth at a flexible postnatal age. BMC Pediatr 2014;14:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roos PM, Lierhagen S, Flaten TP, Syversen T, Vesterberg O, Nordberg M. Manganese in cerebrospinal fluid and blood plasma of patients with amyotrophic lateral sclerosis. Exp Biol Med (Maywood) 2012;237:803–10. [DOI] [PubMed] [Google Scholar]
- 25.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica 1980;48:817. [Google Scholar]
- 26.Santos D, Batoreu C, Mateus L, Marreilha Dos Santos AP, Aschner M. Manganese in human parenteral nutrition: considerations for toxicity and biomonitoring. Neurotoxicology 2014l;43:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fell JM, Reynolds AP, Meadows N, Khan K, Long SG, Quaghebeur G, Taylor WJ, Milla PJ. Manganese toxicity in children receiving long-term parenteral nutrition. Lancet 1996;347:1218–21. [DOI] [PubMed] [Google Scholar]
- 28.Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann N Y Acad Sci 2004;1012:115–28. [DOI] [PubMed] [Google Scholar]
- 29.Takagi Y, Okada A, Sando K, Wasa M, Yoshida H, Hirabuki N. Evaluation of indexes of in vivo manganese status and the optimal intravenous dose for adult patients undergoing home parenteral nutrition. Am J Clin Nutr 2002;75:112–8. [DOI] [PubMed] [Google Scholar]
- 30.Ono J, Harada K, Kodaka R, Sakurai K, Tajiri H, Takagi Y, Nagai T, Harada T, Nihei A, Okada A, et al. Manganese deposition in the brain during long-term total parenteral nutrition. JPEN J Parenter Enteral Nutr. 1995;19:310–2. [DOI] [PubMed] [Google Scholar]
- 31.Bertinet DB, Tinivella M, Balzola FA, de Francesco A, Davini O, Rizzo L, Massarenti P, Leonardi MA, Balzola F. Brain manganese deposition and blood levels in patients undergoing home parenteral nutrition. JPEN J Parenter Enteral Nutr. 2000;24:223–7. [DOI] [PubMed] [Google Scholar]
- 32.Charney PJ; American Society for Parenteral and Enteral Nutrition Aluminum Task Force. Statement on aluminum in parenteral nutrition solutions. Nutr Clin Pract 2004;19:416–7. [DOI] [PubMed] [Google Scholar]
- 33.A.S.P.E.N. Clinical Practice Committee Shortage Subcommittee. A.S.P.E.N. parenteral nutrition trace element product shortage considerations. Nutr Clin Pract 2014;29:249–51. [DOI] [PubMed] [Google Scholar]
- 34.Ruktanonchai D, Lowe M, Norton SA. Zinc deficiency-associated dermatitis in infants during a nationwide shortage of injectable zinc–Washington, DC, and Houston, Texas 2012-2013. MMWR Morb Mortal Wkly Rep 2014;63:35–7\. [PMC free article] [PubMed] [Google Scholar]
- 35.Smith D, Gwiazda R, Bowler R, Roels H, Park R, Taicher C, Lucchini R. Biomarkers of Mn exposure in humans. Am J Ind Med 2007;50:801–11. [DOI] [PubMed] [Google Scholar]
- 36.Lehmann CU, Conner KG, Cox JM. Preventing provider errors: online total parenteral nutrition calculator. Pediatrics 2004;113:748–53. [DOI] [PubMed] [Google Scholar]
- 37.Barnhart JL, Berk RN. Influence of paramagnetic ions and pH on proton NMR relaxation of biologic fluids. Invest Radiol 1986;21:132–6. [DOI] [PubMed] [Google Scholar]
- 38.Kim Y. High signal intensities on T1-weighted MRI as a biomarker of exposure to manganese. Ind Health 2004;42:111–5. [DOI] [PubMed] [Google Scholar]