Abstract
Background: Increased energy intake is consistently observed in individuals consuming sugar-sweetened beverages (SSBs), likely mainly because of an inadequate satiety response to liquid calories. However, SSBs have a high content of fructose, the consumption of which acutely fails to trigger responses in key signals involved in energy homeostasis. It is unclear whether the fructose content of SSBs contributes to the increased energy intake in individuals drinking SSBs.
Objective: We investigated whether the relative amounts of fructose and glucose in SSBs modifies ad libitum energy intake over 8 d in healthy adults without fructose malabsorption.
Design: We conducted 2 randomized, controlled, double-blind crossover studies to compare the effects of consuming 4 servings/d of a fructose-, glucose-, or aspartame-sweetened beverage (study A; n = 9) or a fructose-, glucose-, or high-fructose corn syrup (HFCS)–sweetened beverage (study B; n = 24) for 8 d on overall energy intake. SSBs were provided at 25% of estimated energy requirement, or an equivalent volume of the aspartame-sweetened beverage, and consumption was mandatory. All solid foods were provided at 125% of estimated energy requirements and were consumed ad libitum.
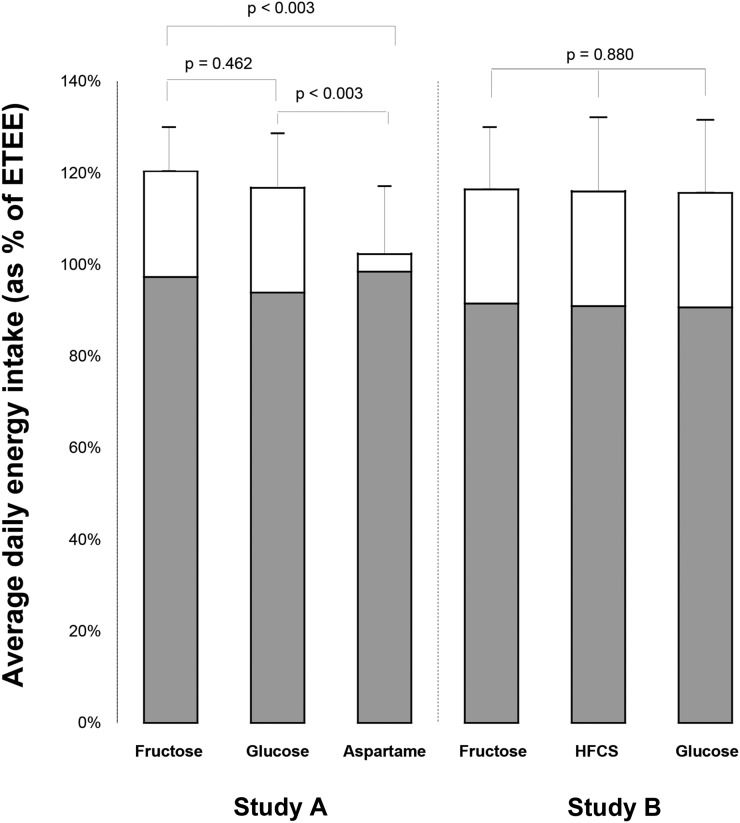
Results: In study A, ad libitum energy intake was 120% ± 10%, 117% ± 12%, and 102% ± 15% of estimated energy requirements when subjects consumed the fructose-, glucose-, and aspartame-sweetened beverages. Energy intake was significantly higher in the fructose and glucose phases than in the aspartame phase (P < 0.003 for each), with no difference between the fructose and glucose phases (P = 0.462). In study B, total energy intake during the fructose, HFCS, and glucose phases was 116% ± 14%, 116% ± 16%, and 116% ± 16% of the subject’s estimated total energy requirements (P = 0.880).
Conclusions: In healthy adults, total 8-d ad libitum energy intake was increased in individuals consuming SSBs compared with aspartame-sweetened beverages. The energy overconsumption observed in individuals consuming SSBs occurred independently of the relative amounts of fructose and glucose in the beverages. These trials were registered at clinicaltrials.gov as NCT00475475 and NCT01424306.
Keywords: sugar-sweetened beverages, energy intake, fructose, HFCS, obesity, overweight, humans
INTRODUCTION
Sugar-sweetened beverages (SSBs) are a significant source of calories in the Western diet. According to current estimates, SSBs account for 8% of daily energy intake in youth aged 2–19 y and 7% of energy intake in adults (1), the latter being equivalent to almost 40 g/d sugar. The cumulative body of evidence from randomized clinical trials and observational studies indicates that SSB consumption leads to excessive energy intake, weight gain, and an increased risk of obesity (2).
Two different mechanisms could explain why SSBs promote weight gain in healthy individuals. First, liquid calories in general (3) and SSBs in particular (4) are sensed differently by energy regulatory systems from solid foods, and humans fail to completely compensate for these calories by decreasing food intake elsewhere in the diet. Early studies on this topic have shown that including different types of caloric beverages, such as beer and soda in a meal, trigger an overall increase in energy intake within that meal (5). Less clearly established, however, is whether the type of sugar used to sweeten these beverages affects energy intake. SSBs are commonly sweetened with sucrose or high-fructose corn syrup (HFCS), both combinations of glucose and fructose in roughly equivalent amounts. Because fructose does not stimulate insulin secretion on ingestion, leptin and ghrelin responses are also blunted (6). This dampened hormonal signaling may result in a failure to fully engage the energy homeostasis system, thereby possibly leading to an increase in total calorie intake. This suggests that fructose may be less satiating than glucose, and perhaps is a major contributing factor in the weight gain–promoting effects of SSBs, considering their high fructose content. Therefore, we sought to determine whether the excess energy intake associated with SSB consumption is a result of the high fructose content of these beverages. This question may have relevance beyond SSBs, because any difference in satiety response between fructose and glucose also may extend to solid foods.
To address this question, we conducted secondary analyses on data from 2 controlled studies designed to determine 1) whether healthy humans can adequately compensate for calories contained in SSBs by decreasing energy intake from solid foods over a longer period of time than most previous studies, and 2) whether the degree of compensation for SSBs differs depending on the fructose content of these beverages. To our knowledge, these are the first well-controlled crossover interventions to provide subjects with all foods for the duration of the study and also to screen for fructose malabsorption. The latter point may be critical, considering that when fructose is consumed in a molar ratio to glucose of >1, it is malabsorbed in as much as 50% of the population (7). Without prior screening, the metabolic and energy homeostatic response to fructose when administered without glucose, as is the case in many artificial research settings, cannot be accurately determined.
METHODS
This article describes the results of 2 dietary interventions that were carried out consecutively at the University of Washington and the Fred Hutchinson Cancer Research Center in Seattle, Washington. For study A, 10 healthy normal-weight adults [BMI (in kg/m2): 20–25; age: 18–25 y] living in the Seattle area were included. Subjects were recruited by fliers and advertisements in the University of Washington campus newspaper, and screened and enrolled between September 2009 and June 2011, with the final subject completing the study in August 2011. For study B, a separate group of 25 healthy adults (BMI: 20–40; age: 18–65 y] were similarly recruited by newspaper advertisements and fliers posted in the Seattle area. Subjects were recruited into 2 categories based on BMI: normal weight (BMI: 20.0–24.9; n = 13) and overweight/obese (BMI: 25.0–39.9; n = 12). Subjects were screened and enrolled between December 2011 and December 2013, with the final subject completing the study in April 2014.
In both studies, all subjects were required to have been weight stable to within 4.5 kg (10 pounds) for the previous 6 mo and within 13.6 kg (30 pounds) of their lifetime maximum weight. Potential subjects had to be willing to consume only food and beverages provided by the research kitchen for 3 periods of 8 d each, and were required to be available for admission to the clinic on up to 6 occasions. Other exclusion criteria included smoking or the use of recreational drugs; alcohol abuse (>2 drinks/d); a history of cardiovascular disease or diabetes mellitus; the presence or history of any chronic inflammatory, autoimmune, or metabolic disease; recent (within 12 mo) pregnancy or breastfeeding; the presence of phenylketonuria, hereditary fructose intolerance, or other malabsorption syndromes; the presence or recent (within 2 mo) history of anemia; and the current or recent (within 3 mo) use of insulin, antidiabetics, β-blockers, glucocorticoids, anabolic steroids, warfarin, antibiotics, probiotics, or nonsteroidal anti-inflammatory drugs (daily use). Before being enrolled, subjects in both studies underwent telephone and in-person screening interviews to assess medical history, obtain a plasma biochemistry panel, and conduct a fructose malabsorption test (8) to assess eligibility. Briefly, during the in-person screening visit, each subject consumed a beverage sweetened with fructose in an amount equivalent to 6.25% of estimated daily calorie intake (representing 1 serving of the study beverage) as estimated by the Mifflin formula (9) and a standardized activity factor of 1.5. The hydrogen content of the exhaled breath was measured at baseline and 0, 30, 60, 90, 120, 150, and 180 min after consumption of the beverage. An increase of >20 ppm above baseline for 2 subsequent time points was considered indicative of fructose malabsorption, for which a subject was excluded from the study. Written informed consent was obtained from all subjects, and studies were approved by the institutional review boards of the University of Washington (study A) and the Fred Hutchinson Cancer Research Center (study B).
Study design and diets
The primary aim of study A was to assess whether glucose- compared with fructose-sweetened beverages differentially influenced overall energy intake compared with aspartame-sweetened beverages. Predetermined secondary endpoints included resting energy expenditure, fasting and postprandial plasma concentrations of satiety and adiposity signals (leptin, ghrelin, adiponectin, insulin, glucagon-like peptide 1, peptide YY, cholecystokinin, amylin, and oxyntomodulin), lipid and lipoprotein concentrations (total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, and free fatty acids), and plasma inflammatory mediators (C-reactive protein and IL-6). Of these, leptin, ghrelin, glucagon-like peptide 1, peptide YY, cholecystokinin, amylin, and oxyntomodulin were never analyzed because of lack of funds. No other data from this trial have been published as yet. The primary aim of study B was to assess whether glucose- compared with fructose- compared with HFCS-sweetened beverages differentially influence markers of systemic inflammation, as assessed by measuring the fasting plasma concentration of C-reactive protein and IL-6. Ad libitum energy intake was a predetermined secondary endpoint of this study, along with fasting plasma adiponectin, zonulin, and lipopolysaccharide-binding protein, urinary lactulose-to-mannitol ratio, and adipose tissue inflammation. No data from this trial have been published to date. Because data from both studies together most comprehensively address the open question of whether ad libitum energy intake is differentially affected by fructose- and glucose-sweetened beverages, data on energy intake from both studies are presented here separately from effects of the interventions on other endpoints.
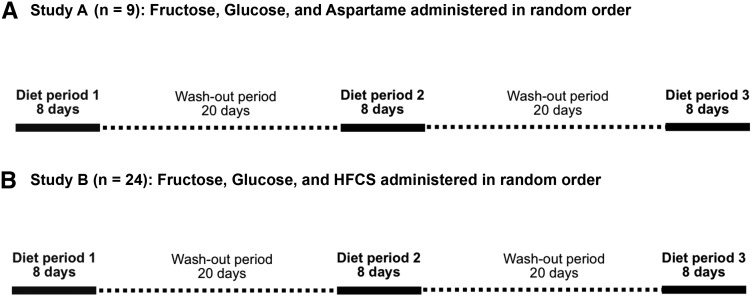
The study designs (Figure 1) were identical in both studies; however, the type of sweetener used during one of the diet periods was different. Study A was designed to investigate whether ad libitum calorie consumption was differentially affected by the intake of fructose-, glucose-, or aspartame-sweetened beverages. The study consisted of 3 separate intervention periods, each lasting 8 d, and assigned in random order. During these periods, subjects consumed 4 servings/d of a beverage sweetened with either fructose, glucose, or a low-calorie sweetener (Equal; Merisant Company) with the use of aspartame as the primary sweetening agent. The sweetener contains 4 kcal energy/g, largely from carbohydrate-based fillers used in the product; however, the amount needed to achieve a sweetness equivalent to glucose or fructose provides a much smaller amount of energy. In the fructose and glucose phases, subjects were given beverages equivalent to 25% of the subject’s estimated energy requirement, and an isovolumetric amount during the aspartame phase. Subjects were instructed to consume the entire beverage each day in 4 separate servings. In addition, subjects were provided with a standardized diet that they consumed ad libitum. The dietary intervention periods were separated by washout periods of 20 d.
FIGURE 1.
Study designs. In both study A and study B, participants completed each of 3 diet periods, during which they consumed standardized solid foods ad libitum, as well as 4 mandatory servings per day of beverages sweetened with fructose, glucose, or aspartame (study A) or fructose, glucose, or HFCS (study B). The order in which the beverages were consumed was randomized, and the intervention periods were separated by washout periods of 20 d each. All foods and beverages were provided, and ad libitum energy intake was assessed by weighing all foods that were not consumed and subtracting those calories from the number of calories in the foods provided. HFCS, high-fructose corn syrup.
Study B was identical to study A except that an HFCS (55% fructose, 41% glucose, and 4% higher saccharides) phase was included in place of the aspartame phase. In both studies, aspartame was included in the glucose and HFCS beverages to match the sweetness of the fructose beverage. This was achieved with the use of a concentration of aspartame in the aspartame-, glucose-, and HFCS-sweetened beverages that, on average, received the same sweetness rating as the fructose-sweetened beverage by a panel of volunteers. The order in which subjects consumed the beverages was randomized, and subjects, kitchen, and study staff were blinded as to which order subjects received the beverages. The randomization scheme was generated by the principal investigator with the use of block randomization, stratified for sex and adiposity group (normal weight compared with overweight/obese). A random number generator produced blocks of 6 numbers consisting of the numbers 1–6 in random orders. These blocks were then randomly grouped and ordered to generate randomization lists for the 4 strata defined by sex and adiposity group. Neither the study coordinators nor the principal investigator were aware of the randomization code, and beverage preparation was conducted by individuals who did not communicate with participants or members of the study or kitchen teams to ensure blinding was maintained until the end of the study.
All foods were prepared and packaged specifically for each subject by the University of Washington Nutrition Research Kitchen in study A and the Fred Hutchinson Cancer Research Center Human Nutrition Laboratory in study B. The solid food diet was designed as a 4-d rotating menu patterned after the average US diet (50% carbohydrate, 34% fat, and 16% protein) and was identical in all 3 phases of both studies. Subjects were provided solid food in an amount equal to 125% of their estimated daily calorie needs, as calculated by the Mifflin formula to determine their estimated resting energy expenditure, with an activity factor based on their habitual physical activity calculated from a modified Blair Physical Activity Questionnaire administered during the screening visit (10). In total, subjects were provided with 150% of their estimated energy requirements (25% mandatory consumption from the sweetened beverages and 125% ad libitum consumption from the standardized solid foods diet), except during the aspartame phase, in which they were provided with 125% of their estimated energy requirements in the form of solid foods and 4% of their estimated energy requirements from the aspartame-sweetened beverage. Subjects were asked to eat from the solid foods provided until they were comfortably full and to return any uneaten foods to kitchen staff.
On day 1 of each intervention period, subjects were given detailed instructions regarding the diet. Subjects were advised to complete a checklist each day and record the foods consumed, with an estimation of the uneaten portions to be returned. Subjects visited the research kitchens a minimum of 2 times per diet period to pick up food and drop off leftovers. Uneaten, returned foods were weighed and recorded, and compared with the subject’s daily checklist for consistency. Any discrepancy between the checklist and the kitchen weigh-back was resolved with the subject immediately. In this manner, subject compliance with the diet and protocol was confirmed. Diets were designed with the use of ProNutra software, and weigh-back of returned food occurred with the use of the ProNESSY software package for ProNutra (version 3.4.0.0; Viocare). Uneaten food portions were weighed in their original containers and the net caloric intake was determined by subtraction for each day of each diet period. The overall total caloric intake was then calculated for each diet period. Subjects were also asked to record any nonstudy foods consumed during each diet period and calories from these foods were calculated as a measure of subject compliance.
Statistical analysis
All statistical analyses were performed with SPSS for Macintosh (versions 16.0 and 20.0; IBM). Distribution of variables was analyzed by checking histograms and normal plots of the data, and normality was tested by means of Kolmogorov-Smirnov and Shapiro-Wilk tests. Because study A was designed to be a pilot study, no sample size calculation was conducted. Study B was powered to detect a clinically significant difference in markers of systemic inflammation between diet groups. The level of significance was set to P < 0.05 for all analyses. We conducted intent-to-treat and per-protocol analyses, both of which yielded identical results in all regards. In the intent-to-treat analysis, we carried the last value forward for those time points for which no data were available. We therefore report only the results of the per-protocol analyses because our primary interest is in the biological effects of the interventions.
Study A
A repeated-measures ANOVA was used to determine whether the within-subjects variable “diet” explained any variation in body weight or the mean energy intake from solid foods or total energy during the three 8-d diet periods. A post hoc paired t test with Bonferroni correction was used to compare caloric intake between the fructose and glucose diet periods, the fructose and aspartame diet periods, and the aspartame and glucose diet periods, and to compare energy intake during the fructose and glucose diet periods expressed as a percentage of energy intake during the aspartame diet period.
Study B
The primary analysis used repeated-measures ANOVA to assess whether the within-subjects variable “diet” explained any variation in mean overall energy intake, calories from solid foods, or physical activity. We also used repeated-measures ANOVA to assess whether there were any changes in body weight and waist circumference between days 1 and 9 of each diet period, and whether this change differed by diet period. The data were then stratified by adiposity category and sex in the repeated-measures ANOVA to assess whether energy intake was differentially affected by the 3 diet periods within these subgroups. We also ran repeated-measures ANOVA stratified by a 4-d menu block to determine whether energy intake differed between the 3 diet periods during the first 4 d compared with the second 4 d of each diet period. All variables as well as residuals from repeated-measures ANOVA were tested for normality by conducting a Shapiro-Wilk test and by checking histograms and normal plots. Variables were log(10)-transformed if they were not normally distributed. This was the case for body weight, waist circumference, overall energy intake, and energy intake within each 4-d diet block.
For sensitivity analyses, we further ran a series of multiple linear regression models to assess whether adjusting for diet order, physical activity (metabolic equivalent task hours per week), subject self-reported minor illness (as a categorical variable defined as either 0 = healthy or 1 = ill), adiposity [in categories defined as 0 = normal weight (BMI <25.0) and 1 = overweight or obese (BMI ≥25.0)], age, or sex affected the relation between diet period and total energy intake (dependent variable). A final model included all variables the inclusion of which changed the β coefficient describing the relation between diet period and energy intake by more than 10%. Because these analyses were conducted as sensitivity analyses, we did not adjust for multiple testing.
RESULTS
Study A
Ten subjects were enrolled in the study; however, one female subject was excluded because of lack of compliance with the study protocol. All analyses were initially conducted as intent-to-treat with the inclusion of the one subject who failed to complete the entire study. The results did not differ whether this subject was included or excluded. Therefore, the per-protocol analysis including only those participants who completed all study procedures is presented herein. Subject characteristics for the 9 subjects who completed all study procedures per protocol between December 2009 and August 2011 are presented in Table 1. Overall, total energy intake was different between the 3 diet groups (P < 0.001 for diet effect in repeated-measures ANOVA) (Table 2), with significantly greater energy intake in both the glucose and fructose phases (P < 0.003 for both sugars compared with aspartame in post hoc t tests, Figure 2). However, there was no difference in total energy intake between the fructose and glucose diet periods (P = 0.462 in post hoc t test, Figure 2). There also was no difference between groups in the amount of solid food calories consumed (P = 0.224 for diet effect in repeated-measures ANOVA) (Table 2). There were no statistically significant differences in body weight between the 3 diet periods, with median (IQR) body weights of 66.1 (59.9, 69.1) kg, 66.5 (61.7, 69.5) kg, and 66.6 (59.7, 70.1) kg at the end of the fructose, glucose, and aspartame diet periods, respectively (P = 0.243).
TABLE 1.
Characteristics of study participants at baseline1
| Study B (n = 24) |
||||
| Study A (n = 9): normal weight | Normal weight | Overweight/obese | All | |
| F/M, n/n | 5/4 | 3/9a | 6/6a | 9/15 |
| Age, y | 21 ± 2.0 | 33 ± 11a | 39 ± 12a | 36 ± 12 |
| BMI, kg/m2 | 22.7 ± 1.3 | 23.7 ± 1.0 | 31.0 ± 4.3 | 27.4 ± 4.8 |
| Fasting glucose, mg/dL | 83 ± 6.9 | 87 ± 10a | 96 ± 8b | 92 ± 10 |
| Physical activity, MET2-h/wk | 44.2 ± 18.7 | 81 ± 54.5a | 56.3 ± 33.1a | 68.7 ± 45.9 |
| Estimated total calorie requirement, kcal/d | 2230 ± 398 | 2610 ± 380a | 2510 ± 370a | 2560 ± 370 |
Values are means ± SDs. For study B, values in the same row with different superscript letters are significantly different from each other, P < 0.05 (post hoc independent samples t tests). No statistical test was performed on BMI, because the adiposity categories were designed to be different.
MET, metabolic equivalent task.
TABLE 2.
Energy intake and diet composition from solid foods, sweetened beverages, and overall during each dietary period in study A1
| Fructose (n = 9) | Glucose (n = 9) | Aspartame (n = 9) | P-diet2 | |
| Solid foods (ad libitum) | ||||
| Energy intake, kcal/d | 2180 ± 520 | 2120 ± 595 | 2220 ± 637 | 0.224 |
| Energy intake, % of estimated energy requirements | 97 ± 9.5 | 94 ± 12 | 99 ± 15 | 0.213 |
| Protein, % of total energy intake | 13.4 ± 0.73a | 13.8 ± 0.83a | 16.3 ± 0.50b | <0.001 |
| Fat, % of total energy intake | 24.4 ± 2.8a | 24.2 ± 2.7a | 29.3 ± 3.2b | <0.001 |
| Carbohydrate, % of total energy intake | 42.8 ± 2.1a | 42.2 ± 2.4a | 50.4 ± 2.9b | <0.001 |
| Sweetened beverages (mandatory) | ||||
| Energy intake, kcal/d | 515 ± 92 | 509 ± 92 | 85 ± 153 | |
| Fructose, % of total energy intake | 19.3 ± 1.6 | 0 | 0 | |
| Glucose, % of total energy intake | 0 | 19.8 ± 2.1 | 0 | |
| Overall energy intake | ||||
| Energy intake, kcal/d | 2698 ± 607a | 2629 ± 682a | 2307 ± 651b | <0.001 |
| Energy intake, % of estimated energy requirement | 120 ± 9.6a | 117 ± 12a | 102 ± 15b | <0.001 |
| Energy intake, % relative to aspartame phase | 119 ± 11a | 115 ± 8.4a | 100 ± 0b | <0.001 |
Values are means ± SDs. Values in the same row with different superscript letters are significantly different from each other, P < 0.05 (post hoc paired t tests).
Reflects an overall comparison of the 3 dietary phases by repeated-measures ANOVA. No statistical tests were performed on sweetened-beverage intake, because these were designed to be different.
Calories from beverages in the aspartame phase provided by carbohydrate fillers included in the sweetener.
FIGURE 2.
Mean ± SD daily energy intake from solid foods (gray portion of the bars) and beverages (white portion of the bars), as a percentage of estimated total energy requirement during each diet period for study A (n = 9) and study B (n = 24). Energy intake from solid foods was not affected by the calorie content of the sweetened beverages or the type of sugar used to sweeten beverages in either study, which led to lower overall energy intake in participants consuming aspartame-sweetened beverages than in participants consuming fructose- or glucose-sweetened beverages in study A. No difference in total energy intake was observed when participants consumed beverages sweetened with fructose, HFCS, or glucose. P values were determined by post hoc paired t tests with Bonferroni correction for multiple testing for study A and by repeated-measures ANOVA for study B. ETEE, estimated total energy expenditure; HFCS, high-fructose corn syrup.
Study B
To meet the predetermined sample size for the primary endpoint (n = 24 to complete the study per protocol), 25 subjects were enrolled in the study. One subject was disenrolled after completion of her first diet period because of inability to complete the required clinic visits for the remaining diet periods. Analyses were initially conducted as intent-to-treat with the inclusion of all 25 subjects who initiated the study. Inclusion or exclusion of the disenrolled subject did not affect the results; therefore, only the per-protocol analysis is presented herein. Subject characteristics for the 24 participants who completed all study procedures between January 2012 and April 2014 are shown in Table 1.
Compliance with study procedures and clinic visits was very good. Only 9 of the 24 subjects consumed some form of solid food that was not provided by the research kitchen. On average, 0.08% of energy was consumed as nonstudy food items, ranging from 0.015% to 0.41% of a subject’s total calorie intake across all 3 diet periods (data not shown). There were no significant changes in body weight or waist circumference within the 8-d diet periods, and no effect from diet group on either variable (Table 3). Physical activity also was not significantly different between the 3 diet periods (P = 0.203). Energy intake was not significantly different between the 3 diet periods, whether expressed as total calorie intake or normalized to each subject’s estimated total calorie requirements (Table 4). Consistent with study A, subjects consumed on average 16% more energy than their estimated energy requirement predicted they would need (Table 4, Figure 2). There were also no differences between the diet groups in the macronutrient composition of the solid foods consumed (Table 4).
TABLE 3.
Changes in anthropometric variables and physical activity during each diet period in study B participants1
| Fructose (n = 24) | HFCS (n = 24) | Glucose (n = 24) | P-time2 | P-diet2 | P–time × diet2 | |
| Body weight, kg | 0.142 | 0.452 | ||||
| Day 1 | 78.6 (74.2, 85.9) | 79.6 (74.6, 85.4) | 79.5 (74.0, 87.3) | |||
| Day 9 | 78.0 (73.7, 86.0) | 79.5 (74.0, 86.3) | 79.3 (74.4, 86.3) | |||
| Waist circumference, cm | 0.208 | 0.346 | ||||
| Day 1 | 94.0 (87.0, 130.0) | 91.5 (85.0, 101.5) | 91.8 (86.5, 101.0) | |||
| Day 9 | 92.5 (86.3, 101.5) | 91.8 (86.0, 103.5) | 93.0 (83.8, 101.5) | |||
| Physical activity, MET-h/wk | 56.1 (19.7, 68.6) | 55.9 (23.2, 80.8) | 50.2 (25.3, 87.3) | 0.203 |
Values are medians; IQRs in parentheses. HFCS, high-fructose corn syrup; MET, metabolic equivalent task.
Reflects an overall comparison of the 3 dietary phases by repeated-measures ANOVA.
TABLE 4.
Energy intake and diet composition from solid foods, sweetened beverages, and overall during each dietary period in study B1
| Fructose (n = 24) | HFCS2 (n = 24) | Glucose (n = 24) | P3 | |
| Solid foods (ad libitum) | ||||
| Energy intake, kcal/d | 2330 ± 421 | 2310 ± 482 | 2300 ± 409 | 0.836 |
| Energy intake, % of estimated energy requirement | 92 ± 14 | 91 ± 16 | 91 ± 16 | 0.925 |
| Protein, % of total energy intake | 14.3 ± 0.64 | 14.4 ± 0.74 | 14.3 ± 0.57 | 0.838 |
| Fat, % of total energy intake | 26.7 ± 2.8 | 26.6 ± 3.2 | 26.7 ± 3.3 | 0.980 |
| Carbohydrate, % of total energy intake | 38 ± 3.1 | 38 ± 3.6 | 38 ± 2.9 | 0.699 |
| Sweetened beverages (mandatory) | ||||
| Energy intake, kcal/d | 638 ± 92 | 640 ± 91 | 641 ± 93 | |
| Fructose, % of total energy intake | 21.6 ± 2.3 | 12.1 ± 1.7 | 0 | |
| Glucose, % of total energy intake | 0 | 9.9 ± 1.4 | 22.0 ± 3.1 | |
| Overall energy intake | ||||
| Energy intake, kcal/d | 2970 ± 482 | 2950 ± 535 | 2940 ± 460 | 0.880 |
| Energy intake, % of estimated energy requirement | 116 ± 13.6 | 116 ± 16.2 | 116 ± 15.9 | 0.880 |
Values are means ± SDs over each 8-d diet period.
HFCS, high-fructose corn syrup.
Reflects an overall comparison of the 3 dietary phases by repeated-measures ANOVA. No statistical tests were performed on sweetened-beverage intake, because these were designed to be different.
Secondary analyses stratified by sex and adiposity found no differential impact of the diets on energy intake in men compared with women or in normal-weight compared with overweight/obese individuals (data not shown). We also ran sensitivity analyses excluding 6 subjects who reported a minor illness (such as a cold) during ≥1 of the diet periods, which did not change the result (data not shown). As another sensitivity analysis, we reran all analyses with the exclusion of 10 subjects whose total energy intake varied by more than 10% of their estimated energy requirement between any 2 diet periods and confirmed that the exclusion of these subjects also did not change the overall finding (data not shown).
Multiple linear regression analyses were conducted as further sensitivity analyses. These analyses confirmed that overall energy intake was not associated with the type of sugar used to sweeten the beverages, and that adjustment for diet order, physical activity, illness, adiposity, age, and sex did not materially change that relation (data not shown).
Because it may be possible that energy homeostatic compensatory mechanisms attenuate any effect of the fructose content of SSBs on energy intake within a few days, we also assessed whether energy intake differed between the 3 dietary periods just in the first 4 d of each diet period, or just in the last 4 d of each diet period. As for the entire 8-d period, we did not detect even a trend for a difference in energy intake between the 3 dietary phases within each of these 4-d periods (data not shown). Last, we tested whether there were any differences in calorie intake between the first and second 4-d diet blocks within each 8-d period. Overall energy intake was significantly greater by 87 ± 507 kcal during the first 4 d of the diet periods than in the second 4 d (P = 0.003 for the difference between the first and second 4-d block in repeated-measures ANOVA). The reduction in energy intake between the first and the second 4-d diet block was not different between the 3 dietary periods (time × diet interaction, P = 0.228). No unexpected adverse events or adverse events that were more severe than “mild” occurred in either study.
DISCUSSION
The major finding from study A was that healthy normal-weight adults consumed significantly more calories when 25% of their estimated energy requirement was provided as a glucose- or fructose-sweetened beverage compared with an isovolumetric amount of an aspartame-sweetened beverage. On average, this caloric excess was ∼17%, demonstrating that participants compensated only for approximately one-third of the calories consumed in the SSB. This finding was supported by study B in that normal and overweight/obese subjects also consistently consumed 16% more energy during each diet phase than their estimated energy requirement predicted they would need to maintain energy homeostasis. The fructose-to-glucose ratio of the beverages did not influence overall energy intake.
Our results are in line with observational studies that consistently show that consumption of SSBs promotes weight gain in children and adults (2). Although our studies did not compare liquid with solid sugar calories, our findings are also consistent with previous data demonstrating failure to fully compensate for liquid sugar calories. For example, DiMeglio and Mattes (11) conducted a crossover study in which healthy subjects consumed 450 kcal in the form of either jelly beans or soda in addition to their ad libitum habitual diet for 4 wk each. During the solid sugar phase, subjects completely compensated for the calories consumed as jelly beans by reducing their intake of solid foods. In contrast, during the soda phase, subjects failed to decrease their intake of solid foods, which remained at an amount equivalent to their habitual intake. Body weight increased significantly during the liquid calorie phase only (11).
A remaining open question was whether differing adipogenic effects are exerted by the glucose and fructose moieties that make up most caloric sweeteners. Meta-analyses showed that fructose does not differentially affect weight when isocalorically exchanged for glucose in energy-matched diets (12). Arguably more relevant, however, is whether calorically matched fructose and glucose may exert a differential effect on overall ad libitum energy intake. Our studies sought to answer this question by making the consumption of varying ratios of fructose compared with glucose mandatory while allowing subjects to eat from a standardized diet ad libitum. Stanhope et al. (13) instructed overweight subjects to consume 25% of their estimated energy intake in the form of a fructose- or glucose-sweetened beverage while also consuming their habitual diet for 10 wk. Subjects in both arms gained a similar amount of weight. This result supports our findings that fructose-sweetened beverages do not differentially affect energy intake compared with glucose-sweetened beverages. However, this study was not randomized, and our studies offer an additional level of confidence because of the strong randomized crossover design, provision of all foods for the entirety of the study periods, and the fact that we included a fructose malabsorption screening test, which we suggest is critical for true assessment of the effect of 100% fructose-sweetened SSBs on energy intake.
Our studies were specifically designed to be medium-term in length to prevent significant weight gain by subjects over the course of the intervention. This avoided confounding of the relation between sweetened beverage type and energy intake by substantial changes in adiposity during the experimental diet periods. There are a number of reasons why we may not have detected even a small increase in weight. First, the study was not designed or powered to detect changes in weight. Second, it is likely that subjects lost some body water during the study diet periods, which we frequently see in the first few days after subjects switch to a controlled study diet with lower sodium content. Thus, it is important not to interpret the lack of weight gain in these moderate-term diet periods as evidence that weight would remain stable in individuals consuming SSBs over longer periods of time.
We believe that the crossover design was a major strength of our study, because it allowed us to compare energy consumption in each person on each diet; however, the relatively short duration of each intervention period is also a possible limitation. Theoretically, it is possible that differences could become apparent if these SSBs were consumed for >8 d. However, substantial differences in key appetite- and satiety-regulating hormones, including insulin, leptin, and ghrelin, are apparent within the first day of consuming a similar amount of fructose- or glucose-sweetened beverages (6). It appears implausible that acute hormonal differences would lead to differences in ad libitum energy intake only >8 d later. Because not even the slightest trend was apparent in our studies, we are confident that the differences in the response of satiety-regulating hormones between fructose- and glucose-sweetened beverages described in previous studies do not translate into differences in energy intake. We did observe a significant decrease in the overall energy consumed during the second 4 d on the diet compared with the first 4 d. This suggests that energy homeostatic mechanisms are engaged to some degree over an 8-d period and that our methodology was sufficiently precise to detect this effect. However, the difference in energy intake during the first compared with the second 4-d period was independent of the fructose content of the beverages. Failure of the fructose content of the SSB to affect this adaptive response to caloric excess further strengthens our central conclusion. We also stratified by sex and adiposity and did not detect a difference in energy intake according to beverage type within these subgroups. Although the sample sizes in these subgroup analyses were relatively small and lack of power could be a concern, energy intake was strikingly similar across all diet phases and not even a trend toward a difference could be detected.
Another potential limitation is that the total number of calories provided to participants in study A differed across study arms, with participants receiving 150% of their estimated total energy requirement in the fructose and glucose arms (125% from solid foods and 25% from SSBs), but only 129% in the aspartame arm (125% from solid foods and 4% from the aspartame-sweetened beverage). This may have contributed to the greater total energy intake in the fructose and glucose arms. However, this design was necessary to conduct this trial in a double-blinded fashion, because providing more solid food in one arm would have unblinded participants partially. There is also a rich literature suggesting that total energy intake is affected by the total volume rather than the total energy content of the food served (14–16). We therefore felt that providing more solid (i.e., visible) food to subjects, and therefore more overall volume, would have been very likely to increase total energy intake in the aspartame arm.
Our findings are strengthened by the fact that we obtained essentially the same result in these paired studies. Another strength of our study was the provision of all foods, which allowed us to accurately assess energy intake and experimentally control other dietary factors that may affect ad libitum energy intake. However, by providing subjects with all foods, we left little opportunity for subjects to self-select food items. Because the study diet did not include highly palatable snack foods high in sugar and fat, we could not test whether the beverages tested here affect the types of solid foods participants would eat if they had the ability to choose, which in return could affect total energy intake.
In conclusion, these studies strengthen the argument that SSBs promote an increase in overall energy intake in healthy adults over the medium term, which over longer periods of time would be expected to result in weight gain. Furthermore, fructose and glucose do not differ in terms of energy intake regulation when consumed in the form of an SSB. Rather, our study shows that both glucose and fructose consumed in liquid form promote excess energy intake by failing to invoke a concomitant reduction of energy from solid foods.
Acknowledgments
We thank Pamela Yang, Michelle Wurscher, Allison Shircliff, Barbara Burke, Peggie Bates, Sara Bennett, Linda Glockling, and David Hatten.
The authors’ responsibilities were as follows—JNK: completed laboratory procedures, statistical analysis of the data, and the first draft of the manuscript; JNK and GC: were responsible for collection of the data; DKH: provided technical assistance; KLB: oversaw preparation of all study meals; CLR: provided input on study design and data analysis and interpretation; KEF-S: served as the physician of record for the study; SEH: provided statistical guidance; HSC: designed and calculated the study diets; DSW: was involved in the design of the studies as well as in data analysis and interpretation; MK: initiated the studies and had overall responsibility for the design and conduct of the studies as well as the data analyses; and all authors: contributed to the preparation of the manuscript and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
REFERENCES
- 1.Kit BK, Fakhouri TH, Park S, Nielsen SJ, Ogden CL. Trends in sugar-sweetened beverage consumption among youth and adults in the United States: 1999-2010. Am J Clin Nutr 2013;98:180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr 2013;98:1084–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf A, Bray GA, Popkin BM. A short history of beverages and how our body treats them. Obes Rev 2008;9:151–64. [DOI] [PubMed] [Google Scholar]
- 4.Pan A, Hu FB. Effects of carbohydrates on satiety: differences between liquid and solid food. Curr Opin Clin Nutr Metab Care 2011;14:385–90. [DOI] [PubMed] [Google Scholar]
- 5.Mattes RD. Dietary compensation by humans for supplemental energy provided as ethanol or carbohydrate in fluids. Physiol Behav 1996;59:179–87. [DOI] [PubMed] [Google Scholar]
- 6.Teff KL, Elliott SS, Tschop M, Kieffer TJ, Rader D, Heiman M, Townsend RR, Keim NL, D’Alessio D, Havel PJ. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab 2004;89:2963–72. [DOI] [PubMed] [Google Scholar]
- 7.Skoog SM, Bharucha AE. Dietary fructose and gastrointestinal symptoms: a review. Am J Gastroenterol 2004;99:2046–50. [DOI] [PubMed] [Google Scholar]
- 8.Keller J, Franke A, Storr M, Wiedbrauck F, Schirra J. [Clinically relevant breath tests in gastroenterological diagnostics–recommendations of the German Society for Neurogastroenterology and Motility as well as the German Society for Digestive and Metabolic Diseases] Z Gastroenterol 2005;43:1071–90 (in German). [DOI] [PubMed] [Google Scholar]
- 9.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51:241–7. [DOI] [PubMed] [Google Scholar]
- 10.Blair SN, Haskell WL, Ho P, Paffenbarger RS Jr, Vranizan KM, Farquhar JW, Wood PD. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol 1985;122:794–804. [DOI] [PubMed] [Google Scholar]
- 11.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord 2000;24:794–800. [DOI] [PubMed] [Google Scholar]
- 12.Sievenpiper JL, de Souza RJ, Mirrahimi A, Yu ME, Carleton AJ, Beyene J, Chiavaroli L, Di Buono M, Jenkins AL, Leiter LA, et al. . Effect of fructose on body weight in controlled feeding trials: A systematic review and meta-analysis. Ann Intern Med 2012;156:291–304. [DOI] [PubMed] [Google Scholar]
- 13.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. . Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolls BJ, Meengs JS, Roe LS. Variations in cereal volume affect the amount selected and eaten for breakfast. J Acad Nutr Diet 2014;114:1411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rolls BJ, Roe LS, Meengs JS. The effect of large portion sizes on energy intake is sustained for 11 days. Obesity (Silver Spring) 2007;15:1535–43. [DOI] [PubMed] [Google Scholar]
- 16.Ello-Martin JA, Ledikwe JH, Rolls BJ. The influence of food portion size and energy density on energy intake: implications for weight management. Am J Clin Nutr 2005;82:236S–41S. [DOI] [PubMed] [Google Scholar]